Abstract
Background
The epidemiology of the gastroparesis is unknown. We aimed to determine the incidence, prevalence and outcome of gastroparesis in the community.
Methods
Using the Rochester Epidemiology Project, a medical records linkage system in Olmsted County, Minnesota, we identified county residents with potential gastroparesis. The complete medical records were reviewed by a gastroenterologist. Three diagnostic definitions were used: 1) definite gastroparesis: delayed gastric emptying by standard scintigraphy and typical symptoms for more than 3 months 2) probable gastroparesis: typical symptoms and food retention on endoscopy or upper GI study 3) possible gastroparesis: typical symptoms alone or delayed gastric emptying by scintigraphy without GI symptoms. Poisson regression was used to assess the association of incidence rates with age, gender and calendar period.
Results
Among 3604 potential cases of gastroparesis, 83 met diagnostic criteria for definite gastroparesis, 126 definite plus probable gastroparesis, and 222 any of the three definitions of gastroparesis. The age-adjusted (to 2000 U.S. whites) incidence per 100,000 person-years of definite gastroparesis for the years 1996–2006 was 2.5 (95% CI, 1.2–3.8) for men, and 9.8 (95% CI, 7.5–12.1) for women. The age-adjusted prevalence of definite gastroparesis per 100,000 person on January 1, 2007 was 9.6 (95% CI, 1.8–17.4) for men and 37.8 (95% CI, 23.2–52.4) for women. Overall survival was significantly lower than the age and gender specific expected survival computed from the Minnesota white population (p<0.05).
Conclusions
Gastroparesis is an uncommon condition in the community, but is associated with a poor outcome.
Keywords: Gastroparesis, Incidence, Prevalence, Survival, Epidemiology
INTRODUCTION
Gastroparesis is a syndrome characterized by delayed gastric emptying1 in the absence of mechanical obstruction of the stomach. Delayed gastric emptying is traditionally associated with nausea, vomiting, postprandial fullness, early satiety, bloating and abdominal pain but asymptomatic cases have been reported.2,3,4 Mild gastroparesis is thought to have a low mortality rate, but patients with decompensated gastroparesis are more likely to develop complications and have related mortality.4 The number of gastroparesis-related hospitalizations has been increasing in the United States, and the economic impact of gastroparesis-related hospitalizations is significant and may be increasing.5
The prevalence of gastroparesis is difficult to estimate due to the relatively poor correlation of symptoms with gastric emptying,3 and the need to apply a diagnostic test in a community setting.4 It is unclear if the majority of patients with gastroparesis seek health care or how often they are referred to gastroenterologists, and therefore the true prevalence of gastroparesis is not known.
The etiology of gastroparesis may be diverse but most cases are classified as idiopathic or occur secondary to diabetes mellitus.4 Population-based studies have shown that 11–18% of patients with diabetes have upper gastrointestinal symptoms,6 and in a referral center, delayed gastric emptying was present in 50–65% of patients with diabetes. 7,8 However, how often delayed gastric emptying explains dyspepsia in diabetes in the community setting is uncertain.1, 7
The natural history of gastroparesis is largely unknown. One study suggested that symptoms and gastric emptying in 20 patients with diabetes were generally stable over 12 years of follow-up.9 In another study of 86 diabetic patients who were followed for at least 9 years, delayed gastric emptying was not related with mortality after adjustment of comorbidities.8 However, other data from a tertiary setting over 6 years follow-up observed that 7% had died and 22% needed long-term parenteral or enteral feeding, suggesting gastroparesis is not a benign condition.4 Community studies of the outcome of gastroparesis are lacking, and studies conducted in tertiary referral centers may not reflect findings encountered in the general population.
Population-based research in Olmsted County, Minnesota, has been made possible using the data resources of the Rochester Epidemiology Project (REP).10 The system allows nearly complete enumeration of the population and has the ability to link individuals with their complete lifetime in- and outpatient medical record. In this study, we aimed to estimate the incidence and prevalence of gastroparesis in the general population. We also evaluated outcomes, including natural history and survival in a community based cohort of subjects identified with gastroparesis.
METHODS
Setting
This study was approved by the Institutional Review Boards of the Mayo Foundation and the Olmsted Medical center.
Olmsted County is approximately 653 square miles and is located in southeastern Minnesota, 90 miles from a major metropolitan center (Minneapolis/St. Paul). The 2000 census population was 124,277 residents, with 24% employed in the health care industry. The urban center of Rochester (2000 population, 85,806) is surrounded by rural countryside. The demographic characteristics very closely resemble the US white population.10
Residents of Olmsted County receive their medical care almost exclusively from two group practices: Mayo Medical Center and Olmsted Medical Center. Mayo Clinic has maintained a common medical record system with its two affiliated hospitals (Saint Mary’s and Rochester Methodist) for over 100 years. Recorded diagnoses and surgical procedures are indexed, including the diagnoses made for outpatients seen in office or clinic consultations, emergency room visits or nursing home care, as well as the diagnoses recorded for hospital inpatients, at autopsy examination or on death certificates. This system was further developed by the Rochester Epidemiology Project (REP), which created similar indices for the records of other providers of medical care to local residents, most notably the Olmsted Medical Group and its affiliated Olmsted Community Hospital (Olmsted Medical Center). Thus, details of the medical care provided to the residents of the County are available for study. Annually, over 80% of the entire population is attended by one or both of these two practices, and 96% are seen at least once during any given four-year period. This provides the capability for population-based studies of disease and outcomes by affording access to the details of the medical care sought by residents of Olmsted County at almost all area medical facilities.
Case ascertainment
All county residents diagnosed between January 1, 1996 and December 31, 2006 with gastroparesis were identified by one of two mechanisms. First, potential cases were identified using the diagnostic index developed by the REP including all Mayo and non-Mayo Clinic patients who were Olmsted County residents. The potential cases were identified as having one or more of the following diagnostic or surgical codes: gastroparesis, H-ICDA-8 5379662 or ICD-9 536; paresis of stomach, H-ICDA-8 5379660; paralysis of stomach, H-ICDA-8 5379661; gastroparesis with diabetes, H-ICDA-8 5379670; impaired gastric emptying, H-ICDA-8 5361242; atonia of stomach, H-ICDA-8 5361130; pseudo-obstruction, H-ICDA-8 5649450; stasis or atonia of intestine, H-ICDA-8 5649120, 5649130, 5649131; insertion of feeding tube, H-ICDA-8 34161131 or ICD-9 V44.1. Second, we linked the list of all patients who had undergone gastric emptying testing by scintigraphy at Mayo Clinic with clinic registration data, to identify all residents of Olmsted County residents who had their gastric emptying assessed. Gastric scintigraphy is only performed at the Mayo Clinic in Olmsted County.
The diagnostic index was designed to be sensitive and thus this broad search should capture all clinically diagnosed cases. However, a detailed chart review was undertaken to improve specificity and ensure these cases actually had gastroparesis.
Case definition
Among the potential cases, three diagnostic definitions were used:
Definite gastroparesis: delayed gastric emptying by standard scintigraphy and symptoms of nausea and/or vomiting, postprandial fullness, early satiety, bloating, or epigastric pain for more than 3 months,
Probable gastroparesis: symptoms as above plus food retention on endoscopy or an upper GI study, but no scintigraphy had been performed,
Possible gastroparesis: typical symptoms alone or delayed gastric emptying by scintigraphy in the absence of GI symptoms.
Scintigraphic techniques were routinely used to evaluate gastric emptying as described in prior studies conducted in our laboratory.11 All patients discontinued their medications 48 hours before testing. After an overnight fast, a 99mTc-sulfur colloid labeled egg meal assessed gastric emptying. The eggs were served with one slice of buttered bread and an 8-oz glass of 1% milk (total calories: 296 kcal, 32% protein, 35% fat, 33% carbohydrate). Anterior and posterior gamma camera images were obtained at 0, 1, 2, and 4 hours after the test meal ingestion. Delayed gastric emptying was defined as a proportion emptied that was slower than two standard deviations below the cutoff in healthy subjects at either the 2 or 4 hour time point.12, 13
We excluded patients with identifiable causes of nausea, vomiting or fullness, related to gastric outlet obstruction or mechanical obstruction. The subjects who had possible gastroparesis symptoms but normal or fast gastric emptying by scintigraphy were excluded, however, the subjects who had retained food on endoscopy or an upper GI study but exhibited normal or fast gastric emptying were classified as probable gastroparesis cases (n=6). Any patients who refused general authorization for review of their medical records for research and thus could not be studied according to Minnesota statute were excluded.
Data collection
We performed several steps to ensure reliability and high quality. All information pertaining to diagnosis was recorded in a specifically designed standardized clinical data form. Demographic data as well as clinical and outcome data were collected from the complete medical records, including all inpatient and outpatient records. Variables were obtained from a thorough review of the doctor’s note, medication history, past medical and surgical history, radiologic studies, including double contrast upper GI X-ray, small bowel and colon studies for exclusion of mechanical obstruction, abdominal-pelvic CT scan, scintigraphy, endoscopy and pathology in electronic and paper chart format. Each clinical record was reviewed by two experienced gastroenterologists (H.J. and R.C). Any uncertainties in data abstraction were discussed with an experienced investigator (G.R.L). For defining an incident case, every case was exhaustively evaluated for prior disease.
Through careful review of the linked medical records, patients were followed from the time of diagnosis of gastroparesis until death or date of last follow-up on January 1, 2007, in the community. Mayo Clinic and Olmsted Medical Group which together provide almost all care in the County maintain an institutional (“registration”) database in which routine follow-up (e.g. correspondence with patients or next of kin or local providers of medical care) is updated periodically, including clinic visits. From the database (which is up to date for all Olmsted county residents), vital status (and, if deceased, date of death) was obtained for all subjects used in this analysis.
An incident case was defined as any new case diagnosed over the 10-year study period. A prevalent case was defined as having one or more following conditions in the 2 year period prior to January 1st, 2007 : 1) any hospital visit related with gastroparesis as an in- and out-patients clinic; 2) on medication related to gastroparesis 3) need for tube feeding related to gastroparesis.
We checked Olmsted County residence status at the index date. Patients who resided outside of Olmsted County at the time of initial diagnosis of gastroparesis were excluded from incidence calculations, but were included in prevalence calculations if they resided in the county. Patients who had a diagnosis of gastroparesis before 1996 were also included in prevalence calculations if they fulfilled the definition of a prevalence case during the two-year period before January 1st, 2007.
Ascertainment of vital status and causes of death were obtained from medical records and death certificates.
The following information was collected from the complete review of medical records:
Demographics; age at time of initial diagnosis and gender.
Date of initial diagnosis, any visit in the prior 2 years before January 1st 2007 and last follow-up date (date of death if deceased).
Body mass index (BMI) at the diagnosis of gastroparesis.
Symptoms, including nausea, vomiting, postprandial fullness, early satiety, bloating, epigastric pain, and weight loss.
Socioeconomic factors: marital status (married or not), employment status (retired, student, employed, unemployed, disability, other) and educational level (professional training, high school or some college, or less than high school).
Cigarettes and alcohol: we checked medical records for the use of cigarettes and alcohol abuse (yes for current use of more the 7 times per week, no for never or not current use).
Possible causes of gastroparesis: we searched the records for evidence of diabetes mellitus, post-surgical gastroparesis related to gastrectomy or fundoplication, connective tissue diseases (such as systemic sclerosis or Sjögren’s syndrome), dermatomyositis, hypothyroidism, malignancy, Parkinson’s disease, and other neuromuscular disease. Idiopathic gastroparesis was defined as no definitive underlying cause and included acute viral illness related to gastroparesis. Co-morbid psychiatric diseasee such as anxiety/depression and eating disorders were also recorded.
Medications: any medications that might possibly induce delayed GI motility, such as narcotics, anti-cholinergic agents, calcium-channel blockers, and tricyclic anti-depressants were recorded.
Complications of gastroparesis: we recorded nutritional support given including parenteral nutrition or tube feeding, hospitalization for gastroparesis, Botulinum toxin injections into the pylorus, balloon dilatation of the gastric outlet, gastric electrical pacing, or surgical treatment (e.g. gastrostomy, jejunostomy, pyloroplasty, partial or total gastrectomy).
Cause of death: primary cause of death in the death certificate or medical record was recorded.
Statistical analysis
The entire population of Olmsted County from 1996 to 2006 was considered at risk for gastroparesis. Age- and sex-specific denominator values were derived from decennial census data for Olmsted County with linear interpolation between census year. Because the Olmsted County population was >90% white during most of the study period, the overall incidence and prevalence estimates were directly age- and age-and sex -adjusted to the population distribution of U.S. whites in the year 2000. The point prevalence of gastroparesis in the community was calculated based on the number of case subjects residing in Olmsted County on January 1, 2007. The 95% confidence intervals (CI) for the incidence and prevalence of gastroparesis were estimated assuming a Poisson distribution of cases. The association of incidence of gastroparesis with age at diagnosis, gender, and calendar year of diagnosis (5-year intervals) were evaluated using Poisson regression. Three separate analyses were examined corresponding to the three overlapping cohorts: 1) the definite gastroparesis cohort, 2) the definite plus probable gastroparesis cohort, and 3) the definite plus probable plus possible gastroparesis cohort.
Overall survival was summarized using the Kaplan-Meier product-limit life table method. The survival of Olmsted County gastroparesis patients was compared with the expected survival of Minnesota whites in 2000 using the log rank test. A Cox proportional hazards regression model examined the association of survival with age at diagnosis, gender, calendar year of diagnosis, and the most likely cause of gastroparesis in each subject.
RESULTS
Case characteristics
The initial query of the diagnostic index and gastric emptying test database yielded 3604 potential cases. We excluded 241 (6.7%) subjects who refused research authorization. As a quality control check we reviewed 100 randomly selected medical records from a list of dyspepsia or dyspepsia related codes in non-Mayo Clinic subjects, and did not identify any eligible cases; therefore all cases with these diagnostic codes (n=1215) were excluded. We also reviewed the 210 randomly selected medical records from a list of patients that had undergone tube feeding, and no definite gastroparesis cases were identified, though one case meeting criteria for possible gastroparesis was found; these diagnostic codes (n=1154) were therefore not further considered. Finally, we reviewed detailed medical records of 994 potential cases identified by the diagnostic index or gastric emptying test roster. On the basis of the criteria mentioned above, 772 patients were excluded for the following reasons: 151 normal or fast gastric emptying patients who did not meet the definition of gastroparesis. 13 of the patients underwent gastric emptying testing more than two times, and we considered these patients to be a gastroparesis case if one of their gastric emptying tests was abnormally delayed. A total of 567 patients had another GI motility disorder or neurogenic bowel without gastroparesis; 20 mechanical obstruction; 6 biliary disease; 5 malignancy including 1 ovarian cancer, 2 pancreatic cancer, 3 carcinomatosis peritoni; active ulcerative colitis or Crohn’s disease in 5; and other reasons in 19 patients.
Among the 222 eligible cases of gastroparesis, 4 patients had a diagnosis of gastroparesis before 1996 and were not included as incidence cases, but were considered as prevalence cases. A total of 83 patients met diagnostic criteria for definite gastroparesis while 44 were probable cases and 95 were possible cases between 1996 and 2006 (Table 1). Overall, 68 out of 83 patients with definite gastroparesis were female (82%). The mean age (±SD) at diagnosis of definite gastroparesis was 44 (±21) years, with the range of 4–86 years.
Table 1.
Clinical Characteristics of Patients with Gastroparesis
| Definite gastroparesis(n=83) | Definite & probable gastroparesis(n=127) | Definite & probable & possible gastroparesis (n=222) | |
|---|---|---|---|
| Age at onset (years, mean ± SD) | 44 ± 21 | 47 ± 20 | 51 ± 21 |
| Gender (No. of female, %) | 68 (81.9%) | 97 (76.4%) | 154 (69.4%) |
| BMI (mean ± SD) (kg/m2) | 24.9 ± 6.8 | 26.2 ± 7.0 | 27.0 ± 7.7 |
| Marital status-married (%) | 33 (39.8%) | 51 (40.2%) | 94 (42.3%) |
| Education -high school/college (%) |
68 (81.9%) | 103 (81.1%) | 165 (74.3%) |
| Work-employed or student (%) | 37 (44.6%) | 52 (40.9%) | 80 (36.0%) |
| Alcohol use (%) | 22 (26.5%) | 34 (26.8%) | 50 (22.5%) |
| Tobacco use (%) | 29 (34.9%) | 43 (33.9%) | 86 (38.7%) |
Three diagnostic definitions were used; definite gastroparesis, delayed gastric emptying and typical symptoms; probable gastroparesis, typical symptoms as above and food retention on endoscopy or upper GI study; possible gastroparesis, typical symptoms alone or delayed gastric emptying without GI symptoms. BMI, body mass index.
Among the patients with definite gastroparesis, nausea and vomiting were the most common symptoms (73.5% and 53%, respectively). Other major contributing symptoms to the clinical presentation were abdominal pain (44.6%), bloating (31.3%), weight loss (30.1%), postprandial fullness (22.9%) and early satiety (28.9%).
Amongst the cases with definite gastroparesis, the cause was identified to be diabetes mellitus in 21 patients (25.3%), drugs in 19 (5 narcotics, 9 antidepressant, 2 antipsychotics, 3 others, 22.9%), connective tissue disease in 9 (10.8%), post-surgical gastroparesis in 6 (7.2%), and malignancy in 2 (familial adenomatous polyposis and myeloproliferative leukemia, 2.4%); idiopathic gastroparesis was determined to be the cause in 41 cases (49.4%), including acute viral illnesses (n=9) (table 2). Co-morbid psychiatric illnesses were identified in 25 patients (20 anxiety/depression, 5 other).
Table 2.
Secondary Causes of Gastroparesis in the Population
| Probable cause of gastroparesis, n (%) | Definite gastroparesis(n=83) | Definite & probable gastroparesis(n=127) | Definite & probable & possible gastroparesis (n=222) |
|---|---|---|---|
| Idiopathic | 41 (49.4%) | 55 (43.3%) | 70 (31.5%) |
| Diabetes mellitus | 21 (25.3%) | 39 (30.7%) | 103 (46.4%) |
| Connective tissue disease | 9 (10.8%) | 12 (9.4%) | 15 (6.8%) |
| Hypothyroidism | 1 (1.2%) | 2 (1.6%) | 5 (2.3%) |
| Malignancy | 2 (8.4%) | 4 (3.1%) | 11 (5.0%) |
| Gastrectomy/fundoplication | 6 (7.2%) | 10 (7.9%) | 12 (5.4%) |
| Provocation drugs | 19 (22.9%) | 29 (22.8%) | 54 (24.3%) |
| End stage renal disease | 4 (4.8%) | 7 (5.5%) | 19 (8.6%) |
Three diagnostic definitions were used; definite gastroparesis, delayed gastric emptying and typical symptoms; probable gastroparesis, typical symptoms and food retention on endoscopy or upper GI study; possible gastroparesis, typical symptoms alone or delayed gastric emptying without GI symptoms. Causes of gastroparesis are not mutually exclusive.
Incidence and prevalence of gastroparesis
The sex specific age-adjusted (to 2000 U.S. whites) incidence per 100,000 person-years for definite gastroparesis over the years 1996–2006 was 9.8 (95% CI, 7.5–12.1) in women and 2.4 (95% CI, 1.2–3.8) in men. The female-to-male age adjusted ratio was thus approximately 4:1 (Table 3). Poisson regression analysis indicated that the incidence of definite gastroparesis increased significantly with advancing age (Fig 1A), with a peak incidence of 10.5 per 100 000 in patients ≥ 60 years of age. The incidence of definite gastroparesis was significantly greater in women than in men (p<0.001), but no effect of calendar period was detected (Fig 2). These findings were replicated in the cohorts of definite plus probable gastroparesis, and all possible gastroparesis subjects.
Table 3.
Incidence of Gastroparesis in Olmsted County, MN, 1996–2006
| Definite gastroparesis | Definite & probable gastroparesis | Definite & probable & possible gastroparesis | |
|---|---|---|---|
| Females (95% CI)a | 9.8 (7.5 – 12.1) | 14.2 ( 11.4 – 17.0) | 22.3 ( 18.7 – 25.9) |
| Males (95% CI)a | 2.4 (1.2 – 3.8) | 5.0 ( 3.2 – 6.9) | 11.8 ( 9.0 – 14.7) |
| Total (95% CI)b | 6.3 (4.9 – 7.7) | 9.8 ( 8.1 – 11.6) | 17.2 ( 14.9 – 19.5) |
Three diagnostic definitions were used; definite gastroparesis, delayed gastric emptying and symptoms of nausea and/or vomiting, postprandial fullness, early satiety, bloating, or epigastric pain for more than 3 months; probable gastroparesis, symptoms and food retention on endoscopy or upper GI study; possible gastroparesis, typical symptoms alone or delayed gastric emptying without GI symptoms.
Incidence per 100,000 person-years, age-adjusted to the population structure of 2000 U.S. whites.
Incidence per 100,000 person-years, age-and gender-adjusted to the population structure of 2000 U.S. whites.
Figure 1. Age-specific incidence of gastroparesis in Olmsted County, Minnesota, 1996–2006.
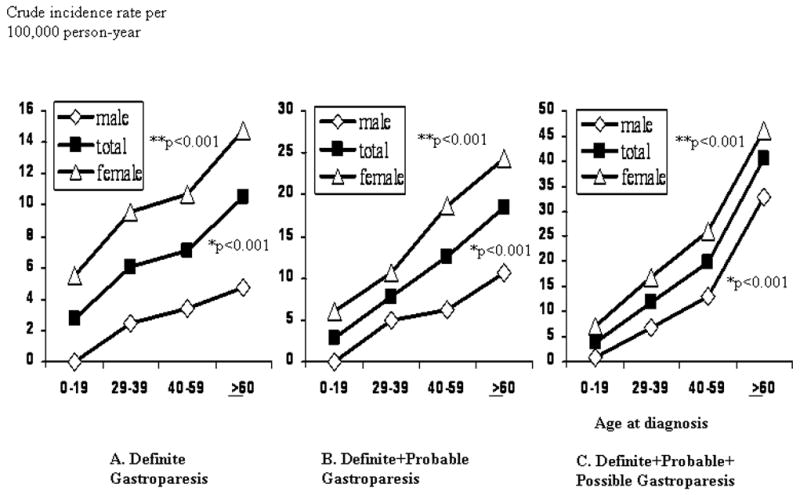
A. Definite gastroparesis B. Definite+probable gastroparesis C. Definite+probable+possible gastroparesis. * Comparison of incidence according to advancing age ** Comparison of incidence according to the gender. Three diagnostic definitions were used; definite gastroparesis, delayed gastric emptying and symptoms of nausea and/or vomiting, postprandial fullness, early satiety, bloating, or epigastric pain for more than 3 months; probable gastroparesis, symptoms and food retention on endoscopy or upper GI study or delayed gastric emptying; possible gastroparesis, typical symptoms alone or delayed gastric emptying without GI symptoms.
Figure 2. Incidence of definite gastroparesis over time among Olmsted County. Minnesota, 1996–2006.
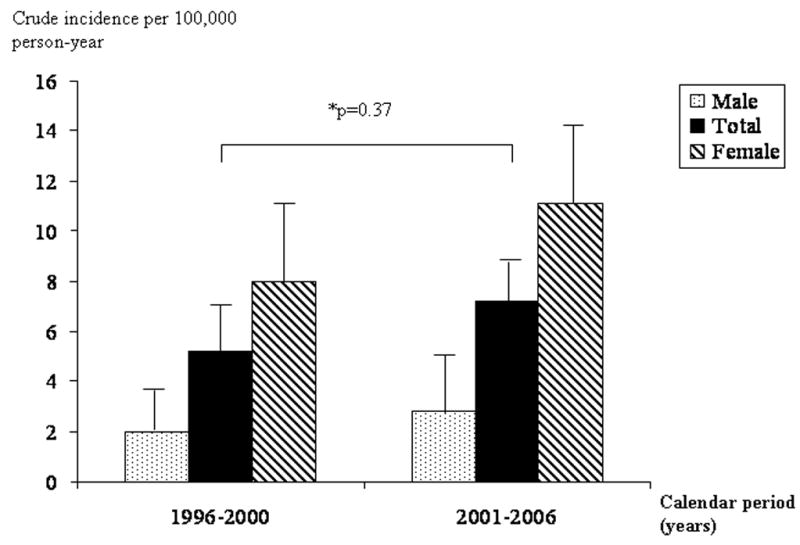
Total incidence was adjusted by age and gender. Gender specific incidence was adjusted by age.
On January 1, 2007, there were 65 Olmsted County residents alive with a diagnosis meeting the criteria for definite, probable or possible gastroparesis. The age-adjusted prevalence of definite gastroparesis per 100,000 persons was 37.8 (95% CI, 23.3–52.4) in women and 9.6 (95% CI, 1.8–17.4) in men (table 4). As expected, prevalence rates were significantly higher among women than men (age-adjusted female to male ratio of 3.9:1).
Table 4.
Age and Gender Specific Prevalence of Gastroparesis in Olmsted County, MN, January 1, 2007
| Definite gastroparesis | Definite & probable gastroparesis | Definite & probable & possible gastroparesis | |
|---|---|---|---|
| Females (95% CI)a | 37.8 (23.3 – 52.4) | 48.9 ( 32.2 – 65.7) | 70.6 ( 50.6 – 90.7) |
| Males (95% CI)a | 9.6 (1.8 – 17.4) | 15.3 ( 5.7 – 24.9) | 27.9 ( 14.4 – 41.4) |
| Total (95% CI)b | 24.2 (15.7 – 32.6) | 33.4 ( 23.3 – 43.4) | 50.5 ( 38.1 – 62.8) |
Three diagnostic definitions were used; definite gastroparesis, delayed gastric emptying and typical symptoms; probable gastroparesis, typical symptoms and food retention on endoscopy or upper GI study; possible gastroparesis, typical symptoms alone or delayed gastric emptying without GI symptoms.
Prevalence per 100,000 person, age-adjusted to the population structure of 2000 U.S. whites.
Prevalence per 100,000 person, age-and gender-adjusted to the population structure of 2000 U.S. whites.
Outcomes
This population-based cohort study was followed up for a median duration of 5 years (range 0–12 years). During these follow-up periods, therapeutic interventions more than medication were needed in 54 patients (24.8%) with definite plus probable plus possible gastroparesis. Among them, 47 patients needed hospitalization for tube feeding or parenteral nutrition, 5 patients required tube feeding or parenteral nutrition without hospitalization, and 2 patients underwent Botox injection in the pylorus for treatment.
Figure 3 shows the survival of the cohort comprising the definite plus probable plus possible gastroparesis, observed from the time of diagnosis in comparison to the expected survival of the Minnesota white population, adjusting for age and gender. The estimated 5-year survival in the cohort was 67% (95% CI, 60%–75%), compared with an expected 81% survival (p<0.01) (Fig. 3). This reduced survival was similarly observed in the definite gastroparesis cohort.
Figure 3. Survival of Gastroparesis inception cohort in Olmsted County, 1996–2006, and expected survival of gender- and age-matched Minnesota whites in 2000 (p= 0.0001).
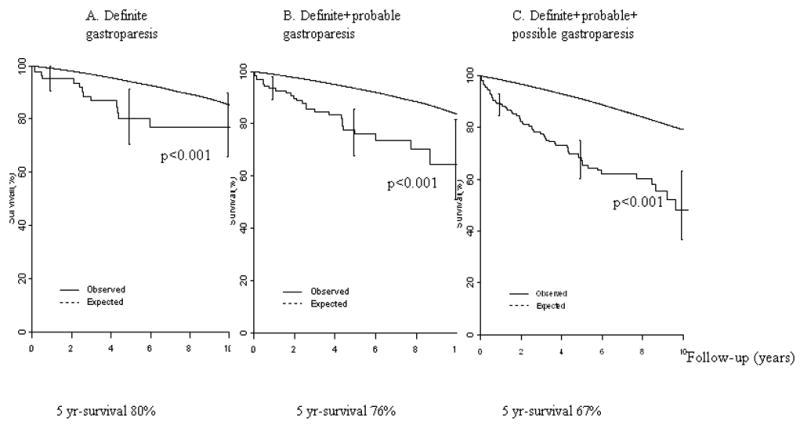
A. Definite gastroparesis B. Definite+probable gastroparesis C. Definite+probable+possible gastroparesis. Three diagnostic definitions were used; definite gastroparesis, delayed gastric emptying and symptoms of nausea and/or vomiting, postprandial fullness, early satiety, bloating, or epigastric pain for more than 3 months; probable gastroparesis, symptoms and food retention on endoscopy or upper GI study or delayed gastric emptying; possible gastroparesis, typical symptoms alone or delayed gastric emptying without GI symptoms.
Among the 69 patients who died, the most common causes of death were cardiovascular disease in 17 cases (24.6%), respiratory failure in 16 (23.2%), malignancy in 11 (15.9%), chronic renal failure in 11 (15.9%), a cerebrovascular accident in 7 (10.1%) and other causes in 7 (10.1%). Among the patients with gastroparesis in this study, 13 patients underwent repeated gastric emptying testing with an interval of between 1 and 6 years; 4 patients whose symptoms improved still had delayed gastric emptying on the second test, while the remaining 9 patients had symptom improvement and normal gastric emptying on repeat testing.
A Cox proportional hazards regression model assessed the association of age at diagnosis, gender, and cause of gastroparesis (idiopathic vs. non-idiopathic) with time to death among the patients with definite plus probable plus possible gastroparesis cohort. As expected, older age at diagnosis was associated with decreased survival (p<0.0001), and male gender was modestly associated with poor survival (p=0.06). Cause of gastroparesis was associated with survival (p=0.009) with idiopathic gastroparesis exhibiting better survival in contrast to non-idiopathic gastroparesis. Overall, 92 out of 222 patients with gastroparesis (definite, probable and possible) had diabetes mellitus; 36 of the diabetic cases were deceased. Non-diabetic gastroparesis was associated (p=0.0007) with better survival than diabetic gastroparesis.
DISCUSSION
This population-based epidemiological study of gastroparesis has determined the incidence and prevalence of gastroparesis, and we observed significantly lower estimates than data based on tertiary hospital settings.4 The age and gender-adjusted incidence of definite gastroparesis ranged from 6.3 to 17.2 cases per 100,000 person-years. Even applying broader criteria for gastroparesis, the incidence and prevalence remained similar, irrespective of the different diagnostic criteria used. Notably, the disease burden of gastroparesis is similar to other important gastrointestinal disease such as inflammatory bowel disease 14, 15 and hence the observed prevalence still represents a substantial disease burden in the United States.
Most studies on the prevalence of gastroparesis have come from tertiary referral centers or have been limited to the diabetic gastroparesis.4,6,7,8 In previous studies, delayed gastric emptying was reported in 30 to 50% of type 1 diabetic patients and 15 to 30% of type 2 diabetics 7, 16, 17, 18, 19, 20 Because of selection bias, the previous studies of gastroparesis might have overestimated the prevalence of gastroparesis. We would argue that our observed prevalence of definite gastroparesis in Olmsted County of 37.8 in women and 9.6 in men per 100,000 persons represents a figure closer to the true disease burden in the USA at least in whites.
Our study showed a distinct female predominance of gastroparesis, which is consistent with prior investigations.4,5,7,8 The incidence and prevalence of gastroparesis in women was four times higher than in men. In a tertiary single-center study, 82% of patients were women, with a mean age of 45 year4 and this finding was similar with the gastroparesis-related hospitalization data from North America.5 It is still unclear whether the higher incidence of gastroparesis in women reflects a specific predisposition to develop delayed emptying. Delayed gastric emptying has been noted to be more common in women compared with men in most other studies.21,22,23,24 Several studies showed that female gender was associated with delayed gastric emptying in both functional dyspeptics and in patients with diabetes.20,22 In few studies, gastrointestinal transit time was significantly prolonged during the luteal phase of the menstrual cycle when progesterone levels were increased compared with the follicular phase.25,26 However, the role of ovarian hormones on gastric emptying is still unclear.24,27 Another possible explanation is a difference in health-care seeking behavior. It is generally accepted that most functional gastrointestinal disorders are more common in women than in men and female patients with IBS and/or dyspepsia seek health care more frequently than males.22 Whether more men have undiagnosed gastroparesis in the community needs to be investigated.
The incidence rate of gastroparesis may be increasing with advancing age. Recent gastroparesis-related hospitalization data in the United States showed increasing number of gastroparesis related admissions from 1995 to 2004.5 These data were based on an all-payer inpatient care database in the United States which covered approximately 20% of U.S. hospitals. They postulated that the increase of gastroparesis-related hospitalizations defined by ICD-9 code was reflective of the increasing prevalence of diabetes mellitus, changes in gastroparesis diagnostic criteria, treatment, or better recognition and diagnosis of this disorder.5 While the incidence rate during 2001–2006 was increased in Olmsted County compared with that during 1996–2000, it didn’t reach the statistical significance but this might reflect a type II error. It is therefore unclear whether the incidence rate of gastroparesis is truly increasing or not.
Data describing the outcome and natural history of gastroparesis are very limited. One study of 146 patients with gastroparesis in an academic referral center observed that three quarters of the patients required long-term medications and 7% of patients died after 6-years of follow-up, suggesting a substantial amount of morbidity and mortality associated with gastroparesis.4 Another study of the natural history of diabetic gastroparesis, however, suggested that upper GI symptoms in patients with diabetes were stable over 12 years of follow-up and delayed gastric emptying was not related with the increased mortality after adjustment for comorbid diseases.7,8,20 Notably, these studies were limited because of relatively small numbers of patients and potential referral bias.4,7,8 Among incident cases of gastroparesis combing all subjects in this study, one third of patients died and another one third required hospitalization, medications or tube feeding related to gastroparesis. Notably, we observed that overall survival in patients with gastroparesis was significantly lower than that of the Minnesota white population. These results indicate that gastroparesis is not a benign condition and more aggressive management strategies need to be devised to reduce mortality. We did note that the survival of the definite gastroparesis cohort was better than that of all cases combined. The underlying causes of gastroparesis include diabetes mellitus, surgery, connective tissue disease, systemic neuromuscular disturbances and Parkinson’s disease4,5,7,8 and the definite gastroparesis cohort was composed of less secondary gastroparesis and more cases of idiopathic gastroparesis, which likely has a better prognosis. Also, patients with more severe co-morbidities may be less likely to be referred for functional assessment of gastric emptying to confirm the diagnosis. We could not, however, formally compare the outcome of idiopathic gastroparesis with secondary gastroparesis because of study power.
The current study has several strengths. We took a population-based approach which should have minimized the effect of referral bias and improved the generalizability of the results. The entire population studied obtains care from indexed local providers and the entire population is covered by a unified diagnostic index. Case ascertainment was based on a community-wide diagnostic index that has an established track record of accuracy,10 and the population at risk was clearly defined because of the geographic demarcation of the community. Furthermore, we specified three specific gastroparesis cohorts including those with definite and possible disease which would be expected to cover all gastroparesis cases in the community. There are also some potential limitations of this study. First, this was a historical cohort study and hence misclassification of gastroparesis may have reduced our estimates of the incidence and prevalence. A key issue may be underreporting or under-recognition of gastroparesis although we took every step to minimize this issue. Second, this study is not representative of all ethnic groups with gastroparesis because our cohort mainly consisted of a white population. Functional dyspepsia in non-referred multiethnic subjects in the United States may have a higher prevalence in African-Americans than in whites, but data on gastroparesis are not available.28 Hence, it is not clear whether ethnic differences influence the epidemiology of gastroparesis or not. Third, we may have underestimated the prevalence of gastroparesis as not everyone with gastroparesis-like symptoms will undergo a gastric emptying test, and there may be some with gastroparesis who are otherwise well and have never presented for care. Therefore, health care seeking behavior or practice patterns may have influenced the prevalence estimates, although we tried to minimize selection bias by applying different diagnostic certainties. Finally, the reproducibility of gastric emptying scintigraphy may vary, and gastric emptying status may have been misclassified because of concurrent medication use. However, all patients who underwent gastric emptying testing followed strict standarized instructions in terms of any medication use permitted in the 48 hours prior to the study. The gastric emptying testing performed at Mayo Clinic has been validated and excellent reproducibility has been documented.11, 29–31 Among the patients with gastroparesis in this study, 13 patients underwent repeated gastric emptying testing with an interval of between 1 and 6 years. Of these cases, 4 patients still had delayed gastric emptying on the second test and notably their symptoms had improved. The remaining 9 patients had symptom improvement and normal gastric emptying on the second test, but this may reflect natural resolution of the disease.
In summary, this is to our knowledge the first, large population-based study to describe the incidence, prevalence and outcome of gastroparesis in the community. Gastroparesis is an uncommon condition in the community, compared with tertiary hospital settings, but still represents a major disease burden. Most patients with gastroparesis need continuous medical care, and this disease has a relatively poor prognosis despite modern management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kassander P. Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum) Ann Intern Med. 1958;48:797–812. doi: 10.7326/0003-4819-48-4-797. [DOI] [PubMed] [Google Scholar]
- 2.Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, Tack J. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–50. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Locke GR, 3rd, Lahr BD, Zinsmeister AR, Tougas G, Ligozio G, Rojavin MA, Tack J. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55:933–9. doi: 10.1136/gut.2005.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 5.Wang YR, Fisher RS, Parkman HP. Gastroparesis-Related Hospitalizations in the United States: Trends, Characteristics, and Outcomes, 1995–2004. Am J Gastroenterol. 2007;103:313–322. doi: 10.1111/j.1572-0241.2007.01658.x. [DOI] [PubMed] [Google Scholar]
- 6.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–96. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 7.Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–9. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 8.Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22:503–7. doi: 10.2337/diacare.22.3.503. [DOI] [PubMed] [Google Scholar]
- 9.Jones KL, Russo A, Berry MK, Stevens JE, Wishart JM, Horowitz M. A longitudinal study of gastric emptying and upper gastrointestinal symptoms in patients with diabetes mellitus. doi: 10.1016/s0002-9343(02)01228-7. [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 11.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–90. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin HC, Prather C, Fisher RS, Meyer JH, Summers RW, Pimentel M, McCallum RW, Akkermans LM, Loening-Baucke V. AMS Task Force Committee on Gastrointestinal Transit. Measurement of gastrointestinal transit. Dig Dis Sci. 2005;50:989–1004. doi: 10.1007/s10620-005-2694-6. [DOI] [PubMed] [Google Scholar]
- 13.Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, McCallum RW, Nowak T, Nusynowitz ML, Parkman HP, Shreve P, Szarka LA, Snape WJ, Jr, Ziessman HA. Consensus Recommendations for Gastric Emptying Scintigraphy: A Joint Report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2007;102:1–11. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 14.Loftus EV, Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Ulcerative colitis in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gut. 2000;46:336–43. doi: 10.1136/gut.46.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loftus EV, Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–8. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 16.Bredenoord AJ, Chial HJ, Camilleri M, Mullan BP, Murray JA. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2003;1:264–272. [PubMed] [Google Scholar]
- 17.Nilsson P. Diabetic gastroparesis: a review. J Diabetes Complications. 1996;10:113–122. doi: 10.1016/1056-8727(96)00001-3. [DOI] [PubMed] [Google Scholar]
- 18.Koch KL. Diabetic gastropathy. Gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061–1075. doi: 10.1023/a:1026647417465. [DOI] [PubMed] [Google Scholar]
- 19.Jebbink HJA, Bravenboer B, Akkermans LMA, van Berge-Henegouwen GP, Smout AJPM. Relationships between dyspeptic symptoms and gastrointestinal motility in patients with type 1 (insulin dependent) diabetes mellitus. Diabetologia. 1993;36:948–954. doi: 10.1007/BF02374478. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz M, Wishart JM, Jones KL, Hebbard GS. Gastric emptying in diabetes: an overview. Diabet Med. 1996;13:S16–22. [PubMed] [Google Scholar]
- 21.Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–68. [PubMed] [Google Scholar]
- 22.Stanghellini V, Tosetti C, Paternico A, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036–1042. doi: 10.1053/gast.1996.v110.pm8612991. [DOI] [PubMed] [Google Scholar]
- 23.Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut . 996;39:299–305. doi: 10.1136/gut.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wald A, Van Thiel DH, Hoechstetter L, Gavaler JS, Egler KM, Verm R, Scott L, Lester R. Gastrointestinal transit: the effect of the menstrual cycle. Gastroenterology. 1981;80:1497–500. [PubMed] [Google Scholar]
- 25.Gill RC, Murphy PD, Hooper HR, Bowes KL, Kingma YJ. Effect of the menstrual cycle on gastric emptying. Digestion. 1987;36:168–74. doi: 10.1159/000199414. [DOI] [PubMed] [Google Scholar]
- 26.Monés J, Carrió I, Calabuig R, Estorch M, Sainz S, Berná L, Vilardell F. Influence of the menstrual cycle and of menopause on the gastric emptying rate of solids in female volunteers. Eur J Nucl Med. 1993;20:600–2. doi: 10.1007/BF00176554. [DOI] [PubMed] [Google Scholar]
- 27.Horowitz M, Maddern GJ, Chatterton BE, Collins PJ, Petrucco OM, Seamark R, Shearman DJ. The normal menstrual cycle has no effect on gastric emptying. Br J Obstet Gynaecol. 1985;92:743–6. doi: 10.1111/j.1471-0528.1985.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 28.Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99:2210–6. doi: 10.1111/j.1572-0241.2004.40052.x. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri M, Zinsmeister AR, Greydanus MP, Brown ML, Proano M. Towards a less costly but accurate test of gastric emptying and small bowel transit. Dig Dis Sci. 1991;36:609–15. doi: 10.1007/BF01297027. [DOI] [PubMed] [Google Scholar]
- 30.Thomforde GM, Camilleri M, Phillips SF, Forstrom LA. Evaluation of an inexpensive screening scintigraphic test of gastric emptying. J Nucl Med. 1995;36:93–6. [PubMed] [Google Scholar]
- 31.Charles F, Camilleri M, Phillips SF, Thomforde GM, Forstrom LA. Scintigraphy of the whole gut: clinical evaluation of transit disorders. Mayo Clin Proc. 1995;70:113–8. doi: 10.4065/70.2.113. [DOI] [PubMed] [Google Scholar]


