Abstract
Background & Aims
Steatosis in patients with nonalcoholic fatty liver disease (NAFLD) is due to an imbalance between intrahepatic triglyceride (IHTG) production and export. The purpose of this study was to evaluate TG metabolism in adipose tissue and liver in NAFLD.
Methods
Fatty acid, VLDL-TG, and VLDL-apolipoprotein B-100 (apoB100) kinetics were assessed by using stable isotope tracers in 14 nondiabetic obese subjects with NAFLD (IHTG, 22.7% ± 2.0%) and 14 nondiabetic obese subjects with normal IHTG content (IHTG, 3.4% ± 0.4%), matched on age, sex, body mass index, and percent body fat.
Results
Compared with the normal IHTG group, the NAFLD group had greater rates of palmitate release from adipose tissue into plasma (85.4 ± 6.6 and 114.1 ± 8.1 µmol/min, respectively; P = .01) and VLDL-TG secretion (11.4 ± 1.1 and 24.3 ± 3.1 µmol/min, respectively; P = .001); VLDL-apoB100 secretion rates were not different between groups. The increase in VLDL-TG secretion was primarily due to an increased contribution from “nonsystemic” fatty acids, presumably derived from lipolysis of intrahepatic and intra-abdominal fat and de novo lipogenesis. VLDL-TG secretion rate increased linearly with increasing IHTG content in subjects with normal IHTG but reached a plateau when IHTG content was ≥10% (r = 0.618, P < .001).
Conclusions
Obese persons with NAFLD have marked alterations in both adipose tissue (increased lipolytic rates) and hepatic (increased VLDL-TG secretion) TG metabolism. Fatty acids derived from nonsystemic sources are responsible for the increase in VLDL-TG secretion. However, the increase in hepatic TG export is not adequate to normalize IHTG content.
Nonalcoholic fatty liver disease (NAFLD) represents a series of liver abnormalities, beginning from simple steatosis and progressing to steatohepatitis, fibrosis, and cirrhosis.1 The mechanism(s) responsible for the accumulation and maintenance of an excessive amount of intrahepatic fat is not clear but must involve an imbalance between the intrahepatic production of triglyceride (TG) (primarily derived from plasma fatty acids delivered to the liver that are not oxidized for fuel) and the removal of intrahepatic TG (primarily exported from the liver within very low-density lipoprotein; VLDL-TG).2
Hepatic secretion of VLDL involves packaging triglycerides, cholesterol, phospholipids, and apolipoproteins into a water-soluble particle, which is secreted into the systemic circulation. Each VLDL particle contains 1 molecule of apolipoprotein B-100 (apoB100), which is required to export VLDL from the liver. The relationship between NAFLD and VLDL kinetics is not clear because of conflicting results from different studies, which have reported decreased VLDL-apoB100 secretion rates in obese subjects with NAFLD3; increased VLDL-apoB100 and VLDL-TG secretion rates in lean, overweight, and obese subjects with NAFLD plus type 2 diabetes4; and normal TG secretion rates in lean, overweight, and obese subjects with NAFLD.5 The reason for these potential discrepancies could be related to differences between the control and NAFLD groups in factors that can independently influence hepatic lipoprotein metabolism, including sex, adiposity, and type 2 diabetes.6–8
The purpose of this study was to evaluate whether adipose tissue lipolytic rates and hepatic lipoprotein kinetics are altered in nondiabetic obese subjects who have NAFLD compared with nondiabetic obese subjects who have normal intrahepatic triglyceride (IHTG) content, when both groups are matched on age, sex, body mass index (BMI), and percent body fat. We hypothesized that hepatic VLDL-TG secretion would be increased in subjects with NAFLD, primarily because of an increased flux of fatty acids derived from lipolysis of subcutaneous adipose tissue and intrahepatic and intra-abdominal fat to VLDL-TG production; however, the increase in VLDL-TG secretion may not be adequate to normalize IHTG content. Stable isotopically labeled tracer infusions, in conjunction with mathematical modeling, were used to determine plasma free fatty acid (FFA) and VLDL-TG and VLDL-apoB-100 kinetics, and proton magnetic resonance spectroscopy (MRS) was used to assess IHTG content.
Materials and Methods
Subjects
Twenty-eight obese subjects participated in this study: 14 subjects had NAFLD (IHTG > 10% of liver volume) and 14 subjects had normal intrahepatic fat content (IHTG ≤5.5% of liver volume)9 (Table 1). Groups with NAFLD and normal IHTG content were matched on age, sex, BMI, and percent body fat. All subjects completed a comprehensive medical evaluation, which included a detailed history and physical examination, routine blood tests, a 12-lead electrocardiogram, and a 2-hour oral glucose tolerance test. No subject had any history or evidence of liver disease other than NAFLD, took medications that affect lipid metabolism or cause hepatic abnormalities, consumed more than 20 g/day of alcohol, had severe hypertriglyceridemia (≥300 mg/dL), or had evidence of diabetes (plasma glucose concentration at 2 hours of the oral glucose tolerance test was 107 ± 7 mg/dL in the group with normal IHTG content and 151 ± 9 mg/dL in the group with NAFLD). In addition, no subject had evidence of other serious illnesses or organ dysfunction. All subjects were weight stable (≤2% fluctuation in body weight) and had been sedentary (exercising <1 hour per week) for at least 3 months before enrollment into the study. Subjects gave their written informed consent before participating in this study, which was approved by the Human Studies Committee and the General Clinical Research Center Advisory Committee of Washington University School of Medicine in St. Louis, MO.
Table 1.
Characteristics of Subjects With Normal Intrahepatic Triglyceride Content and Those With Nonalcoholic Fatty Liver Disease
| Normal IHTG | NAFLD | |
|---|---|---|
| Age (y) | 41 ± 3 | 45 ± 3 |
| Male/female | 3/11 | 3/11 |
| Body weight (kg) | 100 ± 3 | 102 ± 4 |
| Body mass index (kg/m2) | 35.3 ± 1.3 | 36.8 ± 1.2 |
| Body fat mass (% body weight) | 42 ± 2 | 41 ± 1 |
| Subcutaneous abdominal fat (cm3) | 3083 ± 370 | 3360 ± 303 |
| Intra-abdominal fat (cm3) | 1219 ± 263 | 2146 ± 310a |
| Intrahepatic triglyceride (%) | 3.4 ± 0.4 | 22.7 ± 2.0b |
NOTE. Values are means ± SEM.
P < .05
P < .001: Value significantly different from the corresponding value in the Normal IHTG group.
Body Composition Analyses
Body fat and fat-free mass were determined by dual-energy x-ray absorptiometry (Delphi-W densitome densitometer; Hologic, Waltham, MA).10 Intra-abdominal and abdominal subcutaneous adipose tissue volumes were quantified by magnetic resonance imaging (Siemens, Iselin, NJ; ANALYZE 7.0 software, Mayo Foundation, Rochester, MN)11; 8, 10-mm-thick slice images, obtained at the L4–L5 interspace and proximally, were analyzed for intra-abdominal and subcutaneous fat content. IHTG content was measured by using proton MRS (1.5T Siemens Magneton Vision scanner; Siemens, Erlanger, Germany), as we have previously described.12 Three 2 × 2 × 2 cm3 voxels were examined in each subject, and the values were averaged to determine IHTG content. The coefficient of variation of replicate values of the triplicate determinations for 3 voxels was 1.5%.
Isotope Infusion Study
Subjects were admitted to the inpatient unit of the General Clinical Research Center on the evening before the isotope infusion study. At 1800 hours, subjects consumed a meal containing 12 kcal per kg fat-free mass (55% of total energy as carbohydrates, 30% as fat, and 15% as protein). At 1900 hours, subjects ingested a liquid formula snack containing 250 kcal (40 g carbohydrate, 6.1 g fat, and 8.8 g protein; Ensure; Ross Laboratories, Columbus, OH) and then fasted, except for water, until the completion of the isotope infusion study the next day.
At 0500 hours the following morning, a catheter was inserted into a forearm vein to infuse stable isotopically labeled tracers. A second catheter was inserted into a contralateral hand vein, which was heated to 55°C by using a thermostatically controlled box to obtain arterialized blood samples. At 0600 hours, a bolus of [1,1,2,3,3-2H5]glycerol (75 µmol/kg body weight) dissolved in 0.9% NaCl solution was injected, and constant infusions of [2,2-2H2]palmitate (0.024 µmol/kg · min−1) bound to human albumin and [5,5,5-2H3]leucine (0.062 µmol/kg · min−1 and a 4.2 µmol/kg priming dose) dissolved in 0.9% NaCl solution were started and maintained for 12 hours. All isotopically labeled tracers were purchased from Cambridge Isotope Laboratories (Andover, MA). Subjects remained in bed during the entire duration of the isotope infusion study.
Blood samples were obtained before the start of the tracer infusions to determine plasma concentrations of substrates, insulin, and background isotopic enrichments of glycerol, palmitate, and leucine in plasma, VLDL-TG, and VLDL-apoB100. Blood samples were then taken at 5, 15, 30, 60, 90, and 120 minutes and then every hour for 10 hours after the start of the isotope infusion to determine glycerol and palmitate tracer-to-tracee ratio (TTR) in plasma and VLDL-TG and leucine TTR in plasma and VLDL-apoB100. Blood was immediately placed in chilled tubes containing EDTA to determine substrate concentrations and TTRs and in chilled tubes containing EDTA and aprotinin (Trasylol) to determine plasma insulin concentrations. Samples were placed on ice, and plasma was separated by centrifugation within 30 minutes of collection. Aliquots of plasma (2 mL) were refrigerated at 4°C for subsequent isolation of VLDL; the remaining plasma samples were stored at −80°C until final analyses were performed.
Analyses of Blood Samples
VLDL isolation from plasma
Plasma VLDL was prepared as previously described.13 Approximately 1.5 mL plasma was transferred into OptiSeal polyallomer tubes (Beckman Instruments, Palo Alto, CA), overlaid with an NaCl/EDTA solution (1.006 g/mL) and centrifuged at 100,000g for 16 hours at 10°C in an Optima LE-80K preparative ultracentrifuge equipped with a Type 50.4 Ti rotor (Beckman Instruments, Palo Alto, CA). The top layer containing VLDL was removed by tube slicing (CentriTube slicer; Beckman Instruments). ApoB100 concentration was measured immediately in a fresh aliquot of the VLDL fraction; the remaining samples were stored at −80°C until final analyses were performed.
Plasma insulin and substrate concentrations
Plasma insulin concentration was measured by radioimmunoassay (Linco Research, St. Louis, MO). Plasma glucose concentration was measured by using an automated glucose analyzer (Yellow Spring Instruments Co, Yellow Springs, OH). Plasma FFA concentrations were quantified by gas chromatography (Hewlett-Packard 5890-II; Hewlett-Packard, Palo Alto, CA) after adding heptadecanoic acid to plasma as an internal standard.14 Plasma VLDL-TG concentration was determined using a commercially available enzymatic spectrophotometric kit (Sigma Chemical Co, St. Louis, MO). VLDL-apoB100 concentration was measured using a commercially available immunoturbidimetric kit (Wako Chemicals, Richmond, VA). VLDL-TG and VLDL-apoB100 concentrations were averaged for VLDL samples collected at 0, 2, 4, 6, 8, 10, and 12 hours.
TTRs
Plasma glycerol, palmitate, and leucine TTRs were determined by electron impact ionization gas chromatography-mass spectrometry (MSD 5973; Hewlett-Packard) as previously described.13,14 The heptafluorobutyryl derivative was prepared for plasma glycerol, the t-butyldimethylsilyl derivative was prepared for plasma leucine, and plasma palmitate was analyzed as a methyl ester.15 Triglyceride in VLDL was isolated by thin-layer chromatography, and the methyl ester and heptafluorobutyryl derivatives of palmitate and glycerol in VLDL-TG, respectively, were prepared for gas chromatography-mass spectrometry analysis. ApoB100 was isolated from the VLDL fraction as previously described,13 and the N-heptafluorobutyryl n-propyl ester of leucine was formed for gas chromatography-mass spectrometry analysis.
Calculations
Insulin resistance
The computerized homeostasis model assessment (HOMA2), based on fasting plasma glucose and insulin concentrations, was used to provide an index of insulin resistance (www.OCDEM.ox.ac.uk).16 In contrast with the original HOMA version,17 the computerized HOMA2 model allows nonlinear solutions and can account for variations in hepatic and peripheral resistance in glucose effectiveness.16
Fatty Acid Kinetics
Isotopic and physiologic steady states were obtained between 60 and 120 minutes of tracer infusion. Therefore, palmitate rate of appearance (Ra) in plasma was calculated using Steele’s equation for steady-state conditions18 by dividing the tracer infusion rate by the average plasma palmitate TTR between 60 minutes and 120 minutes. Total FFA Ra was determined by dividing the palmitate Ra by the proportional contribution of palmitate to total FFA concentration in plasma.19 The rate of FFA clearance from plasma was calculated by dividing FFA rate of disappearance (which equals FFA Ra during steady state conditions) by plasma FFA concentration.19 The proportion of total FFA released into plasma that was incorporated into VLDL-TG was calculated by dividing the rate of secretion of fatty acids within VLDL-TG that were derived from systemic plasma FFA by whole-body FFA Ra.
VLDL-TG and VLDL-apoB100 kinetics
The fractional turnover rate (FTR) of VLDL-TG (in pools/h) was calculated by fitting the glycerol TTR in plasma and VLDL-TG to a multicompartmental model as previously described.20 The FTR represents the fraction of the VLDL-TG pool that enters/leaves the pool per unit of time. The rate of VLDL-TG secretion into plasma (in µmol/min), which represents the total amount of VLDL-TG secreted by the liver, was calculated by multiplying the FTR of VLDL-TG (in pools/min) by the steady-state pool size of VLDL-TG (in µmol); the latter was calculated as VLDL-TG concentration (in µmol/L) times plasma volume (0.055 L/kg fat-free mass).21 The proportion of fatty acids within VLDL-TG derived from systemic plasma FFA (derived primarily from lipolysis of subcutaneous adipose tissue TG) and nonsystemic fatty acids (derived from lipolysis of intrahepatic and intraperitoneal TG, hepatic lipolysis of circulating TG, and de novo hepatic fatty acid synthesis) was calculated by accounting for isotopic dilution between plasma and VLDL-TG palmitate using a multicompartmental model.13,21
The FTR of VLDL-apoB100 (in pools/h) was determined by fitting the TTR of leucine in plasma and in VLDL-apoB100 to a multicompartmental model, as previously described.13,21 The rate of VLDL-apoB100 secretion into plasma (in nmol/min) was calculated by multiplying the FTR of VLDL-apoB100 (in pools/min) by the steady-state pool size of VLDL-apoB100 (in nmol). The molar ratio of VLDL-TG to VLDL-apoB100 secretion rates, which provides an index of the average TG content and size of newly secreted VLDL particles, was calculated by dividing VLDL-TG secretion rate by VLDL-apoB100 secretion rate.22
Statistical Analysis
All data sets were normally distributed according to Kolmogorov–Smirnov. A Student t test for unpaired samples was used to evaluate the statistical significance of differences between subjects who had normal IHTG content and those with NAFLD. The relationship between variables of interest was assessed by using Pearson correlation and regression analysis. A P value≤.05 was considered statistically significant. Results are presented as means ± SEM.
Results
Demographics and Body Composition
Subjects who had NAFLD and those with normal IHTG content were matched on age, sex, body weight, BMI, and percent body fat (Table 1). The amount of IHTG ranged from 1.3% to 5.4% in the normal IHTG group and from 13.9% to 22.7% in the NAFLD group. Abdominal subcutaneous fat volume was similar between groups, whereas intra-abdominal fat volume was ~75% greater in subjects who had NAFLD than those with normal IHTG (Table 1).
Metabolic and Biochemical Variables
Plasma glucose concentration was not different between groups, but plasma insulin concentration and the HOMA2 index of insulin resistance were greater in subjects with NAFLD than in those with normal IHTG content (Table 2). Plasma FFA and TG concentrations were also greater in subjects with NAFLD, whereas LDL-cholesterol, HDL-cholesterol, and VLDL-apoB100 concentrations did not differ between the 2 groups. Plasma alanine aminotransferase concentration was higher in subjects with NAFLD than in subjects with normal IHTG content.
Table 2.
Metabolic Variables of Subjects With Normal Intrahepatic Triglyceride Content and Those With Nonalcoholic Fatty Liver Disease
| Normal IHTG | NAFLD | |
|---|---|---|
| Glucose (mg/dL) | 95.0 ± 1.6 | 97.8 ± 2.2 |
| Insulin (µU/mL) | 11.3 ± 1.3 | 20.7 ± 2.8a |
| HOMA2-IR | 1.5 ± 0.2 | 2.7 ± 0.3a |
| Free fatty acids (mmol/L) | 0.396 ± 0.035 | 0.510 ± 0.028b |
| LDL cholesterol (mg/dL) | 97 ± 6 | 92 ± 9 |
| HDL cholesterol (mg/dL) | 49 ± 4 | 48 ± 5 |
| Triglyceride (mmol/L) | 1.17 ± 0.10 | 1.90 ± 0.22a |
| VLDL triglyceride (mmol/L) | 0.46 ± 0.07 | 0.85 ± 0.15b |
| VLDL apolipoprotein B-100 (mg/L) | 36 ± 5 | 52 ± 8 |
| Total apolipoprotein B-100 (mg/L) | 677 ± 36 | 736 ± 55 |
| Alanine aminotransferase (IU/L) | 29 ± 3 | 43 ± 6a |
NOTE. Values are means ± SEM.
HOMA2-IR, computerized homeostasis model assessment of insulin resistance.
P < .01
P< .05: Value significantly different from the corresponding value in the Normal IHTG group.
Fatty Acid and Lipoprotein Kinetics
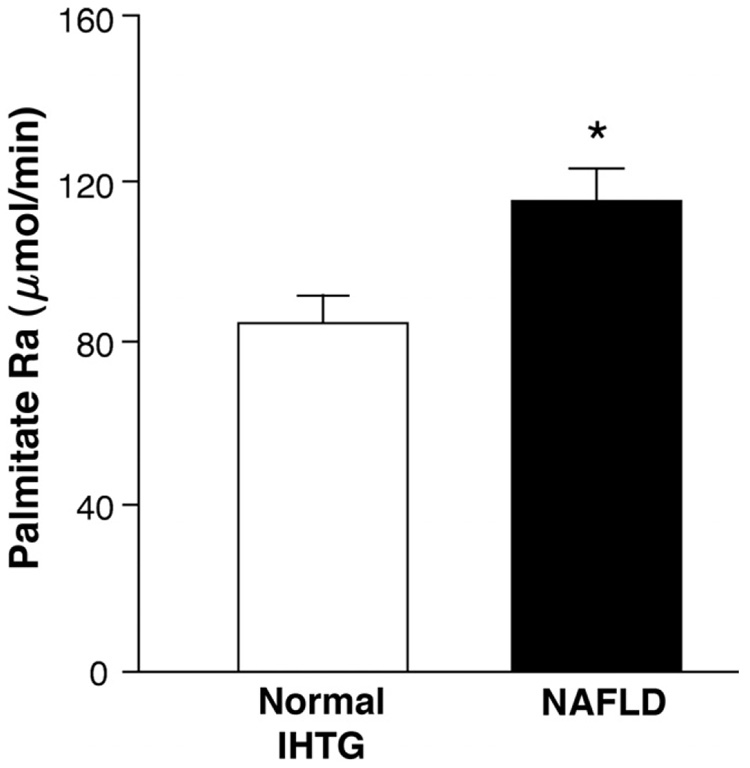
Whole-body palmitate Ra was 34% higher in subjects with NAFLD than in those with normal IHTG content (114.1 ± 8.1 and 85.4 ± 6.6 µmol/min, respectively; P = .01) (Figure 1). However, the proportion of FFA Ra incorporated into VLDL-TG was not significantly different between the NAFLD and normal IHTG groups (8.4% ± 1.4% and 7.7% ± 1.0% of total FFA Ra, respectively; P = .65). The rate of FFA clearance from plasma was also similar in the NAFLD and normal IHTG groups (827 ± 75 mL/min and 820 ± 49 mL/min, respectively; P = .94).
Figure 1.
Whole-body palmitate rate of appearance (Ra) in plasma in subjects with normal intrahepatic triglyceride (IHTG) content and increased IHTG content (nonalcoholic fatty liver disease [NAFLD]). *Value significantly different from the Normal IHTG group value, P < .05.
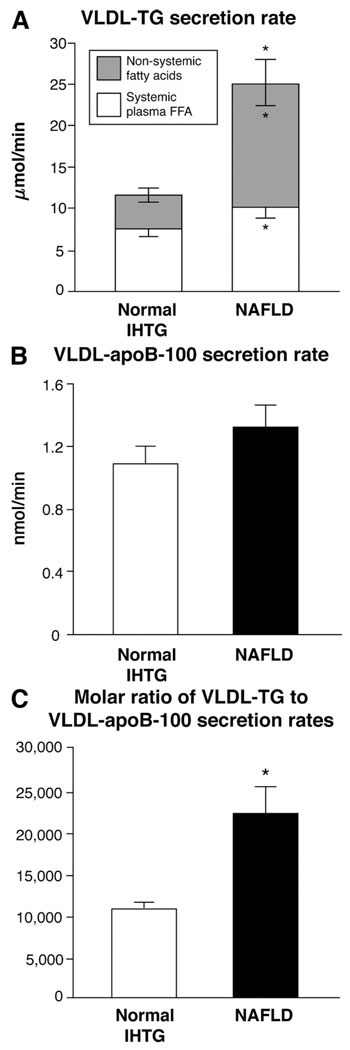
The secretion rate of VLDL-TG was more than 2-fold greater in subjects with NAFLD (24.3 ± 3.1 µmol/min) than in those with normal IHTG content (11.4 ± 1.1 µmol/min) (P = .001) (Figure 2A), whereas the secretion rate of VLDL-apoB100 was not significantly different between groups (NAFLD group: 1.3 ± 0.1 nmol/min; normal IHTG group: 1.1 ± 0.1 nmol/min) (Figure 2B). Therefore, the molar ratio of VLDL-TG to VLDL-apoB100 secretion rates, an index of the TG content of newly secreted VLDL particles, was more than 2-fold greater in subjects with NAFLD (22,100 ± 3500 moles of TG per VLDL particle) than subjects with normal IHTG (10,900 ± 990 moles of TG per VLDL particle) (P = .008) (Figure 2C). No differences were detected between the NAFLD and normal IHTG groups in FTR of either VLDL-TG (0.88 ± 0.21 and 0.62 ± 0.10 pools/h, respectively; P = .280) or VLDL-apoB100 (0.29 ± 0.03 and 0.38 ± 0.06 pools/h, respectively; P = .183).
Figure 2.
(A) Total VLDL-TG secretion rate (sum of shaded and open bars) in subjects with normal and increased (nonalcoholic fatty liver disease [NAFLD]) intrahepatic triglyceride (IHTG) content. Open bars represent fatty acids in VLDL-TG that originated from systemic plasma free fatty acids, presumably derived primarily from lipolysis of subcutaneous fat, whereas shaded bars represent fatty acids in VLDL-TG that originated from nonsystemic fatty acids, presumably derived primarily from lipolysis of intrahepatic and visceral fat and de novo lipogenesis. *Value significantly different from corresponding value in the normal IHTG group, P <.05. (B) VLDL-apoB100 secretion rates in the subjects with normal IHTG content (open bar) and NAFLD (solid bar). Values for each group are not significantly different from each other. (C) Molar ratio of VLDL-TG and VLDL-apoB100 secretion rates, an index of the average TG content of nascent VLDL particles, in subjects with normal IHTG content (open bar) and NAFLD (solid bar). *Value significantly different from value in the normal IHTG group, P < .01.
The relative contribution of systemic plasma FFA and nonsystemic fatty acids to total VLDL-TG secretion was significantly different between groups. Systemic plasma FFA accounted for 66% ± 4% and 43% ± 3% of fatty acids in VLDL-TG in subjects with normal IHTG and subjects with NAFLD, and nonsystemic fatty acids accounted for 34% ± 4% and 57% ± 3% of fatty acids within VLDL-TG in subjects with normal IHTG and subjects with NAFLD, respectively (P < .001). Therefore, the absolute secretion rate of VLDL-TG derived from nonsystemic fatty acids was more than 3-fold greater in subjects with NAFLD than in subjects with normal IHTG content (14.8 ± 2.6 and 4.0 ± 0.7 µmol/min, respectively; P < .001), and the absolute secretion rate of VLDL-TG derived from systemic plasma FFA was 35% greater in subjects with NAFLD than those with normal IHTG (10.0 ± 1.0 and 7.4 ± 0.7 µmol/min, respectively; P = .039) (Figure 2A).
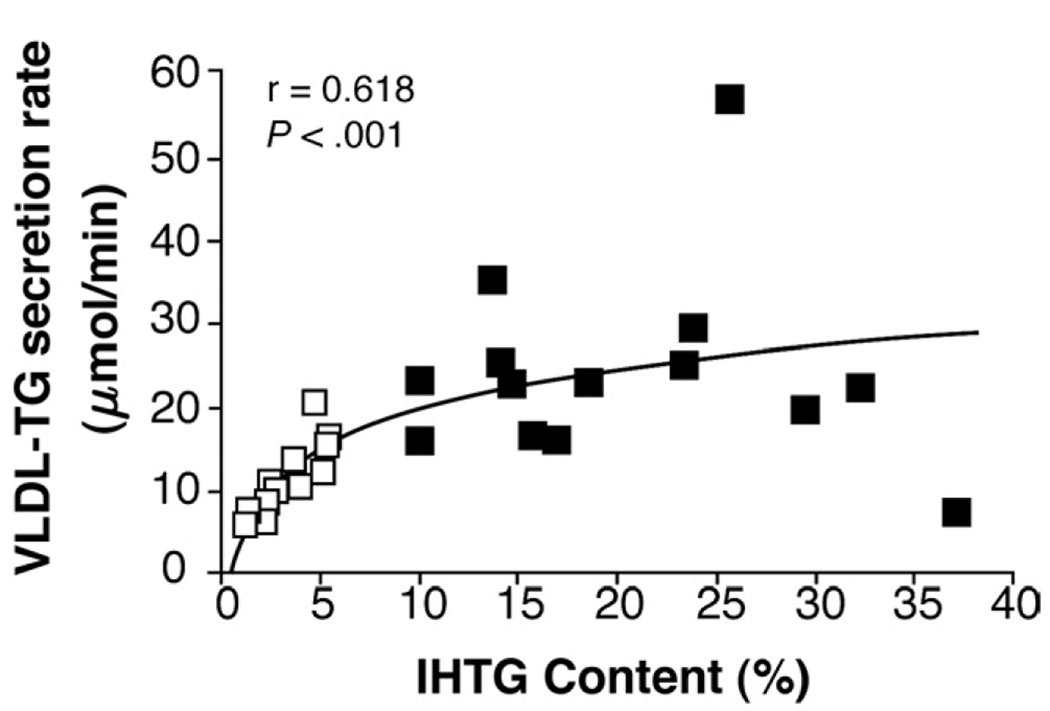
There was a direct correlation between IHTG content and total VLDL-TG secretion rate among subjects with normal IHTG (r = 0.848, P < .001) but not among subjects with NAFLD (r = 0.161, P = .583). Therefore, there was an overall curvilinear, rise-to-plateau relationship between intrahepatic fat and total VLDL-TG secretion (r = 0.618, P < .001) (Figure 3). In all subjects, no significant relationship was detected between IHTG content and VLDL-apoB100 secretion rate. Plasma VLDL-TG concentration was directly correlated with VLDL-TG secretion rate in the group with normal IHTG (r = 0.810, P < .001) but not in the group with NAFLD (r = −0.136, P = .643). Plasma VLDL-apoB100 concentration was directly correlated with VLDL-apoB100 secretion rate in the group with normal IHTG content (r = 0.574, P < .05) and in the group with NAFLD (r = 0.611, P < .05).
Figure 3.
Relationship between VLDL-TG secretion rate and intrahepatic triglyceride content (IHTG) in subjects with normal IHTG (open boxes) and nonalcoholic fatty liver disease (NAFLD) (solid boxes).
Discussion
Alterations in hepatic lipoprotein metabolism are likely involved in the pathogenesis and pathophysiology of NAFLD. However, the relationship between NAFLD and VLDL kinetics is not clear because of conflicting results from different studies. The reason for these discordant findings might be related to differences in study subject characteristics that can independently affect hepatic lipoprotein metabolism. In the present study, we evaluated the key physiologic factors involved in the accumulation of excessive IHTG in nondiabetic obese subjects who had NAFLD and a carefully matched population of subjects with normal IHTG content. The rate of release of fatty acids from adipose tissue into the bloodstream, which is an important source of fatty acids for IHTG synthesis, and the rates of hepatic VLDL-TG and VLDL-apoB100 secretion, which export TG out of the liver, were determined in obese subjects with either normal (~3% IHTG) or increased (~23% IHTG) liver fat content. Both groups had normal fasting plasma glucose concentrations and were matched on age, sex, BMI, and percent body fat to eliminate potential confounding factors that influence fatty acid and VLDL metabolism. We found that the rate of fatty acid release into plasma and the secretion rate of VLDL-TG were much higher in subjects with NAFLD than in those with normal IHTG content. Moreover, the increase in VLDL-TG secretion in the group with NAFLD was primarily caused by a marked increase in the contribution of nonsystemic fatty acids, presumably derived from lipolysis of intrahepatic and visceral fat and de novo lipogenesis, to VLDL-TG secretion. In contrast, the secretion rate of VLDL-apoB100, which reflects the number of secreted VLDL particles, was not different between groups, indicating that VLDL particles produced by subjects with NAFLD should contain more TGs and be larger than those produced by subjects who have normal IHTG content. These data demonstrate that persons with NAFLD have considerable alterations in both adipose tissue and hepatic TG metabolism. The increased rates of fatty acid release into plasma and hepatic VLDL-TG secretion likely contribute to insulin resistance and hypertriglyceridemia that are commonly associated with NAFLD.23,24 Moreover, our data suggest that a major physiologic mechanism responsible for the accumulation and maintenance of steatosis in patients with NAFLD is an increase in fatty acid delivery to the liver and increased TG production, in conjunction with a limited capacity of the liver to increase TG export because of an inability to adequately increase VLDL-apoB100 secretion.
Several mechanisms were responsible for the increase in VLDL-TG secretion rate observed in our subjects with NAFLD. By using mathematical modeling techniques, in conjunction with stable isotopically labeled tracers, we were able to determine the proportion of fatty acids incorporated in VLDL-TG that were derived from systemic and nonsystemic sources. Our data demonstrate that the increase in total VLDL-TG secretion rate in subjects with NAFLD was primarily the result of more than a 3-fold increase in the secretion of VLDL-TG derived from nonsystemic fatty acids, whereas there was only a one-third increase in the contribution of systemic fatty acids to VLDL-TG secretion. The alterations in fatty acid metabolism shifted the contribution of nonsystemic fatty acids to total VLDL-TG secretion from ~35% in subjects with normal IHTG content to ~60% in those with NAFLD. Therefore, our results suggest that nonsystemic fatty acids, presumably derived by lipolysis of intrahepatic and visceral fat and de novo lipogenesis, stimulate the increase in VLDL-TG secretion, whereas the increased availability of systemic plasma FFA, released primarily by lipolysis of subcutaneous adipose tissue, is less important. In fact, only a small percentage (~8%) of total FFA released into systemic plasma was incorporated into VLDL-TG in both groups.
Fatty acids synthesized by the liver de novo are also part of the nonsystemic fatty acid pool and could have contributed to the increased rate of VLDL-TG secretion observed in our subjects with NAFLD. In fact, our results are consistent with data from a previous study that found that the contribution of de novo lipogenesis to VLDL-TG secretion was greater in subjects with NAFLD than in those with normal IHTG content.5 During post-absorptive conditions, de novo lipogenesis accounts for less than 5% of fatty acids incorporated into secreted VLDL-TG in normal subjects but for 15% to 23% of the fatty acids in VLDL-TG in subjects with NAFLD.5,25 Therefore, de novo synthesis of fatty acids could have been responsible for almost half of the nonsystemic fatty acids that were incorporated into VLDL-TG in the NAFLD group.
Hepatic steatosis develops when the rate of IHTG production is greater than the rate of TG disposal (oxidation and secretion) by the liver. Our data demonstrate that hepatic VLDL-TG secretion rate in subjects with NAFLD is approximately double that observed in subjects with normal IHTG content. However, the increase in VLDL-TG secretion was obviously not adequate to compensate fully for the increased rate of IHTG production, so steatosis was maintained. The relationships among IHTG content, VLDL-TG secretion, and VLDL-apoB100 secretion observed in our subjects provide important insights into the physiologic mechanisms responsible for excessive IHTG accumulation. We found that VLDL-TG secretion increased linearly with increasing IHTG content in subjects with normal IHTG content, but appeared to reach a plateau in subjects with NAFLD, independent of IHTG content. In contrast, the rate of VLDL-apoB100 secretion, which represents the number of VLDL particles secreted by the liver, was not different between groups. Therefore, the TG content of nascent VLDL particles was much greater in subjects with NAFLD than in those with normal IHTG content. These results suggest that the accumulation of excessive IHTG is caused by an inadequate increase in the number of secreted VLDL particles (ie, VLDL-apoB100 secretion rate) in response to an increase in IHTG production. The notion that the liver’s limited capacity to increase VLDL-apoB100 secretion rate is involved in the pathogenesis of NAFLD is also consistent with data from previous studies, which found that experimentally induced decreases in hepatic apoB100 secretion are associated with concomitant increases in liver fat content in both human subjects and animal models. 26,27
We did not evaluate hepatic fatty acid oxidation in our study subjects. A defect in hepatic fatty acid oxidation could contribute to the development and maintenance of steatosis by increasing fatty acid availability for esterification to IHTG. However, data from previous studies suggest that subjects with NAFLD actually have increased rates of hepatic fatty acid oxidation28 and an increased capacity to oxidize fatty acids because of mitochondrial uncoupling between fatty acid oxidation and adenosine triphosphate production in the liver.29 These data suggest that the increase in intrahepatic fatty acid availability from both systemic and nonsystemic sources exceeds the liver’s ability to oxidize excess fatty acids, despite an up-regulation of fatty acid oxidation.
Our data demonstrate a dissociation in the regulation of VLDL-TG and VLDL-apoB100 secretion rates in subjects with NAFLD. Although the rate of VLDL-TG secretion was much greater in subjects with NAFLD than in subjects with normal IHTG content, the rate of VLDL-apoB100 secretion was not significantly different between groups. This dissociation in VLDL-TG and VLDL-apoB100 kinetics has also been observed after diet-induced weight loss,13 high-carbohydrate-diet consumption,30 and experimental increases in FFA availability31; in all studies, the experimental perturbation affected VLDL-TG but not apoB100 secretion rates. Therefore, the composite of these data suggest that the secretion of VLDL-TG by the liver is much more responsive to physiologic alterations than is the secretion of VLDL-apoB100.
Compared with subjects who had normal IHTG, our cohort with NAFLD had many of the characteristics of the metabolic syndrome, including increased intra-abdominal fat content, insulin resistance, and increased plasma TG concentrations. Moreover, our kinetic data provide insights into the physiologic mechanisms that help explain why the metabolic syndrome and NAFLD are closely linked. The higher rates of FFA release into the bloodstream and higher plasma FFA concentrations observed in our subjects with NAFLD likely increased the delivery of FFA to the liver and skeletal muscle, which can promote intrahepatic TG accumulation, increase hepatic glucose production,32 and impair insulin-mediated skeletal muscle glucose uptake.33 The increase in VLDL-TG secretion rate likely contributed to the increase in plasma VLDL-TG concentrations observed in subjects with NAFLD.
In summary, excessive IHTG content in obese subjects is associated with alterations in both adipose tissue and hepatic lipid metabolism; subjects with NAFLD have increased rates of adipose tissue TG lipolysis and hepatic VLDL-TG secretion. Moreover, our data suggest that increased IHTG content is not simply a marker of altered hepatic metabolic function but is directly involved in the pathophysiology of NAFLD because the increase in VLDL-TG secretion was caused by an increased incorporation of nonsystemic fatty acids, presumably from lipolysis of intrahepatic and intra-abdominal fat and de novo lipogenesis, into VLDL. Moreover, the increase in VLDL-TG secretion, which is the major source of circulating TG,34 is likely responsible for the increase in serum TG concentrations commonly observed in patients with NAFLD.35,36 The dissociation in VLDL-TG and VLDL-apoB100 kinetics suggests that a failure to increase adequately the secretion rate of apoB100, which provides the framework for TG incorporation into VLDL, limits the liver’s capacity to export TG. These data underscore the complex metabolic interactions associated with NAFLD in obese persons.
Acknowledgments
Supported by National Institutes of Health grants DK 37948, DK 56341 (Clinical Nutrition Research Unit), RR-00036 (General Clinical Research Center), and RR-00954 (Biomedical Mass Spectrometry Resource) and by an AGA-Roche Junior Faculty Clinical Research Award in Hepatology.
The authors thank Jennifer McCrea, Adewole Okunade, Freida Custodio, and Jennifer Shew for their technical assistance; the staff of the General Clinical Research Center for their help in performing the studies; and the study subjects for their participation.
Abbreviations used in this paper
- apoB100
apolipoprotein B-100
- FFA
free fatty acid
- FTR
fractional turnover rate
- IHTG
intrahepatic triglyceride
- NAFLD
nonalcoholic fatty liver disease
- TTR
tracer-to-tracee ratio
- VLDL
very low-density lipoprotein
Footnotes
Conflicts of interest: No conflicts of interests exist.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg IJ, Ginsberg HN. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1343–1346. doi: 10.1053/j.gastro.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Charlton M, Sreekumar R, Rasmussen D, et al. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 4.Adiels M, Taskinen MR, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 5.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 6.Kissebah AH, Alfarsi S, Evans DJ, et al. Integrated regulation of very low-density lipoprotein triglyceride and apolipoprotein-B kinetics in non-insulin-dependent diabetes mellitus. Diabetes. 1982;31:217–225. doi: 10.2337/diab.31.3.217. [DOI] [PubMed] [Google Scholar]
- 7.Mittendorfer B, Patterson BW, Klein S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am J Clin Nutr. 2003;77:573–579. doi: 10.1093/ajcn/77.3.573. [DOI] [PubMed] [Google Scholar]
- 8.Adiels M, Boren J, Caslake MJ, et al. Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler Thromb Vasc Biol. 2005;25:1697–1703. doi: 10.1161/01.ATV.0000172689.53992.25. [DOI] [PubMed] [Google Scholar]
- 9.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 10.Genton L, Hans D, Kyle UG, et al. Dual-energy x-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66–70. doi: 10.1016/s0899-9007(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 11.Abate N, Burns D, Peshock RM, et al. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res. 1994;35:1490–1496. [PubMed] [Google Scholar]
- 12.Frimel TN, Deivanayagam S, Bashir A, et al. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 13.Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–E556. doi: 10.1152/ajpendo.00379.2002. [DOI] [PubMed] [Google Scholar]
- 14.Patterson BW, Zhao G, Elias N, et al. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 15.Klein S, Mittendorfer B, Eagon JC, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–1572. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 19.Mittendorfer B, Liem O, Patterson BW, et al. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–1648. doi: 10.2337/diabetes.52.7.1641. [DOI] [PubMed] [Google Scholar]
- 20.Patterson BW, Mittendorfer B, Elias N, et al. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res. 2002;43:223–233. [PubMed] [Google Scholar]
- 21.Magkos F, Wright DC, Patterson BW, et al. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am J Physiol Endocrinol Metab. 2006;290:E355–E362. doi: 10.1152/ajpendo.00259.2005. [DOI] [PubMed] [Google Scholar]
- 22.Magkos F, Patterson BW, Mohammed BS, et al. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab. 2007;92:1311–1318. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 23.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 24.Bugianesi E, Gastaldelli A, Vanni E, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchiyama S, Shimizu T, Shirasawa T. CuZn-SOD deficiency causes ApoB degradation and induces hepatic lipid accumulation by impaired lipoprotein secretion in mice. J Biol Chem. 2006;281:31713–31719. doi: 10.1074/jbc.M603422200. [DOI] [PubMed] [Google Scholar]
- 27.Cuchel M, Bloedon LT, Szapary PO, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 28.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 29.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 30.Melish J, Le NA, Ginsberg H, et al. Dissociation of apoprotein B and triglyceride production in very-low-density lipoproteins. Am J Physiol. 1980;239:E354–E362. doi: 10.1152/ajpendo.1980.239.5.E354. [DOI] [PubMed] [Google Scholar]
- 31.Lewis GF, Uffelman KD, Szeto LW, et al. Interaction between free fatty acids and insulin in the acute control of very low-density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrannini E, Barrett EJ, Bevilacqua S, et al. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72:1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley DE, Mokan M, Simoneau JA, et al. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest. 1993;92:91–98. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson LA, Ericsson M. Quantitative and qualitative serum lipoprotein analysis. Part 1. Studies in healthy men and women. Atherosclerosis. 1975;21:417–433. doi: 10.1016/0021-9150(75)90054-4. [DOI] [PubMed] [Google Scholar]
- 35.Neuschwander-Tetri BA. Fatty liver and the metabolic syndrome. Curr Opin Gastroenterol. 2007;23:193–198. doi: 10.1097/MOG.0b013e32801421a9. [DOI] [PubMed] [Google Scholar]
- 36.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]