Abstract
We examined whether activation of angiotensin-1 receptors (AT1R) could account for impaired responses of cerebral arterioles during Type 1 diabetes (T1D). First, we measured responses of cerebral arterioles in nondiabetic rats to eNOS-dependent (acetylcholine and adenosine diphosphate (ADP)) and -independent (nitroglycerin) agonists before and during application of angiotensin II. Next, we examined whether losartan could improve impaired responses of cerebral arterioles during T1D. In addition, we harvested cerebral microvessels for Western blot analysis of AT1R protein and measured production of superoxide anion by brain tissue under basal conditions and in response to angiotensin II in the absence or presence of losartan. We found that angiotensin II specifically impaired eNOS-dependent reactivity of cerebral arterioles. In addition, while losartan did not alter responses in nondiabetics, losartan restored impaired eNOS-dependent vasodilatation in diabetics. Further, AT1R protein was higher in diabetics compared to nondiabetics. Finally, superoxide production was higher in brain tissue from diabetics compared to nondiabetics under basal conditions, angiotensin II increased superoxide production in nondiabetics and diabetics, and losartan decreased basal (diabetics) and angiotensin II-induced production of superoxide (nondiabetics and diabetics). We suggest that activation of AT1R during T1D plays a critical role in impaired eNOS-dependent dilatation of cerebral arterioles.
Keywords: Brain, Angiotensin II, Acetylcholine, ADP, Nitroglycerin, Oxidative Stress
1. Introduction
The incidence of cerebrovascular abnormalities, including stroke, is increased in Type 1 diabetes (T1D) (Cooper et al., 1997; Mortel et al., 1990). Over the past several years, a number of studies have implicated an important role for the renin-angiotensin system in the pathogenesis of cardiovascular abnormalities associated with T1D. For example, investigators have shown that tissue and plasma levels of angiotensin converting enzyme (ACE) and angiotensin II are elevated in diabetic subjects and animals (Duntas et al., 1992; Harrison-Bernard et al., 2002; Lieberman and Sastre, 1980; Schernthaner et al., 1984) and treatment of diabetic subjects with ACE inhibitors can improve impaired NOS-dependent responses of large peripheral vessels (Arcaro et al., 1999; Cheetham et al., 2000; O’Driscoll et al., 1997; O’Driscoll et al., 1999). In addition, we (Trauernicht et al., 2003) have shown that treatment of diabetic rats with an ACE inhibitor (enalapril) could alleviate impaired eNOS-dependent responses of cerebral arterioles during T1D. Further, other investigators have shown that treatment of non-insulin dependent diabetic patients with inhibitors of angiotensin type 1 receptors (AT1R) improves endothelial dysfunction (Cheetham et al., 2000). Thus, it appears that treatment of diabetic subjects and animals with ACE inhibitors or AT1R antagonists may be useful therapeutic tools for the prevention of cardiovascular abnormalities associated with T1D. However, there are no data regarding the potential therapeutic benefit of inhibition of AT1R on cerebrovascular dysfunction in T1D.
Mechanisms that account for the effects of ACE inhibitors and AT1R antagonists on vascular dysfunction are not clear but have been reported to be related to effects on insulin sensitivity (Torlone et al., 1993), potentiating the actions of bradykinin (Cachofeiro et al., 1992), an increase in NOS activity (Gonzalez Bosc et al., 2001; Rajagopalan and Harrison, 1996), and/or by inhibiting the influence of angiotensin II on oxidative stress (de Cavanagh et al., 2001; Rajagopalan et al., 1996; Welch and Wilcox, 2001). Support for this latter concept can be found in studies that have shown that stimulation of vascular cells with angiotensin II increases the activity of NAD(P)H oxidase, increases the expression of p47phox and stimulates the production of superoxide anion (Fukui et al., 1997; Griendling et al., 1994; Griendling et al., 2000; Landmesser et al., 2002; Rajagopalan et al., 1996). Further, inhibition of AT1R can decrease angiotensin II-induced increases in superoxide anion production by vascular cells (Berry et al., 2000; Kusaka et al., 2004; Zimmerman et al., 2002). Thus, in the present study we tested the hypotheses that angiotensin II could impair eNOS-dependent reactivity of rat cerebral arterioles and that treatment of diabetic rats with an AT1R antagonist (losartan) would alleviate/prevent impaired eNOS-dependent responses of cerebral arterioles via an influence on oxidative stress.
2. Results
2.1. Responses during angiotensin II
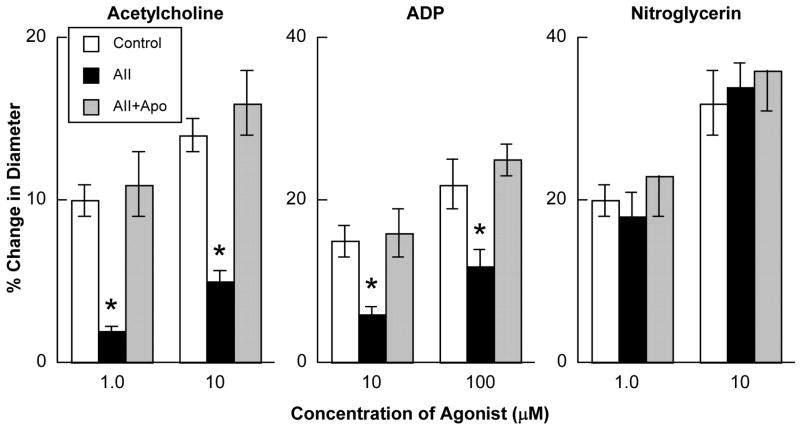
Baseline diameter of cerebral arterioles was 47±5 microns. Prior to superfusion with angiotensin II, acetylcholine, ADP and nitroglycerin produced dose-related dilation of cerebral arterioles in nondiabetic rats (Figure 1). Superfusion with angiotensin II (1.0 μM) for 2 hours did not alter baseline diameter of cerebral arterioles (49±9 microns after 2 hours of superfusion with angiotensin II; p>0.05 versus baseline diameter), but significantly impaired eNOS-dependent vasodilatation in response to acetylcholine and ADP (Figure 1). In contrast, superfusion with angiotensin II did not alter eNOS-independent responses to nitroglycerin (Figure 1). In addition, treatment of the cranial window preparation with apocynin (1 mM) prevented angiotensin II-induced impairment in eNOS-dependent reactivity, without influencing reactivity to nitroglycerin (Figure 1).
Figure 1.
Responses of cerebral arterioles to acetylcholine, ADP and nitroglycerin in nondiabetic rats under control conditions (open bars), following a 2-hour superfusion with angiotensin II (1.0 μM; closed bars) and following a 2-hour superfusion with angiotensin II in the presence of apocynin (1.0 mM; hatched bars). Values are means ± SE. * p < 0.05 versus response under basal conditions and in the presence of apocynin.
2.2. Responses following losartan
There were no significant differences between baseline diameter of cerebral arterioles or mean arterial blood pressure in nondiabetic and diabetic rats (Table 1). In contrast, blood glucose concentration was significantly higher and body weight was lower in diabetic than in nondiabetic rats (Table 1).
Table 1.
Baseline diameter of cerebral arterioles, mean arterial pressure, blood glucose concentration and body weight in nondiabetic and diabetic rats.
| Nondiabetic | Diabetic | |
|---|---|---|
| Baseline diameter (microns) | 48±2 | 46±2 |
| Mean arterial pressure (mmHg) | 107±3 | 106±5 |
| Blood glucose (mg/dl) | 91±8 | 368±17* |
| Body weight (grams) | 395±16 | 229±12* |
Values are means±SE.
p<0.05 versus nondiabetic rats.
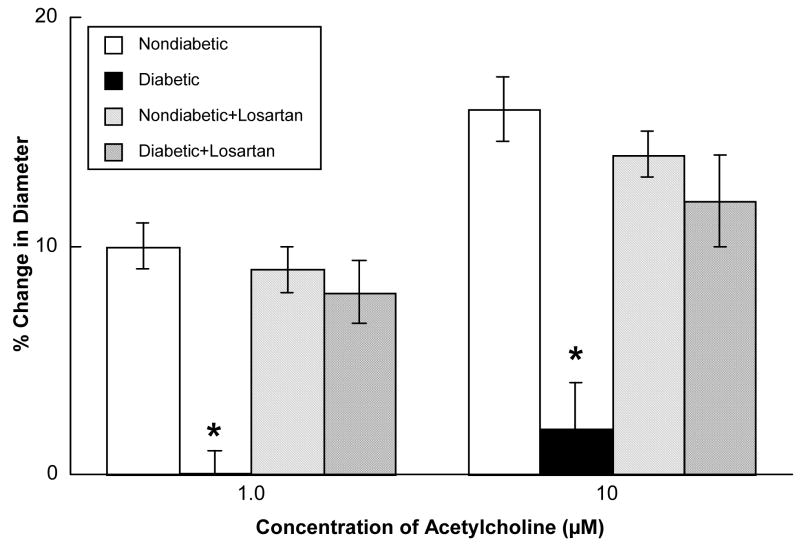
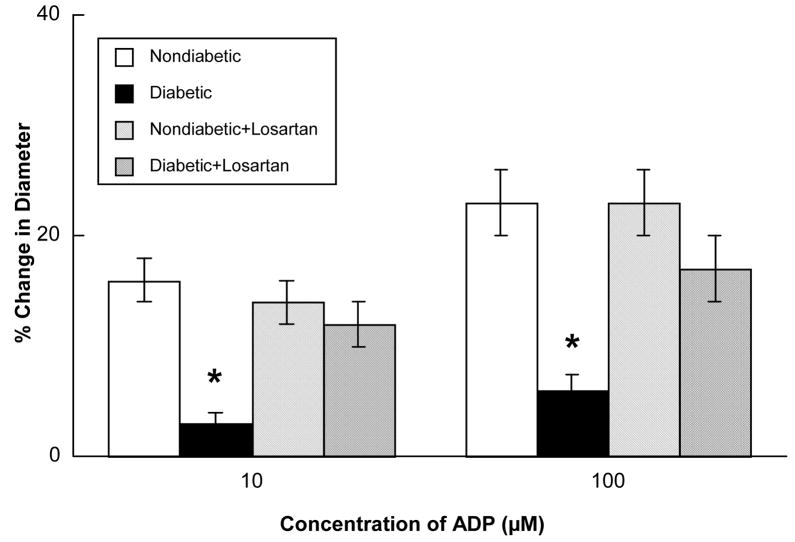
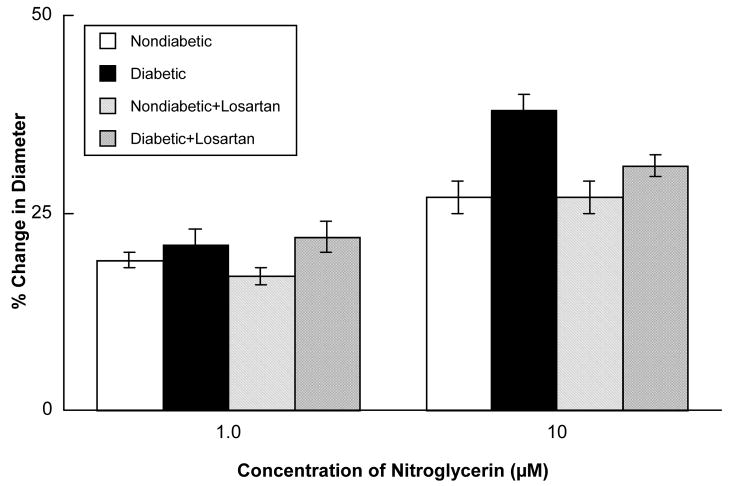
Acetylcholine (Figure 2), ADP (Figure 3) and nitroglycerin (Figure 4) produced dilatation of cerebral arterioles in nondiabetic and diabetic rats. However, the magnitude of vasodilatation in response to acetylcholine and ADP was significantly less in diabetic compared to nondiabetic rats (Figures 2 and 3). In contrast, nitroglycerin produced similar vasodilatation in nondiabetic and diabetic rats (Figure 4).
Figure 2.
Responses of cerebral arterioles to acetylcholine in nondiabetic rats before (open bars; n=6) and during (left hatched bars; n=6) application of losartan (0.1 mM), and in diabetic rats before (closed bars; n=9) and during (cross hatched bars; n=9) application of losartan. Values are means ± SE. * p < 0.05 versus response in nondiabetic rats and diabetic rats after treatment with losartan.
Figure 3.
Responses of cerebral arterioles to ADP in nondiabetic rats before (open bars; n=6) and during (left hatched bars; n=6) application of losartan (0.1 mM), and in diabetic rats before (closed bars; n=9) and during (cross hatched bars; n=9) application of losartan. Values are means ± SE. * p < 0.05 versus response in nondiabetic rats and diabetic rats after treatment with losartan.
Figure 4.
Responses of cerebral arterioles to nitroglycerin in nondiabetic rats before (open bars; n=6) and during (left hatched bars; n=6) application of losartan (0.1 mM), and in diabetic rats before (closed bars; n=9) and during (cross hatched bars; n=9) application of losartan. Values are means ± SE.
Topical application of losartan did not alter baseline diameter of cerebral arterioles in nondiabetic (46±3 microns before versus 44±2 microns during losartan; p>0.05) or diabetic (43±3 microns before versus 41±3 microns during losartan; p>0.05) rats. However, topical application of losartan restored impaired dilatation of cerebral arterioles in diabetic rats in response to acetylcholine (Figure 2) and ADP (Figure 3) to that observed in nondiabetic rats. In contrast, treatment with losartan did not influence reactivity of cerebral arterioles in nondiabetic or diabetic rats in response to nitroglycerin (Figure 4).
2.3. Superoxide anion production and AT1R protein
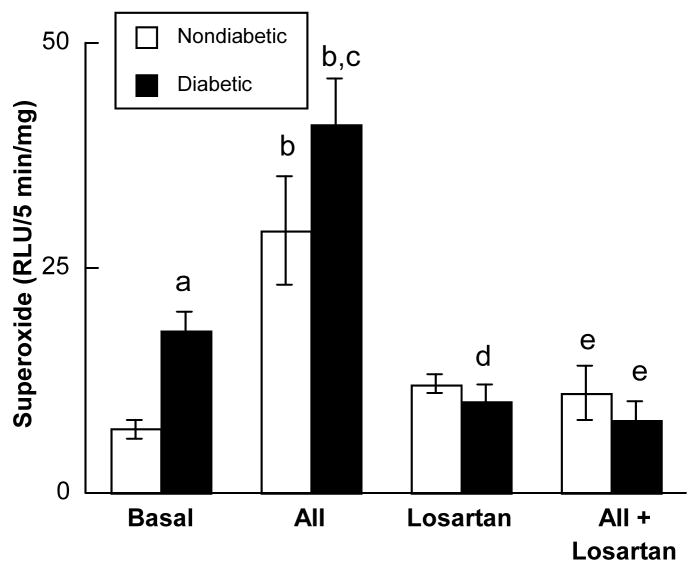
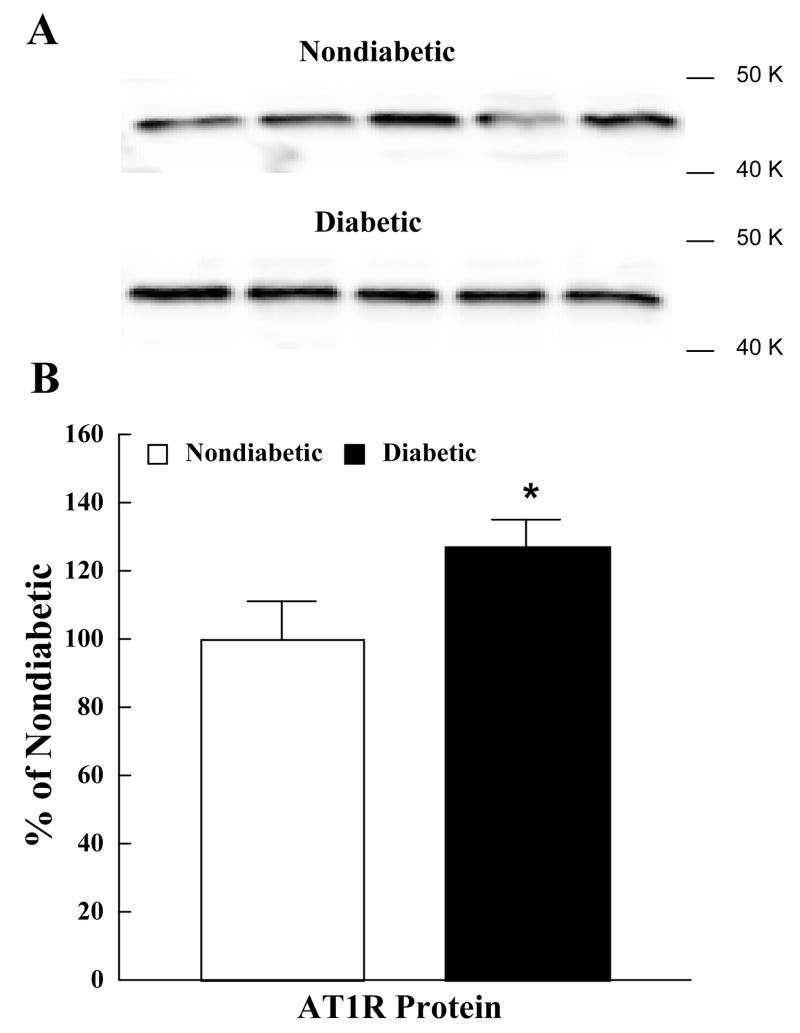
Basal superoxide production was greater in brain tissue obtained from diabetic compared to nondiabetic rats (Figure 5). Exposure to angiotensin II (1.0 μM) produced an increase in superoxide anion production from tissue obtained from nondiabetic and diabetic rats with the magnitude of the increase being greater from tissue obtained from diabetic rats (Figure 5). Losartan did not influence basal production of superoxide anion by brain tissue in nondiabetic rats, but reduced basal superoxide anion production by brain tissue in diabetic rats (Figure 5). In addition, losartan blocked the increase in superoxide anion production by brain tissue in response to angiotensin II in both nondiabetic and diabetic rats (Figure 5). Finally, we found that AT1R protein in cerebral cortex microvessels from diabetic rats was significantly higher than that found in nondiabetic rats (Figure 6).
Figure 5.
Superoxide anion production by parietal cortex tissue in nondiabetic (open bars; n=15) and diabetic (closed bars; n=26) rats under basal conditions (Basal), during exposure to angiotensin II (AII; 1.0 μM), in the presence of losartan (Losartan; 0.1mM), and during exposure to angiotensin II in the presence of losartan (AII+Losartan). Values are means±SE. a p<0.05 versus basal production of superoxide anion in nondiabetic rats, b p<0.05 versus basal levels of superoxide anion, c p<0.05 versus response to angiotensin II in nondiabetic rats, d p<0.05 versus basal production of superoxide anion in diabetic rats, and e p<0.05 versus production of superoxide anion during exposure to angiotensin II.
Figure 6.
AT1R protein in cerebral microvessels obtained from nondiabetic and diabetic rats. The upper panel shows Western immunoblot of AT1R protein in cerebral cortex microvessels (upper lanes are microvessels from nondiabetic rats and lower lanes are microvessels from diabetic rats). The lower panel shows the quantified data for AT1R protein in nondiabetic (open bars) and diabetic (closed bars) rats. Optical density of AT1R protein positive bands was quantified by scanning densitometry and plotted relative to nondiabetic rats. Values are means ± SE. *p<0.05 versus nondiabetic rats.
3. Discussion
There are four findings from the present study. First, angiotensin II impairs eNOS-dependent reactivity of cerebral arterioles in nondiabetic rats via an increase in superoxide anion production by activation of NAD(P)H oxidase. Second, acute treatment with losartan can restore impaired eNOS-dependent responses of cerebral arterioles in diabetic rats to that observed in nondiabetic rats. Third, superoxide anion production is increased under basal conditions in brain tissue from diabetic rats, can be increased in both nondiabetic and diabetic rats by exposure to angiotensin II, and can be inhibited by treatment with losartan. Fourth, AT1R protein is increased in cerebral microvessels from diabetic compared to nondiabetic rats.
3.1. Consideration of methods
Agonists can produce dilatation of cerebral arterioles via the synthesis/release of nitric oxide and/or endothelium-derived hyperpolarizing factor (EDHF). We used ADP, acetylcholine and nitroglycerin to examine eNOS-dependent and -independent reactivity of cerebral arterioles in nondiabetic and diabetic rats. We and others (Ayajiki et al., 1992; Faraci, 1991; Mayhan, 1992b) have shown that ADP and acetylcholine, but not nitroglycerin, dilate cerebral arterioles via the synthesis/release of nitric oxide. Other investigators (Marrelli et al., 1999; You et al., 1999), however, have suggested that relaxation of the rat middle cerebral artery to purines is related to the synthesis/release of nitric oxide and EDHF. We did not examine a role for EDHF in responses of cerebral arterioles to ADP, acetylcholine or nitroglycerin in the present study, and thus we cannot exclude the possibility that EDHF may contribute to dilatation of cerebral arterioles to these agonists. However, others (Brayden, 1991; Chrissobolis et al., 2000; Faraci and Heistad, 1993; Taguchi et al., 1995) also have suggested that activation of potassium channels, presumably by EDHF, does not play a significant role in dilatation of cerebral vessels to the agonists used in the present study. Thus, we suggest that dilatation of cerebral arterioles to the agonists used in the present study primarily involves the synthesis/release of nitric oxide, presumably via activation of eNOS.
We measured superoxide anion production from sample of parietal cortex tissue. Thus, it may not be entirely clear how superoxide anion generated in this sample of brain tissue might influence reactivity of cerebral arterioles. One must be aware that our sample of cortical tissue contains blood vessels as well as other components of brain tissue, and we cannot be certain of the source(s) of superoxide anion. In addition, previous studies that have shown that exogenous production of superoxide anion by enzymatic methodologies can not only impair responses of cerebral arteries and arterioles to nitric oxide synthase-dependent agonists, but can produce morphological changes in these vessels (Kontos, 1985; Rosenblum, 1983; Wei et al., 1985). Therefore, it is conceivable that the production of superoxide anion, if it occurs predominately in cortical tissue, can influence reactivity of cerebral arterioles.
We examined the influence of local treatment with angiotensin II on reactivity of cerebral arterioles in nondiabetic rats. We found that superfusion with angiotensin II impaired eNOS-dependent, but not –independent, responses of cerebral arterioles, and that pretreatment with apocynin prevented angiotensin II-induced impairment in cerebrovascular reactivity. We used apocynin, as others have (Beswick et al., 2001; Hamilton et al., 2001; Hamilton et al., 2002; Rey et al., 2002), to inhibit NAD(P)H oxidase. Based upon our previous study (Mayhan et al., 2006) in which we found an increase in the protein expression of subunits for NAD(P)H oxidase, it seemed reasonable to examine the possibility that angiotensin II may alter reactivity of cerebral arterioles via the production of superoxide anion via activation of NAD(P)H oxidase. A recent study (Heumuller et al., 2008) suggests that apocynin may not be an inhibitor of NAD(P)H oxidase in vascular tissue, but may merely act as an antioxidant. While it was beyond the scope of the present study to examine the precise mechanism by which apocynin affects vascular cells, as discussed by Touyz (Touyz, 2008) many additional studies will be necessary to determine the complex nature by which apocynin influences vascular cells.
A previous study reported that superfusion with angiotensin II inhibited reactivity of rabbit cerebral arteries in response to bradykinin, but not to sodium nitroprusside (Didion and Faraci, 2003). In addition, these investigators report that pretreatment with tiron, a scavenger of superoxide anion, or DPI, an inhibitor of NAD(P)H oxidase could prevent the effects of angiotensin II on eNOS-dependent responses of rabbit cerebral arteries. In another study, investigators found that infusion of angiotensin II for five days via an osmotic minipump produced a decrease in eNOS-dependent relaxation of the aorta in rats, presumably via an increase in superoxide anion production (Rajagopalan et al., 1996). Further, treatment of rats with losartan for two days prevented the impairment in eNOS-dependent relaxation of the aorta and increase in superoxide anion production (Rajagopalan et al., 1996). The findings of the present study complement and extend that of previous studies (Didion and Faraci, 2003; Rajagopalan et al., 1996) by examining the influence of angiotensin II on rat cerebral resistance arterioles, by measuring the production of superoxide anion during exposure to angiotensin II and by demonstrating that acute treatment with losartan can inhibit production of superoxide anion during exposure to angiotensin II.
We examined AT1R protein in cerebral microvessels from nondiabetic and diabetic rats. While one might predict that under a situation where circulating levels of angiotensin II may be elevated, i.e., T1D, that AT1R would be downregulated. In fact, studies have shown that angiotensin receptor density is decreased under conditions of high circulating levels of angiotensin II and increased when circulating levels of angiotensin II are low (Gunther et al., 1982; Osborn and Camara, 1997). In contrast, others have shown that during chronic increases in the levels of angiotensin II that there is an increase in AT1R in the brain (Liu et al., 2006; Moellenhoff et al., 2001). Further, a previous study has shown that AT1R protein expression is increased in the kidney of diabetic rats (Harrison-Bernard et al., 2002). The findings from the present study extend previous findings by reporting an increase in AT1R protein in cerebral microvessels obtained from diabetic rats. We suggest that this increase in AT1R protein in diabetic rats may have important implications for the production of superoxide anion, and thus impairment in reactivity of cerebral arterioles.
3.2. Studies of AT1R inhibition on vascular function
Although no studies that we are aware of have specifically examined the effects of AT1R antagonists on reactivity of cerebral arterioles during T1D, studies (Cheetham et al., 2000; Welch and Wilcox, 2001) have examine the role of AT1R in vascular dysfunction during a variety of disease states, including type 1 and type 2 diabetes and atherosclerosis. A study by Cheetham et al (Cheetham et al., 2000) found that chronic (1 month) treatment of type 1 diabetic subjects with losartan improved impaired NOS-dependent changes in forearm blood flow. However, mechanisms that accounted for the effects of losartan on NOS-dependent vasoreactivity were not examined. Another study by Welch and Wilcox (Welch and Wilcox, 2001) reports that chronic treatment of hypertensive rats with candesartan for two weeks diminishes oxidative stress and restores impaired nitric oxide signaling in the kidney. The mechanism for the effects of candesartan was suggested to be related to a decrease in oxidative stress. Further, a study by Prasad et al (Prasad et al., 2000) reports that chronic oral administration of losartan for eight weeks or acute (10 minute intra-arterial infusion) of losartan enhanced impaired flow mediated dilation in human subjects with atherosclerosis. In addition, these authors (Prasad et al., 2000) report a significant increase in serum nitric oxide containing compounds during treatment with losartan. They conclude that the effects of losartan on vascular reactivity were related to an improvement in NO bioavailability by either AT1R mediated reduction in oxidative stress and/or an angiotensin type 2 receptor (AT2R) mediated increase in NO synthesis (Prasad et al., 2000). The results of the present study complement and extend the findings of previous studies. We report that acute treatment with losartan can improve impaired eNOS-dependent responses of cerebral resistance arterioles via a mechanism that appears to be related to an influence on oxidative stress.
In summary, peripheral and cerebral vascular disease are major contributing factors to morbidity and mortality observed during T1D. Recent evidence suggests that angiotensin II plays a critical role in vascular complications of the peripheral circulation. However, the precise role of angiotensin II in cerebrovascular dysfunction during T1D is not clear. In the present study, we examined contribution of angiotensin II and stimulation of AT1R in T1D-induced impairment in eNOS-dependent dilatation of cerebral arterioles. Based upon the results of the present study, we suggest that impaired eNOS-dependent responses of cerebral arterioles observed in T1D may be related to stimulation of AT1R (presumably by an increase in circulating levels of angiotensin II), which in turn leads to activation of NAD(P)H oxidase with the subsequent production of superoxide anion that serves to inactivate nitric oxide and/or uncouple eNOS. We speculate that treatment of diabetic patients with AT1R antagonists may be a useful therapeutic tool for the prevention of T1D-induced cerebrovascular abnormalities, including stroke.
4. Experimental Procedures
4.1. Preparation of animals
All rats were housed in an animal care facility at the University of Nebraska Medical Center that is approved by the American Association for the Accreditation of Laboratory Animal Care (AAALAC), and all protocols were reviewed and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (200–220 grams) were randomly assigned to nondiabetic or diabetic groups. Nondiabetic rats were injected with vehicle (sodium citrate buffer) and diabetic rats were injected with streptozotocin (50 mg/kg IP).
4.2. Functional responses of cerebral arterioles
On the day of the experiment (14 ± 0.2 weeks after injection of streptozotocin or vehicle), rats were anesthetized (thiobutabarbital (Inactin); 100 mg/kg body weight, i.p.), and a tracheotomy was performed. The animals were ventilated mechanically with room air and supplemental oxygen. Supplemental anesthesia was administered at a dose of 10–20 mg/kg/hr intravenously, as needed. A catheter was placed into a femoral vein for injection of supplemental anesthesia, and a femoral artery was cannulated for measurement of arterial blood pressure and to obtain blood samples for the determination of blood glucose concentration.
To visualize the microcirculation of the cerebrum, a craniotomy was prepared over the left parietal cortex (Mayhan and Heistad, 1985). The cranial window was suffused with artificial cerebral spinal fluid that was bubbled continuously (95% nitrogen and 5% carbon dioxide). Temperature of the suffusate was maintained at 37±1° C. The cranial window was connected via a three-way valve to an infusion pump, which allowed infusion of agonists into the suffusate. This method, which we have used previously (Mayhan, 1989; Mayhan, 1992a), maintained a constant temperature, pH, pCO2, and pO2 of the suffusate during infusion of agonists. Arterial blood gases were monitored and maintained within normal limits.
Diameter of cerebral arterioles was measured using a video image-shearing device (model 908, Instrumentation for Physiology and Medicine, Inc.). In each rat, we examined responses of the largest arteriole exposed by the craniotomy. Diameter of arterioles was measured immediately before application of agonists and every minute for 5 minutes during application of agonists. Steady state responses were reached within 2–3 minutes after starting application of agonist and the diameter returned to baseline within 3 minutes after stopping application of the agonist.
4.3. Experimental Protocol
In the first series of studies, we examined the influence of superfusion with angiotensin II on reactivity of cerebral arterioles in nondiabetic rats (n=6). In these studies, the cranial window was superfused with artificial cerebral spinal fluid for 30–45 minutes prior to testing responses of arterioles to the agonists. Then, we examined responses of arterioles in nondiabetic rats to eNOS-dependent agonists: acetylcholine (1 and 10 μM) and 5′-adenosine diphosphate (ADP; 10 and 100 μM), and an eNOS-independent agonist: nitroglycerin (1.0 and 10 μM). After this initial examination of reactivity, we started a continuous superfusion with angiotensin II (1.0 μM). Two hours after starting superfusion with angiotensin II, we again examined responses of cerebral arterioles to acetylcholine, ADP and nitroglycerin. In another group of nondiabetic rats (n=4), we examined whether prior treatment of the cerebral microcirculation with an inhibitor of NAD(P)H oxidase (apocynin) could alleviate/prevent the influence of angiotensin II on reactivity of cerebral arterioles. Thus, in these studies we examined responses to acetylcholine, ADP and nitroglycerin in nondiabetic rats. Then, we started a continuous superfusion with apocynin (1 mM). Thirty minutes after starting superfusion with apocynin, we started superfusion with angiotensin II. Two hours later, we again examined responses of arterioles to acetylcholine, ADP and nitroglycerin in the presence of apocynin and angiotensin II.
In a second series of functional studies, we examined the influence of losartan on reactivity of cerebral arterioles to eNOS-dependent and –independent agonists in nondiabetic (n=6) and diabetic (n=9) rats. In these studies, we examined responses of cerebral arterioles to acetylcholine, ADP and nitroglycerin in nondiabetic and diabetic rats, as described above. Then, we started a continuous superfusion with losartan (0.1 mM). Thirty minutes after starting superfusion with losartan, we again examined responses of cerebral arterioles to acetylcholine, ADP and nitroglycerin.
4.4. Western blot analysis
In separate groups of nondiabetic (n=5) and diabetic (n=5) rats, brain tissue (cerebrum) was harvested, rinsed with a phosphate-buffer solution (PBS), frozen on dry ice, and stored at −80 °C until isolation of cerebral microvessels. Cerebral microvessels from nondiabetic and diabetic rats were isolated using procedures described previously (McNeill et al., 1999; Sun et al., 2001; Sun et al., 2002). Once isolated, cerebral microvessels were homogenized separately in 20% (weight/volume) ice-cold buffer containing 10 mM Tris-HCl, pH 7.4; 1% SDS; 1mM sodium vanadate; 10 μg/ml aprotinine; 10 μg/ml leupeptin; and 1mM phenylmethylsulfonyl fluoride. The homogenates were centrifuged at 12000 g for 20 min at 4 °C and protein concentration was determined by the Bradford method (Bio-Rad, Richmond, CA) with BSA as the standard. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed and the proteins were transferred onto a polyvinylidene difluoride membrane. Immunoblot analysis was performed using an anti-AT1R receptor antibody. The bound antibody was detected using an ECL kit and quantified by scanning densitometry. The amount of protein was expressed as percent relative to that in nondiabetic rats, as described in previously (Sun et al., 2001; Sun et al., 2002).
4.5. Superoxide anion measurement
In other groups of nondiabetic (n=15) and diabetic (n=26) rats, superoxide production was measured using lucigenin-enhanced chemiluminescence. After the rat was exsanguinated, the brain was removed and immersed in a modified Krebs-HEPES buffer containing (in mmol/L): 118 NaCl, 4.7 KCl, 1.3 CaCl2, 1.2 MgCl2, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES, 5 glucose for samples from nondiabetic rats and 20 glucose for samples from diabetic rats (pH 7.4). Tissue samples from the parietal cortex were placed in polypropylene tubes containing 5 μmol/L lucigenin, then read in a Fentomaster FB12 (Zytox) luminometer, which reports relative light units (RLU) emitted integrated over 30 second intervals for 5 minutes. Data were corrected for background activity and normalized to tissue weight.
4.6. Statistical analysis
Analysis of variance with Fischer’s test for significance was used to compare values between nondiabetic and diabetic rats before and during treatment with angiotensin II and losartan, and for superoxide anion production. An unpaired t test was used to compare differences between nondiabetic and diabetic rats regarding AT1R protein. A p value of 0.05 or less was considered to be significant.
Acknowledgments
This study was supported by National Institutes of Health Grants HL-40781, AA-11288, and DA-14258, and funds from the University of Nebraska Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arcaro G, Zenere BM, Saggiani F, Zenti MG, Monauni T, Lechi A, Muggeo M, Bonadonna RC. ACE inhibitors improve endothelial function in type 1 diabetic patients with normal arterial pressure and microalbuminuria. Diabetes Care. 1999;22:1536–42. doi: 10.2337/diacare.22.9.1536. [DOI] [PubMed] [Google Scholar]
- Ayajiki K, Okamura T, Toda N. Involvement of nitric oxide in endothelium-dependent, phasic relaxation caused by histamine in monkey cerebral arteries. Japanese Journal of Pharmacology. 1992;60:357–362. doi: 10.1254/jjp.60.357. [DOI] [PubMed] [Google Scholar]
- Berry C, Hamilton CA, Brosnan MJ, Magill FG, Berg GA, McMurray JJ, Dominiczak AF. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation. 2000;101:2206–12. doi: 10.1161/01.cir.101.18.2206. [DOI] [PubMed] [Google Scholar]
- Beswick RA, Dorrance AM, Leite R, Webb RC. NADH-NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- Brayden JE. Hyperpolarization and relaxation of resistance arteries in response to adenosine diphosphate. Circulation Research. 1991;69:1415–1420. doi: 10.1161/01.res.69.5.1415. [DOI] [PubMed] [Google Scholar]
- Cachofeiro V, Sakakibara T, Nasjletti A. Kinins, nitric oxide, and the hypotensive effect of captopril and ramiprilat in hypertension. Hypertension. 1992;19:138–145. doi: 10.1161/01.hyp.19.2.138. [DOI] [PubMed] [Google Scholar]
- Cheetham C, Collis J, O’Driscoll G, Stanton K, Taylor R, Green D. Losartan, an angiotensin type 1 receptor antagonist, improves endothelial function in non-insulin-dependent diabetes. Journal of the American College of Cardiology. 2000;36:1461–1466. doi: 10.1016/s0735-1097(00)00933-5. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Ziogas J, Chu Y, Faraci FM, Sobey CG. Role of inwardly rectifying K+ channels in K+-induced cerebral vasodilatation in vivo. American Journal of Physiology. 2000;279:H2704–H2712. doi: 10.1152/ajpheart.2000.279.6.H2704. [DOI] [PubMed] [Google Scholar]
- Cooper ME, Gilbert RE, Jerums G. Diabetic vascular complications. Clinical and Experimental Pharmacology and Physiology. 1997;24:770–775. doi: 10.1111/j.1440-1681.1997.tb02130.x. [DOI] [PubMed] [Google Scholar]
- de Cavanagh EMV, Inserra F, Toblli J, Stella I, Fraga CG, Ferder L. Enalapril attenuates oxidative stress in diabetic rats. Hypertension. 2001;38:1130–1136. doi: 10.1161/hy1101.092845. [DOI] [PubMed] [Google Scholar]
- Didion SP, Faraci FM. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke. 2003;34:2038–42. doi: 10.1161/01.STR.0000081225.46324.AA. [DOI] [PubMed] [Google Scholar]
- Duntas L, Keck FS, Haug C, Hetzel W, Wolf CF, Rosenthal J, Pfeiffer EF. Serum angiotensin-converting enzyme activity and active renin plasma concentrations in insulin-dependent diabetes mellitus. Diabetes Research and Clinical Practice. 1992;16:203–208. doi: 10.1016/0168-8227(92)90118-b. [DOI] [PubMed] [Google Scholar]
- Faraci FM. Role of endothelium-derived relaxing factor in cerebral circulation: Large arteries vs. microcirculation. American Journal of Physiology. 1991;261:H1038–H1042. doi: 10.1152/ajpheart.1991.261.4.H1038. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Role of ATP-sensitive potassium channels in the basilar artery. American Journal of Physiology. 1993;264:H8–H13. doi: 10.1152/ajpheart.1993.264.1.H8. [DOI] [PubMed] [Google Scholar]
- Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circulation Research. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- Gonzalez Bosc LV, Kurnjek ML, Muller A, Terragno NA, Basso N. Effect of chronic angiotensin II inhibition on the nitric oxide synthase in the normal rat during aging. J Hypertens. 2001;19:1403–9. doi: 10.1097/00004872-200108000-00008. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circulation Research. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase. Role in cardiovascular biology and disease. Circulation Research. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Gunther S, Alexander RW, Atkinson WJ, Gimbrone MA., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982;92:289–98. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging. A common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, Al-Benna S, Berg G, Dominiczak AF. NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension. 2002;40:755–62. doi: 10.1161/01.hyp.0000037063.90643.0b. [DOI] [PubMed] [Google Scholar]
- Harrison-Bernard LM, Imig JD, Carmines PK. Renal AT1 receptor protein expression during the early stage of diabetes mellitus. Int J Exp Diabetes Res. 2002;3:97–108. doi: 10.1080/15604280214483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–7. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Kontos HA. Oxygen radicals in cerebral vascular injury. Circulation Research. 1985;57:508–516. doi: 10.1161/01.res.57.4.508. [DOI] [PubMed] [Google Scholar]
- Kusaka I, Kusaka G, Zhou C, Ishikawa M, Nanda A, Granger DN, Zhang JH, Tang J. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 2004;286:H2442–51. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–5. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J, Sastre A. Serum angiotensin-converting enzyme: elevations in diabetes mellitus. Annals of Internal Medicine. 1980;93:825–826. doi: 10.7326/0003-4819-93-6-825. [DOI] [PubMed] [Google Scholar]
- Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Neuronal angiotensin II type 1 receptor upregulation in heart failure: activation of activator protein 1 and Jun N-terminal kinase. Circ Res. 2006;99:1004–11. doi: 10.1161/01.RES.0000247066.19878.93. [DOI] [PubMed] [Google Scholar]
- Marrelli SP, Khorovets A, Johnson TD, Childres WF, Bryan RM., Jr P2 purinoceptor-mediated dilations in the rat middle cerebral artery after ischemia-reperfusion. Am J Physiol. 1999;276:H33–41. doi: 10.1152/ajpheart.1999.276.1.H33. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Heistad DD. Permeability of blood-brain barrier to various sized molecules. American Journal of Physiology. 1985;248:H712–H718. doi: 10.1152/ajpheart.1985.248.5.H712. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Impairment of endothelium-dependent dilatation of cerebral arterioles during diabetes mellitus. American Journal of Physiology. 1989;256:H621–H625. doi: 10.1152/ajpheart.1989.256.3.H621. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Impairment of endothelium-dependent dilatation of the basilar artery during diabetes mellitus. Brain Research. 1992a;580:297–302. doi: 10.1016/0006-8993(92)90957-b. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Endothelium-dependent responses of cerebral arterioles to adenosine 5′-diphosphate. Journal of Vascular Research. 1992b;29:353–358. doi: 10.1159/000158951. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Arrick DM, Sharpe GM, Patel KP, Sun H. Inhibition of NAD(P)H oxidase alleviates impaired NOS-dependent responses of pial arterioles in Type 1 diabetes mellitus. Microcirculation. 2006;13:567–575. doi: 10.1080/10739680600885194. [DOI] [PubMed] [Google Scholar]
- McNeill AM, Kim N, Duckles SP, Krause DN. Chronic estrogen treatment increases levels of endothelial nitric oxide synthase protein in rat cerebral microvessels. Stroke. 1999;30:2186–2190. doi: 10.1161/01.str.30.10.2186. [DOI] [PubMed] [Google Scholar]
- Moellenhoff E, Blume A, Culman J, Chatterjee B, Herdegen T, Lebrun CJ, Unger T. Effect of repetitive icv injections of ANG II on c-Fos and AT(1)-receptor expression in the rat brain. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1095–104. doi: 10.1152/ajpregu.2001.280.4.R1095. [DOI] [PubMed] [Google Scholar]
- Mortel KF, Meyer JS, Sims PA, McClintic K. Diabetes mellitus as a risk factor for stroke. So Med J. 1990;83:904–911. doi: 10.1097/00007611-199008000-00014. [DOI] [PubMed] [Google Scholar]
- O’Driscoll G, Green D, Rankin J, Stanton K, Taylor R. Improvement in endothelial function by angiotensin converting enzyme inhibition to insulin-dependent diabetes mellitus. Journal of Clinical Investigation. 1997;100:678–684. doi: 10.1172/JCI119580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll G, Green D, Maiorana A, Stanton K, Colreavy F, Taylor R. Improvement in endothelial function by angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. Journal of the American College of Cardiology. 1999;33:1506–1511. doi: 10.1016/s0735-1097(99)00065-0. [DOI] [PubMed] [Google Scholar]
- Osborn JL, Camara AK. Renal neurogenic mediation of intracerebroventricular angiotensin II hypertension in rats raised on high sodium chloride diet. Hypertension. 1997;30:331–6. doi: 10.1161/01.hyp.30.3.331. [DOI] [PubMed] [Google Scholar]
- Prasad A, Tupas-Habib T, Schenke WH, Mincemoyer R, Panza JA, Waclawin MA, Ellahham S, Quyyumi AA. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation. 2000;101:2349–2354. doi: 10.1161/01.cir.101.20.2349. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Harrison DG. Reversing endothelial dysfunction with ACE inhibitors. A new trend. Circulation. 1996;94:240–3. doi: 10.1161/01.cir.94.3.240. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations in vascular tone. Journal of Clinical Investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey FE, Li XC, Carretero OA, Garvin JL, Pagano PJ. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation: role of gp91(phox) Circulation. 2002;106:2497–502. doi: 10.1161/01.cir.0000038108.71560.70. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI. Effects of free radical generation on mouse pial arterioles: probable role of hydroxyl radicals. American Journal of Physiology. 1983;245:H139–H142. doi: 10.1152/ajpheart.1983.245.1.H139. [DOI] [PubMed] [Google Scholar]
- Schernthaner G, Schwarzer C, Kuzmits R, Muller MM, Klemen U, Freyler H. Increased angiotensin-converting enzyme activities in diabetes mellitus: analysis of diabetes type, state of metabolic control and occurrence of diabetes vascular disease. J Clin Pathol. 1984;37:307–312. doi: 10.1136/jcp.37.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Patel KP, Mayhan WG. Tetrahydrobiopterin, a cofactor for NOS, improves endothelial dysfunction during chronic alcohol consumption. American Journal of Physiology. 2001;281:H1863–H1869. doi: 10.1152/ajpheart.2001.281.5.H1863. [DOI] [PubMed] [Google Scholar]
- Sun H, Patel KP, Mayhan WG. Impairment of neuronal nitric oxide synthase-dependent dilatation of cerebral arterioles during chronic alcohol consumption. Alcoholism: Clinical and Experimental Research. 2002;26:663–670. [PubMed] [Google Scholar]
- Taguchi H, Heistad DD, Kitazono T, Faraci FM. Dilatation of cerebral arterioles in response to activation of adenylate cyclase is dependent on activation of Ca2+-dependent K+ channels. Circulation Research. 1995;76:1057–1062. doi: 10.1161/01.res.76.6.1057. [DOI] [PubMed] [Google Scholar]
- Torlone E, Britta M, Rambotti AM, Perriello G, Santeusanio F, Brunetti P, Bolli GB. Improved insulin action and glycemic control after long-term angiotensin-converting enzyme inhibition in subjects with arterial hypertension and type II diabetes. Diabetes Care. 1993;16:1347–1355. doi: 10.2337/diacare.16.10.1347. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension. 2008;51:172–4. doi: 10.1161/HYPERTENSIONAHA.107.103200. [DOI] [PubMed] [Google Scholar]
- Trauernicht AK, Sun H, Patel KP, Mayhan WG. Enalapril prevents impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in diabetic rats. Stroke. 2003;34:2698–2703. doi: 10.1161/01.STR.0000092121.62649.DC. [DOI] [PubMed] [Google Scholar]
- Wei EP, Christman CW, Kontos HA, Povlishock JT. Effects of oxygen radicals on cerebral arterioles. American Journal of Physiology. 1985;248:H157–H162. doi: 10.1152/ajpheart.1985.248.2.H157. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Wilcox CS. AT1 receptor antagonist combats oxidative stress and restores nitric oxide signaling in the SHR. Kidney International. 2001;59:1257–1263. doi: 10.1046/j.1523-1755.2001.0590041257.x. [DOI] [PubMed] [Google Scholar]
- You J, Johnson TD, Marrelli SP, Mombouli JV, Bryan RM. P2u receptor-mediated release of endothelium-derived relaxing factor/nitric oxide and endothelium-derived hyperpolarizing factor from cerebrovascular endothelium in rats. Stroke. 1999;30:1125–1133. doi: 10.1161/01.str.30.5.1125. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circulation Research. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]