Abstract
Background
Even low levels of residual symptoms are known to increase the risk of relapse and early recurrence of major depression. It is not known if ongoing psychotherapy lessens this risk. We therefore examined the impact of persistent symptoms, including mood, insomnia, and anxiety symptoms, on time to recurrence in women receiving maintenance interpersonal psychotherapy (IPT-M) for recurrent depression.
Method
We analyzed data on 131 women aged 20 to 60 from a 2-year randomized trial of weekly vs. twice-monthly vs. monthly IPT-M. Participants achieved remission with IPT alone (n=99) or IPT plus sequential antidepressant medication (n=32). Medications were tapered before starting maintenance treatment. Residual symptoms were assessed with the Hamilton Rating Scale for Depression (HRSD; total score and subscales); insomnia was also assessed in 76 women with the Pittsburgh Sleep Quality Index (PSQI). Data analyses used Cox proportional hazards regression models.
Results
Neither overall burden of residual symptoms (HRSD total score), nor HRSD mood and anxiety subscale scores predicted recurrence during ongoing IPT-M. In contrast, persistent insomnia measured both by the HRSD-17 insomnia subscale and the PSQI predicted recurrence. Women with persistent insomnia who required sequential pharamacotherapy had the highest recurrence rate (65%) compared to women requiring sequential treatment without insomnia (13%), or women who had recovered with IPT alone but had persistent insomnia (21%) or no insomnia (18%).
Conclusions
Persistent insomnia following the recovery from an episode of recurrent major depression is associated with increased risk of recurrence despite maintenance psychotherapy, particularly for those withdrawn from antidepressant medication. govIdentifier: NCT00227981
Keywords: Depressive Disorder, Depressive Symptoms, Sleep Initiation and Maintenance Disorders, Sleep, Anxiety, Recurrence
INTRODUCTION
Complete recovery is the goal of depression treatment. However, numerous studies (Judd et al. 1998; Kanai et al. 2003; Karp et al. 2004; Paykel et al. 1995; Thase et al. 1992; Van Londen et al. 1998) and clinical practice show that many of the patients who recover from an episode of depression experience persisting symptoms, which may put them at risk for recurrence. These residual symptoms have been likened to the mirror image of a prodrome, suggesting that the onset and the resolution (“roll-back”) of the depressive episode may be related (Fava 1999). Alternatively, residual symptoms may be seen as long-term psychological sequelae of a depressive episode (“depressive scar hypothesis”) (Shea et al. 1996).
While the negative prognostic significance of residual symptoms has been established, it is unclear how clinicians should approach them. Are certain symptoms more ominous than others and should be targeted preferentially? In older patients with depression, persistent anxiety rather than the core depressive symptoms of low mood, guilt, suicidal thoughts, and anergia/anhedonia herald poorer long-term outcomes (Dombrovski et al. 2007). Persistently poor sleep quality, assessed both objectively (Buysse et al. 1996; Kupfer et al. 1990) and subjectively (Buysse et al. 1996; Reynolds et al. 1997), also appears to be a marker of unfavorable long-term course. The development of sleep problems may also be a prodromal symptom of recurrence (Perlis et al. 1997).
The problem of residual symptoms may be of particular relevance for patients who prefer a psychological treatment to antidepressant medications. A recent study examined rates of residual insomnia following acute treatment with fluoxetine or cognitive behavior therapy (CBT), finding a prevalence of 51%, with no significant differences between the treatments (Carney et al. 2007). To our knowledge, no study has assessed the extent of residual symptoms following acute treatment with another commonly used time-limited therapy for depression, interpersonal psychotherapy (IPT), or, more importantly, their long-term impact.
We recently reported the results of a two-year trial of maintenance interpersonal psychotherapy (IPT) in women aged 20 to 60 with recurrent depression (Frank et al. 2007). Maintenance IPT (IPT-M) therapy sessions, provided at frequencies as low as once per month, were relatively effective in preventing depression recurrence among patients treated to remission with IPT alone; despite having a median of 4 previous episodes, only 26% had a recurrence of depression. IPT-M was less effective for patients who required adjunctive medication treatment to achieve remission of the acute depressive episode; after the medication was tapered, these women displayed a 50% recurrence rate during maintenance treatment. In this study, in addition to replicating and extending previous findings on the long-term impact of residual symptoms (Dombrovski et al. 2007; Flint and Rifat 1997; Kupfer et al. 1990; Perlis et al. 1997; Reynolds et al. 1997), we had the opportunity to examine the role of these symptoms in two groups of women: those who recovered from depression with psychotherapy alone and those who required the addition of an antidepressant to achieve remission. We hypothesized that participants with higher overall levels of residual symptoms, and persistent anxiety and insomnia in particular, would be more likely to suffer a recurrence.
METHODS
Study design and participant flow
All participants provided written informed consent as required by the University of Pittsburgh Institutional Review Board. Between 1992 and 1999, 233 women aged 20 to 60 and accepting non-pharmacologic treatment for their depression entered the study. All had recurrent major depression diagnosed using the Research Diagnostic Criteria (18) as extracted from either the Schedule for Affective Disorders and Schizophrenia (SADS) (18) or the Structured Clinical Interview for DSM-IV. Participants were required to have a score of 15 or above on the Hamilton Rating Scale for Depression 17-item version (HRSD-17) (Hamilton 1960) and a score 7 or above on the Raskin Severity of Depression Scale. Patients with co-occurring Axis I disorders other than anxiety disorders, hypomania, adult-onset dysthymia, eating disorders (NOS); antisocial or borderline personality disorder; history of alcohol or substance abuse/dependence within the past 2 years; history of a manic episode; or any significant or unstable medical condition were excluded.
In the acute treatment phase participants received weekly IPT alone for 12–24 weeks, or until achieving depression remission, defined as 3 consecutive weeks with an HRSD-17 score ≤ 7. Of 112 patients who achieved remission, 99 remained well for the requisite 5 additional weeks of continuation therapy and were then entered into the maintenance phase. Eighty-six IPT non-responders agreed to the addition of a selective serotonin reuptake inhibitor (SSRI), among whom 58 (67%) remitted. SSRI remitters received an additional 17 weeks of continuation phase treatment, after which the SSRI was tapered over 1 to 4 weeks, followed by 4 to 6 weeks of IPT alone; 32/58 participants remained in stable remission. During the continuation phase, 1/32 women in this group received zolpidem and 2/32, trazodone, for the treatment of insomnia. Thus, 99 women who remitted with IPT alone and 32 who remitted with the IPT/SSRI combined treatment were randomly assigned to one of three frequencies (weekly, twice-monthly, or monthly) of IPT-M sessions for a period of 2 years or until recurrence.
Measures
Recurrence was defined as two consecutive HRSD-17 scores of 15 or more and meeting DSM IV criteria for major depression, confirmed by an independent psychiatrist. We assessed residual symptoms at the time of random assignment to maintenance treatment. We used the HRSD-17 to measure the total burden or residual symptoms. We defined the presence of residual core mood symptoms as a score of ≥1 on the HRSD-17 core symptom subscale including the depressed mood (item 1), guilt (item 2), suicide (item 3), and anergia/anhedonia (item 7). Persistent insomnia was defined as a score of ≥1 on the HRSD-17 sleep subscale including the early (item 4), middle (item 5), and late (item 6) insomnia items. In addition to the HRSD-17 subscale, insomnia was assessed with the Pittsburgh Sleep Quality Index (Buysse et al. 1989). Persistent anxiety was defined as a score of ≥2 on the HRSD-17 anxiety subscale including the agitation (item 9), psychic (item 10) and somatic (item 11) anxiety, and hypochondriasis (item 15). These factor-analytically derived subscales have been shown to explain similar proportions of overall symptom variability (Dombrovski et al. 2006). Their construction was confirmed by the results of a recent meta-analysis of the HRSD-17 factor structure (Shafer 2006). We chose the above cutoffs rather than continuous subscale scores because of the limited internal consistency of the HRSD-17 subscales (Dombrovski et al. 2006; Dombrovski et al. 2007), probably reflecting insufficient unidimensionality of the scale as a whole (Bagby et al. 2004). We chose cutoffs of 1 on the core mood symptoms subscale (range 0–16) because any symptoms of depressed mood or anhedonia are clinically significant in a remitted patient, and on the sleep subscale, because sleep items are scored from 0 to 2, resulting in lower ratings overall (range 0–6). The cutoff of 2 on the anxiety subscale (range 0–14) was chosen because the psychic anxiety, somatic anxiety, and hypochondriasis items are scored from 0 to 4 and ratings of 1 correspond to doubtful symptoms or physiological phenomena resembling somatic anxiety. Our examination of score distributions at the start of maintenance treatment confirmed these cutoffs.
Data Analyses
We included data on all 131 randomized participants; PSQI scores were available on 76 (the scale was added later in the study). We used Cox proportional hazards regression models stratifying by SSRI treatment to examine the relationship between residual symptoms and subsequent recurrence. Symptom measures included the total residual symptom burden (HRSD-17 total score at the time of randomization), residual core mood, insomnia, and anxiety symptom subsets (HRSD-17 subscales and PSQI score at the time of randomization). Data were censored at the time of discontinuation for patients who terminated from the study. Next, we tested whether identified predictors would remain significant after accounting for other known predictors of recurrence using a multiple Cox proportional hazards regression model. These predictors included age, number of previous episodes, and duration of the index episode.
RESULTS
Table 1 presents participant characteristics at the beginning of maintenance treatment. There were no differences between groups in overall severity of residual symptoms (t(129)=0.63, p=0.53), prevalence of core mood symptoms (Fisher’s exact p=0.31), insomnia (p=0.31), or anxiety symptoms (p=1.0) among participants requiring sequential treatment (IPT + SSRI) compared to participants recovered with IPT alone.
Table 1.
Characteristics of women who started maintenance treatment
| Mean (SD) unless otherwise specified | ||
|---|---|---|
| Remitted with IPT alone N=99 | Remitted with IPT+SSRI N=32 | |
| Demographics | ||
| Age | 37.8 (10.4) | 38.3 (9.9) |
| Race: n(%) white | 87 (88%) | 28 (88%) |
| Education in years | 15.2 (1.8) | 14.9 (2.0) |
| Married: n(%) | 35 (35%) | 17 (53%) |
| Employed fulltime: n(%) | 59 (60%) | 17 (53%) |
| Clinical Measures | ||
| Duration of current episode, wks | 26.1 (20.8) median=20.0 |
29.9 (21.6) median=24.5 |
| Number of previous episodes | 4.6 (3.1) median=4 |
5.2 (2.6) median=5 |
| Age of first lifetime onset | 25.6 (9.1) | 23.5 (8.8) |
| Baseline Scores | ||
| Hamilton Rating Scale for Depression-17 item | 18.1 (2.7) | 18.8 (3.2) |
| Global Assessment Scale | 56.1 (4.7) | 54.9 (5.2) |
| Beck Depression Inventory | 24.7 (6.6) | 26.1 (6.6) |
| Treatment | ||
| Duration of acute and continuation treatment, weeks | 26.9 (4.8) | 56.3 (11.9) |
| Number of psychotherapy sessions before start of maintenance | 22.0 (3.5) | 47.3 (7.4) |
| Final dose of fluoxetine, mg/d (N=31)* | 28.6 (15.3) | |
| Residual symptoms at the start of maintenance treatment | ||
| HRS17 total | 3.24 (2.71) | 3.59 (2.79) |
| %Core ≥ 1 | 45% | 56% |
| %Sleep ≥ 1 | 42% | 53% |
| %Anxiety ≥ 2 | 32% | 31% |
| Pittsburgh Sleep Quality Index | 4.14 (2.20) | 3.82 (1.59) |
One patient received sertraline 100 mg/d
The HRSD total score was not significantly related to the hazard of recurrence (hazard ratio [HR]: 1.12, confidence interval [CI]: [0.99–1.26], p=0.064). Likewise, neither persistent core mood symptoms (HR: 1.09, CI: 0.54–2.21, p=0.81) nor persistent anxiety symptoms (HR: 1.39, CI: 0.68–2.81, p=0.37) significantly predicted recurrence risk.
By contrast, patients with persistent insomnia measured both by the HRSD-17 subscale (HR: 2.33, CI: 1.13–4.81, p=0.022) and the PSQI (HR: 1.25, CI: 1.02–1.53, p=0.030) were more likely to suffer a recurrence. The survival plot (Figure 1) illustrates that women with persistent insomnia who required sequential pharamacotherapy had the highest recurrence rate (65%, n=11/17, CI: 42%–87%) compared to women requiring sequential treatment without insomnia (13%, n=2/15, CI: 0–30%), women who had recovered with IPT alone but had persistent insomnia (21%, n=9/42, CI: 9%–34%) or no insomnia (18%, n=10/57, CI: 8%–27%). Persistent insomnia measured by the HRSD-17 subscale (HR: 2.48, CI: 1.19–5.17, p=0.015) and the PSQI (HR: 1.97., CI: 1.01–3.86, p=0.047) remained a significant predictor in a Cox multiple proportional hazards regression model, controlling for the duration of current episode and the number of previous episodes.
Figure 1.
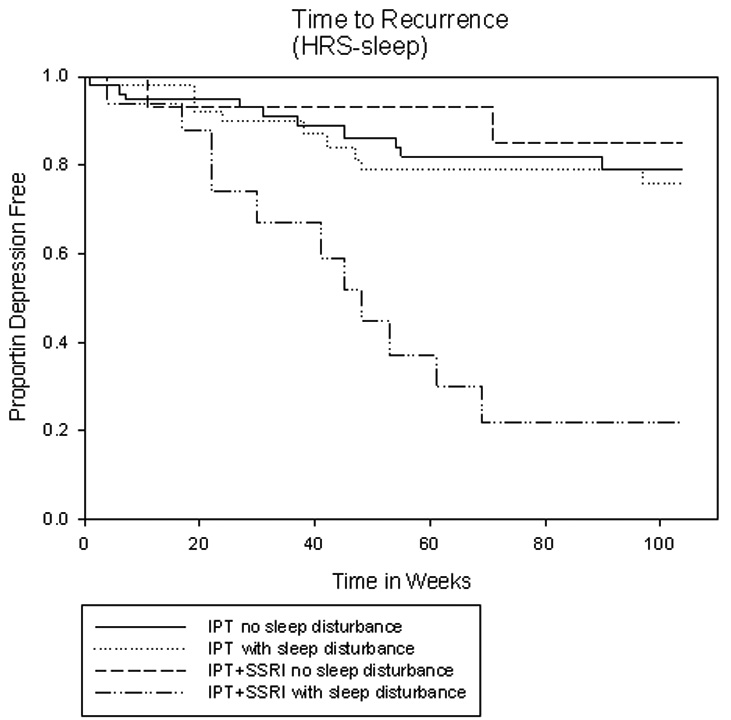
Legend: Kaplan-Meyer depression-free survival plots illustrate that women who required sequential acute treatment and suffered from persistent insomnia measured by the HRSD-17 subscale had the highest recurrence rate (65%, n=17, CI: 42%–87%) compared to women requiring sequential treatment without insomnia (13%, n=15, CI: 0–30%), and to women who had recovered with IPT alone with (21%, n=42, CI: 9%–34%) or without persistent insomnia (18%, n=57, CI: 8%–27%).
Having observed the long-term impact of insomnia, we examined post hoc whether it was present among women requiring sequential treatment before their antidepressant medication was tapered. The prevalence of insomnia was 47– before and 53– after the antidepressant medication was discontinued (Fisher’s exact p=0.80).
DISCUSSION
Persistent insomnia, but not core depressive symptoms such as depressed mood, guilt, suicidal thoughts, and anergia/anhedonia, predicted depression-free survival during two-year maintenance treatment with interpersonal psychotherapy in women recovered from an episode of recurrent major depression. Contrary to our hypothesis, persistent anxiety symptoms had no such impact on long-term course.
Randomized controlled design, two-year follow-up, and comprehensive clinical characterization add confidence in our findings. The relatively low recurrence rate in our sample (26% percent in 99 remitters with IPT monotherapy and 50% in 32 remitters with sequential treatment) presumably reflect the protective effect of ongoing treatment; this may have limited our power to detect differences in time to recurrence between patients. Our use of HRSD-17 subscales to assess residual symptoms can be seen as another limitation, because of their relative lack of unidimensionality, characteristic of the HRSD-17 in general (Bagby et al. 2004; Dombrovski et al. 2007). To address this issue, we used clinically determined cutoffs to define significant levels of residual symptoms. The PSQI provided a more comprehensive assessment of sleep disturbance, independently validating our sleep results; however both instruments rely on self-report, and objective polysomnographic recordings were not obtained in our study. Our results can only be generalized to women with a history of recurrent depression, but no serious psychiatric comorbidities (other than anxiety disorders) treated in a specialty psychiatric clinic.
Effects of persistent insomnia on mood
Our finding of early recurrence of depression in women with persistent sleep problems agrees with previous clinical studies (Buysse et al. 1996; Dombrovski et al. 2007; Perlis et al. 1997; Reynolds et al. 1997) and with findings of insomnia preceding incident depression in community samples [(Johnson et al., 2006; Neckelmann et al., 2007; Weissman et al. 1997), ing, older studies reviewed in (Riemann and Voderholzer 2003)], underscoring the clinical importance of restoring normal sleep in depression. It is clear that in a subgroup of patients, even a combination of psychotherapy and SSRI does not achieve this goal; half of those women in our study reported some sleep problems following recovery from depression. Of even greater clinical interest may be the observation that IPT alone was not sufficient to maintain remission among patients who required medication treatment in addition to psychotherapy to recover from the depressive episode AND who still displayed persistent insomnia. This is in line with previous findings of poorer response to psychotherapy in depressed patients with abnormal sleep (Thase et al. 1996). It is possible that the need for acute antidepressant drug treatment and sleep problems that persist following remission are clinical markers of a biologically distinct depressive subtype that is characterized by hyperarousal and a recurrent or chronic course. Hyperarousal is a state of abnormally high alertness in the sleep environment characterized by the failure to appropriately modulate the metabolic activity of the prefrontal cortex (Nofzinger et al. 2004) and the brain’s electrical activity during sleep (Krystal et al. 2002; Merica et al. 1998; Perlis et al. 2001), by the sympathetic cardiovascular response (Bonnet and Arand 1997), hypercortisolemia (Rodenbeck et al. 2002; Vgontzas et al. 2001), and increased systemic metabolism (Bonnet and Arand 1995). Hyperarousal and, more broadly, disruption of the circadian cycle, appears to play a particularly important role in depressed women (Armitage 2007); it may interfere with mood regulation and thus precipitate new depressive episodes in these patients.
Role of persistent anxiety
In our study, women aged 20 to 60 with residual anxiety were not more likely to suffer an early depressive recurrence, unlike older participants in previous trials (Dombrovski et al. 2007; Flint and Rifat 1997). Our power to detect a possible relationship between persistent anxiety and recurrence of depression in this sample was limited by both the low rate of recurrence and the fact that stringent remission criteria (HRSD-17 ≤ 7) may have precluded some women with residual anxiety symptoms from being classified as remitters. Furthermore, as we have previously reported, patients with anxiety symptoms are less likely to respond acutely to IPT (Frank et al. 2000) and are probably under-represented in our sample. In addition, the 4-item HRSD-17 subscale may not be sensitive enough to all anxiety symptoms. However, this observation may point to actual differences in the phenomenology and course of depression across the lifespan: anxiety may play a greater role in old age than in mid-life, as noted by Kraepelin a century ago (Diefendorf 1915).
Treatment implications
Half of the women who did not initially respond to IPT alone in our study continued to suffer from poor sleep after the addition of fluoxetine. We do not know whether treating their sleep problems would have prevented early recurrence of depression. Future studies might test alternative treatment strategies for patients with depression complicated by persistent insomnia. Although tricyclic antidepressants (TCAs) have been largely replaced by newer drug classes because of their autonomic and cardiovascular side effects, their ability to improve not only insomnia (Haskell et al. 1975; Vaisanen et al. 1978; Young et al. 1976), but also sleep quality (Buysse et al. 1996; Feuillade et al. 1992; Hajak et al. 2001; Roth et al. 1982; Shipley et al. 1985) is well established. “Second-generation” sedating antidepressants trazodone and nefazodone (Manber et al. 2003; Thase et al. 2002) also improve sleep quality and provide another antidepressant monotherapy option for these patients, although their benefits need to be weighed against possible cardiovascular side effects of trazodone and the risk of hepatic failure, which resulted in the boxed warning for nefazodone. The new 5HT-2C antagonist agomelatine, which has shown antidepressant efficacy in initial short-term clinical trials (Kennedy and Emsley 2006; Loo et al. 2002; Pierre Olie and Kasper 2007), appears to exert effects on sleep quality and circadian rhythm mediated by its action on melatonin MT-1 and MT-2 receptors. Agomelatine may prove to be a useful antidepressant for patients with enduring sleep problems. The addition of a GABA-ergic hypnotic such as zolpidem (Asnis et al. 1999) or escopiclone (Fava et al. 2006) also appears to improve both sleep and mood outcomes in the short term, although the possibly increased incidence of depression on hypnotics (Kripke, 2007) warrants caution. Finally, cognitive behavioral therapy for insomnia (CBT-I), which combines behavioral approaches (relaxation training, stimulus control, sleep restriction) with a cognitive intervention targeting negative beliefs about sleep, is effective and may be superior to hypnotics in primary insomnia (Jacobs et al., 2004; Sivertsen et al., 2006). There is accumulating evidence, albeit so far limited to small studies (Kuo et al., 2001; Lichstein et al., 2000; Taylor et al. 2007), of its efficacy in insomnia associated with depression.
CONCLUSION
In summary, we found that women who required the addition of an antidepressant medication to achieve remission, and reported poor sleep following the recovery from an episode of recurrent major depression and cessation of the antidepressant, are less likely to remain well with maintenance psychotherapy alone. Future studies will be needed to determine the optimal acute and maintenance treatment regime for such patients; however, our findings suggest that maintenance psychotherapy alone is unlikely to be sufficient in this group.
Acknowledgments
Supported by: grants MH049115 and MH030915, and the John A. Hartford Foundation.
Footnotes
DECLARATION OF INTEREST
Drs. Dombrovski, Andreescu, and Mallinger and Ms. Houck do not have any potential conflict to acknowledge. Dr. Cyranowski has received research support from Eli Lilly. Dr. Mulsant has received honoraria and/or research support from Bristol-Myers Squibb, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Lundbeck, and Pfizer. Dr. Frank has served on advisory boards of Pifzer, Inc., Servier, and Eli Lilly & Company, has been a consultant for Pifzer, Inc., Eli Lilly & Company, and Novartis, and has received an investigator-initiated grant from Forest Research Institute. Dr. Buysse serves as a consultant for Actelion, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, Sanofi-Aventis, Servier, Sepracor, and Takeda. Dr. Thase has been a consultant for AstraZeneca, Bristol-Myers Squibb Company, Cephalon, Inc., Cyberonics, Inc., Eli Lilly & Co., GlaxoSmithKline, Janssen Pharmaceutica, MedAvante, Inc., Neuronetics, Inc., Novartis, Organon, Inc., Sepracor, Inc., Shire US Inc.,Supernus Pharmaceuticals, and Wyeth Pharmaceuticals, is on the speakers bureau of AstraZeneca, Bristol-Myers Squibb Company, Cyberonics, Inc., Eli Lilly & Co., GlaxoSmithKline, Organon, Inc., Sanofi Aventis, and Wyeth Pharmaceuticals, and holds equity in MedAvante, Inc. This work was performed at the University of Pittsburgh, prior to Dr. Mallinger’s official duties as a Government employee. The views expressed in this paper do not necessarily represent the views of the NIMH, NIH, or the United States Government.
This study is registered at www.clinicaltrials.gov under the title “Maintenance Interpersonal Psychotherapy for Sustaining Remission of Depression” (govIdentifier: NCT00227981, Study ID Numbers: R01 MH49115; DSIR 950309–9905). All criteria as stated in the Clinical Trial Registration policy have been met.
References
- Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand. 2007;433 Suppl:104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Asnis GM, Chakraburtty A, DuBoff EA, Krystal A, Londborg PD, Rosenberg R, Roth-Schechter B, Scharf MB, Walsh JK. Zolpidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiatry. 1999;60(10):668–676. doi: 10.4088/jcp.v60n1005. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? American Journal of Psychiatry. 2004;161(12):2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18(7):581–588. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr Clin Neurophysiol. 1997;102(5):390–396. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Hoch CC, Houck PR, Kupfer DJ, Mazumdar S, Frank E. Longitudinal effects of nortriptyline on EEG sleep and the likelihood of recurrence in elderly depressed patients. Neuropsychopharmacology. 1996;14(4):243–252. doi: 10.1016/0893-133X(95)00114-S. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007;68(2):254–260. doi: 10.4088/jcp.v68n0211. [DOI] [PubMed] [Google Scholar]
- Diefendorf R. New York, London: The Macmillan Company; 1915. Clinical psychiatry; a text-book for students and physicians, abstracted and adapted from the 7th German ed. of Kraepelin's “Lehrbuch der psychiatrie” by A. Ross Diefendorf. New York, London. [Google Scholar]
- Dombrovski AY, Blakesley-Ball RE, Mulsant BH, Mazumdar S, Houck PR, Szanto K, Reynolds CF. Speed of improvement in sleep disturbance and anxiety compared to core mood symptoms during acute treatment of depression in old age. American Journal of Geriatric Psychiatry. 2006;14(6):550–554. doi: 10.1097/01.JGP.0000218325.76196.d1. [DOI] [PubMed] [Google Scholar]
- Dombrovski AY, Mulsant BH, Houck PR, Mazumdar S, Lenze EJ, Andreescu C, Cyranowski JM, Reynolds CF., 3rd Residual symptoms and recurrence during maintenance treatment of late-life depression. J Affect Disord. 2007;103(1–3):77–82. doi: 10.1016/j.jad.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava GA. Subclinical symptoms in mood disorders: pathophysiological and therapeutic implications. Psychol Med. 1999;29(1):47–61. doi: 10.1017/s0033291798007429. [DOI] [PubMed] [Google Scholar]
- Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J, Amato D, Roth T. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59(11):1052–1060. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Feuillade P, Pringuey D, Belugou JL, Robert P, Darcourt G. Trimipramine: acute and lasting effects on sleep in healthy and major depressive subjects. J Affect Disord. 1992;24(3):135–145. doi: 10.1016/0165-0327(92)90061-a. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Rifat SL. Two-year outcome of elderly patients with anxious depression. Psychiatry Res. 1997;66(1):23–31. doi: 10.1016/s0165-1781(96)02964-2. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Buysse DJ, Swartz HA, Pilkonis PA, Houck PR, Rucci P, Novick DM, Grochocinski VJ, Stapf DM. Randomized trial of weekly, twice-monthly, and monthly interpersonal psychotherapy as maintenance treatment for women with recurrent depression. Am J Psychiatry. 2007;164(5):761–767. doi: 10.1176/appi.ajp.164.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Shear MK, Rucci P, Cyranowski JM, Endicott J, Fagiolini A, Grochocinski VJ, Houck P, Kupfer DJ, Maser JD, Cassano GB, JD, et al. Influence of panic-agoraphobic spectrum symptoms on treatment response in patients with recurrent major depression. Am J Psychiatry. 2000;157(7):1101–1107. doi: 10.1176/appi.ajp.157.7.1101. [DOI] [PubMed] [Google Scholar]
- Hajak G, Rodenbeck A, Voderholzer U, Riemann D, Cohrs S, Hohagen F, Berger M, Ruther E. Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. Journal of Clinical Psychiatry. 2001;62(6):453–463. doi: 10.4088/jcp.v62n0609. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell DS, DiMascio A, Prusoff B. Rapidity of symptom reduction in depressions treated with amitriptyline. Journal of Nervous & Mental Disease. 1975;160(1):24–33. doi: 10.1097/00005053-197501000-00005. [DOI] [PubMed] [Google Scholar]
- Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. 2004;164(17):1888–1896. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700–708. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB, TI, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2–3):97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Kanai T, Takeuchi H, Furukawa TA, Yoshimura R, Imaizumi T, Kitamura T, Takahashi K. Time to recurrence after recovery from major depressive episodes and its predictors. Psychol Med. 2003;33(5):839–845. doi: 10.1017/s0033291703007827. [DOI] [PubMed] [Google Scholar]
- Karp JF, Buysse DJ, Houck PR, Cherry C, Kupfer DJ, Frank E. Relationship of variability in residual symptoms with recurrence of major depressive disorder during maintenance treatment. Am J Psychiatry. 2004;161(10):1877–1884. doi: 10.1176/ajp.161.10.1877. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Emsley R. Placebo-controlled trial of agomelatine in the treatment of major depressive disorder. Eur Neuropsychopharmacol. 2006;16(2):93–100. doi: 10.1016/j.euroneuro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Kripke DF. Greater incidence of depression with hypnotic use than with placebo. BMC Psychiatry. 2007;7:42. doi: 10.1186/1471-244X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25(6):630–640. [PubMed] [Google Scholar]
- Kuo T, Manber R, Loewy D. Insomniacs with comorbid conditionsdepression achieved comparable improvement in a cognitive behavioral group treatment program as insomniacs without comorbid depression. Sleep. 2001;14(A62) [Google Scholar]
- Kupfer DJ, Frank E, McEachran AB, Grochocinski VJ. Delta sleep ratio. A biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry. 1990;47(12):1100–1105. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Wilson NM, Johnson CT. Psychological treatment of secondary insomnia. Psychol Aging. 2000;15(2):232–240. doi: 10.1037//0882-7974.15.2.232. [DOI] [PubMed] [Google Scholar]
- Loo H, Hale A, D'Haenen H. Determination of the dose of agomelatine, a melatoninergic agonist and selective 5-HT(2C) antagonist, in the treatment of major depressive disorder: a placebo-controlled dose range study. Int Clin Psychopharmacol. 2002;17(5):239–247. doi: 10.1097/00004850-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Manber R, Rush AJ, Thase ME, Amow B, Klein D, Trivedi MH, Korenstein SG, Markowitz JC, Dunner DL, Munsaka M, Borian FE, Martin, Keller B, M, et al. The effects of psychotherapy, nefazodone, and their combination on subjective assessment of disturbed sleep in chronic depression. Sleep. 2003;26(2):130–136. doi: 10.1093/sleep/26.2.130. [DOI] [PubMed] [Google Scholar]
- Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10(5):1826–1834. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30(7):873–880. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42(2–3):209–212. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- Pierre Olie J, Kasper S. Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder. Int J Neuropsychopharmacol. 2007:1–13. doi: 10.1017/S1461145707007766. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Frank E, Houck PR, Mazumdar S, Dew MA, Cornes C, Buysse DJ, Begley A, Kupfer DJ. Which elderly patients with remitted depression remain well with continued interpersonal psychotherapy after discontinuation of antidepressant medication? Am J Psychiatry. 1997;154(7):958–962. doi: 10.1176/ajp.154.7.958. [DOI] [PubMed] [Google Scholar]
- Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? Journal of Affective Disorders. 2003;76(1–3):255–259. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- Rodenbeck A, Huether G, Ruther E, Hajak G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci Lett. 2002;324(2):159–163. doi: 10.1016/s0304-3940(02)00192-1. [DOI] [PubMed] [Google Scholar]
- Roth T, Zorick F, Wittig R, McLenaghan A, Roehrs T. The effects of doxepin HCl on sleep and depression. J Clin Psychiatry. 1982;43(9):366–368. [PubMed] [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62(1):123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Shea MT, Leon AC, Mueller TI, Solomon DA, Warshaw MG, Keller MB. Does major depression result in lasting personality change? Am J Psychiatry. 1996;153(11):1404–1410. doi: 10.1176/ajp.153.11.1404. [DOI] [PubMed] [Google Scholar]
- Shipley JE, Kupfer DJ, Griffin SJ, Dealy RS, Coble PA, McEachran AB, Grochocinski VJ, Ulrich R, Perel JM. Comparison of effects of desipramine and amitriptyline on EEG sleep of depressed patients. Psychopharmacology (Berl) 1985;85(1):14–22. doi: 10.1007/BF00427316. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, Nielsen GH, Nordhus IH. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295(24):2851–2858. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Lichstein KL, Weinstock J, Sanford S, Temple JR. A pilot study of cognitive-behavioral therapy of insomnia in people with mild depression. Behavior Therapy. 2007;38(1):49–57. doi: 10.1016/j.beth.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Thase ME, Rush AJ, Manber R, Kornstein SG, Klein DN, Markowitz JC, Ninan PT, Friedman ES, Dunner DL, Schatzberg AF, Borian FE, Trivedi MH, Keller MB, AF, et al. Differential effects of nefazodone and cognitive behavioral analysis system of psychotherapy on insomnia associated with chronic forms of major depression. J Clin Psychiatry. 2002;63(6):493–500. doi: 10.4088/jcp.v63n0605. [DOI] [PubMed] [Google Scholar]
- Thase ME, Simons AD, McGeary J, Cahalane JF, Hughes C, Harden T, Friedman E. Relapse after cognitive behavior therapy of depression: potential implications for longer courses of treatment. Am J Psychiatry. 1992;149(8):1046–1052. doi: 10.1176/ajp.149.8.1046. [DOI] [PubMed] [Google Scholar]
- Thase ME, Simons AD, Reynolds CF., 3rd Abnormal electroencephalographic sleep profiles in major depression: association with response to cognitive behavior therapy. Arch Gen Psychiatry. 1996;53(2):99–108. doi: 10.1001/archpsyc.1996.01830020013003. [DOI] [PubMed] [Google Scholar]
- Vaisanen E, Naarala M, Kontiainen H, Merilainen V, Heikkila L, Malinen L. Maprotiline and doxepin in the treatment of depression. A double-glind multicentre comparison. Acta Psychiatrica Scandinavica. 1978;57(3):193–201. doi: 10.1111/j.1600-0447.1978.tb06885.x. [DOI] [PubMed] [Google Scholar]
- Van Londen L, Molenaar RP, Goekoop JG, Zwinderman AH, Rooijmans HG. Three- to 5-year prospective follow-up of outcome in major depression. Psychol Med. 1998;28(3):731–735. doi: 10.1017/s0033291797006466. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86(8):3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. General Hospital Psychiatry. 1997;19(4):245–250. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- Young JP, Hughes WC, Lader MH. A controlled comparison of flupenthixol and amitriptyline in depressed outpatients. British Medical Journal. 1976;1(6018):1116–1118. doi: 10.1136/bmj.1.6018.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]


