Abstract
Purpose:
The aims of this study were to investigate telomere function in normal and Barrett's esophageal adenocarcinoma (BEAC) cells purified by laser capture microdissection (LCM) and to evaluate the impact of telomerase inhibition in cancer cells in vitro and in vivo.
Experimental Design:
Epithelial cells were purified from surgically resected esophagi. Telomerase activity was measured by modified “Telomeric Repeat Amplification Protocol” and telomere length determined by Real-Time PCR assay. To evaluate the impact of telomerase inhibition, adenocarcinoma cell lines were continuously treated with a specific telomerase inhibitor (GRN163L) and live cell number determined weekly. Apoptosis was evaluated by annexin labeling and senescence by beta-galactosidase staining. For in vivo studies, SCID-mice were subcutaneously inoculated with adenocarcinoma cells and following appearance of palpable tumors, injected intraperitoneally with saline or GRN163L.
Results:
Telomerase activity was significantly elevated whereas telomeres were shorter in BEAC cells relative to normal esophageal epithelial cells. The treatment of adenocarcinoma cells with telomerase inhibitor, GRN163L, led to loss of telomerase activity, reduction in telomere length, and growth arrest through induction of both the senescence and apoptosis. GRN163L induced cell death could also be expedited by addition of chemotherapeutic agents, doxorubicin and ritonavir. Finally, the treatment with GRN163L led to a significant reduction in tumor volume in a subcutaneous tumor model.
Conclusions:
We show that telomerase activity is significantly elevated whereas telomeres are shorter in BEAC and suppression of telomerase inhibits proliferation of adenocarcinoma cells both in vitro and in vivo.
Keywords: Telomerase, Telomere, Laser Capture, Barrett's, Adenocarcinoma
Introduction
The ends of eucaryotic chromosomes associate with specific DNA binding proteins to form specialized nucleoprotein structures which play a vital role in maintaining genomic integrity by preventing exonucleolytic degradation and end-to-end fusion of chromosomal DNA (1). In humans the telomeric DNA is comprised of few hundred to several thousand repeats of a conserved DNA sequence “TTAGGG” (2-4), which is associated with at least six proteins TRF1, TRF2, TIN2, hRap, POT1 and TPP (5), each with a specific role in the maintenance of telomeric DNA length and genomic integrity of a cell.
Telomere length in normal somatic cells shortens with each cell division. Since DNA polymerases require an RNA primer to initiate DNA synthesis, they are unable to replicate the 5′ end of lagging strand of DNA, leading to a loss of 50-100 base pairs of telomeric DNA with each cell division in normal human cells (6). When telomere length reaches below the critical limit of 2kb, the critically short telomeres are recognized as DNA damage and may lead to either apoptosis and/or cellular senescence (7-9). The length of telomeric DNA may therefore act as a mitotic clock (10) which determines the life span of normal human somatic cells. Although telomeres play a vital role in the maintenance of genomic integrity and cellular health (11-14), telomere dysfunction or excessive telomere shortening caused by mutation or inherited disorder may result in genetic instability (15) and development of cancer (16). Consistent with these findings, it has been demonstrated that telomere length may serve as a marker for progression and/or prognosis for cancers such as neuroblastoma (17), prostate, colon, breast, brain, head and neck, and lung (18).
Telomere length is maintained by telomerase, an enzyme with an RNA component (TERC) containing a short template for the synthesis of TTAGGG telomere repeats (19), and a polypeptide component with a reverse transcriptase activity (TERT). Telomerase extends telomere length by adding TTAGGG sequences to guanine-rich strand of telomeric DNA. Activity of telomerase depends on posttranslational and posttranscriptional modifications of the protein component (hTERT) including phosphorylation, assembly into telomerase holoenzyme, and its association with other proteins, such as p23, and hsp90 etc (6, 20). Telomerase activity is present in human germ-line cells which maintain their telomere length (21). Conversely, telomerase activity is low or undetectable in normal somatic tissues in which telomeres are not extended and therefore undergo progressive shortening with cell division (22). Telomerase knock-out mice show a number of abnormalities including hair graying, alopecia, higher incidence of skin lesions, reduced body weight, delayed healing, diminished hematopoietic reserve, and atrophy of small intestinal cells by generation three (23). Additionally they show hypoproliferative defects in lymphoid, hematopoietic, and gonadal cells by generation six (24). Conversely, the induction of telomerase in normal human cells increases their life-span in culture (25) and oncogenic conversion in the presence of SV40 T antigen and H-ras (26). Consistent with this, telomerase is re-activated in most immortalized cells(27-31), and cancers.(30)
Although majority of immortal and cancer cells have elevated telomerase activity, a subset of immortal and cancer cell lines lack detectable telomerase activity, and maintain their telomeres through an alternative (ALT) mechanism (32). Consistent with this, Opitz et al. (33) have demonstrated the immortalization of human primary oral squamous epithelial cells through a mechanism independent of telomerase. However it has also been shown that human cells can utilize both telomere maintenance mechanisms, telomerase and ALT, at the same time (34).
In cancer cells, telomerase activity is elevated and telomeres are shorter but stable (35, 36). Since telomeres are shorter in cancer cells relative to normal cells whereas telomerase activity is elevated in most cancers but absent or low in normal somatic cells (21, 29, 37), the inhibitors of telomerase activity have a strong potential to be used as anti-cancer therapeutics which may inhibit proliferation of tumor cells while having little or no effect on normal cells. Low levels of telomerase activity is also detected in some normal somatic cells such as hematopoietic, peripheral blood, and gastroesophageal cells; however, transient telomerase inhibition may not affect them as telomeres in normal cells are significantly longer than those in cancer. Consistent with this, we have shown that treatment of various cancer (8, 9, 38-40) and immortal (human cell lines) (41, 42) with a variety of telomerase inhibitors results in the loss of telomerase activity, telomere shortening, and reversal of immortality.
Morales et al.(43) have demonstrated that expression of RNA component of telomerase (hTR) is increased in surgical specimens of Barrett's esophagus (BE) and further elevated in high grade dysplasia and esophageal adenocarcinoma. Consistent with this report, studies by Lord et al.(44) indicate that transcript levels of catalytic subunit of telomerase (hTERT) are also elevated early at Barrett's esophagus stage with a further upregulation in specimens of high grade dysplasia and adenocarcinoma. These studies may indicate that telomerase expression is increased early at BE stage, however levels of hTR and hTERT do not reflect telomerase activity, the ability of enzyme to add telomeric repeats. Although hTERT mRNA expression correlates with telomerase activity in many cancers, it does not measure telomerase activity,(45) which depends on posttranscriptional and posttranslational modifications of hTERT including phosphorylation of hTERT protein, assembly into telomerase holoenzyme, and its association with other proteins, such as hsp90 and p23 etc.(6, 20)
The aims of this study were to: 1) Assess telomerase activity and telomere length in primary human cells (normal and cancer; BEAC) purified by laser capture microdissection; and 2) Evaluate the efficacy of a specific telomerase inhibitor GRN163L in vitro and in vivo. We show that Barrett's esophageal adenocarcinoma cells purified from surgical specimens by LCM have significantly elevated telomerase activity and markedly reduced telomeres, relative to normal esophageal epithelial cells. The treatment of adenocarcinoma cells with a specific and potent telomerase inhibitor, GRN163L, led to loss of telomerase activity, further reduction in telomere length, and inhibition of cell growth through induction of both the senescence and apoptosis. GRN163L induced cell death could also be expedited by combination treatment with doxorubicin (a DNA interacting drug) or ritonavir (a protease inhibitor). Addition of these drugs to cultures pretreated with GRN163L had a significant additive effect on GRN163L-induced cancer cell death. Efficacy of GRN163L was also tested in a murine model in which SCID-mice were subcutaneously inoculated with SEG-1 adenocarcinoma cells and following appearance of palpable tumors, treated with either PBS or GRN163L. A significant reduction in tumor volume was seen in mice treated with the drug.
MATERIALS AND METHODS
Human tissues
Tissue samples that were immediately processed and frozen at −80° C from surgically resected esophagi bearing various Barrett's esophagus related lesions including adenocarcinoma were used for this study. Protocol for this study is already approved by IRB of University of MI, Ann Arbor, MI. The tissue specimens were coded without use of any of patient private identifiers. For all specimens, only the mucosa containing the columnar epithelium, or cancer or normal squamous mucosa was selected by macro dissection. The presence of goblet cells was diagnostic for Barrett's mucosa. The specimens of five normal, one Barrett's, and five Barrett's esophageal adenocarcinoma were used for this study and the BE specimen was from patient who had cancer. Populations of normal and abnormal cells were isolated by laser capture microdissection (LCM).
Acquisition of target cells with laser capture micro-dissection (LCM)
Target cells from fresh-frozen tissue sections were obtained using the laser capture microdissection (LCM) technique as following: Frozen tissue sections (8 μm) were cut, mounted onto glass slides, fixed (70% ethanol for 3 min), and stained with Hematoxylin. The stained sections were examined under a microscope by an experienced gastrointestinal pathologist and the targeted area including various Barrett's esophagus related lesions was identified according to the standard histopathological criteria (46). Specialized intestinal metaplasia (SIM) was defined as specialized columnar epithelium containing well formed goblet cells whereas BEAC was defined with the break down of the basement membrane by dysplastic glands that present within the laminar propria and beyond. Once the targeted glands were identified, the area was marked with a marker and the slide re-positioned for laser capture micro-dissection using the Pixcell II LCM System (Arcturus Inc., Mountain View, CA ), according to the standard protocol (47). The captured cells were placed in an appropriate buffer for subsequent analysis.
Telomere Length Analysis
Genomic DNA was isolated from captured cells using the QIAamp DNA Micro Kit (Qiagen, Valencia, CA) Average relative telomere length as represented by the telomere repeat copy-number to single-copy gene copy-number (T/S) ratio, was determined using a modified version of a previously described Real-Time PCR assay on an Applied Biosystems 7900HT Thermocycler (48). Briefly, 5 ng of genomic DNA was dried down in a 384-well plate and resuspended in 10μL of either the telomere or 36B4 qPCR reaction mixture. Primer sequences of Tel-1, Tel-2, 36B4-U and 36B4-D were previously described (48). The telomere reaction mixture consisted of 1x Qiagen Quantitect Sybr Green Master Mix, 2.5mM of DTT, 270nM of Tel-1, and 900nM of Tel-2 primer. The reaction proceeded for 1 cycle at 95°C for 5:00, followed by 40 cycles at 95°C for 0:15, and 54C for 2:00. The 36B4 reaction consisted of 1x Qiagen Quantitect Sybr Green Master Mix, 300nM of 36B4U primer, and 500nM of 36B4D primer. The 36B4 reaction proceeded for 1 cycle at 95°C for 5:00, followed by 40 cycles at 95°C for 0:15, and 58°C for 1:10. All samples for both the Telomere and 36B4 reactions were performed in triplicate. In addition to the samples, each 384-well plate contained a 6-point standard curve from 1.25ng to 30ng using pooled buffy-coat derived genomic DNA. The slope of the standard curve for both the telomere and 36B4 reactions was −3.6 +/− 0.2 and the linear correlation coefficient (R2) value for both reactions was 0.98 and 0.99 respectively. The T/S ratio for each sample was calculated by subtracting the mean 36B4 Ct value from the mean Telomere Ct value. The T/S ratio values were then linearized to 2−(−dCt).
Assay of Telomerase Activity
For telomerase activity assays, the cells were immediately lysed in CHAPS lysis buffer (Chemicon International, Inc.Temecula, CA) at 50 cells/μl and stored at −150°C. Telomerase activity was measured by an improved version of the original Telomeric Repeat Amplification Protocol, using the TRAPeze XL Telomerase Detection Kit (Chemicon International, Inc.Temecula, CA), as previously reported (8, 9, 39). TRAPeze XL is a fluorescence-based highly sensitive assay and provides quantitative analysis of telomerase activity. Utilizing this assay, telomerase activity can be detected in extracts from as few as one hundred telomerase-positive cells. In triplicate, cell lysates were mixed with TRAPeze XL reaction mix containing Amplifuor primers, and incubated at 30°C for 30 minutes. Amplified telomerase products were quantitated with FLUOstar OPTIMA Fluorescence Plate Reader (BMG LABTECH, Durham, NC). Telomerase activity (in TPG units) was then calculated by comparing the ratio of telomerase products to an internal standard for each lysate, as described by the manufacturer. The assay has a linear range of 1 to 300 TPG units, equivalent to telomerase activity from approximately 30 to 10,000 control cells.
Telomerase inhibitor GRN163L
GRN163L is a palmitoyl (C16) lipid – attached oligonucleotide (5′-Palm-TAGGGTTAGACAA 3′), targeting RNA component of telomerase and has N3′-P5′ thio-phosphoramidate back bone.
Cell lines and treatments
Adenocarcinoma cell lines BIC-1, SEG-1, and FLO-1 have been described previously (49). Cells were cultured in DMEM with 10% FBS, as described previously (49). Constant numbers of cells were plated in multiple 100 mm dishes, treated with GRN163L at 0.5 – 2.0 μM and evaluated weekly for cell viability, telomere length, and telomerase activity. Briefly, every week the cells were harvested, counted, and viable cell number confirmed by trypan blue exclusion and MTT assays. Aliquots of cells were separated for various molecular analyses and the remaining cells replated at the same cell number and at the same concentration of the inhibitor. For combination studies, cells were pre-treated with GRN163L for 10 days. Test agents were then added at various concentrations and live cell number determined at alternate days.
Apoptosis
Following exposure to GRN163L, apoptotic cells were detected by annexin labeling using FITC-Annexin Apoptosis Detection Kit (Oncogene Research Products, Boston, MA). Briefly, the cells treated with GRN163L were harvested, 0.5 ml of cells (1 × 106 cells/ml) were mixed with FITC-annexin in “Binding Buffer” and incubated for 15 minutes at room temperature (RT). A portion of cell suspension (50 μl) was placed onto a glass slide, covered with a cover slip and viewed immediately using a fluorescence microscope equipped with FITC (green) filter. Approximately two hundred cells, representing at least five distinct microscopic fields, were analyzed to assess the fraction of FITC labeled cells for each sample.
Senescence
One day before this assay, the treated cells were plated on Lab-Tek slides in the presence of mismatch or match (GRN163L) oligonucleotide and the attached cells were stained for β-galactosidase expression, a marker for cellular senescence (50). Briefly, the cells were rinsed three times with PBS and fixed in 2% formaldehyde and 0.2% glutaraldehyde solution in PBS. The cells were then washed again as described above and stained overnight in solution containing 1 mg/ml X-gal, 40 mM citric acid/sodium phosphate (pH 6), 5 mM potassium ferrocyanide, 150 mM NaCl, and 2 mM MgCl2. The stain was then removed, cells were rinsed with PBS, and staining was viewed under a fluorescence microscope (Olympus).
Murine subcutaneous tumor model
SCID mice were purchased from the National Cancer Institute-Frederick Animal Production area (Frederick, MD). Maintenance of the animals was carried out following guidelines of the Institutional Animal Care and Use Committee (IACUC) and all experimental procedures were approved by the IACUC and the Occupational Health and Safety Department of the University of Alabama at Birmingham. The mice were acclimatized for a week and 3.0×106 SEG-1 cells in 100 μl saline were injected subcutaneously (s.c) in the interscapular area. Following appearance of palpable tumors, mice were injected intraperitoneally with normal saline alone or GRN163L at concentrations described in the Figure Legends. Animals were sacrificed when tumors reached 2cc in volume or when paralysis or major compromise in their quality of life occurred.
RESULTS
Laser capture microdissection (LCM) significantly enhances the sensitivity of telomerase activity assay
Epithelial cells were purified from tissue specimens of normal and Barrett's esophagi as shown in Figure 1A and telomerase activity was measured in these purified cells and also in tissue extracts of the same specimens, using same amount of total protein. Telomerase activity in tissue extracts of normal and Barrett's esophagus was only 3.1 ± 0.9 and 19 ± 4.6 TPG units, respectively. However the activity in LCM purified normal and Barrett's esophageal cells was 37.7 ± 5.5 and 197 ± 23.1 TPG units, respectively (Figure 1 B). These data show that telomerase activity is ∼ 10-fold higher in LCM derived cells relative to that in tissue extracts of the same samples. To investigate if the low activity detected in tissue extracts was due to PCR inhibitors, both the tissue extracts and lysates of LCM purifed BE cells were diluted and evaluated for telomerase activity. A 10-fold dilution of tissue extract and the lysate of LCM purified cells of BE led to 11.4- and 9.2-fold reduction in telomerase activity, respectively.
Figure 1. Telomerase activity in tissue extracts and esophageal epithelial cells isolated by laser capture microdissection (LCM).
A. Isolation of esophageal epithelial cells by LCM. Panel I shows epithelial cells in a microscopic field before LCM; Panel II is the same microscopic field after LCM and shows that epithelial cells have been removed; Panel III shows the captured epithelial cells. B. Telomerase activity in tissue extract vs LCM purified cells. Three normal and three BEAC surgical specimens were processed for evaluation of telomerase activity in LCM and tissue extracts. Each surgical specimen was cut into two portions; one processed for LCM purification of epithelial cells and the other used for making tissue extract. Telomerase activity is shown in tissue extract, equivalent of 0.6 μg protein, of normal esophagus (lane 1); tissue extract, equivalent of 0.6 μg protein, of Barrett's esophagus (lane 2); diluted tissue extract, equivalent of 0.06 μg protein, of Barrett's esophagus (lane 3); lysate of LCM purified normal esophageal epithelial cells, equivalent of 0.6 μg protein (lane 4); lysate of LCM purified Barrett's esophagus cells, equivalent of 0.6 μg protein (lane 5); diluted lysate, equivalent of 0.06 μg protein, of LCM purified Barrett's esophagus cells (lane 6). C. Telomerase activity in defined primary normal and Barrett's adenocarcinoma (BEAC) cells derived by laser capture microdissection. The activity was measured in the lysates (equivalent of 0.6 μg protein) of normal primary esophageal epithelial cells purchased from ScienCell (HEEC), epithelial cells purified from surgical specimens of normal esophagus from three different patients, and epithelial cells purified from surgical specimens of Barrett's esophageal adenocarcinoma from three different patients, using LCM.
Telomerase activity is markedly elevated in Barrett's esophageal adenocarcinoma (BEAC) relative to normal esophageal epithelial cells
Epithelial cells purified from tissue specimens of normal, Barrett's adenocarcinoma (BEAC), and commercially obtained primary normal esophageal epithelial cells (HEEC; ScienCell) were lysed at same cell concentration and evaluated for telomerase activity using TRAPeze XL Telomerase Detection Kit (Chemicon International). Telomerase activity in LCM purified normal esophageal epithelial cells from three different donors was 41±8 TPG units; consistent with this the activity in commercially obtained primary normal esophageal epithelial cells (HEEC) was also low (37±3 TPG units) (Figure 1C). A markedly elevated telomerase activity was detected in BEAC (275±20 TPG units; Figure 1C). Relative to normal esophageal epithelial cells, the activity in BEAC was 7.0±0.5-fold elevated (P=0.004). These data indicate that telomerase activity is significantly elevated in primary BEAC cells relative to normal esophageal epithelial cells.
Telomeres are consistently shorter in BEAC relative to normal esophageal epithelial cells
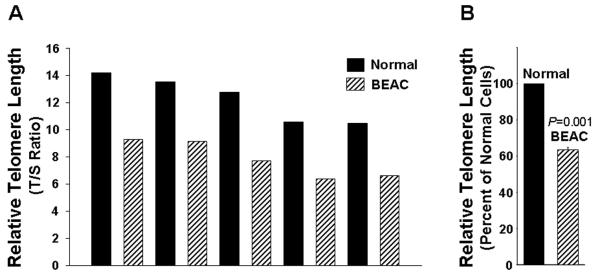
We next analyzed telomere length in a panel of LCM purified normal and BEAC cells. Genomic DNA was isolated using QIAamp DNA Micro Kit (Qiagen, Valencia, CA) and telomere length measured by real time kinetic quantitative polymerase chain reaction (48). Briefly the PCR reactions for telomere specific (T) and β2-globin control gene specific (S) amplification were set up in separate 96-well plates. Except primers, other conditions including reagent concentrations, DNA amount, and sample order were the same for both plates. To monitor the efficiency of PCR reactions, a standard curve spanning at least 0.3 to 5 ng/μl of reference DNA was produced in each 96-well plate. DNA from each sample and reference DNA was tested in triplicate using a total of 12.5 ng DNA/well. PCR amplification reactions were performed in a PRISM 7900 Sequence Detection System (Applied Biosystems) using conditions described by Cawthon (2005) (48). Analysis of data and determination of telomere length were done using software (Applied Biosystems), as described (48). The ratio of telomere specific (T) and single control gene specific (S) real time kinetic PCR reactions (T/S ratio) indicated the relative telomere length. The T/S ratio, indicating relative telomere length, ranged from 10.3 – 14.3 in normal and 6.2 – 9.2 in BEAC cells, indicating that telomeres in all BEAC specimens were shorter than those in normal samples (Figure 2A). Average TL was reduced by 38% (P=0.001) in BEAC relative to the length in normal epithelial cells (Figure 2 B). These results provided a further rationale for investigating the importance of telomerase as a target for the treatment of Barrett's esophageal adenocarcinoma.
Figure 2. Telomere length in esophageal epithelial cells isolated from normal and Barrett's adenocarcinoma speimens by LCM.
Normal epithelial and BEAC cells were isolated by LCM, genomic DNA was extracted, and telomere length determined by qPCR. A. Relative Telomere length in primary normal and BEAC cells purified by LCM. B. Average telomere length in five normal and five BEAC specimens is shown as percent of telomere length in normal cells.
GRN163L is taken up by adenocarcinoma cells without transfection and inhibits telomerase activity
GRN163L is a Palmitoyl (C16) lipid – conjugated oligonucleotide N3′-P5′-thio-phosphoramidate targeting template region of RNA component of telomerase (hTR) (Figure 3A). We treated SEG-1 cells with TAMRA-labeled GRN163L at various concentrations and monitored the uptake and intracellular localization at 24 hrs using a confocal microscope. As seen by TAMRA (red) fluorescence in Figure 3B, the drug was efficiently taken by SEG-1 cells without any need of a transfection procedure or reagent. The fluorescence was predominantly nuclear. An efficient uptake of GRN163L without any need for transfection reagents was also observed for other adenocarcinoma cell lines BIC-1 and FLO-1 (not shown).
Figure 3. GRN163L inhibits telomerase activity in adenocarcinoma cells.
A. Structure of GRN163L, a Palmitoyl (C16) lipid – attached oligonucleotide targeting RNA component of telomerase. B. Uptake of GRN163L in SEG-1 cells without any need of transfection. Cells were treated with TAMRA-labeled GRN163L for 24 hrs and examined by a multiphoton fluorescence microscope. Uptake can be seen as red fluorescence. C – D. Telomerase activity in cells treated with a mismatch control oligonucleotide (MM) or GRN163L. SEG-1 (C) and FLO-1 (D) were treated with GRN163L at various concentrations for 24 hrs and evaluated for telomerase activity using TRAPeze XL Telomerase Detection Kit.
We next evaluated if the uptake of GRN163L was also associated with the inhibition of telomerase activity. We therefore treated SEG-1, FLO-1, and BIC-1 cells with the drug at 0.1, 0.5, 1.0, and 2.0 μM concentrations for 24 hrs and evaluated for telomerase activity using TRAPezeR XL Telomerase Detection Kit (Intergen). GRN163L at 1 and 2 μM led to near complete inhibition of telomerase activity in SEG-1 cells (Figure 3C). Mismatch control oligonucleotide thio-phosphoramidate 5′-Palm – TAGGTGTAAGCAA (mismatch bases are underlined, designated as GRN140833) had no effect on growth of these cells at either concentration. Similarly, the treatment with 1 μM of GRN163L led to >95% inhibition of telomerase activity in FLO-1 (Figure 3 D) and BIC-1 cells (not shown). No significant inhibition of telomerase activity was seen at 0.1 μM concentration in any cell line.
Inhibition of telomerase by GRN163L leads to induction of growth arrest in adenocarcinoma cells
Cells were treated with GRN163L or mismatch oligonucleotide GRN140833 and the substrate attached viable cell number was counted every week. Cell viability was further confirmed by trypan blue exclusion and/or MTT assays. A marked arrest of cell proliferation was observed in all cell lines following treatment with the inhibitor, leading to a gradual decline in viable cell number by 79-82% in a span of 2 – 3 weeks. Treatment of SEG-1 cells with the drug at 1 and 2 μM concentrations induced 45% and 80% cell death respectively, in a period of 21 days (Figure 4A). Similar induction of cell death was observed following three week treatment of FLO-1 (Figure 4B) and BIC-1 (not shown). Consistent with telomerase activity data 1 μM GRN163L was enough to induce a marked (80%) growth inhibition in FLO-1 cells (Figure 4B).
Figure 4. Effect of GRN163L on growth and telomere length of adenocarcinoma cells.
A – B. Cells were cultured in regular growth medium containing GRN163L or mismatch control oligonucleotide at concentrations shown and live cell number determined at different time points as indicated. The growth curves show the mean of three independent experiments, with S.E.M. Panels: (A) SEG-1 cells; (B) FLO-1 cells; C – D. Telomere shortening in cells treated with GRN163L. Cells were treated with mismatch control oligonucleotide or GRN163L at 1 μM for 3 weeks and telomere length examined by qPCR. (C) Relative Telomere Length in control and GRN163L treated SEG-1 cells; (D) Relative Telomere Length in control and GRN163L treated FLO-1 cells.
GRN163L induced inhibition of telomerase activity is associated with reduction in telomere length
We also analyzed telomere length following exposure of cells to GRN163L using the same real time kinetic quantitative polymerase chain reaction as described above (48). Telomere length in GRN163L treated SEG-1 and FLO-1 cells was reduced by 38% and 67% respectively, relative to cells treated with mismatch control oligonucleotide (Figure 4C and D). These data indicate that inhibition of telomerase and growth arrest following treatment with GRN163 was also associated with reduction in telomere length.
The nature of cell death following GRN163L treatment
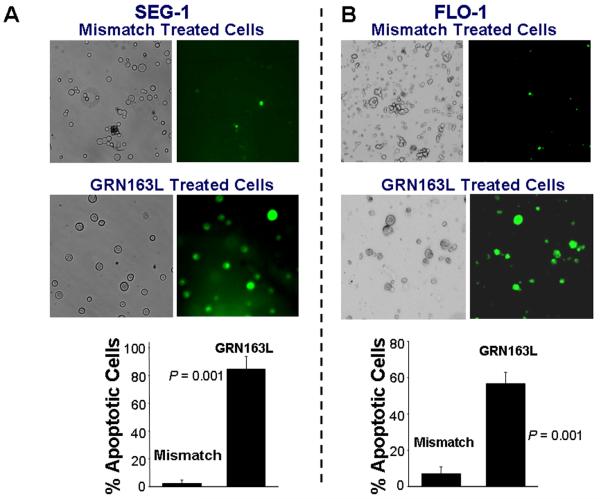
Cell lines were treated with 1 μM GRN163L and evaluated for apoptosis and senescence. Apoptotic cells were detected using an Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, San Jose, CA). Following two weeks treatment with 2 μM GRN163L, 85±9% of SEG-1 cells stained positive for annexin V, whereas only 2.4±2% annexin V positive cells were detected when the cells were treated with mismatch oligonucleotide (Figure 5A). Annexin labeling was also detected in majority (57±3%) of FLO-1 cells treated with drug (Figure 5B). These data indicate that telomerase inhibition and telomere shortening was associated with induction of apoptosis in treated cells.
Figure 5. Apoptosis and senescence in adenocarcinoma cells treated with GRN163L.
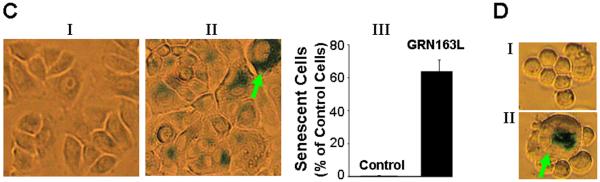
A – B. Apoptotic cell death in cells treated with GRN163L. The cells treated with control oligonucleotide or GRN163L were harvested, 0.5 ml of cells (1 × 106 cells/ml) were mixed with FITC-annexin and incubated for 15 minutes at room temperature (RT). A portion of cell suspension (50 μl) was placed onto a glass slide, covered with a cover slip and FITC-labeled apoptotic cells within the same microscopic field were viewed and photographed by phase contrast (PC) or by fluorescence emitted at 518 nm (FITC filter). Apoptotic cells appear bright green. (A) SEG-1 cells treated with 2 μM control oligonucleotide or GRN163L for three weeks; (B) FLO-1 cells, treated with 1 μM control oligonucleotide or GRN163L for two weeks. Approximately 200 – 300 cells representing five different microscopic fields were evaluated to assess percentage of apoptotic cells, shown as bar graphs in Panels A and B. C – D. Cells treated with GRN163L were evaluated for expression of β-galactosidase, a marker of cell senescence. C. β-galactosidase staining is shown in SEG-1 cells treated with 2 μM control oligonucleotide (I) or GRN163L (II) for three weeks. Bar graph showing percentage of senescent SEG-1 cells is also shown (III). D. FLO-1 cells treated with 1 μM control oligonucleotide (I) or GRN163L (II) for two weeks. Cells with typical senescent morphology in C and D are shown with arrows.
GRN163L treated cells were also evaluated for expression of β-galactosidase, a marker for cell senescence. Interestingly, a large fraction (64±7%) of SEG-1 cells treated with GRN163L also stained positive for β-galactosidase. Moreover, a subset of GRN163L treated cells also showed typical senescent morphology and became large in size. Such cells, indicated with red arrows, could be seen in SEG-1 (Figure 5C), FLO-1 (Figure 5D), and also BIC-1 cells (not shown). These data indicate that GRN163L induced both the senescence and apoptosis in adenocarcinoma cells.
Effect of combining GRN163L mediated telomerase inhibition with other agents
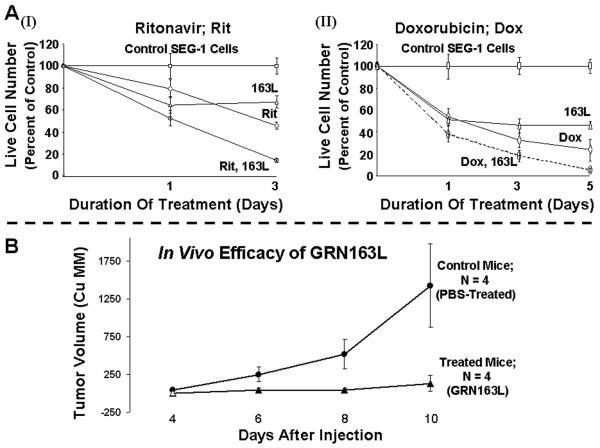
We have also evaluated the impact of other novel agents on GRN163L induced adenocarcinoma cell death. Of several agents tested, a DNA interacting drug “doxorubicin” and a protease inhibitor “ritonavir” had a significant additive effect on GRN163L-induced cell death. Addition of ritonavir and doxorubicin to cultures pretreated with GRN163L for 10 days led to ≥80% cell death within three days of addition (Figure 6A), compared to significantly higher viability of cells treated with either drug alone. These data indicate that cancer cell death following inhibition of telomerase can also be expedited by addition of other agents which may affect telomere length by other mechanisms.
Figure 6. Efficacy of GRN163L in combination with other agents and in a subcutaneous tumor model.
(A) SEG-1 cells were treated with 2 μM mismatch control oligonucleotide or GRN163L for ten days and the cells in each treatment flask were then divided into two aliquots. Cells were then cultured either in the presence of mismatch or match (GRN163L) oligonucleotides alone or with addition of ritonavir (2 μM) (I) or doxorubicin (2 nM) (II). Live cell number was determined at different time points. (B) In vivo efficacy of GRN163L in a subcutaneous tumor model. SCID mice were inoculated subcutaneously in the interscapular area with 3.0 × 106 SEG-1 cells and following appearance of tumors, the mice were treated intraperitoneally with saline alone or GRN163L 45mg/kg/per day.
Telomerase Inhibitor GRN163L inhibits adenocarcinoma cell growth in vivo
Efficacy of GRN163L was demonstrated in a subcutaneous tumor model. Briefly, the SCID-mice were subcutaneously inoculated in the interscapular area with 3.0 × 106 SEG-1 cells and following appearance of palpable tumors, mice were injected intraperitoneally with saline alone or GRN163L at 45 mg/kg/day. The tumor size in mice treated with 163L was significantly smaller than control (average tumor size in treated mice being > 10-fold smaller; p = 0.03), indicating a marked efficacy in vivo.
Discussion
In this manuscript, we studied telomere maintenance in primary human Barrett's esophageal adenocarcinoma (BEAC) and normal esophageal epithelial cells isolated by laser capture microdissection, and evaluated the impact of telomerase inhibition in three different adenocarcinoma cell lines and in a subcutaneous tumor model using SCID mice. We have shown that: 1) Telomerase activity assays conducted in tissue extracts can produce inaccurate results whereas the assays conducted in LCM purified specimens are more accurate and reliable; 2) Telomerase activity is significantly elevated whereas telomeres are significantly shorter in BEAC relative to normal esophageal epithelial cells; 3) GRN163L, a lipid -conjugated oligonucleotide telomerase template antagonist, is efficiently taken up by adenocarcinoma cells without any transfection and leads to inhibition of telomerase activity within 24 hrs; 4) Inhibition of telomerase activity is associated with growth arrest, reduction in telomere length, and induction of both the senescence and apoptosis; 5). A significant additive effect of doxorubicin and ritonavir was observed on GRN163L induced growth inhibition; and 6) Intraperitoneal injections of GRN163L caused a significant reduction in tumor size in vivo.
A large discrepancy has been reported for telomerase activity in normal and Barrett's esophagi (51-54) and for the activity observed in surgical and endoscopic biopsy specimens (51). The major cause of this controversy is the fact that these studies have been conducted on tissue extracts, contaminated with varying proportions of connective tissue cells. These contaminating cells do not only dilute the lysate but may also have an inhibitory effect on telomerase activity. Bachor et al. (51) have also proposed that the discrepancy arises because of contaminating connective tissue, which is more in surgically obtained specimens and less in superficial biopsies. Consistent with this, it has been shown that removal of dermis from epidermis led to an easier detection of telomerase activity in human skin (55). In an attempt to resolve the discrepancy, we have evaluated and compared telomerase activity in tissue extracts and LCM purified primary epithelial cells from normal and Barrett's esophagi. As shown in Figure 1, the telomerase activity was ∼ 10-fold higher in LCM derived cells relative to that in tissue extracts of the same samples, containing same amount of protein. The most likely explanation of this observation is that the activity in tissue extracts is substantially diluted because of the contaminating (connective tissue, blood vessels, nerve fibers, and smooth muscle) cells in mucosal tissue. Alternatively, the low telomerase activity detected in tissue extracts could also be due to the presence of PCR inhibitors. However, if this is true, the dilution of the samples should lead to a rise in activity. We therefore diluted both the tissue extracts and LCM samples and evaluated for telomerase activity. The dilution did not increase but decreased telomerase activity and the extent of decline was consistent with the linear standard curve, generated from dilutions of a control template. It is therefore less likely that the low telomerase activity detected in tissue extracts in these experiments was due to the presence of PCR inhibitors. We therefore conclude that telomerase assays conducted in tissue extracts, containing varying proportions of contaminating cells, may produce inaccurate results because of dilution effect. Purification of the specific cells under investigation can avoid all these problems and variables. We would also like to caution that the data shown in Figure 1B indicate the importance of LCM purification but may not confirm that telomerase activity is elevated in Barrett's esophagi, in general, because of insufficient sample size.
We therefore studied both the telomerase activity and telomere length in laser captured cells. Normal squamous epithelial cells were used as control for BEAC. It has been debatable as to what the appropriate control cells are for BE? Although BE cells are columnar they do not arise from either gastric or intestinal cells. We elected normal squamous epithelial cells as controls and found that telomerase activity is significantly elevated in BEAC. This is consistent with earlier reports of increased telomerase RNA (43) and catalytic subunit of telomerase (44) in Barrett's adenocarcinoma. Our data also show that telomeres are consistently shorter in BEAC relative to normal esophageal epithelial cells. This is also consistent with the observations of Finley et al.(56) indicating shorter telomeres in BEAC relative to those in epithelial cells from GERD patients. Elevated telomerase activity and shorter telomeres have also been reported in multiple myeloma (Shammas et al. In Revision), B-cell chronic lymphocytic leukemia (57), non-hematopoietic cancers such as hepatocellular carcinoma (58), breast cancer (59), and prostate cancer (60).
Although a variety of agents have been shown to inhibit telomerase activity and block proliferation of cancer cells (8, 9, 38, 42, 61-66), very few have been proven to be specific and also suitable for in vivo delivery and utilization. The telomerase inhibitor (GRN163L) used in this study is a thio-phosphoramidate oligonucleotide complementary to the template region of the RNA subunit of telomerase (hTERC). This oligonucleotide has a lipid (C16) moiety attached to it, which facilitates the uptake of this molecule in human cells without any need of a transfection procedure or reagent. Using TAMRA-labeled GRN163L, we have shown that this drug is efficiently taken up by human adenocarcinoma cells without any transfection within 24 hrs of treatment. Uptake of drug was associated with loss of telomerase activity and growth arrest after a lag period of 3-4 weeks. Although for cancers such as cervical, the inhibition of telomerase induces apoptosis within a few days without any requirement of telomere shortening (67), the cell death in majority of cancer cells occurs after a lag period of few weeks which is probably required for reduction of telomere length below critical limit (8, 9, 38, 39, 42, 68). Since the treatment of adenocarcinoma cells with GRN163L was associated with both the lag period and reduction in telomere length, it seems that growth arrest was probably induced by short telomeres.
Critically short telomeres are recognized as DNA damage and therefore activate mechanisms leading to apoptotic cell death or replicative senescence. Consistent with this, the inhibitors of telomerase have been shown to induce apoptotic cell death (9, 38, 39, 69, 70) or both the apoptosis and senescence (8, 69) in different cancer cell lines. In our study the adenocarcinoma cells treated with GRN163L stained positive for not only annexin-V, but also for β-galactosidase, indicating that growth inhibition in these cells was associated with induction of both the senescence and apoptosis. Induction of both the senescence and apoptosis in adenocarcinoma cells following inhibition of telomerase is consistent with our earlier observations (39). However we would caution that the data does not demonstrate that both the senescence and apoptosis were induced simultaneously. Senescence may have preceded apoptosis following inhibition of telomerase, as observed by Herbert at al. (Herbert et al. 2002), in immortal breast epithelial cell line. This is quite consistent with our data showing senescence in 64% and apoptosis in 85% of treated SEG-1 cells.
We have also shown that growth inhibition following exposure of adenocarcinoma cells to a telomerase inhibitor can be significantly expedited by combination therapy with other agents affecting telomerase or telomeres. The rationale of doing combination study was to first initiate the destruction of telomeric DNA in cancer cells by treating with a specific inhibitor (GRN163L) and then expedite the degradation of vulnerable telomeres by a very brief exposure to an agent, which may be less specific, but could significantly expedite the destruction of already eroding telomeres through different mechanisms. We therefore chose a number of potential agents and evaluated their effect on SEG-1 cells pre-treated with GRN163L. Ritonavir and doxorubicin were found to give a significant additive effect, indicating the possibility of combination therapy with GRN163L. The in vivo efficacy of GRN163L was demonstrated in a murine tumor model in which SCID-mice were inoculated in the interscapular area with SEG-1 adenocarcinoma cells and following appearance of palpable tumors, mice were injected intraperitoneally with either PBS alone or GRN163L. The dose (45 mg/kg/day) of GRN163L used has been demonstrated by us previously to be non-toxic and effective in two different tumor models of SCID mice (Manuscript In press). Average tumor size in the mice treated with the drug was > 10-fold smaller than that in control mice (p = 0.03), without evidence of toxicity, indicating a remarkable efficacy in vivo.
In summary, we have shown that telomerase activity assays conducted in tissue extracts may produce inaccurate results and propose that the activity assays should be conducted in the lysates of cells purified by LCM. We have also shown that telomerase activity is significantly elevated whereas telomere length is significantly shorter in primary epithelial cells derived from tissue specimens of Barrett's esophageal adenocarcinoma relative to normal esophageal epithelial cells. GRN163L, a lipid–conjugated oligonucleotide thio-phosphoramidate targeting RNA component of telomerase, is efficiently taken up by human adenocarcinoma cells without any need of a transfection reagent/procedure, inhibits telomerase activity, induces telomere shortening, and growth inhibition by induction of both cellular senescence and apoptosis. The growth inhibition following treatment with GRN163L could also be expedited by a combination therapy with other drugs. Finally, the efficacy of GRN163L has also been demonstrated in vivo. These data show that telomerase is a potential target for Barrett's esophageal adenocarcinoma and GRN163L is a potent and specific telomerase inhibitor which, either alone or in combination with agents, such as ritonavir and doxorubicin, is suited for in vivo utilization in human clinical trials.
ACKNOWLEDGEMENTS
This work was supported in part by grants from NIH-P50-100007 Developmental Research Award to MAS, from the Dept. of Veterans Affairs Merit Review Awards Research Service and Dept. of Veterans Affairs Merit Review Awards Epidemiology Service, and from the National Institutes of Health Grants P50-100007 and PO1-78378 to NCM, and by funding from Karmanos Cancer Institute, Detroit, MI.
REFERENCES
- 1.Day JP, Marder BA, Morgan WF. Telomeres and their possible role in chromosome stabilization. Environ Mol Mutagen. 1993;22(4):245–9. doi: 10.1002/em.2850220411. [DOI] [PubMed] [Google Scholar]
- 2.Allshire RC, Gosden JR, Cross SH, et al. Telomeric repeat from T. thermophila cross hybridizes with human telomeres. Nature. 1988;332(6165):656–9. doi: 10.1038/332656a0. [DOI] [PubMed] [Google Scholar]
- 3.de Lange T, Shiue L, Myers RM, et al. Structure and variability of human chromosome ends. Mol Cell Biol. 1990;10(2):518–27. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moyzis RK, Buckingham JM, Cram LS, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988;85(18):6622–6. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matulic M, Sopta M, Rubelj I. Telomere dynamics: the means to an end. Cell Prolif. 2007;40(4):462–74. doi: 10.1111/j.1365-2184.2007.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66(3):407–25. doi: 10.1128/MMBR.66.3.407-425.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akiyama M, Hideshima T, Munshi NC, Anderson KC. Telomerase inhibitors as anticancer therapy. Curr Med Chem Anticancer Agents. 2002;2(5):567–75. doi: 10.2174/1568011023353778. [DOI] [PubMed] [Google Scholar]
- 8.Shammas MA, Koley H, Batchu RB, et al. Telomerase inhibition by siRNA causes senescence and apoptosis in Barrett's adenocarcinoma cells: mechanism and therapeutic potential. Mol Cancer. 2005;4:24. doi: 10.1186/1476-4598-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shammas MA, Koley H, Beer DG, Li C, Goyal RK, Munshi NC. Growth arrest, apoptosis, and telomere shortening of Barrett's-associated adenocarcinoma cells by a telomerase inhibitor. Gastroenterology. 2004;126(5):1337–46. doi: 10.1053/j.gastro.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256(26):271–82. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 11.Blasco MA, Lee HW, Hande MP, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA [see comments] Cell. 1997;91(1):25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 12.Collins K. Mammalian telomeres and telomerase. Curr Opin Cell Biol. 2000;12(3):378–83. doi: 10.1016/s0955-0674(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 13.Ducray C, Pommier JP, Martins L, Boussin FD, Sabatier L. Telomere dynamics, end-to-end fusions and telomerase activation during the human fibroblast immortalization process. Oncogene. 1999;18(29):4211–23. doi: 10.1038/sj.onc.1202797. [DOI] [PubMed] [Google Scholar]
- 14.McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annu Rev Genet. 2000;34:331–58. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 15.De Lange T. Telomere-related genome instability in cancer. Cold Spring Harb Symp Quant Biol. 2005;70:197–204. doi: 10.1101/sqb.2005.70.032. [DOI] [PubMed] [Google Scholar]
- 16.Meeker AK. Telomeres and telomerase in prostatic intraepithelial neoplasia and prostate cancer biology. Urol Oncol. 2006;24(2):122–30. doi: 10.1016/j.urolonc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Ohali A, Avigad S, Ash S, et al. Telomere length is a prognostic factor in neuroblastoma. Cancer. 2006;107(6):1391–9. doi: 10.1002/cncr.22132. [DOI] [PubMed] [Google Scholar]
- 18.Bisoffi M, Heaphy CM, Griffith JK. Telomeres: prognostic markers for solid tumors. Int J Cancer. 2006;119(10):2255–60. doi: 10.1002/ijc.22120. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn EH. Telomerases. Annu Rev Biochem. 1992;61:113–29. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- 20.Aisner DL, Wright WE, Shay JW. Telomerase regulation: not just flipping the switch. Curr Opin Genet Dev. 2002;12(1):80–5. doi: 10.1016/s0959-437x(01)00268-4. [DOI] [PubMed] [Google Scholar]
- 21.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer [see comments] Science. 1994;266(5193):2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 22.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph KL, Chang S, Lee HW, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96(5):701–12. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 24.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392(6676):569–74. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 25.Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells [see comments] Science. 1998;279(5349):349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 26.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements [see comments] Nature. 1999;400(6743):464–8. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 27.Avilion AA, Piatyszek MA, Gupta J, Shay JW, Bacchetti S, Greider CW. Human telomerase RNA and telomerase activity in immortal cell lines and tumor tissues. Cancer Res. 1996;56(3):645–50. [PubMed] [Google Scholar]
- 28.Counter CM, Avilion AA, LeFeuvre CE, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. Embo J. 1992;11(5):1921–9. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shay JW. Telomerase in human development and cancer. J Cell Physiol. 1997;173(2):266–70. doi: 10.1002/(SICI)1097-4652(199711)173:2<266::AID-JCP33>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 30.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33(5):787–91. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 31.Shay JW, Wright WE. The reactivation of telomerase activity in cancer progression. Trends Genet. 1996;12(4):129–31. doi: 10.1016/0168-9525(96)30018-8. [DOI] [PubMed] [Google Scholar]
- 32.Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. Embo J. 1995;14(17):4240–8. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Opitz OG, Suliman Y, Hahn WC, Harada H, Blum HE, Rustgi AK. Cyclin D1 overexpression and p53 inactivation immortalize primary oral keratinocytes by a telomerase-independent mechanism. J Clin Invest. 2001;108(5):725–32. doi: 10.1172/JCI11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerone MA, Londono-Vallejo JA, Bacchetti S. Telomere maintenance by telomerase and by recombination can coexist in human cells. Hum Mol Genet. 2001;10(18):1945–52. doi: 10.1093/hmg/10.18.1945. [DOI] [PubMed] [Google Scholar]
- 35.Engelhardt M, Martens UM. The implication of telomerase activity and telomere stability for replicative aging and cellular immortality (Review) Oncol Rep. 1998;5(5):1043–52. doi: 10.3892/or.5.5.1043. [DOI] [PubMed] [Google Scholar]
- 36.Wai LK. Telomeres, telomerase, and tumorigenesis--a review. MedGenMed. 2004;6(3):19. [PMC free article] [PubMed] [Google Scholar]
- 37.Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A. 1995;92(20):9082–6. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shammas MA, Shmookler Reis RJ, Li C, Koley H, Hurley LH, Anderson KC, Munshi NC. Telomerase Inhibition and Cell Growth Arrest Following Telomestatin Treatment in Multiple Myeloma. Clinical Cancer Research. 2003 doi: 10.1158/1078-0432.ccr-0793-03. In Press. [DOI] [PubMed] [Google Scholar]
- 39.Shammas MA, Reis RJ, Li C, et al. Telomerase inhibition and cell growth arrest after telomestatin treatment in multiple myeloma. Clin Cancer Res. 2004;10(2):770–6. doi: 10.1158/1078-0432.ccr-0793-03. [DOI] [PubMed] [Google Scholar]
- 40.Shammas MA, Shmookler Reis RJ, Akiyama M, et al. Telomerase inhibition and cell growth arrest by G-quadruplex interactive agent in multiple myeloma. Molecular Cancer Therapeutics. 2003;2(9):825–33. [PubMed] [Google Scholar]
- 41.Shammas MA, Liu X, Gavory G, Raney KD, Balasubramanian S, Shmookler Reis RJ. Targeting the single-strand G-rich overhang of telomeres with PNA inhibits cell growth and induces apoptosis of human immortal cells. Experimental Cell Research. 2004;295(1):204–14. doi: 10.1016/j.yexcr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Shammas MA, Simmons CG, Corey DR, Reis RJ. Telomerase inhibition by peptide nucleic acids reverses ‘immortality’ of transformed human cells. Oncogene. 1999;18(46):6191–200. doi: 10.1038/sj.onc.1203069. [DOI] [PubMed] [Google Scholar]
- 43.Morales CP, Lee EL, Shay JW. In situ hybridization for the detection of telomerase RNA in the progression from Barrett's esophagus to esophageal adenocarcinoma. Cancer. 1998;83(4):652–9. [PubMed] [Google Scholar]
- 44.Lord RV, Salonga D, Danenberg KD, et al. Telomerase reverse transcriptase expression is increased early in the Barrett's metaplasia, dysplasia, adenocarcinoma sequence. J Gastrointest Surg. 2000;4(2):135–42. doi: 10.1016/s1091-255x(00)80049-9. [DOI] [PubMed] [Google Scholar]
- 45.Swiggers SJ, Nibbeling HA, Zeilemaker A, Kuijpers MA, Mattern KA, Zijlmans JM. Telomerase activity level, but not hTERT mRNA and hTR level, regulates telomere length in telomerase-reconstituted primary fibroblasts. Exp Cell Res. 2004;297(2):434–43. doi: 10.1016/j.yexcr.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 46.Huang Q, Yu C, Klein M, Fang J, Goyal RK. DNA index determination with Automated Cellular Imaging System (ACIS) in Barrett's esophagus: comparison with CAS 200. BMC Clin Pathol. 2005;5:7. doi: 10.1186/1472-6890-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohyama H, Zhang X, Kohno Y, et al. Laser capture microdissection-generated target sample for high-density oligonucleotide array hybridization. Biotechniques. 2000;29(3):530–6. doi: 10.2144/00293st05. [DOI] [PubMed] [Google Scholar]
- 48.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aggarwal S, Taneja N, Lin L, Orringer MB, Rehemtulla A, Beer DG. Indomethacin-induced apoptosis in esophageal adenocarcinoma cells involves upregulation of Bax and translocation of mitochondrial cytochrome C independent of COX-2 expression. Neoplasia. 2000;2(4):346–56. doi: 10.1038/sj.neo.7900097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bachor C, Bachor OA, Boukamp P. Telomerase is active in normal gastrointestinal mucosa and not up-regulated in precancerous lesions. J Cancer Res Clin Oncol. 1999;125(89):453–60. doi: 10.1007/s004320050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barclay JY, Morris A, Nwokolo CU. Telomerase, hTERT and splice variants in Barrett's oesophagus and oesophageal adenocarcinoma. Eur J Gastroenterol Hepatol. 2005;17(2):221–7. doi: 10.1097/00042737-200502000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Going JJ, Fletcher-Monaghan AJ, Neilson L, et al. Zoning of mucosal phenotype, dysplasia, and telomerase activity measured by telomerase repeat assay protocol in Barrett's esophagus. Neoplasia. 2004;6(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- 54.Yoneyama K, Aoyama N, Koizumi H, Tamai S. Telomerase activity in esophageal carcinoma and lesions unstained with Lugol's solution. Nippon Rinsho. 1998;56(5):1181–5. [PubMed] [Google Scholar]
- 55.Harle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci U S A. 1996;93(13):6476–81. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finley JC, Reid BJ, Odze RD, et al. Chromosomal instability in Barrett's esophagus is related to telomere shortening. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1451–7. doi: 10.1158/1055-9965.EPI-05-0837. [DOI] [PubMed] [Google Scholar]
- 57.Bechter OE, Eisterer W, Pall G, Hilbe W, Kuhr T, Thaler J. Telomere length and telomerase activity predict survival in patients with B cell chronic lymphocytic leukemia. Cancer Res. 1998;58(21):4918–22. [PubMed] [Google Scholar]
- 58.Ohashi K, Tsutsumi M, Kobitsu K, et al. Shortened telomere length in hepatocellular carcinomas and corresponding background liver tissues of patients infected with hepatitis virus. Jpn J Cancer Res. 1996;87(5):419–22. doi: 10.1111/j.1349-7006.1996.tb00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Odagiri E, Kanada N, Jibiki K, Demura R, Aikawa E, Demura H. Reduction of telomeric length and c-erbB-2 gene amplification in human breast cancer, fibroadenoma, and gynecomastia. Relationship to histologic grade and clinical parameters. Cancer. 1994;73(12):2978–84. doi: 10.1002/1097-0142(19940615)73:12<2978::aid-cncr2820731215>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 60.Sommerfeld HJ, Meeker AK, Piatyszek MA, Bova GS, Shay JW, Coffey DS. Telomerase activity: a prevalent marker of malignant human prostate tissue. Cancer Res. 1996;56(1):218–22. [PubMed] [Google Scholar]
- 61.Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999;274(11):7264–71. doi: 10.1074/jbc.274.11.7264. [DOI] [PubMed] [Google Scholar]
- 62.Nakajima A, Tauchi T, Sashida G, et al. Telomerase inhibition enhances apoptosis in human acute leukemia cells: possibility of antitelomerase therapy. Leukemia. 2003;17(3):560–7. doi: 10.1038/sj.leu.2402825. [DOI] [PubMed] [Google Scholar]
- 63.Seimiya H, Oh-hara T, Suzuki T, et al. Telomere shortening and growth inhibition of human cancer cells by novel synthetic telomerase inhibitors MST-312, MST-295, and MST-1991. Molecular Cancer Therapeutics. 2002;1(9):657–65. [PubMed] [Google Scholar]
- 64.Munshi NC, Hideshima T, Carrasco D, et al. Identification of genes modulated in multiple myeloma using genetically identical twin samples. Blood. 2004;103(5):1799–806. doi: 10.1182/blood-2003-02-0402. [DOI] [PubMed] [Google Scholar]
- 65.Sumi M, Tauchi T, Sashida G, et al. A G-quadruplex-interactive agent, telomestatin (SOT-095), induces telomere shortening with apoptosis and enhances chemosensitivity in acute myeloid leukemia. Int J Oncol. 2004;24(6):1481–7. [PubMed] [Google Scholar]
- 66.Tauchi T, Shin-Ya K, Sashida G, et al. Activity of a novel G-quadruplex-interactive telomerase inhibitor, telomestatin (SOT-095), against human leukemia cells: involvement of ATM-dependent DNA damage response pathways. Oncogene. 2003;22(34):5338–47. doi: 10.1038/sj.onc.1206833. [DOI] [PubMed] [Google Scholar]
- 67.Yatabe N, Kyo S, Kondo S, et al. 2-5A antisense therapy directed against human telomerase RNA inhibits telomerase activity and induces apoptosis without telomere impairment in cervical cancer cells. Cancer Gene Ther. 2002;9(7):624–30. doi: 10.1038/sj.cgt.7700479. [DOI] [PubMed] [Google Scholar]
- 68.Akiyama M, Hideshima T, Shammas MA, et al. Effects of oligonucleotide N3′-->P5′ thio-phosphoramidate (GRN163) targeting telomerase RNA in human multiple myeloma cells. Cancer Research. 2003;63(19):6187–94. [PubMed] [Google Scholar]
- 69.Herbert BS, Pongracz K, Shay JW, Gryaznov SM. Oligonucleotide N3′-->P5′ phosphoramidates as efficient telomerase inhibitors. Oncogene. 2002;21(4):638–42. doi: 10.1038/sj.onc.1205064. [DOI] [PubMed] [Google Scholar]
- 70.Herbert BS, Sanders BG, Kline K. N-(4-hydroxyphenyl)retinamide activation of transforming growth factor-beta and induction of apoptosis in human breast cancer cells. Nutr Cancer. 1999;34(2):121–32. doi: 10.1207/S15327914NC3402_1. [DOI] [PubMed] [Google Scholar]