Abstract
The purpose of this study was to examine individual differences in the activation and regulation of the Hypothalamic-Pituitary-Adrenal (HPA) Axis in pre-pubertal children after exposure to two different stress modalities, and to evaluate the utility of an individual differences approach to the examination of HPA-axis functioning. After a 30 minute controlled baseline period, 73 seven-year-old children (40 males and 33 females) were randomly assigned to a validity check condition, or one of two experimental tasks designed to elicit fear or frustration. This was followed by a 60-minute controlled regulation phase. A total of 17 saliva samples were collected, including 12 post-stress samples at 5-minute intervals. There was a significant stress modality effect, with children exposed to the fear condition reaching peak cortisol levels at 25 minutes post-stress, while those exposed to the frustration condition reached peak levels at 45 minutes post-stress. There was no difference in peak cortisol levels between the stress modalities. Individual variability across conditions was significant with subjects reaching peak levels as early as 10 minutes post-stress and as late as 60 minutes post-stress. Our data suggest that analysis of individual curves prior to making group level comparisons may improve the explanatory power of HPA-axis-behavior models.
Keywords: Cortisol, Stress, HPA, Fear, Frustration, Children
The study of salivary cortisol has been adopted by behavioral researchers interested in examining a wide range of behaviors and disorders, such as depression (Burke, Davis, Otte, & Mohr, 2005), post-traumatic stress disorder (de Kloet et al., 2006), aggression (Gordis, Granger, Susman, & Trickett, 2006), and social anxiety (Beaton, Schmidt, Ashbaugh, Santesso, & Martin, 2006). Researchers have traditionally observed cortisol responses to laboratory stress tasks in order to examine neuroendocrine reactivity. However, most studies, especially those using pediatric populations, have examined HPA-axis responses to social anxiety paradigms and thus our understanding of cortisol responses to different stress modalities is limited. Furthermore, there is a scarcity of research on individual differences in HPA-axis reactivity in children, and extant studies have usually not examined individual differences as a possible key source of variability.
While peak cortisol concentrations can be found in saliva within 30 minutes post-stress (Kirschbaum & Hellhammer, 2000), significant variability in peak timings have been observed from 10 to 45 minutes post-stress (e.g., Bremner, Vythilingam, & Vermetten, 2003; Gordis et al., 2006). Although such variability can be partly explained by differences in the duration of the stressors used or the nature of the population studied (e.g., psychiatric vs. community; see for example Furlan, DeMartinis, & Schweizer, 2001), there is evidence to suggest that stress modality may have an impact on HPA-axis reactivity. For example, most studies observing peak cortisol levels 25 minutes post-stress have used the 15-minute-long Trier Social Stress Task (e.g., Ditzen et al., 2007; Gordis et al., 2006; Nater et al., 2007; Newman, O'Connor, & Conner, 2007; Rimmele et al., 2007). In contrast, studies using other stress modalities have shown more variability in cortisol response curves. For example, Bremner et al. (2003) observed average peak cortisol concentrations at 45 minutes after exposure to a cognitive challenge task among healthy adults. Similarly, Nejtek (2002) observed peak levels 40 minutes after watching a stressful film among college students. Yet, only a few adult studies have directly compared HPA-axis responses to different stress modalities (e.g., Furlan, DeMartinis, & Schweizer, 2001).
Research on individual variability in HPA-axis activation is also extremely limited. Kirschbaum and Hellhammer (2000) noted significant individual variability in the time to reach peak cortisol levels in saliva. It is unknown however, if such variability is significant enough to preclude the use of samples obtained at standardized times (e.g., 30 minutes post-stress) to answer specific individual and group-level questions, or whether researchers should instead utilize an individual differences approach, in which key cortisol indices (e.g., peak cortisol levels) are determined based on each individual's activation curve prior to group comparisons. At an individual level, cortisol levels measured at exact times may reflect different moments within each individual's activation curve so that levels taken at 45 minutes after the stressor may reflect peak activation levels for one individual and post-peak regulation for another. Thus, cortisol estimations of activation and regulation that are based on predetermined time points (e.g., 25 and 45 minutes, respectively) may not accurately reflect the individual's cortisol response. Yet, most published studies, including those using multiple post-stress samples, have compared mean cortisol levels obtained at specific intervals using repeated measures ANOVA without modeling individual variability (see for example Blackhart, Eckel, & Tice, 2007; Bremner et al., 2003; Ditzen et al., 2007). Furthermore, when trends in cortisol responses are clustered within groups, such as when one group tends to reach peak levels later than a second group, analysis of individual differences may help obtain more accurate group comparisons. For example, group comparisons at pre-determined time points may be useful in providing information on how groups differ in key factors of the dynamics of the cortisol response, such as how do the groups differ exactly 20 minutes after the stressor. However, for questions regarding other aspects of the cortisol response, such as how do the groups differ in time to peak, peak levels, or total cortisol production, analysis of individual variability prior to group comparisons may be more appropriate.
There is also significant evidence suggesting that the development of the HPA-axis extends from infancy through adolescence leading to endocrine differences between pre-pubertal children, post-pubertal children, and adults. For example, studies have shown that diurnal saliva cortisol levels increase during middle childhood and adolescence (e.g., Kiess et al., 1995; Legro, Lin, Demers, & Lloyd, 2003; Tornhage, 2002), and that individual variability in diurnal saliva samples also differs in pre-pubertal and post-pubertal children (Kiess et al., 1995). Similar age effects have been found for post-stress HPA-axis functioning (Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004). However, despite these developmental findings, there are no published studies directly assessing individual variability in cortisol peak timing in pre-pubertal children, or the effects of stress modality on HPA-axis responses in this population.
Given the apparent variability in endocrine responses to different stress modalities and our limited understanding of such variability in middle childhood, we chose to study endocrine reactivity to fear and frustration/anger in children. Although a comprehensive review of the phenomenology of fear and frustration/anger is beyond the scope of this article, difficulty regulating these emotions in middle childhood has been associated with several negative outcomes such as anxiety disorders (e.g., Kagan & Snidman, 1999; Margot Prior, Smart, Sanson, & Oberklaid, 2000; Rosenbaum et al., 1993) and conduct problems (e.g., Cole, Teti, & Zahn-Waxler, 2003; Frick & Morris, 2004), respectively. Attempts to examine individual differences in the phenomenology of these emotions have typically been focused on cognitive/attentional biases and autonomic lability (Boyce et al., 2001; Eckhardt & Cohen, 1997; Kindt, Brosschot, & Everaerd, 1997; Van Honk, Tuiten, de Haan, Hout, & Stam, 2001). However, endocrine reactivity has also been examined as a characteristic of fearful temperament (Buss et al., 2003), as well as personality styles prone to frustration/anger (Sher, 2005 ). Yet, our understanding of endocrine reactivity in response to fear or frustration eliciting situations in middle childhood is limited.
Furthermore, we chose to examine fear and frustration/anger because they represent distinct brain processes that have been extensively studied (e.g., Fox & Davidson, 1988; Kalin, Shelton, & Davidson, 2004; Kimbrell et al., 2008), suggesting different pathways for the activation of the HPA-axis. Specifically, fear responses are governed by more ‘primitive’ centers of the brain including the amygdala and brainstem, while frustration/anger responses are associated with higher cortical structures, specifically the pre-frontal cortex (e.g., Fox & Davidson, 1988; Kalin et al., 2004; Kimbrell et al., 2008). In addition, our choice of stress modalities reflects the differences in cortisol activation curves suggested by the literature. That is, shorter peak latencies have been found in studies looking at social anxiety and fear (e.g., Domes, Heinrichs, & Reichwald, 2002) while longer activation peaks have been found in studies looking at cognitive and physical challenges (e.g., Bremner et al., 2003).
Therefore, in the current study we examined the HPA-axis reactivity to fear or frustration/anger stimuli among 7-year-old children with and without early signs of externalizing behavior problems. These children were participating in a larger examination of the association between endocrine reactivity and externalizing behavior problems. Results regarding such association are reported elsewhere (Lopez-Duran, Olson, Hajal, Felt, & Vazquez, 2009). Instead, in this article we focus on individual differences in endocrine reactivity, paying especial attention to individual variability in endocrine activation to different stressors in middle childhood and associated implications for endocrine research. We hypothesize that we will find a significantly different pattern of HPA-axis activation between the two stress modalities, with fear producing a faster and stronger cortisol response than frustration. We further hypothesize that we will observe great individual variability in activation curves suggesting a need to control for individual differences prior to making group-level comparisons. An in-depth understanding of the individual variability in HPA-axis activation to different stress modalities will likely facilitate and improve both the methodologies used in HPA-axis research and our understanding of the relationship between HPA-axis functioning and related behavioral and affective processes in children.
Methods
Participants
Participants included a total of 73 children (40 males and 33 females) ranging from 6 to 7 years of age, recruited from families that had previously participated in a NIMH-funded longitudinal study of typically developing children and children at risk for school-age conduct problems by virtue of displaying high levels of externalizing problems in early childhood (NIMH Grant R01MH57489). Please see Lopez-Duran et al. (2009) for a more detailed description of recruitment procedures. This community-based sample represented the full range of symptom severity on the Child Behavior Checklist/2-3 (Achenbach, 1992), with an oversampling of toddlers in the medium to high range of the Externalizing Problems scale (T > 60; 44%). However, by the time of our current study (age 7) the sample was highly representative of the local community with only 10% of the sample scoring in the high range of the Externalizing Problems scale. A total of 260 families were recruited to participate in the original longitudinal study.
We contacted all eligible families (N = 203) approximately one year after the end of the final wave of the longitudinal study. Eligible families included those who had agreed to be contacted for future studies and who still lived in the area. During the phone conversation the first author explained the nature of the study, the time commitment, and offered a $25 gift certificate to a large retail toy store as compensation for their participation. Seventy-eight families agreed to participate. Five of these participants did not complete all necessary tasks to be included in the study. The final sample did not differ from the available sample (N =203) in levels of behavior problems at the time of the original study intake (p = .68), externalizing or internalizing problems at the time of the current study (p = .44, p = .93), and socio-economic-status (p =.44). Theethnic distribution of the final sample was nearly identical to that of the original study with 5.1% African American, 3.8% Hispanic American, and 2.5% Asian American. The median annual family income was $52,000, ranging from $20,000 to over $100,000.
Measures and Procedures
Children participated in a 90-minute laboratory session consisting of a 30-minute baseline phase, a 3-minute stress task, and a 60-minute regulation phase. All sessions were conducted at 3:00 p.m. or 4:30 p.m., during non-school days at a Children's Center of a large Midwestern University. Families were asked not to eat within one hour prior to arrival to the laboratory.
Baseline
The 30-minute baseline phase was included in order to control for any stressors that occurred prior to their arrival to the laboratory. Upon arrival each child was introduced to an undergraduate student working as a research assistant (RA). The assistant guided the child into a playroom where the child was allowed choose one out of four baseline activities (Legos, drawing, a castle, or a puzzle). During the baseline procedure, the RAs were instructed to play with the child based on the child interactive style. That is, with shy children the assistant simply did parallel play and asked simple questions in order to build a rapport. With children that were more extroverted the assistant engaged in cooperative play. Physical activity was kept to a minimum. A total of 6 research assistants were used during the study (5 females).
Stress Tasks
Prior to arrival, participants were randomly assigned to one of two conditions: Fear or Frustration. After the baseline play session ended, the RA escorted the child to a different room and proceeded to conduct the stress task.
Fear Condition
Thirty-three (33) children were exposed to a fear task using a modified version of Calkins' protocol (see Calkins, Graziano, Berdan, Keane, & Degnan, 2008). Each child was taken into a semi-dark room, slowly and quietly approached a terrarium (empty fish tank). Inside the terrarium there was a realistic looking snake that was partially covered with mulch. The child was asked to stay away from the terrarium while the RA removed a blanket that was covering the top of the tank, and the child was then asked to approach the tank. Then the RA abruptly took the rubber snake out of the tank while simultaneously indicating that the snake was fake. The child was then allowed to play with the toy for a few moments if requested. The RA verbalizations during this task were based on a script that included: “I have something that I want to show you. It's inside that tank. Let's be quiet so it doesn't wake up.” The fear task took approximately 3 minutes to complete. At the end of the task the children were provided with a prize identical to that used in the frustration condition (see below).
Frustration Condition
Thirty-two (32) children were exposed to a novel Frustration condition also based on Calkins' (1997) protocol. A visually appealing Toys-R-Us gift certificate was placed inside a clear plastic Tupperware™ box. Each child was told that he/she would receive the prize if able to open the container while keeping his/her hands inside tennis socks. The RA modeled the task and made challenging verbalizations such as, “This is so easy even a baby can do it.” These verbalizations were standardized across all participants. The RA discreetly switched boxes and gave the child an identical box that was glued shut. The child was told that he/she had one minute to open the box before the RA returned to the room. Upon returning, the RA explained that the box was probably broken and proceeded to give the child the prize. This task took approximately 3 minutes to complete.
Validity Check
Eight (8) participants were assigned to a validity check group. These children completed the entire protocol but were not exposed to any stressful condition.
Post Stress Phase
After the stress task, each participant was escorted to a different classroom equipped with chairs, cushions, and a TV/VCR. The RA then played two 30-minute episodes of the children's series “Wallace and Gromit” from Aardman Animations. The episodes were “A Grand Day Out” and “A Close Shave.” The videos were selected because they were entertaining enough to keep the child's attention for extended periods of time but had only moderate levels of emotionally inducing stimuli.
Cortisol Sampling
A total of 17 saliva samples per child were obtained. The first sample was collected exactly −30 minutes prior to stress. A digital stopwatch was activated at that time and all samples were collected based on a strict time schedule. Additional baseline samples were taken at −20, −10, and −5 minutes prior to stress, immediately after the stress condition (time 0), and at 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 minutes after the initiation of the stressor.
Although we intended to use a cotton dental roll for saliva collection, most children chose to spit directly into the salivette tube, which shortened the collection time from 30-45 seconds to under 10 seconds. We experienced no difficulties with saliva production, and no agents were used to facilitate saliva production (sugar crystals, gum, etc.). All samples were stored within 4 hours of collection at −80 C. until assayed. Samples were assayed in duplicate and averaged using a commercial enzyme immunoassay kit (Salimetrics™ LLC, PA). The sensitivity of the assay was 0.01 μg/dL. The inter-assay and intra-assay coefficients of variability were 7.6 and 6.2, respectively. To decrease inter-assay variability all samples from the same child were assayed in the same batch. Duplicates varying more than 15% were re-assayed.
Analytical Approach
Cortisol values were log transformed prior to multivariate analysis. When appropriate, non-log values are provided to facilitate interpretability. Five HPA-axis functioning indicators were calculated at an individual level prior to making any group level analysis. Peak Cortisol Concentration for each individual was obtained by selecting the highest cortisol sample within each individual's activation curve. Time from Exposure to Peak for each individual was calculated by selecting the time interval from time 0 until the individual's peak level. Slope of Activation was calculated from time 0 to peak level. Total Cortisol Production was obtained by calculating each individual's Area Under the Curve (AUC) using trapezoidal aggregation (Matthews, Altman, Campbell, & Royston, 1990). Slope of Regulation was calculated from peak level to time 60.
For simple mean comparisons between groups we used independent sample or paired sample t-tests as applicable. Time-of-day and Stress Type effects were tested using a simple Analysis of Variance. Stress × Gender effects of all cortisol determinations were examined via a 2×2 Multiple Analysis of Variance.
Results
Methodological Considerations
The baseline procedure was effective in eliciting a significant drop in cortisol, validating the use of time 0 as basal level. Cortisol levels dropped from 0.103 ug/dl at arrival to 0.069 ug/dl at time 0, t(74) = 10.17, p < .001. Sixty-six participants (90%) had a reduction in cortisol concentration from the time they arrived at the laboratory to the end of the baseline procedures. We found no differences in cortisol levels at time 0 based on the research assistant used, F(5, 66) = 1.09, p = .37, or the toy selected during the baseline, F(3, 69) = 0.02, p = .99.
Forty-six participants (63%) were exposed to the experimental condition at 3 p.m., and 26 participants (47%) at 4:30 p.m. A one-way ANOVA revealed no significant differences between these groups in regards to all of our variables of interest (i.e., time from exposure to peak, mean post-stress cortisol increase, slope of activation, total cortisol production, and slope of regulation).
We examined the activation curve among the 8 validity check participants in order to determine whether the film used during the post-stress phase was free of stress-inducing stimuli. No significant increase in cortisol during the film was observed among these participants (average individual peak level = 0.062 ug/dl compared to baseline = 0.063 ug/dl, t(7) = .336, p = .747). The flat cortisol curve observed in the validity check participants during the film procedures suggests that the film did not result in a noticeable HPA-axis activation. While it is still possible that film impacted the HPA-axis functioning of the kids exposed to the Fear and Frustration conditions, theoretically, any impact of the film on the HPA-axis would have been equivalent among all participants since the film procedures were standardized across all conditions.
We assessed whether the stress tasks were effective in producing a cortisol response. Cortisol levels at exposure and at post-stress peak for participants exposed to the Fear condition were 0.0704 ug/dl and 0.111 ug/dl, respectively, t(32) = 11.681, p = .001. Cortisol levels at exposure and at post-stress peak for participants exposed to the Frustration condition were 0.068 ug/dl and 0.107 ug/dl, respectively, t(31) = 9.226, p < .001.
Finally, we assessed sex differences in cortisol responses to fear and frustration. No sex differences were observed in peak cortisol levels, AUC, peak timing, or regulation slope. However, there was a significant difference in slope of activation with boys (M =.0014, SD = .001) showing a steeper activation curve than girls (M =.0008, SD = .001), t(63) = 3.441, p < .01. There was no interaction between sex and stress type.
Effects of Stress Type on Cortisol Activation
Table 1 presents general descriptive statistics for our 5 main variables of interest. The fear condition elicited cortisol peaks on average at 25.15 minutes after exposure, while frustration elicited peaks on average at 44.69 minutes after exposure. This difference was significant, F(1,63) = 67.68, p < .001. There was also a significant difference in the rate of activation after exposure, F(1, 63) = 21.468, p < .001, with fear producing a significantly steeper activation slope than frustration. There were no differences in cortisol peak levels, AUC, or regulation slope between the groups. However, stress modality differences in latency to peak levels after exposure may limit our ability to see differences in AUC and the regulation slopes. Specifically, 82% (N = 27) of participants exposed to the fear condition returned to baseline cortisol values by time 60. In contrast, only 29% (N= 9) of those exposed to the frustration condition returned to baseline.
Table 1.
General descriptive statistics of cortisol measurements for participants exposed to two experimental conditions.
| Measurement | Condition | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| Time to peak | Fear | 33 | 25.152 | 7.755 | 10 | 40 |
| Frustration | 32 | 44.688 | 11.140 | 20 | 60 | |
| Peak Value | Fear | 33 | 0.112 | 0.023 | 0.064 | 0.145 |
| Frustration | 32 | 0.107 | 0.023 | 0.063 | 0.142 | |
| Activation Slope | Fear | 33 | 0.002 | 0.001 | 0.000 | 0.003 |
| Frustration | 32 | 0.001 | 0.001 | 0.000 | 0.002 | |
| AUC | Fear | 33 | 4.592 | 0.952 | 2.913 | 6.410 |
| Frustration | 32 | 4.527 | 1.026 | 3.183 | 6.700 | |
| Regulation Slope | Fear | 33 | −0.002 | 0.001 | −0.004 | −0.001 |
| Frustration | 32 | −0.001 | 0.005 | −0.004 | 0.026 | |
Individual Differences
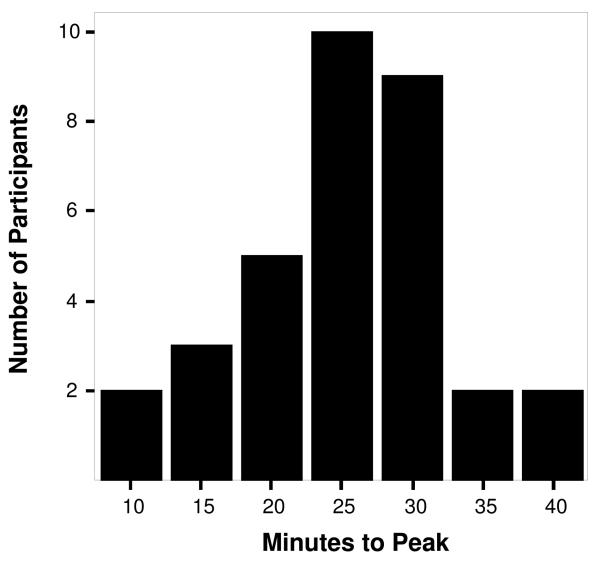
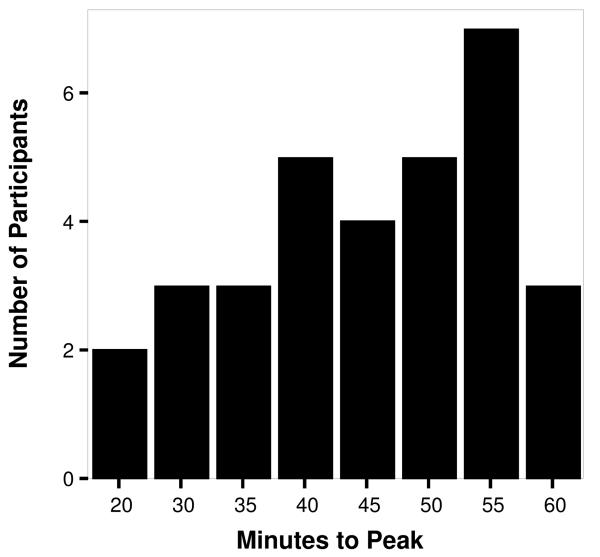
Individual variability in peak latency between and within conditions was noticeable (see Figures 1 and 2). For the fear task, peak latency ranged from 10 to 40 minutes post-stress. The mode of the distribution was at 25 minutes when 30% (N = 10) of the sample reached peak. For the frustration task, peak times ranged from 20 to 60 minutes post-stress. The mode of this distribution was at 55 minutes post-stress when 21% (N = 7)of the sample reached peak.
Figure 1.
Number of participants reaching peak cortisol levels at specific time intervals after exposure to the fear condition.
Figure 2.
Number of participants reaching peak cortisol levels at specific time intervals after exposure to the frustration condition.
Given this large variability, we assessed the utility of conducting group comparisons based on individually obtained peak levels (regardless of timing) as opposed to comparing groups at specific time points, namely: 20, 25, 30, 35 and 40 minutes post-stress, as these represent a common sampling range used in numerous studies (e.g., Ashman, 2002; Kivlighan & Granger, 2006; Nejtek, 2002; O'Leary, Loney, & Eckel, 2007; Wolf, Schommer, Hellhammer, McEwen, & Kirschbaum, 2001). First, using a series of one-tailed paired-sample t-tests, we compared the individually obtained peak level against samples obtained during the five time points (20, 25, 30, 35 and 40). One tailed t-tests were used because the individually selected peak levels could never be lower than the maximum of the different time points. For the total sample, the mean individually selected peak sample (M= 0.109, SD = .023) was significantly higher than that of each of the five time points examined, namely at 20min (M=.078, SD = .029), t(63) = −9.76, p <.01, at 25 minutes (M=.082, SD = .03), t (63) = −8.3, p< .01; at 30 minutes (M=.081, SD = .029), t(63) = −8.6, p< .01; at 35 minutes (M=.079, SD = .021), t (63) = −11, p< .01; and at 40 minutes (M=.078, SD =.02), t(63) = −10, p< .01. This effect was consistent regardless of condition.
We then used sex and stress type as variables of interest in order to compare individually selected peaks with cortisol values obtained at 25 and 45 minutes as these represent the average peak time for both stress conditions. There were no significant sex differences for cortisol levels at 25 minutes, t(63) = .775, p = .44 (Males M =0.085, Females M =0.079), 45 minutes, t(63) = .40, p = .69 (Males M =0.077, Females M=0.075), or for individually selected peak levels, t(63) = 1.505, p = 0.137 (Males M =0.113, Females M =0.104). However, an improvement in effect size change was observed from .20 and .08 for cortisol determinations obtained at 25 and 45 minutes, respectively, to .41 for individually selected peak levels. Sex differences in slope of activation were found only when the slope was calculated using individually selected peak levels, t(63) = 3.23, p <.01.
There was a significant cortisol difference between the Fear and Frustration condition when comparing these conditions at 25 minute t(63) = 4.931, p <.001), and 45 minutes t(36) = −3.54, p <.001). However, when comparing the individually selected peak cortisol levels there was no difference between Fear (M = 0.1115,SD = 0.0234) and Frustration (M =0.1072; SD = 0.0226) conditions, t(63) = .75, p = 46.
Discussion
Our study examined individual differences in cortisol responses to two stress modalities in pre-pubertal children while using a dense cortisol sampling procedure. We found significant individual differences in the activation patterns between and within conditions. Our data suggest that our fear paradigm results in a short but intense HPA-axis response. In contrast, our frustration task results in a more gradual activation of the HPA-axis.
The rapid cortisol responses observed after the fear task are consistent with our understanding of the physiology of fear. Fear-relevant stimuli produce a rapid activation of the thalamus, anterior cingulate cortex, and basolateral nucleus of the amygdala (Das et al., 2005), and connections from the central nucleus of the amygdala to the paraventricular nucleus of the hypothalamus contribute to the rapid activation of the HPA-axis during events of fear (Gunnar & Vazquez, 2006). In contrast, the more gradual response observed after the frustration task may be the result of how these stressors may differentially activate the HPA-axis regulatory mechanism. That is, inhibition of the HPA-axis system is primarily governed by the activation of glucocorticoid (GR) and mineral corticoid (MR) receptors. The fear condition resulted in the rapid release of cortisol, which would saturate available MR and GR receptors, leading to the rapid down-regulation of the HPA-axis system (see Lopez, Vazquez, & Olson, 2004, for a review of the role of GR and MR receptors). However, during the frustration condition, cortisol levels were released slowly, which is less effective in deactivating the system. This is consistent with the notion that the deactivation of the HPA-axis may be less efficient during slow activating situations such as depressive states and rumination (see Checkley, 1996).
It is also possible that the apparent slow response elicited by frustration was not ‘slow’, but instead was the result of cognitive processes occurring during the film portion of the protocol. That is, once the children exposed to the frustration condition began watching the film, they may have engaged in cognitive processes (e.g., self-evaluation, rumination, etc.) that activated their HPA-axis system. This possibility is highly speculative, for we do not have measures of any cognitive process and cannot provide evidence that such cognitive factors may be at play. Yet, rumination has been associated with increases in cortisol under experimental conditions (McCullough, Orsulak, Brandon, & Akers, 2007) and has been found to be often used by pre-pubertal children (Abela, Aydin, & Auerbach, 2007; Abela, Brozina, & Haigh, 2002 ).
We also found significant individual variability within stress modalities, with children reaching peak levels from 10 to 60 minutes post-stress. Our data show that an individual-differences approach provides a clearer, more robust, and likely more accurate picture of HPA-axis functioning when compared to cortisol levels obtained at any single time point. For example, when comparing peak levels of fear vs. frustration at 25 and 45 minutes, a major stress effect is found. However, this reflects differences in activation profiles of the two stress modalities and not differences in strength (peak values). After using an individual differences approach, no stress modality effect was found, which more accurately reflects the nature of the HPA-axis response to these stressors. Our data indicates that for key theoretical questions, group comparisons should be made after the cortisol indices of interest (e.g., peak levels) are calculated based on individual profiles. Although ideally these profiles should be obtained from samples obtained using dense sampling methods (e.g., Fiocco, Joober, & Lupien, 2007; Kumsta, Entringer, Hellhammer, & Wust, 2007), such ideal methodology may not always be feasible due to the associated costs and logistical challenges. However, we argue that in order to obtain data that accurately reflect the dynamics of individual and group differences in cortisol responses, researchers should obtain samples at least at 10-15 minute intervals for no less than 60 minutes post stress, and should not assume, without examining individual profiles, that a sample obtained at a pre-determined time is a reflection of any aspect of the cortisol response, such as when a sample obtained 40 minutes post-stress is assumed to represent regulation levels.
Two additional findings are of note. First, a significant sex difference was found in the rate of cortisol activation post-stress, with boys showing a steeper activation curve than girls. Research has produced conflicting results regarding the role of sex on HPA axis functioning. However, some evidence for a steeper activation curve among males has been reported (Kirschbaum, Wust, & Hellhammer, 1992; Wolf et al., 2001). While estrogen and the menstrual cycle have been examined as possible modulator of HPA axis functioning (Uhart, Chong, Oswald, Lin, & Wand, 2006), Kirschbaum et al. (1992 ) have argued that the greater reactivity found among males is not necessarily due to an attenuated female response and is most likely impacted by sex differences in cognitive factors in anticipation, or response to, the stressor. Our results indicate that these sex differences can be found as early as middle childhood, further suggesting that these differences may not be related, or at least dependent upon, post-pubertal hormonal sex differences.
Finally, our data showed a consistent and intense decline in cortisol levels from arrival at the laboratory to the time of stress exposure. This decline was significantly more intense than that expected during similar times of the day under normal conditions. Ninety percent of the participants experienced a decline from arrival to exposure, and five of the control participants, who were not exposed to any stressor, continued to show a cortisol decline throughout the duration of the study. Although the elevated levels of cortisol found at arrival could be the result of numerous factors, such as food intake, physical exercise, or conflicts prior to arrival, the consistency of this finding among most participants suggests that, at least for this age group, coming to a laboratory session is associated with high levels of anticipatory anxiety. Our data expand on similar declines found in adolescents and adults (e.g., Fiocco et al., 2007; Gordis et al., 2006; Roy, Kirchbaum, & Steptoe, 2001), supporting the need for carefully controlled baseline procedures in laboratory-based HPA-axis studies using pre-pubertal children. In addition, the lowest cortisol values were observed between 30 to 40 minutes after arrival to the laboratory, indicating that baseline procedures should be long enough to allow participants to return to baseline.
In conclusion, our study contributes to our understanding of individual variability in HPA-axis responses to different stressors in pre-pubertal children. Our results show that HPA-axis activation after stress appears to be highly sensitive to stress modality, reflecting possible variability in the regulation of the HPA-axis to different types of stressors. In addition, our data suggest that individual variability is large and that cortisol levels obtained at any particular time can reflect different aspects of each child's activation curve, suggesting that researchers should conduct group comparisons after careful analyses of individual activation curves.
Acknowledgements
Funding for this project was provided by NIMH grants R01MH57489 awarded to Dr. Sheryl Olson, NIMH P50 and MH59396 awarded to Dr. Arnold Sameroff and Dr. Delia M. Vazquez, and by the University of Michigan C.S. Mott Research Fund awarded to Dr. Delia M. Vazquez.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abela J, Aydin C, Auerbach R. Responses to depression in children: Reconceptualizing the relation among response styles. Journal of Abnormal Child Psychology. 2007;35:913–927. doi: 10.1007/s10802-007-9143-2. [DOI] [PubMed] [Google Scholar]
- Abela J, Brozina K, Haigh E. An examination of the response styles theory of depression in third- and seventh-grade children: A short-term longitudinal study. Journal of Abnormal Child Psychology. 2002;30(5):515–527. doi: 10.1023/a:1019873015594. [DOI] [PubMed] [Google Scholar]
- Achenbach T. Manual for the Child Behavior Checklist/2-3 and 1992 Profile. University of Vermont; Burlington: 1992. [Google Scholar]
- Ashman SB. Stress hormone levels of children of depressed mothers. Development and Psychopathology. 2002;14(2):333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Beaton EA, Schmidt LA, Ashbaugh AR, Santesso DL, Martin M. Low salivary cortisol levels among socially anxious young adults: Preliminary evidence from a selected and a non-selected sample. Personality and Individual Differences. 2006;41(7):1217–1228. [Google Scholar]
- Blackhart GC, Eckel LA, Tice DM. Salivary cortisol in response to acute social rejection and acceptance by peers. Biological Psychology. 2007;75(3):267–276. doi: 10.1016/j.biopsycho.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Boyce W, Quas J, Alkon A, Smider N, Essex M, Kupfer D. Autonomic reactivity and psychopathology in middle childhood. The British Journal of Psychiatry. 2001;179(2):144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Bremner J, Vythilingam M, Vermetten E. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28(6):733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Burke H, Davis M, Otte C, Mohr D. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30(1):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Buss K, Schumacher J, Dolski I, Kalin N, Goldsmith H, Davidson R. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003;117(1):11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Calkins S. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31(2):125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Berdan LE, Keane SP, Degnan KA. Predicting cardiac vagal regulation in early childhood from maternal-child relationship quality during toddlerhood. Developmental Psychobiology. 2008;50(8):751–766. doi: 10.1002/dev.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley S. The neuroendocrinology of depression. International Review of Psychiatry. 1996;8(4):373–378. [Google Scholar]
- Cole P, Teti L, Zahn-Waxler C. Mutual emotion regulation and the stability of conduct problems between preschool and early school age. Development and Psychopathology. 2003;15(01):1–18. [PubMed] [Google Scholar]
- Das P, Kemp A, Liddell B, Brown K, Olivieri G, Peduto A, et al. Pathways for fear perception: Modulation of amygdala activity by thalamo-cortical systems. NeuroImage. 2005;26(1):141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Neumann I, Bodenmann G, von Dawans B, Turner R, Ehlert U, et al. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32(5):565–574. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Reichwald U. Hypothalamic-pituitary-adrenal axis reactivity to psychological stress and memory in middle-aged women: High responders exhibit enhanced declarative memory Performance. Psychoneuroendocrinology. 2002;27(7):843–853. doi: 10.1016/s0306-4530(01)00085-3. [DOI] [PubMed] [Google Scholar]
- Eckhardt CI, Cohen DJ. Attention to anger-relevant and irrelevant stimuli following naturalistic insult. Personality and Individual Differences. 1997;23(4):619–629. [Google Scholar]
- Fiocco A, Joober R, Lupien S. Education modulates cortisol reactivity to the Trier Social Stress Test in middle-aged adults. Psychoneuroendocrinology. 2007;32(810):1158–1163. doi: 10.1016/j.psyneuen.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Fox NA, Davidson RJ. Patterns of brain electrical activity during facial signs of emotion in 10-month-old infants. Developmental Psychology. 1988;24(2):230–236. [Google Scholar]
- Frick PJ, Morris AS. Temperament and Developmental Pathways to Conduct Problems. Journal of Clinical Child & Adolescent Psychology. 2004;33(1):54–68. doi: 10.1207/S15374424JCCP3301_6. doi: Article. [DOI] [PubMed] [Google Scholar]
- Furlan P, DeMartinis N, Schweizer E. Abnormal salivary cortisol levels in social phobic patients in response to acute psychological but not physical stress. Biological Psychiatry. 2001;50(4):254–259. doi: 10.1016/s0006-3223(00)01126-4. [DOI] [PubMed] [Google Scholar]
- Gordis E, Granger DA, Susman E, Trickett P. Asymmetry between salivary cortisol and alpha -amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31(8):976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Vazquez D. Developmental Psychopathology, Vol 2: Developmental Neuroscience. Vol. 2. John Wiley & Sons Inc; Hoboken, NJ: 2006. Stress neurobiology and developmental psychopathology; pp. 533–577. [Google Scholar]
- Kagan J, Snidman N. Early childhood predictors of adult anxiety disorders. Biological Psychiatry. 1999;46(11):1536–1541. doi: 10.1016/s0006-3223(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Kalin N, Shelton S, Davidson R. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer R, Schriever K, Kessler U, Konig A, et al. Salivary cortisol levels throughout childhood and adolescence: Relation with age, pubertal stage, and weight. Pediatric Research. 1995;37(4):502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Kimbrell T, George M, Parehk P, Ketter T, Podell D, Danielson A, et al. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biological Psychiatry. 2008;46(4):454–465. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- Kindt M, Brosschot JF, Everaerd W. Cognitive Processing Bias of Children in a Real Life Stress Situation and a Neutral Situation. Journal of Experimental Child Psychology. 1997;64(1):79–97. doi: 10.1006/jecp.1996.2336. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosomatic Medicine. 1992;54(6):648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer D. Encyclopedia of Stress. Academic Press; 2000. Salivary Cortisol; pp. 379–383. [Google Scholar]
- Kivlighan K, Granger D. Salivary alpha -amylase response to competition: Relation to gender, previous experience, and attitudes. Psychoneuroendocrinology. 2006;31(6):703–714. doi: 10.1016/j.psyneuen.2006.01.007. [DOI] [PubMed] [Google Scholar]
- de Kloet C, Vermetten E, Geuze E, Kavelaars A, Heijnen C, Westenberg H. Assessment of HPA axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatric Research. 2006;40(6):550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kudielka B, Buske-Kirschbaum A, Hellhammer D, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology. 2004;29(1):83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Entringer D, Hellhammer D, Wust S. Cortisol and ACTH responses to psychosocial stress are modulated by corticosteroid binding globulin levels. Psychoneuroendocrinology. 2007;32(810):115–1157. doi: 10.1016/j.psyneuen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Legro R, Lin H, Demers L, Lloyd T. Urinary free cortisol increases in adolescent caucasian females during perimenarche. Journal of Clinical Endocrinology and Metabolism. 2003;88(1):215–219. doi: 10.1210/jc.2002-020256. [DOI] [PubMed] [Google Scholar]
- Lopez N, Vazquez D, Olson S. An integrative approach to the neurophysiological substrates of social withdrawal and aggression. Development and Psychopathology. 2004;16(1):69–93. doi: 10.1017/s0954579404044414. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Olson SL, Hajal NJ, Felt BT, Vazquez DM. Hypothalamic Pituitary Adrenal Axis Functioning in Reactive and Proactive Aggression in Children. Journal of Abnormal Child Psychology. 2009;37(2):169. doi: 10.1007/s10802-008-9263-3. [DOI] [PubMed] [Google Scholar]
- Margot Prior P, Smart D, Sanson A, Oberklaid F. Does Shy-Inhibited Temperament in Childhood Lead to Anxiety Problems in Adolescence? Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(4):461. doi: 10.1097/00004583-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Matthews J, Altman D, Campbell M, Royston P. Analysis of serial measurements in medical research. British Medical Journal. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater U, Moor C, Okere U, Stallkamp R, Martin M, Ehlert U, et al. Performance on a declarative memory task is better in high than low cortisol responders to psychosocial stress. Psychoneuroendocrinology. 2007;32(6):758–763. doi: 10.1016/j.psyneuen.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Nejtek V. High and low emotion events influence emotional stress perceptions and are associated with salivary cortisol response changes in a consecutive stress paradigm. Psychoneuroendocrinology. 2002;27(3):337–352. doi: 10.1016/s0306-4530(01)00055-5. [DOI] [PubMed] [Google Scholar]
- Newman E, O'Connor D, Conner M. Daily hassles and eating behaviour: The role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32(2):125–132. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- O'Leary M, Loney B, Eckel LA. Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology. 2007;32(2):183–191. doi: 10.1016/j.psyneuen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Zellweger B, Marti B, Seiler R, Mohiyeddini C, Ehlert U, et al. Trained men show lower cortisol, heart rate and psychological responses to psychosocial stress compared with untrained men. Psychoneuroendocrinology. 2007;32(6):627–635. doi: 10.1016/j.psyneuen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Bolduc-Murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, et al. Behavioral Inhibition in Childhood: A Risk Factor for Anxiety Disorders. Harvard Review of Psychiatry. 1993;1(1):2–16. doi: 10.3109/10673229309017052. [DOI] [PubMed] [Google Scholar]
- Roy M, Kirchbaum C, Steptoe A. Psychological, cardiovascular, and metabolic correlates of individual differences in cortisol stress recovery in young men. Psychoneuroendocrinology. 2001;26(4):375–391. doi: 10.1016/s0306-4530(00)00061-5. [DOI] [PubMed] [Google Scholar]
- Sher L. Type D personality: the heart, stress, and cortisol. QJM. 2005;98(5):323–329. doi: 10.1093/qjmed/hci064. [DOI] [PubMed] [Google Scholar]
- Tornhage C. Reference values for morning salivary cortisol concentrations in healthy school-aged children. Journal of Pediatric Endocrinology and Metabolism. 2002;15(2):197–204. doi: 10.1515/jpem.2002.15.2.197. [DOI] [PubMed] [Google Scholar]
- Uhart M, Chong R, Oswald L, Lin P, Wand G. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31(5):642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Van Honk J, Tuiten A, de Haan E, Hout M, Stam H. Attentional biases for angry faces: Relationships to trait anger and anxiety. Cognition & Emotion. 2001;15(3):279–297. [Google Scholar]
- Wolf O, Schommer N, Hellhammer D, McEwen B, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26(7):711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]