Abstract
Context
Inconsistent findings have been reported regarding improved health-related quality of life (HRQOL) following weight loss.
Objective
To test the efficacy of a weight management program for improving HRQOL in overweight/obese adults diagnosed with type 2 diabetes.
Design
Randomized multi-site clinical trial with two treatment arms and blinded measurements at baseline and end of Year 1.
Setting
Study was conducted at 16 outpatient research centers.
Participants
Total of 5,145 participants (mean age = 58.7±6.9 yr; mean BMI = 36.0±5.9; % women = 59.5%; % white = 63.3%) were randomized to two treatment arms.
Intervention
The two treatment arms were: Intensive Lifestyle Intervention 1 and Diabetes Support and Education (DSE).
Main Outcome Measures
SF-36, physical (PCS) and mental health (MCS) summary scores, and Beck Depression Inventory-II (BDI-II) scores. Baseline means were: PCS = 47.9±7.9; MCS = 54.0±8.1; and BDI-II = 5.7±5.0.
Results
HRQOL, as measured by PCS and BDI-II scores, improved (p<0.001) in the ILI arm compared to the DSE arm. The largest effect was observed for PCS (difference = −2.91, 99% CI: −3.44 ~ −2.37). HRQOL improved greatest in participants with the lowest baseline levels of quality of life. Changes in weight (ILI = −8.77±8.2 kg; DSE = −0.86±5.0 kg), improved fitness, and improved physical complaints mediated treatment effects associated with BDI-II and PCS.
Conclusions
HRQOL was significantly improved in overweight adults diagnosed with type 2 diabetes by enrollment in a weight management program that yielded significant weight loss, improved physical fitness, and reduced physical complaints. Trial Registration: NCT00017953
The prevalence of obesity, with its associated medical conditions (e.g., type 2 diabetes), has increased substantially in recent years.1, 2 Weight loss studies have found that successful weight management is associated with improvements in comorbid medical conditions.3 A consensus has been reached that sustained weight loss, achieved by healthy diet and physical activity, is a primary goal for improving health in overweight and obese individuals.4, 5 In recent years, the definition of improved health has expanded to include patient-reported outcomes,6 most notably health-related quality of life (HRQOL).7
An important question in the field of obesity is: Does successful weight loss result in improved HRQOL? 8 A related question is: Do intensive lifestyle interventions result in improved HRQOL? Investigations of these questions 8 have tested the effects of a variety of weight management strategies on HRQOL.9–13 These investigations have defined HRQOL using a variety of self-report questionnaires.14 In a recent meta-analytic review of this research literature, Maciejewski, Patrick, and Williamson15 reported that the most frequently used measures of HRQOL were the SF-3616 and the Beck Depression Inventory.17 Maciejewski, Patrick, and Williamson15 concluded that HRQOL was not consistently improved in randomized controlled trials (RCT) of weight loss.
The Look AHEAD trial18 is a 16-center RCT in overweight and obese individuals with type 2 diabetes designed to evaluate the long-term effects (up to 11.5 years) of an intensive weight loss intervention on the time to incidence for major cardiovascular events. This study provides an opportunity to investigate the impact of behavioral weight loss program on HRQOL. The current report examines changes in HRQOL after the first year of the Look AHEAD trial. We hypothesized that HRQOL would be significantly improved in the weight management arm in comparison to the control arm and that improvement in HRQOL would be mediated by weight loss, improved physical fitness, and reduction of physical complaints.
Method
Description of the Look AHEAD project
The Look AHEAD (Action for Health in Diabetes) study is a multi-center RCT, designed to determine whether intentional weight loss reduces cardiovascular morbidity and mortality in overweight individuals with type 2 diabetes. Participants were randomly assigned to an Intensive Lifestyle Intervention (ILI) or to an enhanced usual care control condition, called Diabetes Support and Education (DSE).
Participants
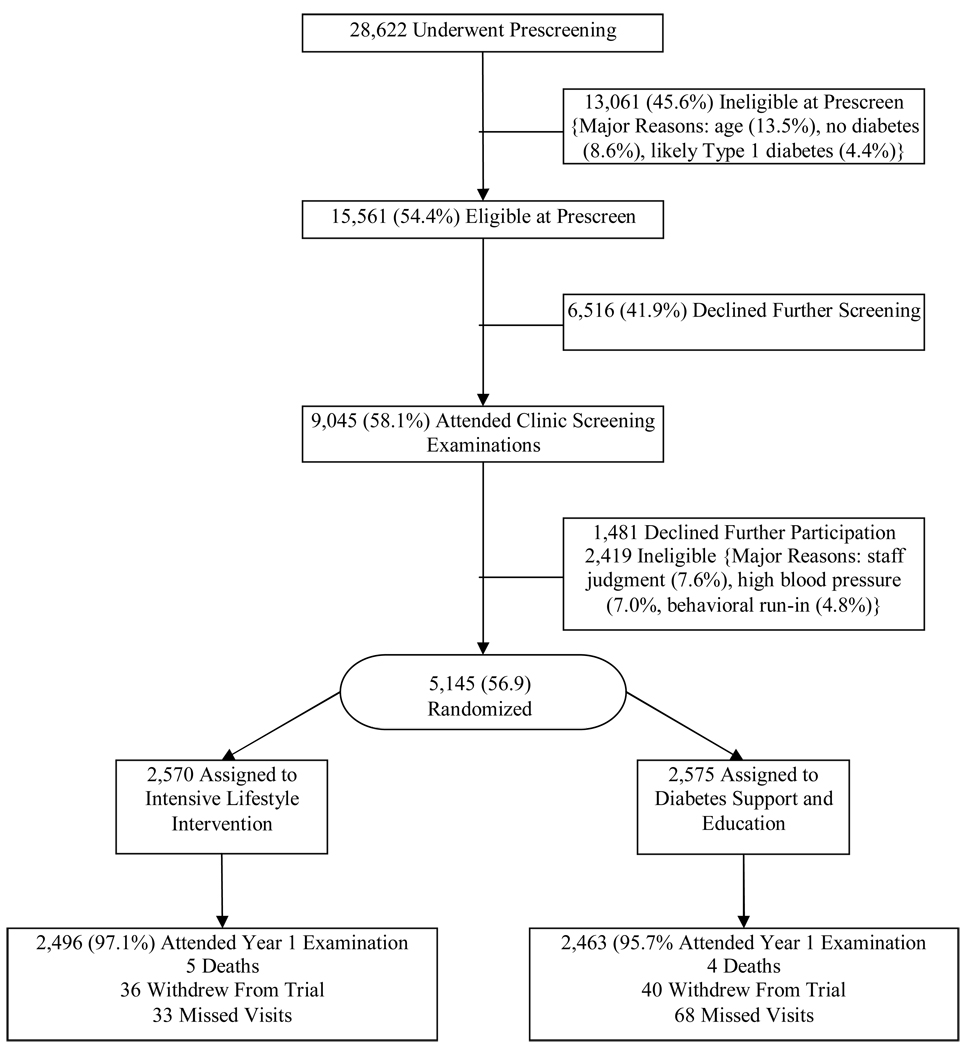
The sample included 5145 overweight or obese persons (a BMI ≥ 25 kg/m2 or ≥ 27 kg/m2 if currently taking insulin) with type 2 diabetes. It included 2082 men and 3063 women, all between the ages of 45 and 74 years. Major exclusions included HbA1c >11%, blood pressure > 160/100 mmHg, triglycerides > 600 mg/dl, inadequate control of comorbid conditions, and underlying diseases likely to limit lifespan or affect safety of the interventions. Potential participants were also excluded if they failed to pass a baseline graded exercise stress test. A CONSORT flow diagram that summarizes enrollment of participants into the clinical trial is shown in Figure 1.
Figure 1.
CONSORT flow chart showing enrollment of participants into the Look AHEAD study.
Assessment Measures
Measures of HRQL
HRQOL was assessed with the Medical Outcomes Survey, Short Form 36 (SF-36) and the Beck Depression Inventory-II (BDI-II). They were the only measures of HRQOL in the Look AHEAD study. The SF-36 is a commonly used measure of health status/HRQOL that consists of two norm-based composite T-scores (mean = 50, SD = 10): the physical component score (PCS) and the mental component score (MCS). Higher scores on the two SF-36 component summary scores indicate more favorable quality of life. The SF-36 also provides eight subscale scores that are described in Table 1. Extensive psychometric data support the reliability and validity of the SF-36.16
Table 1.
Participant characteristics at Baseline.
| Characteristic | |
|---|---|
| Number | 5145 |
| Average age (y) | 58.7 ± 6.9 |
| Number (%) Female | 3003 (59.5%) |
| Race/Ethnicity | |
| African American/Black (not Hispanic) | 803 (15.6%) |
| American Indian/Native American/Alaskan Native | 258 (5.0%) |
| Asian/Pacific Islander | 50 (1.0%) |
| White | 3246 (63.3%) |
| Hispanic | 677 (13.2%) |
| Other | 98 (1.9%) |
| Marital Status | |
| Never Married | 386 (7.5%) |
| Married/Marriage-like Relationship | 3461(67.3%) |
| Widowed | 359 (7.0%) |
| Divorced/Separated | 934 (18.2%) |
| Mean Body Mass Index (Kg/m2) | 36.0 ± 5.9 |
| Number (%) taking anti-depressant medications | 848 (16.5%) |
| Average Maximal MET value | 7.2 ± 2.0 |
| Average Maximal MET value (at 80% HR) | 5.2 ± 1.5 |
| Average SF-36 Physical Composite Score (PCS) | 47.9 ± 7.9 |
| Average SF-36 Mental Composite Score (MCS) | 54.0 ± 8.1 |
| Average Beck Depression Inventory II (BDI-II) Score | 5.7 ± 5.0 |
| Number (%) with BDI-II scores > 19 | 94 (1.8%) |
| SF-36 Subscale Scores | |
| Mean Physical Functioning | 48.5 ± 7.9 |
| Mean Physical Role Functioning | 50.2 ± 8.1 |
| Mean Bodily Pain | 50.7 ± 8.8 |
| Mean General Health | 47.2 ± 8.9 |
| Mean Vitality | 53.0 ± 9.0 |
| Mean Social Functioning | 52.2 ± 7.6 |
| Mean Emotional Role | 51.5 ± 7.9 |
| Mean Mental Health | 53.7 ± 8.0 |
The BDI-II,19 a revision of the BDI,17 is one of the most widely used and well validated self-report measures of depressive symptoms. Higher scores indicate more severe symptoms of depression. Scores on the BDI-II can be interpreted within the context of the following categories: 0–13 = minimal depression, 14–19 = mild depression, 20–28 = moderate depression, and >28 = severe depression.19
Demographics
Demographic characteristics, all from self-report, included age, sex, and race/ethnicity.
Disease burden/fitness
Self-reported measures of disease burden and fitness included diabetes severity, number of physical complaints, and comorbid medical conditions. BMI (kg/m2), and peak metabolic equivalents (MET) capacity were also measured. Physical symptoms were measured by a frequency count of 19 different physical complaints. The number of comorbid medical conditions was based on self-report of arthritis, cardiovascular disease (CVD) history, hypertension, stroke, and emphysema. Disease severity was defined by three categories: (1) no diabetes related drugs, (2) oral diabetes drugs but no insulin, and (3) insulin use with or without oral diabetes drugs. Assessment of height and weight to calculate BMI was obtained by standardized procedures during one of the baseline clinical assessments. Maximal MET capacity was estimated from performance on a graded exercise treadmill test (~10 minutes) that included heart rate measurement.
Procedures
Informed consent was obtained from all participants before screening. Key components of Look AHEAD’s intensive lifestyle intervention (ILI) have been described in a recent paper.20 The treatment protocol combined multiple diet and exercise approaches in an effort to maximize the number of participants who met the study’s weight and activity goals. The two principal intervention goals were to induce a mean loss ≥ 7% of initial weight and to increase participants’ moderately-intense physical activity to ≥ 175 minutes a week. For the first 6 months, participants attended one individual and three group sessions per month and were encouraged to replace two meals and one snack a day with liquid shakes and meal bars. From months 7–12, they attended one individual and two group meetings per month and continued to replace one meal per day. Participants in the ILI arm were instructed to self-monitor energy intake and physical activity, and their body weight was measured at each individual and group counseling session. The control arm, DSE, involved three educational group sessions per year that focused on one of three topics: nutrition, physical activity, and support. Participants assigned to the DSE arm were not given goals for weight loss or caloric intake, were not instructed to monitor energy intake or physical activity, and were not weighed at group meetings.
Statistical Methods
All analyses were performed using SAS version 9.1. Differences between ILI and DSE participants in 1-year changes of HRQOL measures were compared using analysis of covariance (ANCOVA), with adjustment for baseline values and for clinical center, the stratification factor used during randomization. Effect size estimates were summarized using the Glass’ Δ with the corresponding 99% confidence intervals. The experiment-wise alpha level was adjusted due to the use of three different outcomes in the HRQOL battery via a Bonferroni procedure, and resulted in an alpha level for testing main effects of treatment of p < .017.
Separate linear regression models were fitted to test the moderator effects. The dependent variables were the 1-year changes in PCS, MCS, and BDI-II scores, respectively. The independent variables included clinical center, treatment group, main effect for potential moderator, and the interaction effect of treatment by potential moderator. Additionally, in the models testing for the moderator effects (of age, gender, race, BMI, diabetes severity, number of comorbid conditions, and number of physical complaints), baseline HRQOL score corresponding to the dependent variable was also included as an independent variable. The presence of moderator effects was interpreted based on the significance of the treatment-by-moderator interaction effects*.
To test whether weight loss, fitness improvement, or changes in physical complaints during the first year of intervention accounted (fully or partially) for differential treatment effects on the one-year changes in HRQOL measures, mediator analyses were performed in three steps according to the widely used approach of Baron and Kenny.21 This approach requires that the following be established: (1) significant relationships exist between the independent variable, treatment group, and the dependent variables; 2) the treatment is related to the potential mediators and the mediators are related to the dependent variables; and 3), the addition of the mediators to the regression models used in step 1 reduces or removes the significance relationship between treatment and the dependent variables. The Sobel test was used to assess the statistical significance of mediating effects. Mediation analyses were restricted to the participants who had complete data for change in HRQOL measures, changes in weight, fitness, and physical complaints in order to assess the changes in regression coefficients. For moderator and mediator analyses, the type I error rate was fixed at 5%†.
Intent-to-treat analyses were computed including all randomized participants. Missing year 1 data in HRQOL measures, weight, fitness, and physical complaints were imputed using baseline scores.
Results
Description of the Sample at Baseline
The description of the sample has been reported in detail by The Look AHEAD Research Group.22 Table 1 summarizes the characteristics of the participants at baseline.
Effects of Treatment Assignment on HRQOL
Changes in the SF-36 PCS differed as a function of treatment arm (p<0.001; difference = −2.91, 99% CI: −3.44 ~ −2.37). As shown in Figure 2a, in addition to treatment arm differences, assignment to ILI was associated with improvement of PCS (1.65±7.94, p < .001); whereas assignment to DSE was associated with worsening of PCS (−1.27±7.44, p < .001). Baseline PCS was associated with change scores such that greater improvement was associated with lower baseline scores (indicating lower HRQOL related to physical health; Spearman’s ρ= −0.34, p < 0.001). Furthermore, as shown in Figure 3a, the effects of treatment were moderated by baseline PCS such that participants in the ILI arm with lower baseline PCS scores experienced greater improvement in comparison to participants in the DSE arm with lower baseline PCS scores. Participants in both treatment arms with relatively high baseline PCS scores did not show improvement after one year of the interventions. Baseline BMI also moderated the treatment effects on PCS, (p = 0.003). This moderator effect is shown in Figure 4. Participants in the ILI arm had increased PCS scores across all BMI ranges, but in the DSE arm, changes in PCS were negatively associated with baseline BMI such that participants with very high BMI at baseline (~ 35 kg/m2 or greater) did not show improvement. The effect size for the treatment effect related to PCS was 0.39.
Figure 2.
Changes in measures of HRQOL as a function of treatment arm. Figure a depicts changes for PCS; Figure b depicts changes in MCS: Figure c depicts changes in BDI-II. Error bars reflect 99% confidence intervals.
Figure 3.
Moderating effects of baseline scores for PCS (Figure a), MCS (Figure b), and BDI-II (Figure c) upon treatment effects related to changes in HRQOL. The two line graphs in each figure represent the regression lines depicting the relationship between baseline values and changes in HRQOL variables as a function of treatment arm.
Figure 4.
Moderating effects of baseline BMI on the treatment effects related to changes in PCS. The two line graphs in Figure 4 represent the regression lines depicting the relationship between BMI and changes in PCS as a function of treatment arm.
Average changes in SF-36 MCS did not differ as function of treatment arm (p>0.05; difference = −0.46, 99% CI: −1.04 ~ 0.12). This non-significant finding is depicted in Figure 2b. The effect size for the treatment arm effect related to MCS was quite small, 0.08. After one year, there were no changes in MCS scores from baseline for the ILI group (0.03±8.75, p = 0.21), nor the DSE group (−0.60±8.32, p = 0.13). As observed for PCS, baseline MCS was associated with change scores such that greater improvement was associated with lower baseline scores (Spearman’s ρ= −0.39, p < 0.001). As shown in Figure 3b, the effect of treatment on MCS was moderated by baseline MCS scores (p < 0.001) such the greatest improvement was observed in ILI participants with the lowest baseline MCS scores.
Change scores for BDI-II also differed between the two treatment arms (p<0.001; difference = 0.55, 99% CI: 0.24 ~ 0.86). This treatment arm effect is depicted in Figure 2c. BDI-II scores for ILI (−0.83±4.86, p < 0.001) and for DSE (−0.23±4.63, p < 0.001) decreased from baseline. Correlation analysis indicated that higher scores at baseline were associated with greater reductions in the symptoms of depression (Spearman’s ρ= −0.45, p < 0.001). As shown in Figure 3c, the treatment effect was moderated by baseline BDI-II scores (p = 0.015). Participants in the ILI arm with higher BDI-II scores at baseline had the greatest reduction in depressive symptoms at the end of the one-year intervention period. The effect size for the treatment arm effect related to BDI-II changes was 0.13.
Moderating Effects of Comorbid Conditions, Disease Severity, and Physical Complaints
When the baseline number of comorbid medical conditions, disease severity, and physical complaints were entered into the regression models for PCS, MCS, and BDI to test for moderation of the effects of treatment arm, there were no significant interaction effects between treatment group and comorbid conditions or physical complaints (all p values > 0.10). Also, age, gender, and race did not interact with treatment (all p values > 0.10) and therefore were not interpreted as significant moderators of the treatment effects on measures of HRQOL.
Effects of Treatment Assignment on Body Weight, Fitness, and Physical Complaints
The differential effects of treatment arm on weight changes after one-year of intervention have been described in detail by The Look AHEAD Research Group.23 The one-year measurement of outcome was attended by 97.1% of the ILI arm and 95.7% of the DSE arm. Participants in the ILI arm achieved significantly greater reductions in body weight, −8.77±8.2 kg (M = −8.58%, SD = 7.72%) in comparison to the DSE arm, −0.86±5.0 kg (M = −0.81%, SD = 4.76%), p < 0.001. Also participants in the ILI arm improved fitness (M = 20.72%, SD = 29.11%) to a greater extent than the participants in the DSE arm (M = 5.83%, SD = 22.02%), p < 0.001. Furthermore, participants in the ILI arm reported a reduction in physical complaints (M = −0.28, SD = 2.99) in comparison to an increase for those in the DSE arm (M = 0.56, SD = 3.06), p < 0.001.
Mediating Effects of Changes in Body Weight, Fitness, and Physical Complaints
Figure 5 provides an illustration of tests for mediation as a companion to Table 2. As noted earlier, all of the putative mediators, i.e., changes in body weight (p < 0.001), physical fitness (p < 0.001), and physical complaints (p < 0.001) differed as a function of treatment arm. Treatment effects for MCS were not observed; thus, mediation effects were not tested for this variable. The direct effects of treatment on the proposed mediators are presented as path a in Table 2. Each of the mediators was significantly related to PCS and BDI-II; these effects are described under the heading path b in Table 2. Path c, the effects of the treatment on the HRQOL measures, illustrate that both PCS and BDI-II passed this criterion for tests of mediation. To test for mediation of treatment effects on PCS and BDI-II by changes in weight, fitness, and physical complaints, the putative mediators were entered into the regression models and the results are summarized in Table 2 as c′. The Sobel test (shown as P-value in Table 2) was used to test whether the indirect effect of treatment on the outcome through the mediator, was statistically significantly. The mediating effects of changes in weight, fitness, and physical complaints were statistically significant (p<0.0001) but did not completely attenuate the treatment effects attributable to ILI for PCS and BDI-II with one exception; weight changes completely mediated the treatment effects on BDI-II scores (as indicated by the non-significant treatment effect under path c′.
Figure 5.
A model for analyzing mediator effects using the approach described by Baron and Kenny. 21 Note that the lower case letters (a, b, and c) refer to the following effects: a = treatment to mediator, b = mediator to outcome, and c = treatment to outcome. A lower case c′ depicts the effect of the treatment on the dependent variables with the mediator included in the model which parallels step 3 of the Baron and Kenny model.21
Table 2.
Summary of mediation analyses related to changes of body weight, physical fitness, and physical complaints. (Based on models without the interaction effect of treatment group by baseline HRQOL scores)
| HRQOL Outcome of Interest | Putative Mediators | Spearman partial correlation coefficient | P-value from Sobel Test | |||
|---|---|---|---|---|---|---|
| Regression parameter estimate ± standard error | ||||||
| Path a Treatment effect on Mediator | Path b Effect of Mediator on HRQOL | Path c Treatment effect on HRQOL w/o mediation | Path c′ Treatment effect on HRQOL with mediation | |||
| PCS | weight change | −0.61** | −0.17 ** | 0.23** | 0.08 ** | <0.001 |
| −17.78 ± 0.46** | −0.069 ± 0.0069** | 2.84 ± 0.21** | 1.62 ± 0.24** | |||
| fitness change | 0.30 ** | 0.12 ** | 0.19 ** | <0.001 | ||
| 0.76 ± 0.038** | 0.70 ± 0.084** | 2.31 ± 0.21** | ||||
| physical complaint change | −0.14** | −0.20 ** | 0.21 ** | <0.001 | ||
| −0.84 ± 0.091** | −0.45 ± 0.034** | 2.47 ± 0.20** | ||||
| BDI-II | weight change | −0.62** | 0.07 ** | −0.07** | −0.01 ns | <0.001 |
| −17.78 ± 0.46** | 0.023 ± 0.004** | −0.54 ± 0.12** | −0.12 ± 0.14ns | |||
| fitness change | 0.30 ** | −0.07** | −0.05* | <0.001 | ||
| 0.76 ± 0.038** | −0.27 ± 0.05** | −0.33 ± 0.13* | ||||
| physical complaint change | −0.14** | 0.13 ** | −0.05* | <0.001 | ||
| −0.84 ± 0.091** | 0.21 ± 0.021** | −0.36 ± 0.12* | ||||
Table Note: Three statistical models were tested for each putative mediator: Model 1: Change in PCS (or BDI) = treatment arm + baseline PCS (or BDI score); Model 2: Change in mediator = treatment arm; Model 3: Change in PCS (or BDI) = treatment arm + baseline PCS (or BDI score) + Mediator. The designation of paths follows the illustration shown in Figure 5: Path a: ‘treatment arm’ effect from Model 2; Path b: ‘mediator’ effect from Model 3; Path c: ‘treatment arm’ effect from model 1. Path c ′: ‘treatment arm’ effect from Model 3. Three statistics are provided for all tests: Spearman partial correlation coefficient, regression parameter estimate, and standard error. Statistical significance of effects is shown by:
p<0.001
p<0.02
p>0.05.
The last column summarizes the p-value from the Sobel test for the significance of the indirect effect ab, i.e., the amount of mediation. Results show that changes in weight, fitness, and physical complaints reduced the effects of treatment on PCS and BDI-II as indicated by the reduction in both regression parameter estimates from c to c ′ and Spearman partial correlation coefficients. In conclusion, changes in weight, fitness, and physical complaints partially mediated the effects of treatment on PCS and BDI-II, with one exception: changes in weight completely mediated treatment effects on BDI-II, as evidenced by the non-significant treatment effect under path c ′.
Intent-to treat analyses
Intent-to-treat analyses were also performed assuming no change over the one year for those participants with missing values for HRQOL changes, weight change, fitness change, and physical complaints changes. Results were essentially the same as those reported above.
Discussion
The findings of this study support the hypothesis that participation in an intensive lifestyle behavior modification intervention for weight management was associated with significant improvement in HRQOL (relative to the control group), as measured by the PCS score of the SF-36 and the BDI-II. Assignment to ILI was associated with significant improvements in PCS and BDI-II scores, whereas assignment to DSE was associated with worsening in PCS. The overall treatment effect on PCS (0.39) was greater than the relatively small effect size (0.13) that was observed for BDI-II, but was not sufficiently large to be considered clinically significant.24,25 The study also found that individuals with worse HRQOL at baseline derived the greatest benefits from the weight management intervention. Similar results were reported in an uncontrolled study26 of changes in HRQOL following pharmacologic and lifestyle treatment for obesity. This observation is consistent with statistical phenomenon called “regression to the mean”. It is noteworthy (see Figure 3) that for all three measures of HRQOL, the greatest benefits of ILI were observed for participants with baseline SF-36 scores indicative of poor HRQOL and baseline BDI-II scores indicative of mild to moderate depression‡. We also tested for moderation of treatment effects by baseline BMI, baseline levels of pain complaints, disease severity, severity of baseline comorbid conditions, age, gender, and race. Baseline BMI, moderated the differential effects of the two treatment arms on PCS, but did not moderate the effects on MCS or BDI-II scores.
This study is one of the first to evaluate the potential mediation of treatment effects on HRQOL. We tested three putative mediators:21 changes in weight, fitness, and physical complaints. Mediation analyses (see Table 2) indicated that the improvement in PCS associated with participation in the ILI arm was only partially mediated by the improvements in body weight, physical fitness, and reductions in physical complaints. Treatment effects related to BDI-II scores were partially mediated by changes in fitness and physical complaints and completely mediated by changes in body weight. The finding that changes in body weight, physical fitness, and physical complaints accounted for only a portion of the changes in PCS attributed to treatment suggests that other factors (e.g., counseling and group support, changes in the social and/or family environment, improved metabolic parameters, or improved functional abilities) may be critical variables that account for improved HRQOL that occurs with successful lifestyle modification related to weight management in overweight/obese adults diagnosed with type 2 diabetes.
These results significantly inform the medical professions about changes in HRQOL and lifestyle behavior change related to weight management. The research literature on this topic has yielded conflicting findings.15 This study addresses some of the primary shortcomings of previous studies:15 1) loss to follow-up for this study was quite low (2.9% for ILI and 4.3% for DSE) relative to earlier studies, 2) the sample size was large; 3) study assessors were blind to treatment assignment; 4) mediation of changes in HRQOL by changes in body weight, fitness, and physical complaints was tested; and 5) statistical analyses were adjusted for multiple comparisons.
The results suggest that the strongest effects of lifestyle modification were on HRQOL related to physical health. Furthermore, the largest changes in HRQOL were observed for participants with poorest quality of life at baseline. In this context, it is not surprising that the smaller-scale and less well-controlled investigations have yielded conflicting results pertaining to the effect of lifestyle weight management programs on HRQOL.15
Given the initial positive findings of the impact of participation in a lifestyle behavior modification program for obesity on HRQOL, it will be very important to observe the stability of improvement in HRQOL over longer periods of time. It is highly probable that a substantial portion of the participants in the ILI arm will regain some weight over the 11-year duration of the Look AHEAD trial.20 Only one uncontrolled study26 has reported on changes in HRQOL associated with weight regain, and this paper reported deterioration of improvements in HRQOL following weight regain.
The primary limitations of the study are the relatively brief duration of the study (i.e., 1 year) and the focus upon a highly selected population of overweight/obese adults who had been diagnosed with type 2 diabetes. These findings should not be generalized to other (less highly selected) populations and future reports on this sample are needed to determine if these changes in HRQOL persist over much longer periods of time.
In summary, participation in a 1-year ILI for weight loss improved HRQOL in overweight/obese adult type 2 diabetic participants. The greatest effects were observed for improvements in HRQOL related to physical functioning. For all three indicators of HRQOL, participants with the lowest quality of life at baseline derived the greatest HRQOL benefit from participation in the ILI. Improvement in HRQOL for physical health (PCS) was partially mediated by weight loss, improved fitness, and reductions in complaints related to physical problems. It is likely that other factors may account for additional variance in the beneficial changes in HRQOL associated with participation in a weight management program.
Footnotes
The total number of participants in the statistical analyses for treatment effects and moderator effects were 4772 for PCS and MCS and 4799 for BDI-II.
The total number of participants in the statistical models testing mediator effects was 4222 for PCS and MCS and 4223 for BDI-II.
Secondary analyses were conducted on participants with baseline scores indicative of poor HRQOL (SF-36 scores < 40 and BDI-II scores > 13). A significant (p < 0.0001, n = 779) treatment arm effect was found for PCS (effect size = .56), but not for MCS (p = 0.13, n = 325, effect size = .17) or for BDI-II (p = 0.24, n = 353, effect size = .11). The magnitude of this effect size (.56) for PCS meets accepted criteria24,25 for clinically significant improvement.
References
- 1.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Williamson DF. The Relationship of Intentional Weight Loss to Disease Incidence and Mortality. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York: Guilford Press; 2002. pp. 125–143. [Google Scholar]
- 4.National Task Force on the Prevention and Treatment of Obesity. Overweight, obesity, and health risk. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Geneva, Switzerland: World Health Organization; Obesity: Preventing and Managing the Global Epidemic: Technical Report Series 894. 2000 [PubMed]
- 6.Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient's perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health. 2003;6:522–531. doi: 10.1046/j.1524-4733.2003.65309.x. [DOI] [PubMed] [Google Scholar]
- 7.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2:173–182. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 8.Wadden TA, Womble LG, Stunkard AJ, Anderson DA. Psychosocial consequences of obesity and weight loss. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York: Guilford Press; 2002. pp. 144–169. [Google Scholar]
- 9.Rejeski WJ, Focht BC, Messier SP, Morgan T, Pahor M, Penninx B. Obese, older adults with knee osteoarthritis: weight loss, exercise, and quality of life. Health Psychol. 2002;21:419–426. doi: 10.1037//0278-6133.21.5.419. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)--an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord. 1998;22:113–126. doi: 10.1038/sj.ijo.0800553. [DOI] [PubMed] [Google Scholar]
- 11.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Health-related quality of life in WHO class II-III obese men losing weight with very-low-energy diet and behaviour modification: a randomised clinical trial. Int J Obes Relat Metab Disord. 2002;26:487–495. doi: 10.1038/sj.ijo.0801953. [DOI] [PubMed] [Google Scholar]
- 12.Kaukua JK, Pekkarinen TA, Rissanen AM. Health-related quality of life in a randomised placebo-controlled trial of sibutramine in obese patients with type II diabetes. Int J Obes Relat Metab Disord. 2004;28:600–605. doi: 10.1038/sj.ijo.0802591. [DOI] [PubMed] [Google Scholar]
- 13.Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA. 2003;289:1792–1798. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- 14.Williamson DA, O'Neil PM. Obesity and Quality of Life. In: Bray G, Bouchard C, editors. Handbook of Obesity. 2nd edition ed. New York: Marcel Dekker; 2004. pp. 1005–1022. [Google Scholar]
- 15.Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. J Clin Epidemiol. 2005;58:568–578. doi: 10.1016/j.jclinepi.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: New England Medical Center; 1994. [Google Scholar]
- 17.Beck AT, Brown GK, Steer RA. Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- 18.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT. Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 20.The Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 22.The Look AHEAD Research Group. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3:202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson RJ, Robinson AB, Splaine M. Use of the reliable change index to evaluate clinical significance in SF-36 outcomes. Qual Life Res. 2005;11:509–516. doi: 10.1023/a:1016350431190. [DOI] [PubMed] [Google Scholar]
- 25.Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J Biopharm Stat. 2004;14:73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 26.Engel SG, Crosby RD, Kolotkin RL, et al. Impact of weight loss and regain on quality of life: mirror image or differential effect? Obes Res. 2003;11:1207–1213. doi: 10.1038/oby.2003.166. [DOI] [PubMed] [Google Scholar]