Cardiac dysfunction following acute myocardial infarction (MI) is a major cause of death in the world and there is compelling need for new therapeutic strategies. In this report we demonstrate that a direct cardiac injection of drug-loaded microparticles, formulated from the polymer, poly(cyclohexane-1,4-diyl acetone dimethylene ketal) (PCADK), improves cardiac function following MI. Drug delivery vehicles have great potential to improve the treatment of cardiac dysfunction by sustaining high concentrations of therapeutics within the damaged myocardium. PCADK is unique from currently used polymers in drug delivery in that its hydrolysis generates neutral degradation products. We show here that PCADK causes minimal tissue inflammatory response, thus enabling PCADK for the treatment of inflammatory diseases, such as cardiac dysfunction. PCADK holds great promise for treating MI and other inflammatory diseases given its neutral, biocompatible degradation products and its ability to deliver a wide range of therapeutics.
The development of drug delivery vehicles that can improve cardiac dysfunction following MI remains a major challenge in the field of biomaterials. Following acute MI, an excessive inflammatory response is initiated in the myocardium causing chronic elevation of inflammatory cytokines and reactive oxygen species, resulting in cardiac dysfunction1,2,3,4. A large number of clinically approved small molecule inhibitors have been identified that can suppress inflammation and have great potential for improving cardiac dysfunction. However, delivery remains a challenge as many of these drugs require large doses and daily injections for efficacy and cause toxicity at these high doses5,6,7. Thus, drug delivery vehicles that can sustain effective doses of therapeutics within the myocardium for weeks have the potential to slow or halt the progression of cardiac dysfunction8. Although biomaterials have been developed for treating cardiac dysfunction, these materials have been designed to deliver protein therapeutics and cells, and are not well suited for the controlled release of hydrophobic drugs, such as small molecule inhibitors, because of their large pore sizes9. Polyester-based microparticles do have the potential for delivering hydrophobic anti-inflammatory molecules; however their use in cardiac drug delivery has not been fully investigated.
In this work, we demonstrate that microspheres formulated from the polymer, PCADK, which encapsulate the p38-inhibitor SB239063, can improve the treatment of MI. PCADK is a recently developed, acid-sensitive (supplemental Figure 1A) polymer that has great potential for treating inflammatory diseases, such as myocardial infarction, because it degrades into the neutral, excretable, FDA-approved compounds 1,4-cyclohexanedimethanol (approved by the FDA as an indirect food additive) and acetone (an endogenous compound with potential antioxidant properties) and thus should not exacerbate any existing inflammation10,11 (Figure 1A). Using an emulsion/solvent evaporation procedure, we were able to encapsulate SB239063 using a single emulsion to produce microparticles of various sizes (Figure 1B and supplemental Figure 1B) due to the compound’s hydrophobicity (theoretical log P = 3.28, ACD/Labs Software, SciFinder Scholar). We focused on inhibiting the p38 MAPK pathway because of its central importance in activating macrophages and its role in inducing apoptosis in cardiomyocytes12,13,14,15,16,17. We therefore hypothesized that a direct injection of large PCADK-SB239063 microparticles, 10–20 microns in diameter, would be retained in the myocardium and slowly release the drug over the course of weeks, inhibiting p38 activation in many cell types. Additionally, this strategy would also be effective in inhibiting p38 activation in macrophages, as macrophages can phagocytose small particles and fragments of larger particles, thus releasing the inhibitor intracellularly (Figure 1C).
Figure 1. Polyketal microparticles – non-inflammatory polymer chemistry for drug delivery.
(a) Poly(cyclohexane-1,4-diyl acetone dimethylene ketone) (PCADK) degrades to neutral, non-toxic products through the acid catalyzed hydrolysis of the ketal linkage (dotted circle). Acetone is an endogenous metabolite, while 1,4-cyclohexanedimethanol is FDA-approved as an indirect food additive. (b) A single emulsion/solvent evaporation technique was used to produce large (~20 µm) microparticles loaded with SB239063. Particles were imaged using SEM (scale bar: 20 µm). (c) The left descending coronary artery was permanently ligated to create an infarcted zone. Microparticles were injected intramyocardially where they released the encapsulated inhibitor within the infarct zone.
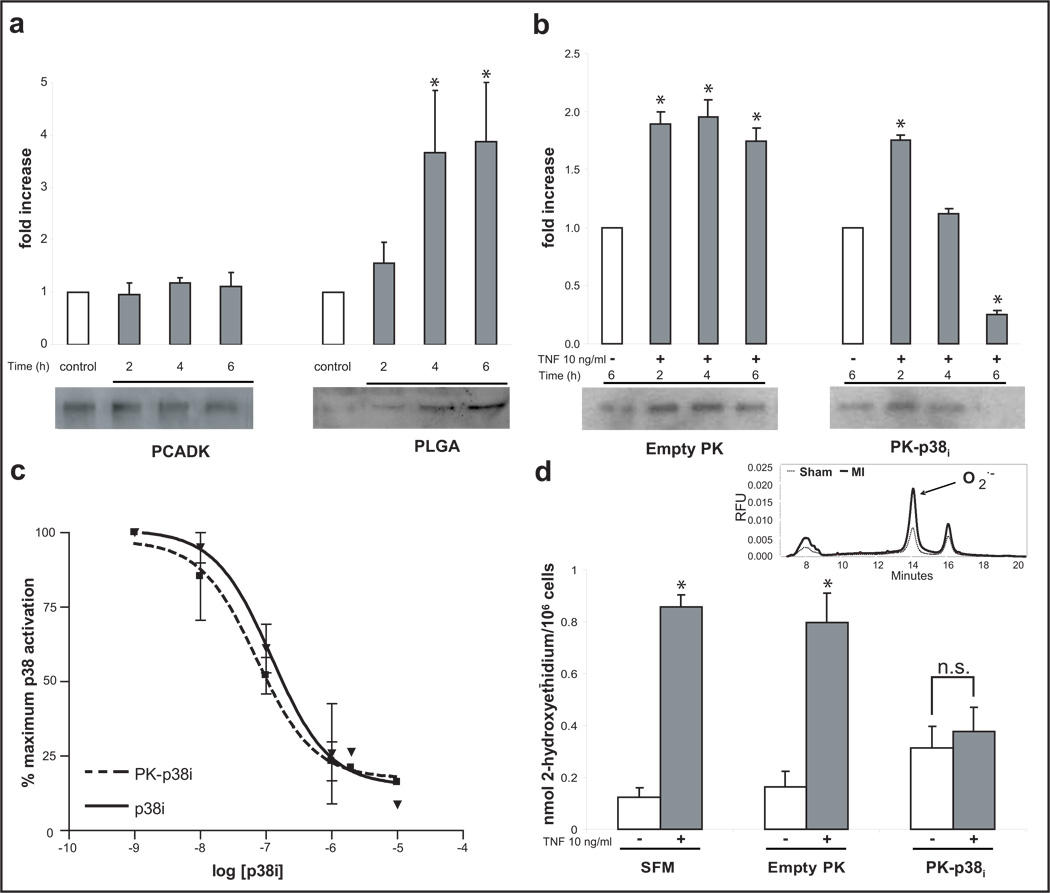
Delivery vehicles, and their degradation byproducts, can potentially cause the activation of inflammatory pathways in macrophages and is a critical issue limiting the use of microparticles for the treatment of inflammatory diseases. We therefore investigated if degradation of PCADK microparticles by macrophages caused the activation of p38 itself and compared this to the response to poly(lactic-co-glycolic acid) (PLGA) microparticles as a control. Macrophages were incubated with PCADK or PLGA microparticles (PCADK: 19.2 ± 7.3 µm, PLGA: 17.9 ± 6.7 µm) for 2, 4, or 6 hours, and examined for p38-phosphorylation by Western blotting. Figure 2A demonstrates that PLGA causes a time-dependent increase in p38-phosphorylation, suggesting elicitation of inflammatory pathways, whereas PCADK did not. This data agrees with published reports showing PLGA treatment increases inflammatory cytokine production in cultured cells18,19, and also demonstrates that PCADK has potential as a drug delivery vehicle designed to deliver anti-inflammatory drugs.
Figure 2. Macrophages are activated by PLGA microspheres in vitro, while PK-p38i treatment inhibits p38 activation.
(a) RAW 264.7 macrophages were treated with empty PCADK and PLGA particles for 2, 4, and 6 hours. Densitometric evaluation (mean + SEM; n=3) of Western blots (representative blot for phospho-p38 shown) demonstrate that PCADK did not elevate p38-phosphorylation at any time point. PLGA increased p38-phosphorylation in a time dependent manner, resulting in a four-fold increase in activation at 4 and 6 hours (*p<0.05 vs. all groups; ANOVA followed by Tukey-Kramer post test). (b) Macrophages were treated with PK-p38i particles for the indicated time and stimulated with tumor necrosis factor-alpha (TNF-α). A time-dependent inhibition of phosphorylation was observed with complete inhibition occurring at 4–6h, and no effect seen with empty PK treatment (mean ± SEM, n=4, *p<0.05 vs. control, ANOVA followed by Tukey-Kramer post test). (c) Inhibition curves demonstrating similar dose-response profiles of the encapsulated and free inhibitor. Macrophages were stimulated with TNF-α following incubation with increasing doses of free or encapsulated inhibitor. There was no difference between treatments at any dose, and both exhibited similar IC50 values. (d) Superoxide production, a downstream effect of p38 activation, was measured using DHE-HPLC. HPLC traces were used to quantify the superoxide-specific oxidation product of dihydroethidium, 2-hydroxyethidium (sample trace from sham and infarcted animals shown, inset). No effect is seen with empty PK treatment, while PK-p38i reduced superoxide levels following TNF-α stimulation (mean ± SEM, n=4, n.s.=not significant *p<0.05 vs. control; ANOVA followed by Tukey-Kramer post test; SFM = serum free medium).
We therefore investigated the ability of PCADK microparticles to encapsulate the p38-inhibitor SB239063, and the bioactivity of these microparticles (PK-p38i). The encapsulation efficiency of SB239063 was 44.4 ± 6.0%, and PK-p38i microparticles had 3–5 µg of inhibitor per mg of PCADK. To test bioactivity, macrophages were treated with either PK-p38i or empty PCADK particles (PK) for 2, 4, or 6 hours, washed and stimulated for 20 minutes with tumor necrosis factor-alpha (TNF-α). As the representative blot and grouped data demonstrate, PK-p38i but not PK, prevented p38 phosphorylation by TNF-α stimulation in a time-dependent manner (Figure 2B). Additionally, both the free inhibitor and PK-p38i demonstrated similar dose-response profiles, suggesting little loss of SB239063 activity in PK-p38i microparticles (Figure 2C). The release half life of SB239063 from PCADK microparticles is 7 days at pH 7.4, and thus based on these release kinetics only 1.9% of encapsulated SB239063 (120 pmol) would be released into the extracellular media in our 6 hour cell culture experiment. These results suggest that macrophages play an active role in accelerating the release of SB239063 from PK-p38i microparticles, either through phagocytosis of the microparticles, leading to intracellular release of the inhibitor, or through fusion of their phagosomes with the microparticles, leading to extracellular release of the inhibitor. Similar experiments with cultured neonatal cardiac myocytes, non-phagocytic cells, showed no inhibition of TNF-α−stimulated p38-phosphorylation suggesting passive hydrolysis alone cannot account for this inhibitory effect (supplemental Figure 2).
We also investigated if PK-p38i microparticles could reduce downstream inflammatory effectors, in particular superoxide. Cultured macrophages were incubated with PK-p38i microparticles or empty PK microparticles, stimulated with TNF-α and then assayed for extracellular superoxide production. Superoxide production was quantified by measuring accumulation of a superoxide-specific oxidation product of dihydroethidium, 2-hydroxyethidium by high-performance liquid chromatography (DHE-HPLC)20,21. As the data in Figure 2D demonstrate, PK-p38i pretreatment reduced TNF-α-induced superoxide production while PK had no effect. These data demonstrate that PK-p38i is active in reducing p38-phosphorylation, as well as clinically relevant p38-dependent second messenger generation.
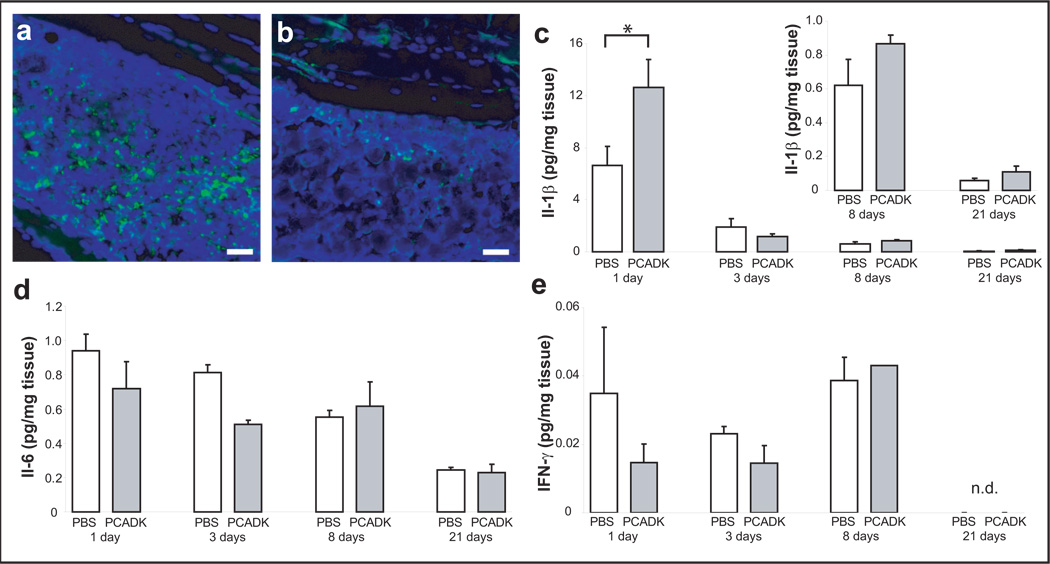
A key issue involving microparticle drug delivery systems is the foreign body response by the host immune system; in general immune cells are recruited to the area of the microparticles and remain there until the microparticles are metabolized and phagocytosed, thus compromising the microparticle’s function. The foreign body response is particularly severe with particles that have acidic degradation products. In contrast, PCADK may minimize the foreign body response because it generates neutral degradation products. We therefore compared the tissue biocompatibility of PCADK and PLGA microparticles. Mice were subjected to an intramuscular injection of size matched PLGA or PCADK microparticles (50 mg/mL), sacrificed at 3 days, and histological sections of the injection site were made and stained for CD45, an inflammatory cell marker. Figure 3A demonstrates that PLGA microspheres generated a large influx of CD45-positive cells (Figure 3A), in agreement with prior studies demonstrating in vivo inflammation associated with PLGA delivery22. In contrast, PCADK microparticles caused very little recruitment of CD45-positive cells (Figure 3B). We further investigated the inflammatory response to PCADK microparticles with quantitative cytokine analysis. Mice were subjected to an intramuscular injection of either saline (vehicle) or PCADK microparticles (10mg/mL) and leg muscles were harvested at acute and chronic time points (1, 3, 8, and 21 days). We found that while PCADK briefly increased levels of interleukin-1β (IL-1β) levels at day 1, there was no significant increase subsequently (Figure 3C). Additionally, there was no significant increase in IL-6 (Figure 3D) or interferon-gamma (Figure 3E) at any time point, while levels of TNF-α and IL-12 were below detection limits (data not shown). These data suggest that PCADK microparticles do not induce an inflammatory response at concentrations far exceeding those needed for drug delivery applications, and can therefore be used as a delivery vehicle for the treatment of inflammatory diseases.
Figure 3. PCADK microparticles demonstrate little inflammatory response following intramuscular injections.
Rats were injected with a high dose (50 mg/mL) of empty polymer microparticles. Histological sections were made and stained with DAPI for nuclei (blue) and for CD-45 (green), an inflammatory cell-specific marker. Tissue injected with PLGA microspheres (a) show a large influx of inflammatory cells, while tissue injected with PCADK microspheres (b) have little CD-45-positive staining. In a separate study, cytokine levels were measured in muscle tissue following an injection of 1 mg of PCADK particles or phosphate buffered saline (PBS) using a multiplex assay. (c) Injection of 1 mg of PCADK particles into the leg of rats leads to a slight elevation of interleukin-1β (IL-1β) at day one. At days 8, 14, 21 following surgery, PCADK particles do not elevate IL-1 β above basal levels. (d) PCADK particles do not elevate IL-6 levels at all time points following injection of 1 mg of PCADK particles. (e) Interferon gamma (IFN-γ) levels are not affected by PCADK particles. IFN-γ was not detected by the Bioplex assay at 21 days (n.d.). (mean + SEM, *p<0.05 vs. PBS, Student’s t-test).
PK-p38i microparticles were tested in a rat model of MI, as described in the methods23. In preliminary experiments, we determined that PCADK microparticles with diameters of 15–20 µm remained in the myocardium for several days, most likely because their size precluded them from being carried away in the microcirculation (supplemental Figure 3). Following coronary artery ligation, treatments were injected intramyocardially in a randomized and double-blinded fashion (100 µL). Treatment conditions were vehicle, free inhibitor (2 µM), PK (0.5 mg particle/mL), or PK-p38i (0.5 mg/mL corresponding to 2 µM SB239063) injected directly into the left ventricular free wall. At three and seven days following ligation, treatment with PK-p38i significantly inhibited p38-phosphorylation within the infarct zone, while free inhibitor and PK had no effect (Figure 4A). These data suggest that PK-p38i is able to provide sustained p38-inhibition in the myocardium for at least seven days. Free SB239063 injections were not effective in inhibiting p38-phosphorylation since the free inhibitor likely diffused away rapidly. Because chronic p38-inhibition has been shown to reduce levels of superoxide during heart failure24, infarct zone tissue from three day samples was also analyzed for the production of superoxide using DHE-HPLC. As the grouped data in Figure 4B demonstrate, PK-p38i treatment significantly reduced superoxide levels while free inhibitor and PK had no effect. Another downstream product of p38 activation, TNF-α production, was measured and similarly, only PK-p38i treatment was able to significantly reduce levels of this inflammatory cytokine (Figure 4C).
Figure 4. PK-p38i particles inhibit p38 phosphorylation, superoxide production, and TNF-α production in vivo following infarction.
(a) PK-p38i treatment inhibited phosphorylation of p38 at three and seven days in the infarct zone while free inhibitor (p38i) or empty particles had no effect on phosphorylation at either time point (mean ± SEM, n ≥ 4, *p<0.05 vs. other treatment groups, ANOVA followed by Tukey-Kramer post test; MI = myocardial infarction). (b) Infarct zone tissue at three days was analyzed for superoxide using DHE-HPLC. MI alone, free inhibitor, and empty particles had significantly greater superoxide levels compared to sham, while PK-p38i decreased the amount of superoxide produced (mean ± SEM, n ≥ 4, *p<0.05 vs. other treatment groups, ANOVA followed by Tukey-Kramer post test). (c) The inflammatory cytokine TNF-α was measured in the infarcted zone by ELISA three days post-surgery. MI alone, free inhibitor, and empty particles had significantly greater amounts of TNF-α, while PK-p38i treatment reduced TNF-α levels by nearly two-fold (mean ± SEM, n≥4; *p<0.05 vs. other treatment groups, ANOVA followed by Tukey-Kramer post test).
In addition to in vivo findings supporting our in vitro biochemical data, we also measured the functional outcome of microparticle treatments in separate randomized and double-blinded studies. We compared several treatment groups in these functional experiments: Sham, MI, PK, PK-p38i; as well as a separate randomized and blinded study with PLGA and PLGA microparticles loaded with SB239063 (PLGA-p38i). PLGA and PCADK delivery vehicles were compared in order to determine if microparticle chemistry had an influence on therapeutic efficacy in vivo. PLGA-p38i and PK-p38i microparticles were similar in size (mean and distribution), loading efficiency, and release kinetics (supplemental Figure 4). Cardiac function was assessed using MRI and echocardiography 7 and 21 days post-infarction and dimensions of the heart were measured in a double-blinded manner. While our biochemical data would suggest an immediate effect on function, we found no significant improvement with any treatment at seven days post-infarction (Figure 5A; white bars). At 21 days however, there was a significant improvement in fractional shortening in PK-p38i treated rats, while no effect was seen with free inhibitor, PK, PLGA, or PLGA-p38i particles when compared to 7 day data (Figure 5A; gray bars). Additionally, raw fractional shortening data on day 21 was significantly higher with PK-p38i treatment compared to MI alone and all other treatment groups (PK, p38i, PLGA, PLGA-p38i). To highlight this improvement over time, we expressed this as a difference in fractional shortening from 7 to 21 days, demonstrating that while there was no initial improvement, sustained p38-inhibition slowed the progression of the dysfunction, even demonstrating improvement over time (Figure 5B). We believe this can be explained by the fact that significant damage from MI by apoptosis and necrosis is done within the first 24–48 hours25. As the half-life of our particles is approximately 7 days, it is likely that the inhibitor was not released fast enough to affect this early time point.
Figure 5. PK-p38i therapy results in improved cardiac function and reduced fibrosis.
(a) Dimensions of the left ventricle were taken at systole and diastole using MRI at day seven and day 21 post-occlusion. PK-p38i showed a statistically significant difference between days 7 and 21, whereas all other treatments did not reach significance (mean + SEM, n>4, *p<0.05 repeated measures ANOVA). In addition, PK-p38i fractional shortening was significantly higher on day 21 compared to MI alone and all other treatment groups (ANOVA followed by Tukey-Kramer post-test). (b) Fractional shortening was measured and expressed as an absolute percent difference between days 7 and 21. A positive value represents an improvement in cardiac function between days 7 and 21, while negative values represent progression of cardiac dysfunction. PK-p38i treatment showed a significant 10% improvement (absolute value) in cardiac function while PLGA-p38i treatment did not inhibit cardiac dysfunction (mean ± SEM, n ≥ 4, **p<0.001 vs. all groups). (c) The left-ventricular free wall was analyzed histologically in at least 3 serial sections for fibrosis using a collagen-specific Picrosirius red stain. MI alone, free inhibitor, and empty particle treatments had significant increases in fibrosis compared to sham operation. PK-p38i treatment reduced fibrotic area by more than half, though not completely to sham levels (mean + SEM, n≥4, *p<0.05 vs. other treatment groups, ANOVA followed by Tukey-Kramer post test). (d–g) Representative Picrosirius red images of (d) MI, (e) MI + free inhibitor, (f) MI + PK, and (g) MI + PK-p38i treatment are shown (scale bar: 200 µm).
Given that the effect on function was not seen early, we hypothesized that prolonged p38-inhibition was having an effect on the development of fibrosis26,27. Histological sections were made from the rats at 21 days post-infarction and stained for collagen using Picrosirius Red. Digital images of the sections were captured and quantified manually in a blinded manner (Figure 5C). Untreated MI, free inhibitor, and PK particle treatment had significantly more fibrosis than sham operated animals (Figures 5D–5F). Treatment with PK-p38i however, resulted in significantly less fibrosis in comparison to other treatment conditions (Figure 5G). These data, taken together with the functional data, suggest that a single injection of PK-p38i microparticles reduced fibrosis and reversed progression of cardiac dysfunction following myocardial infarction.
There is a compelling need for development of new biomaterials that can improve the treatment of cardiac dysfunction. In this letter, we demonstrate that the polyketal PCADK has the biocompatibility and controlled release properties needed for treating inflammatory diseases. PCADK microparticles do not induce an inflammatory response in vitro or in vivo, and can therefore reside in inflamed tissue and act as a controlled release reservoir for SB239063, having a release half-life of 7 days for SB239063 at neutral pH values. We also demonstrate that delivery of PCADK microparticles loaded with SB239063 results in prolonged reduction of inflammatory signaling and eventual increased functional outcome from a single injection. In a direct comparison with PLGA, only loaded PCADK microparticles significantly improved function over time. Based on these results, we anticipate numerous applications of PCADK for treatment of myocardial infarction and other inflammatory diseases.
Materials and Methods
Polyketal synthesis and particle preparation
Poly(cyclohexane-1,4-diyl acetone dimethylene ketal) (PCADK) was synthesized as described in Lee, et al (2007). Briefly, 1,4-cyclohexanedimethanol was reacted with 2,2-dimethoxypropane in an acetal exchange reaction. First, 1,4-cyclohexanedimenthanol was dissolved in benzene and brought to 100°C with constant stirring. A solution of p-toluenesulfonic acid in ethyl acetate was added to the reaction flask in order to catalyze the reaction. The ethyl acetate was allowed to boil off, and distilled 2,2-dimethoxypropane was added to the benzene solution in an equimolar ratio to 1,4-cyclohexanedimethanol, initiating the polymerization reaction. Additional doses of 2,2-dimethoxypropane and benzene were subsequently added to the reaction dropwise to the reaction flask via a metering funnel to compensate for the 2,2-dimethoxypropane and benzene that had been distilled off. After 8 hours, the reaction was stopped by addition of 500 µL of triethylamine. The polymer was isolated by precipitation in cold hexane (stored at −20°C) followed by vacuum filtration. The molecular weight of the resulting polymer was approximately 6 kDa with a mean polydispersity (PDI) of 1.923. All reagents were purchased from Sigma-Aldrich. 2,2-dimethoxypropane and benzene were distilled prior to use. p-Toluenesulfonic acid was recrystallized prior to use. All other reagents were used as received.
Polyketal particles loaded with SB239063 (Axxora) (PK-p38i) were generated using an emulsion-solvent evaporation technique. Five-hundred µg of inhibitor and 50 mg of PCADK were dissolved in 500 µl of dichloromethane. The polymer solution was then added to 5 mL of 4% PVA and homogenized at a low speed for 60s. The resulting emulsion was transferred to 30 mL of 1% PVA and stirred at approximately 100 rpm for 4h to allow for evaporation of dichloromethane and solidification of microparticles. The particles were then centrifuged and washed with deionized water three times to remove residual PVA. The suspension was then frozen in liquid nitrogen and lyophilized to produce a free flowing powder.
PLGA properties
PLGA (Resomer RG 503 H, 48:52 lactide:glycolide, 35.4 kDa, PDI 2.5, Boehringer Ingelheim) was used as received. Microparticles containing SB239063 were made with the same protocol for PCADK particles and generated particles with similar inhibitor content, sizes, and release kinetics.
SB239063 loading and release characterization
PCADK particles containing SB239063 and corresponding empty particles were hydrolyzed overnight in 1N HCl at 65°C. The resulting solution was measured at 320 nm and loading efficiencies calculated from a previously determined standard curve (r2=0.99).
Release studies were conducted in vitro by suspending particles at a concentration of 1 mg/ml in PBS (pH 7.4) or 100 mM acetic acid (pH 4.5). One ml aliquots were made in microcentrifuge tubes and kept at 37°C under constant agitation. At the designated time points, tubes were centrifuged and 200 µl of the supernatant withdrawn for analysis. Fresh buffer was replaced before centrifuging to resuspend the particles. At the end of the release studies, the remaining polymers were centrifuged, supernatant removed, and 1N HCl added in order to hydrolyze the microparticles. Unreleased SB239063 was quantified spectrophotometrically and used to calculate the percentage of inhibitor released at each time point.
Macrophage culture
RAW264.7 macrophages were maintained in DMEM (Fisher) supplemented with 10% fetal bovine serum (Hyclone), l-glutamine, and penicillin/streptomycin (Invitrogen). For experiments involving TNF-α stimulation, cells were plated at confluence 18 hours prior to experiments in serum free media (SFM, DMEM supplemented with l-glutamine and penicillin/streptomycin). Media was then aspirated and replaced with treatment media containing either SB239063 dissolved in DMSO, neat DMSO as a control, or polyketal microparticles. Cells were preincubated with the treatment for the indicated time and washed with fresh SFM media before being exposed to 10 ng/mL TNF-α (Sigma).
Bioplex cytokine analysis and inflammation studies
C57BL6 adult mice (Charles River) were anesthetized under isoflurane (1–3%, Charles River). Thighs were shaved and skin was incised to expose the muscle. For cytokine measurements, muscle was injected with 1 mg of polymer (100 µl of 10 mg/mL solution in sterile saline). The contralateral leg was injected with saline as a control. For immunohistochemical analysis 5 mg (100 µl of 50 mg/mL solution in sterile saline) was injected into the muscle. At the designated intervals, mice were sacrificed by CO2 and the leg muscles exposed. Approximately 50 mg of muscle was removed at the injection site and processed.
For cytokine analysis, tissue was frozen in liquid nitrogen and homogenized in Cell Lysis Buffer (Bio-Rad) according to manufacturer’s instructions. Briefly, tissue was homogenized in 0.5 mL of lysis buffer and sonicated to ensure full disruption of tissue. The homogenate was centrifuged and supernatant incubated with X-Plex multiplex beads (Bio-Rad) according to manufacturer’s instructions and analyzed on a Bio-Rad Bioplex system.
For immunohistochemical analysis, tissue was fixed in 4% paraformaldehyde (Sigma) and embedded in paraffin. Histological sections were made and stained with FITC-conjugated anti-CD-45 antibody (eBiosciences) using standard immunohistochemical techniques. DAPI was used to visualize nuclei in the sections. Slides were imaged under fluorescence microscopy and digital images of the sections saved.
Rat myocardial infarction model
Adult male Sprague-Dawley rats (obtained from Charles River) weighing 250 grams were subjected to myocardial infarction/injection surgeries in a randomized and double-blinded manner. Briefly, the animals were anesthetized (1–3% isoflurane (Webster Veterinary) and, following tracheal intubation, hearts were exposed by separation of the ribs. Myocardial infarction was performed by ligation of the left anterior descending coronary artery. For PK injection or cell therapy studies, immediately after coronary artery ligation, polyketals (100 µL) were injected into the infarct zone through a 30-gauge needle while the heart was beating. Following injection, the chests were closed and animals allowed to recover on a heating pad. At the indicated time points, magnetic resonance imaging (MRI) was performed and data analyzed in a blinded manner. For immunohistological evaluation, hearts were harvested and fixed in 4% paraformaldehyde. Following dehydration, hearts were embedded in paraffin and 5 µm sections were made.
Western analysis
Cell (250 µg) or tissue (500 µg) protein homogenates were incubated with an antibody against total p38 (Cell Signaling) overnight at 4°C, prior to 2 hour incubation with Protein-A agarose beads (Sigma). Beads were washed and boiled in sample buffer prior to loading on a 12% polyacrylamide-SDS gel. Proteins were transferred to a PVDF membrane (Bio-Rad) and blots probed with an antibody for phospho-specific p38 (Cell Signaling). Films were scanned and quantified using Image J software.
Quantification of Superoxide via DHE-HPLC
To determine levels of superoxide in cells and tissues, a novel method described recently was used. Dihydroethidium (DHE) is a commonly used immunohistochemical marker to measure superoxide in tissues. However, when DHE is oxidized by superoxide, it becomes impermeable to cells. Therefore, any DHE oxidized in the extracellular space will remain in the incubation medium. This oxidation product, 2-hydroxyethidium, has a different retention that ethidium and can be quantified by high performance liquid chromatography (HPLC) with fluorescence detection.28
To detect tissue superoxide following myocardial infarction in rats, the left ventricular free wall was harvested at the indicated time point and equal sized pieces were incubated at 37°C in 1 mL of Krebs/Hepes buffer (KHB; pH= 7.35) containing 50 µM dihydroethidium (Invitrogen) for 30 minutes. Following incubation, buffer was syringe filtered and 100 µL placed in a tube containing 300 µL methanol. The sample was loaded on a C18 column for reverse-phase HPLC analysis using an acetonitrile gradient and data were normalized to wet tissue weight.
For cell culture experiments, macrophages were incubated with polyketals for the indicated time period. Following polyketal pre-treatment, cells were incubated with 10 ng/mL TNF-α for 5 minutes in KHB at 37°C, prior to addition of 25 µM dihydroethidium for 20 minutes. Buffer was collected and analyzed in a manner similar to tissue studies.
TNF-α measurement
Protein homogenates were evaluated for TNF-α levels by ELISA (eBiosciences) according to manufacturer’s protocol and normalized to protein levels determined by Bradford assay.
Collagen evaluation
Collagen deposition was determined by Picrosirius Red (Sigma) staining as previously described.29
Statistical evaluation
Treatment groups were analyzed for statistical significance with GraphPad Prism software using post-test analysis where necessary.
Supplementary Material
Acknowledgements
The authors wish to thank Dr. Melissa Kemp for her assistance with Bioplex assays for cytokine analysis.
This work was supported by a seed grant from Emtech Biotechnology Development, Inc. (MED), the Georgia Tech/Emory Center for the Engineering of Living Tissues (funded by NSF-EEC-9731643) (NM), NIH UO1 HL80711-01 (NM), NIH R21 EB006418 (NM), J&J/GT Health Care Innovation Seed Grant Proposal (NM), and the Department of Homeland Security (DHS) Scholarship and Fellowship Program, administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and DHS (JCS). ORISE is managed by Oak Ridge Associated Universities (ORAU) under DOE contract number DE-AC05-06OR23100. All opinions expressed in this paper are the author's and do not necessarily reflect the policies and views of DHS, DOE, or ORAU/ORISE.
Literature Cited
- 1.Anversa P. Myocyte death in the pathological heart. Circ Res. 2000;86(2):121. doi: 10.1161/01.res.86.2.121. [DOI] [PubMed] [Google Scholar]
- 2.Anversa P, Leri A, Kajstura J. Cardiac regeneration. J Am Coll Cardiol. 2006;47(9):1769. doi: 10.1016/j.jacc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R. Oxygen-derived free radicals and myocardial reperfusion injury: an overview. Cardiovasc Drugs Ther. 1991;5 Suppl 2:249. doi: 10.1007/BF00054747. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R, et al. Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci U S A. 1989;86(12):4695. doi: 10.1073/pnas.86.12.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2(9):717. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 6.Lee JC, et al. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology. 2000;47(2–3):185. doi: 10.1016/s0162-3109(00)00206-x. [DOI] [PubMed] [Google Scholar]
- 7.Peifer C, Wagner G, Laufer S. New approaches to the treatment of inflammatory disorders small molecule inhibitors of p38 MAP kinase. Curr Top Med Chem. 2006;6(2):113. doi: 10.2174/156802606775270323. [DOI] [PubMed] [Google Scholar]
- 8.Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005;97(1):8. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48(5):907. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Heffernan MJ, Murthy N. Polyketal nanoparticles: a new pH-sensitive biodegradable drug delivery vehicle. Bioconjug Chem. 2005;16(6):1340. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, et al. Polyketal microparticles: a new delivery vehicle for superoxide dismutase. Bioconjug Chem. 2007;18(1):4. doi: 10.1021/bc060259s. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, et al. Selective inhibition of p38alpha MAPK improves cardiac function and reduces myocardial apoptosis in rat model of myocardial injury. Am J Physiol Heart Circ Physiol. 2006;291(4):H1972. doi: 10.1152/ajpheart.00043.2006. [DOI] [PubMed] [Google Scholar]
- 13.Liu YH, et al. Inhibition of p38 mitogen-activated protein kinase protects the heart against cardiac remodeling in mice with heart failure resulting from myocardial infarction. J Card Fail. 2005;11(1):74. doi: 10.1016/j.cardfail.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Minamino T, et al. MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proc Natl Acad Sci U S A. 1999;96(26):15127. doi: 10.1073/pnas.96.26.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porras A, et al. P38 alpha mitogen-activated protein kinase sensitizes cells to apoptosis induced by different stimuli. Mol Biol Cell. 2004;15(2):922. doi: 10.1091/mbc.E03-08-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren J, et al. Role of p38alpha MAPK in cardiac apoptosis and remodeling after myocardial infarction. J Mol Cell Cardiol. 2005;38(4):617. doi: 10.1016/j.yjmcc.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 17.See F, et al. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44(8):1679. doi: 10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 18.Ding T, Sun J, Zhang P. Immune evaluation of biomaterials in TNF-alpha and IL-1beta at mRNA level. Journal of materials science. 2007;18(11):2233. doi: 10.1007/s10856-007-3014-9. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki Y, et al. Reduction of surface-induced inflammatory reaction on PLGA/MPC polymer blend. Biomaterials. 2002;23(18):3897. doi: 10.1016/s0142-9612(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes DC, et al. Analysis of dihydroethidium-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00188.2006. [DOI] [PubMed] [Google Scholar]
- 21.Gongora MC, et al. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48(3):473. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 22.Kim MS, et al. An in vivo study of the host tissue response to subcutaneous implantation of PLGA-and/or porcine small intestinal submucosa-based scaffolds. Biomaterials. 2007;28(34):5137. doi: 10.1016/j.biomaterials.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Davis ME, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103(21):8155. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widder J, et al. Vascular endothelial dysfunction and superoxide anion production in heart failure are p38 MAP kinase-dependent. Cardiovasc Res. 2004;63(1):161. doi: 10.1016/j.cardiores.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhao ZQ, Vinten-Johansen J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res. 2002;55(3):438. doi: 10.1016/s0008-6363(02)00442-x. [DOI] [PubMed] [Google Scholar]
- 26.Clerk A, Sugden PH. Inflame my heart (by p38-MAPK) Circ Res. 2006;99(5):455. doi: 10.1161/01.RES.0000241053.89089.c3. [DOI] [PubMed] [Google Scholar]
- 27.Sugden PH, Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxidants & redox signaling. 2006;8(11–12):2111. doi: 10.1089/ars.2006.8.2111. [DOI] [PubMed] [Google Scholar]
- 28.Fink B, et al. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287(4):C895. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 29.Sanada S, et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117(6):1538. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.