Abstract
The short-term aftereffects of a bout of moderate aerobic exercise were hypothesized to facilitate children’s executive functioning as measured by a visual task-switching test. Sixty-nine children (mean age = 9.2 years) who were overweight and inactive performed a category-decision task before and immediately following a 23-min bout of treadmill walking and, on another session, before and following a nonexercise period. The acute bout of physical activity did not influence the children’s global switch cost scores or error rates. Age-related differences in global switch cost scores, but not error scores, were obtained. These results, in concert with several studies conducted with adults, fail to confirm that single bouts of moderately intense physical activity influence mental processes involved in task switching.
Keywords: exercise psychology, pediatrics, cognition, information processing
Exercise-induced physical arousal has been shown to facilitate specific types of mental functioning in adults (see reviews by Brisswalter, Collardeau, & Arcelin, 2002; McMorris & Graydon, 2000; Tomporowski, 2003b); however, very few studies have assessed the short-term aftereffects of acute bouts of physical activity on children’s cognitive function, and most of these studies have focused on children with developmental disabilities (Tomporowski, 2003a). Research conducted with children without development disorders has been designed, for the most part, to determine whether bouts of physical activity performed as part of school curricula would have a detrimental impact on children’s classroom behavior and academic performance. The results of three field-based studies indicated that bouts of physical activity improved, rather than debilitated, children’s cognitive performance (Caterino & Polak, 1999; Gabbard & Barton, 1979; McNaughten & Gabbard, 1993), and another study (Raviv & Low, 1990) found no evidence to support anecdotal observations that recess and physical education activities overly excite children. Only one laboratory-based experiment has evaluated normal children’s cognitive function immediately following a bout of steady-state exercise. Zervas, Apostolos, and Klissouras (1991) recruited 9 pairs of monozygotic twin boys (age range = 11–14 years) and assigned one twin to a 25-week structured aerobic physical fitness training program and the other twin to a traditional physical education class. Following training, the children performed a design-matching task before and 15 min following a strenuous 20-min treadmill run (speed range = 12–14 kph). The pre- and postexercise cognitive performance of trained and untrained twins was compared with the performance of eight age-matched children who did not complete the treadmill run. The acute bout of exercise resulted in improved design-matching accuracy of both trained and untrained twins, whose postexercise task accuracy was significantly better than that of control children’s.
The relation between arousal and cognitive function has been described in terms of an inverted-U function in which mental performance is enhanced with moderate, but not high, levels of arousal (McMorris & Graydon, 2000). The facilitative effects of acute bouts of exercise in adults have been explained in terms of the changes in central nervous system neurotransmitter systems that modulate brain activity. Specifically, physical movements lead to a cascade of metabolic responses that signal brainstem nuclei that are central to the activation of noradrenergic and serotonergic systems that modulate brain activity, particularly in the prefrontal and parietal cortices (Robbins, 1997). Increased arousal is purported to enhance cognitive processing by altering the signal-to-noise ratio of neurological systems, which, in turn, enhances attention, stimulus selection, and decision making (Hasselmo & Linster, 1999). The effects of acute bouts of exercise on the brain have been compared with those produced by psychostimulant drugs (Tomporowski, 2003b).
There is evidence for the facilitative effects of psychostimulant drugs on children’s performance during task-switching tests, which provide an index of executive function (Cepeda, Cepeda, & Kramer, 2000; Kempton et al., 1999; Kramer, Cepeda, & Cepeda, 2001). The task-switching paradigm is designed to isolate mental operations involved in stopping the computational processes required to perform one task and to initiate processes required to perform a different, now relevant, task. Successful performance involves a reconfiguration of mental operations to match current task set requirements (Rogers & Monsell, 1995). The reconfiguration of a task set is believed to be a resource demanding control process that results in slower response times on switch trials than on nonswitch trials. The residual switch cost is often used as an index of the efficiency of executive processing, and several explanations for residual switch costs have been offered (Meiran, Chorev, & Sapir, 2000; Vandierendonck, 2000). Cepeda et al. (2000) observed that task-switching behavior of 16 unmedicated children with attention-deficit hyperactivity disorder (ADHD; mean age = 8.9 years) was less controlled than in 16 age-matched children without ADHD; importantly, psychostimulant medication improved the task-switching performance of children with ADHD to a level comparable to that of children without ADHD. Medication selectively improved ADHD children’s ability to inhibit inappropriate responses. That is, medication ameliorated the underarousal characteristic of ADHD. The task-switching test developed by Cepeda et al. (2001) was employed in the current study to evaluate the aftereffects of a brief bout of physical activity on overweight children’s executive functioning. The first goal of the current study was to determine whether the arousal effects produced by acute exercise would lead to reductions in children’s switch costs.
Exercise psychologists have gained considerable understanding of the effects of exercise on older adults (Tomporowski, 2006); however, little is known of the effects of physical activity on children’s cognition, which is a topic of both scientific and applied importance (Tomporowski, Davis, Miller, & Naglieri, 2007). One challenge facing exercise psychologists interested in assessing the impact of physical activity on children’s cognition is the fact that children undergo rapid, process-specific changes in cognitive development. Some processes, such as exogenously driven attentional control, are fully developed by age 7 (Rueda, Posner, & Rothbart, 2005), whereas others, such as executive function, emerge gradually as a child moves into adolescence (Diamond, 2000). The second goal of the current study was to determine whether the effects of acute exercise on the children’s task-switching performance are influenced by children’s age. The majority of studies of age-related changes in task switching have focused on young and older adults; only a few studies have evaluated the development of task-switching performance in children. Davidson and colleagues (2006) assessed global switch-task cost, which is an index of the time required to perform a task under conditions that do not change across successive trials versus conditions in which processing demands change across successive trials, in children ranging from 4 to 13 years of age and adults (mean age = 26 years). Global switch costs decreased with age in those children 9 to 13 years of age. In the context of a lifespan study, Reimers and Maylor (2005) reported linear age-related reductions in children’s response times and global switch-cost indices from age 10 to 16 years. Likewise, in another lifespan study, Cepeda et al. (2001) contrasted switch costs of children aged 7–9, 10–12, and 13–20 years. The authors found that response times and switch costs decreased systematically from young childhood to young adulthood and that error rates were higher for young children than for adults. Two age groups were constituted in the current study: children between 7 and 9 years of age and children between 9 and 11 years of age.
The present study was conducted as part of a larger experiment that evaluated the effects of exercise training on overweight children’s cognitive function (Davis et al., 2007). Over a third of school-age children in the United States are overweight, with potential implications for school performance (Datar, Sturm, & Magnabosco, 2004; Ogden et al., 2006; Taras & Potts-Datema, 2005). Children who are overweight tend to be physically inactive; as such, a single bout of aerobic exercise would be predicted to elicit a robust physiological response. Children were selected randomly from the parent study to participate in the present experiment, which involved assessing each child’s task-switching performance before and immediately following either treadmill walking or watching a video. The task-switching test was identical to one developed by Cepeda et al. (2000) and provided indices of switch costs on trials in which responses were made under compatible and incompatible stimulus conditions. The exercise bout was hypothesized to facilitate children’s executive functioning and result in reduced switch costs and fewer response errors under both levels of processing demands compared with measures taken before exercise and compared with measures obtained during a nonexercise control condition. Further, age-related differences in performance were predicted with younger children evidencing longer switch times and more response errors compared with older children.
Method
Participants
Sixty-nine children between 7 and 11 years of age (mean = 9.2, SD = 1.2) participated. There were 36 girls and 33 boys (55% African American and 45% Caucasian). Their cognitive function, as measured by the Cognitive Assessment System (CAS; Naglieri & Das, 1997), was within the normal range (CAS Full Scale = 102; SD = 12). The children’s average BMI was 25.3, SD = 5.0, and their average age- and sex-adjusted BMI z-score was 2.01, SD = 0.45. Children and parents completed written informed consent/assent. The study was reviewed and approved by the Human Assurance Committee of the Medical College of Georgia. The study was conducted in concert with a larger study that evaluated the effects of exercise training on cognitive function and metabolism in overweight children. In the parent study, 163 children (7–11 years of age) were recruited from local elementary schools. Participants were overweight (≥85th percentile body mass index [BMI], for age and sex), inactive (did not participate in a regular physical activity program more than 1 hr per week), and had no medical condition that limited physical activity. Six children diagnosed with attention disorder maintained their medication regimes during assessment; the remaining children did not take medications that would affect study results. Selected in cohorts of 30–40, children were assigned randomly either to a low-dose exercise treatment, which consisted of 20 min of aerobic exercise per session; a high-dose exercise treatment, which consisted of 40 min of exercise; or a no-exercise control condition. Children assigned to exercise treatments attended programs that met 5 days per week for up to 15 weeks. Those children assigned to daily exercise regimens received an average of 7.1 weeks (range = 4–11) of exercise training before beginning the present experiment. Details of the exercise protocols are provided by Davis et al. (2007).
Procedure
Each child attended two laboratory test sessions. Each session consisted of three phases: First, the child was taken to a private room and trained to perform a visual switching task before completing three blocks of test trials; second, the child was taken to a room in which he/she walked on a motorized treadmill for 23 min or remained in the testing room and was presented an age-appropriate video for 23 min; third, the child completed a single block of test trials in the testing room. All phases of each session were administered by a single investigator. The order of the exercise treatment and the no-exercise control treatment were counterbalanced, and children or parents were not given advance information concerning the order of treatments. Laboratory personnel were unaware of children’s assignment to treatment conditions. Sessions were separated by an average of 5.5 days (SD =1.2; range = 4–8 days) to avoid possible carryover effects of the exercise protocol. Tests were conducted at the same time of day for each participant and each child was tested individually.
The Switch Task Protocol
The cognitive task was modeled after one developed by Cepeda et al. (2000). Children were seated in front of a laptop computer and instructed to respond to four possible stimulus conditions presented on the computer monitor by depressing a mouse response key. On each trial, either a single digit (1 or 3) or three digits (1 1 1 or 3 3 3) were presented at the center of the screen. The words, “What number?” or “How many?” appeared above each digit stimulus. Under the “What number?” condition, the child was asked to press the right response key if the number 1 (or 1 1 1) was presented and the left key if the number 3 (or 3 3 3) was presented. Under the “How many?” condition, the child was instructed to depress the right response key if one number (1 or 3) was presented and to depress the left key if three numbers (1 1 1 or 3 3 3) were presented. Thus, two response-mapping conditions were constituted: For the “What number?” condition, the number 1 was compatible whereas the number 3 was incompatible; for the “How many?” condition, 3 3 3 was compatible and 1 1 1 was incompatible.
Pretreatment testing began with a description of the task, which was presented on the computer screen and read aloud to the child by the investigator. A series of 20 practice trials under the “What Number?” condition was provided, followed by a block of 24 trials. A series of 20 practice trials under the “How Many?” condition was then provided, followed by a block of 24 “How Many?” trials. Each child’s response times during the two 24-trial blocks were averaged and constituted a measure of his or her baseline performance under no-switch conditions. The third test block contained 72 trials in which the “What Number?” and “How Many?” trials alternated after every second trial (Switch Block). Trials were subject paced and the interval between a response and the next trial varied randomly between 300 and 600 ms. An auditory tone was present following response errors. Children completed the pretreatment test in approximately 11 min. Posttreatment testing consisted of a single 72-trial switch block, which was completed in approximately 5 min. To reduce practice effects, two unique pre-treatment and posttreatment tests were created and assigned to children in a counterbalanced order. The cognitive task was created using SuperLab Version 2.2 (Cedrus Corp., San Pedro, CA) software that ensured 1-ms accuracy of recorded response times. Response times were omitted from analysis if the response was incorrect, less than 150 ms, or greater than 3 standard deviations from the mean for a block of trials. Global switch costs were calculated for each of four trial conditions: switch-compatible, switch-incompatible, no-switch-compatible, and no-switch-incompatible. Global switch costs were derived by subtracting each child’s average response time during switch blocks from his or her average response time during baseline, no-switch blocks. Global switch costs were selected for analyses in the current study rather than local switch costs, which are derived by subtracting average response times on switch trials from average response times on no-switch trials during a block of trials in which switching is required. A recent, large-scale study provides evidence that global switch cost measures are more sensitive to children’s development than are local switch costs (Reimers & Maylor, 2005). The conclusions drawn by these investigators were supported in the current study. Preliminary analysis of local-switch cost scores revealed considerable intrasubject variability, which remained following applications of techniques suggested by Salthouse and Hedden (2002) and Bush, Hess, and Wolford (1993) to reduce response-time variability (e.g., median score and z-score transformation).
The Physical Activity Bout
Immediately following administration of the cognitive test, the child was taken to the exercise laboratory and provided test instructions. Exercise was performed on a SensorMedic (Model #2000) motorized treadmill. All children, as part of the larger experiment, had prior experience walking on the treadmill. The exercise protocol began with a 4-min warm-up period during which the child walked at 2.5 mph and 0% grade. The treadmill speed was increased to 3 mph and the grade was elevated to 3% for 15 min. The 23-min protocol terminated following a 4 min cool-down period during which the child’s walking speed was reduced to 2.5 mph on a level grade. The investigator provided verbal encouragement throughout the exercise period. The intensity and duration of the treadmill exercise protocol were selected on the basis of previous studies conducted to assess the effects of acute bouts of exercise on children’s cognitive function (Tantillo, Kesick, Hynd, & Dishman, 2002; Zervas et al., 1991) and on pilot testing conducted with overweight children. The exercise bout was designed to ensure that the physical activity demands were sufficient to elicit an aerobic response from children who engage in limited physical activity. All children completed the protocol. Water was made available after the exercise period.
The Control Condition Protocol
Immediately following administration of the cognitive test, children remained in the cognitive testing laboratory and viewed “Standard Deviants: Learned Nutrition,” an age-appropriate health and nutrition DVD (Cerebellum Corporation, 2004). The video was presented for 23 min. Immediately following the video and water break (if requested), the child completed the second cognitive test. The time between the termination of exercise and video interventions and the beginning of cognitive testing ranged between 1 and 3 min.
Statistical Analyses
Separate mixed-model ANOVAs were used to assess the effects of an acute bout of exercise on global-switch cost scores and on the proportion of response errors. The model employed was a 2 (Conditions: Exercise; Video) × 2 (Age: 7–8 years; 9–11 years) × 2 (Time: Pre; Post) × 4 (Tasks: Switch-Compatible; Switch-Incompatible; No-switch-Compatible; No-switch-Incompatible) ANOVA in which age was a between-subjects factor and conditions and time and tasks were within-subject factors. For all ANOVAs, post hoc analyses were conducted for significant main effects and relevant interactions, and adjusted for multiple comparisons. Effect sizes expressed as partial eta-square (ηp2) are reported.
Results
Global Switch Costs
Analyses of global switch costs, which are summarized in Table 1, revealed a main effect for the task factor, indicating that children’s performance differed among the four task conditions, F(3,201) = 27.95, p < .001, η2 = .29, with significantly smaller switch costs on non-switch-compatible trials than on the switch-compatible trials and smaller switch costs on non-switch-incompatible trials than on switch-incompatible trials. An antecedent bout of exercise or the presentation of a video before cognitive testing did not differentially influence children’s task-switching performance (See Table 2). Separate statistical analyses that included children’s exercise training assignment and cohort as factors revealed that neither factor interacted with variables of interest. The results of separate statistical analyses in which the scores of six children diagnosed with ADHD were excluded did not differ substantively from statistical outcomes reported. The performance of younger children differed significantly from that of older children, F(1, 67) = 6.13, p < .05, ηp2 = .08. The time taken by children between 7 and 8 years of age to switch from one task to another was slower than the time taken by children between 9 and 11 years of age.
Table 1.
Global Switch Costs and Error Rates Before and Following Exercise and Nonexercise Treatment
| Test Conditions |
|||||
|---|---|---|---|---|---|
| Exercise |
Video |
||||
| Group | Task Condition | Pretest | Posttest | Pretest | Posttest |
| Switch Costs | |||||
| Younga | Switch Compatible | 1111 (139) | 1188 (175) | 1152 (113) | 1083 (107) |
| Switch Incompatible | 1198 (115) | 1225 (166) | 1334 (109) | 1414 (107) | |
| Non-Switch Compatible | 982 (102) | 842 (96) | 1004 (102) | 836.94 (94) | |
| Non-Switch Incompatible | 1113 (101) | 906 (122) | 1329 (115) | 1077 (101) | |
| Olderb | Switch Compatible | 1094 (127) | 922 (160) | 820 (104) | 794 (98) |
| Switch Incompatible | 1126 (106) | 1112 (152) | 876 (100) | 713 (98) | |
| Non-Switch Compatible | 791 (93) | 731 (88) | 688 (93) | 639 (87) | |
| Non-Switch Incompatible | 977 (93) | 941 (112) | 834 (106) | 777 (92) | |
| Error Percentage | |||||
| Young | Switch Compatible | 6.9 (1.9) | 8.0 (1.4) | 5.6 (1.7) | 5.4 (1.6) |
| Switch Incompatible | 16.1 (2.4) | 12.9 (2.6) | 9.6 (1.9) | 9.7 (1.6) | |
| Non-Switch Compatible | 5.9 (1.7) | 3.9 (1.2) | 5.0 (1.8) | 3.7 (1.3) | |
| Non-Switch Incompatible | 16.5 (2.3) | 11.2 (2.4) | 12.8 (2.0) | 8.7 (2.0) | |
| Older | Switch Compatible | 11.4 (1.7) | 4.6 (1.3) | 10.0 (1.5) | 7.7 (1.5) |
| Switch Incompatible | 12.1 (2.2) | 9.4 (2.4) | 16.3 (1.8) | 11.0 (1.4) | |
| Non-Switch Compatible | 5.0 (1.6) | 3.0 (1.1) | 8.1 (1.7) | 3.6 (1.2) | |
| Non-Switch Incompatible | 15.7 (2.1) | 6.8 (2.2) | 12.4 (1.8) | 9.2 (1.9) | |
Note. Switch cost data presented in mean milliseconds (SE)
7–8 year olds (n = 32).
9–10 year olds (n = 37).
Table 2.
Response Times Before and Following Exercise and Nonexercise Treatment
| Test Conditions |
|||||
|---|---|---|---|---|---|
| Exercise |
Video |
||||
| Group | Task Condition | Pretest | Posttest | Pretest | Posttest |
| Younga | Switch Compatible | 2224 (135) | 2329 (180) | 2228(112) | 2210 (143) |
| Switch Incompatible | 2338(121) | 2393 (164) | 2410(110) | 2549(115) | |
| Non-Switch Compatible | 2064 (104) | 1955 (101) | 2047 (99) | 1948 (98) | |
| Non-Switch Incompatible | 2205 (106) | 2025 (123) | 2409 (123) | 2204 (105) | |
| Olderb | Switch Compatible | 2012(122) | 1822 (162) | 1788(101) | 1726 (102) |
| Switch Incompatible | 2041 (109) | 1976 (148) | 1846 (99) | 1672(104) | |
| Non-Switch Compatible | 1692(95) | 1620(91) | 1632 (89) | 1585 (88) | |
| Non-Switch Incompatible | 1923 (96) | 1823(111) | 1814(111) | 1697 (95) | |
Note. Response time data presented in mean milliseconds (SE)
7–8 year olds (n = 32).
9–10 year olds (n = 37).
Response Errors
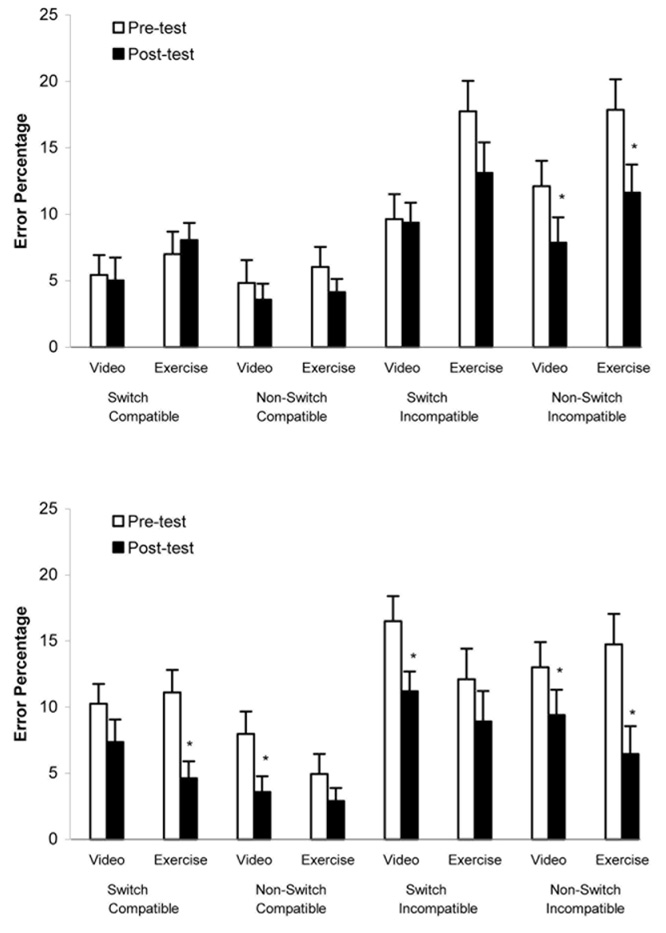
Analyses of children’s response errors, which are summarized in Table 1, revealed that the percentage of errors made by children did not differ as a function of age. There was, however, a significant Condition × Age × Time × Task interaction, F(3, 201) = 2.95, p < .05, ηp2 = .04. Separate ANOVAs were conducted on children’s error scores made during the exercise session and the video-control session. Analysis of children’s performance before and following exercise revealed a significant interaction between time and task type, F(3, 201) = 3.52, p < .05, η2 =.05; results of paired t tests used to compare before and after scores for each task type indicated that error scores decreased significantly for all task types. Analysis of children’s performance during the video condition revealed only a significant effect for time, F(1, 67) = 18.4, p < .001, η2 = .21. As seen in Figures 1a and 1b, young and older children made fewer errors following both exercise and video-control conditions; further, they made significantly more errors on incompatible-stimulus trials than on compatible-stimulus trials (p < .001).
Figure 1. Mean percentage of errors (+SE) by task type and condition.
Figure 1a describes the performance of children 7–8 years of age (n = 32); Figure 1b describes the performance of children 9–11 years of age (n = 37). *p < .05 (pretest-to-posttest comparisons).
Discussion
A brief bout of moderately intense physical activity was hypothesized to facilitate children’s executive functioning as measured by reduction in the processing time required to switch performance of one task to another. Evaluation of children’s global switch-cost scores showed the expected slowing of response time on trials when task requirements were altered; however, there was no evidence that antecedent physical activity influenced children’s test performance. Global switch costs allegedly reflect both the capacity to maintain in working memory those actions required to be performed as task sets change compared with conditions in which task set does not change (Kray & Lindenberger, 2000) and the ability to inhibit incorrect motoric responses (Hillman, Kramer, Belopolsky, & Smith, 2006). It remains to be determined whether the short-term aftereffects of physical activity influence all or only select components of executive functioning. Several studies have failed to detect changes in switch-task performance following acute bouts of exercise. Tomporowski and Ganio (2006) reported that young adults’ switch-task response-time performance following 40 min of ergometer cycling at 60% percent maximal capacity did not differ from performance following a period of quiet rest. A systematic replication of this study revealed that young adults’ switch-task performance measured in terms of local switch costs did not differ following 40 min of cycling or two nonexercise control conditions (Coles & Tomporowski, 2007). Similarly, Kubesch et al. (2003) employed a battery of executive functioning tests to assess the effects of 30 min of ergometer cycling at 40% or 60% of their 4-mmol/L lactic acid workload level on clinically depressed and nondepressed men. Exercise had no effect on participants’ task-switching performance but it did facilitate depressed men’s performance on tests that measured response inhibition (Stroop, and go/no-go test paradigms).
There is a general consensus among researchers that executive function is not a unitary process; rather, it reflects a number of more elemental underlying processes. Purported component processes include updating, which is closely linked to working memory and the need to monitor its representation; inhibition, which involves the deliberate suppression of a prepotent response; and switching, which requires individuals to disengage the processing operations of an irrelevant task and to engage operations involved in a relevant task (Miyake & Shah, 1999). Specific neurological systems have been implicated in the component processes that underlie executive functioning among adults (Miyake et al., 2000). A theory-based review of studies that assessed the effects of acute bouts of exercise on cognition proposed that on simple tasks, with predictable stimulus-response mappings, exercise primes individuals to respond to incoming sensory information and increases response speed without reductions in accuracy (Tomporowski, 2003b). It is clear that additional research designed to isolate specific executive processes will be required to determine how physical activity affects the components of executive processing.
Physical activity did not differentially influence young and older children’s switch-task performance. However, consistent with results of studies that have evaluated age-related differences in executive functioning (Cepeda, Kramer, & Gonzalez de Sather, 2001; Davidson et al., 2006; Reimers & Maylor, 2005), the task-switching performance of 9- to 10-year-old children was significantly faster than that of 7- to 8-year-old children. Behavioral changes in processing speed have been linked to the maturation of cortical areas that underlie executive functioning (Casey et al., 1997). Children’s response errors did not differ as a function of age, however. Davidson et al.’s (2006) evaluation of age-related changes in task-switching performance led them to conclude that response strategies change with development and over the life span. Adults, compared with children, select speed–accuracy tradeoff strategies that result in low error rates, seldom exceeding error rates of 2%. The few studies that have assessed children’s switch-task error rates report them to be considerably higher than those of adults (Cepeda et al., 2000; Kramer et al., 2001). The frequency and pattern of errors made by children in the current study were similar to those previously reported. Children most often produced errors when presented incompatible stimulus conditions, regardless whether the incompatible stimulus occurred on a switch or nonswitch trial. These findings support the view that children, when compared with adults, have difficulty resisting impulses and controlling motoric responses (Cepeda et al., 2001). Evaluation of children’s speed–accuracy tradeoff may provide researchers with an opportunity to assess the impact of both acute and chronic exercise interventions on the development of response strategies.
Several researchers have proposed that acute bouts of physical activity influence brain systems via the activation of neurotransmitters and that the effects are similar to those produced by stimulant drugs (Davranche & Audiffren, 2004; Tomporowski, 2003b). The results obtained by Kramer et al. (2001) provide compelling evidence that methylphenidate facilitates task-switching processing in children diagnosed with ADHD. Further, Cepeda et al. (2000) observed that the switch costs of children with ADHD decreased to a level comparable to those of children without ADHD following administration of clinically prescribed medication. The task used in both of these studies was the same used in the current study. One interpretation for the lack of consistency among studies is that neurological changes that occur during and immediately following physical activity do not mimic those produced by psychostimulant medication. There are alternative explanations, however. Children with attention disorders may be particularly sensitive to the effects of interventions that regulate neural regulatory systems. It is unknown whether psychostimulant drugs facilitate task switching in children who do not have attentional deficits. In addition, children who are not overweight may respond to bouts of physical activity differently than their leaner peers.
There are factors that limit the interpretation of the results obtained in the present experiment. First, the children who participated were overweight (body mass index greater than the 85th percentile for age and sex). It is plausible that the exercise stimulus may have been experienced differently by overweight and inactive children than it would have been by leaner children. Little is known of the role that postexercise affective responses have on subsequent cognitive performance; however, evidence obtained with young adults suggests that that individuals who experience positive affect following a bout of exercise alter their perceptions concerning the mental demands of cognitive tasks (Tomporowski & Ganio, 2006). In addition, physiological responses of the children during and following treadmill walking were not assessed and it is possible that changes in arousal levels were not ideal for altering cognitive function. The intensity and duration of the exercise stimulus was similar to other studies that have evaluate the effects of an acute bout of exercise on cognitive function (Tantillo et al., 2002; Zervas et al., 1991) and all children completed the treadmill protocol. Based on the inverted-U hypothesis, which predicts that cognitive functioning will be enhanced with moderate, but not high, levels of arousal (McMorris & Graydon, 2000), the exercise demands placed on children in the current study were predicted to facilitate switch-task performance. It should be recognized, however, that the acute exercise stimuli (i.e., type, intensity, and duration) selected for laboratory studies of children have been based primarily on research conducted with adults. Physical activity patterns differ between children and adults, with children engaging in briefer bouts of activity than adults (Trost, Kerr, Ward, & Pate, 2001). It remains to be determined whether the physiological and psychological responses elicited in children by adultlike exercise protocols provide a satisfactory basis for assessing the effects of acute exercise on their cognitive function.
Considerable progress has been made recently in verifying linkages between physical activity training and cognition, particularly executive function, in older adults (Colcombe & Kramer, 2003), adults (Pereira et al., 2007), and children (Tomporowski et al., 2007). Challenges facing exercise psychologists include determining how acute bouts of physical activity performed over multiple sessions contribute to changes in cognitive function, the time course of those changes, and whether the selective effects of acute bouts of exercise are predictive of specific changes in cognition brought about by long-term training interventions.
References
- Brisswalter JB, Collardeau M, Arcelin R. Effects of acute physical exercise on cognitive performance. Sports Medicine (Auckland, N.Z.) 2002;32:555–566. doi: 10.2165/00007256-200232090-00002. [DOI] [PubMed] [Google Scholar]
- Bush LK, Hess U, Wolford G. Transformation for within-subject designs: A Monte Carlo investigation. Psychological Bulletin. 1993;113(3):566–579. doi: 10.1037/0033-2909.113.3.566. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Caterino MC, Polak ED. Effects of two types of activity on the performance of second, third, and fourth-grade students on a test of concentration. Perceptual and Motor Skills. 1999;89:245–248. doi: 10.2466/pms.1999.89.1.245. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Cepeda ML, Kramer AF. Task switching and attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2000;28:213–226. doi: 10.1023/a:1005143419092. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JCM. Changes in executive control across the life span: examination of task-switching performance. Developmental Psychology. 2001;37:715–730. [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Coles K, Tomporowski PD. Bouts of exercise influence select executive function and memory processes. Journal of Sports Sciences. 2007;26(3):333–344. doi: 10.1080/02640410701591417. [DOI] [PubMed] [Google Scholar]
- Datar A, Sturm R, Magnabosco JL. Childhood overweight and academic performance: national study of kindergartners and first-graders. Obesity Research. 2004;12:58–68. doi: 10.1038/oby.2004.9. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive function from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, Boyle CA, Waller JL, Miller PH, Naglieri JA, et al. Effects of aerobic exercise on overweight children’s cognitive functioning: A randomized controlled trial. Research Quarterly for Exercise and Sport. 2007;78(5):510–519. doi: 10.1080/02701367.2007.10599450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davranche K, Audiffren M. Facilitating effects of exercise on information processing. Journal of Sports Sciences. 2004;22:419–428. doi: 10.1080/02640410410001675289. [DOI] [PubMed] [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development. 2000;71(1):44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Gabbard C, Barton J. Effects of physical activity on mathematical computation among young children. The Journal of Psychology. 1979;103:287–288. [Google Scholar]
- Hasselmo ME, Linster C. Acetylcholine and frontal cortex “signal-to-noise ratio”. In: Miller BL, Cummings JL, editors. The human frontal lobes: Functions and disorders. New York: The Guilford Press; 1999. pp. 139–158. [Google Scholar]
- Hillman CH, Kramer AF, Belopolsky AV, Smith DP. A cross-sectional examination of age and physical activity on performance and event-related potentials in a task switching paradigm. International Journal of Psychophysiology. 2006;59:30–39. doi: 10.1016/j.ijpsycho.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kempton S, Vance A, Maruff E, Luk E, Costin J, Pantelis C. Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychological Medicine. 1999;29:527–538. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Cepeda NJ, Cepeda ML. Methylphenidate effects on task-switching performance in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(11):1277–1284. doi: 10.1097/00004583-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychology and Aging. 2000;15(1):126–147. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- Kubesch S, Bretschneider V, Freudenmann R, Weidenhammer N, Lehmann M, Spitzer M, et al. Aerobic endurance exercise improves executive functions in depressed patients. The Journal of Clinical Psychiatry. 2003;64:1005–1012. doi: 10.4088/jcp.v64n0905. [DOI] [PubMed] [Google Scholar]
- McMorris T, Graydon J. The effect of incremental exercise on cognitive performance. International Journal of Sport Psychology. 2000;31:66–81. [Google Scholar]
- McNaughten D, Gabbard C. Physical exertion and the immediate mental performance of sixth-grade children. Perceptual and Motor Skills. 1993;77:1159. doi: 10.2466/pms.1993.77.3f.1155. [DOI] [PubMed] [Google Scholar]
- Meiran N, Chorev Z, Sapir A. Component process in task switching. Cognitive Psychology. 2000;41:211–253. doi: 10.1006/cogp.2000.0736. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Miyake A, Shah P. Toward unified theories of working memory. In: Miyake A, Shah P, editors. Models of working memory. Cambridge, UK: Cambridge University Press; 1999. pp. 442–481. [Google Scholar]
- Naglieri JA, Das JP. Cognitive assessment system. Itasca, IL: Riverside Publishing; 1997. [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Journal of the American Medical Association. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv S, Low M. Influences of physical activity on concentration among junior and high-school students. Perceptual and Motor Skills. 1990;70:67–74. doi: 10.2466/pms.1990.70.1.67. [DOI] [PubMed] [Google Scholar]
- Reimers S, Maylor EA. Task switching across the life span: effects of age on general and specific switch costs. Developmental Psychology. 2005;41:661–671. doi: 10.1037/0012-1649.41.4.661. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biological Psychology. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Rogers DR, Monsell S. Costs of a predictable switch between simple tasks. Journal of Experimental Psychology. General. 1995;124:207–231. [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK. The development of executive attention: Contributions to the emergence of self-regulation. Developmental Neuropsychology. 2005;28:573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Hedden T. Interpreting reaction time measures in between-group comparisons. Journal of Clinical and Experimental Neuropsychology. 2002;24:858–872. doi: 10.1076/jcen.24.7.858.8392. [DOI] [PubMed] [Google Scholar]
- Tantillo M, Kesick CM, Hynd GW, Dishman RK. The effects of exercise on children with attention-deficit hyperactivity disorder. Medicine and Science in Sports and Exercise. 2002;34:203–212. doi: 10.1097/00005768-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Taras H, Potts-Datema W. Obesity and student performance at school. The Journal of School Health. 2005;75(8):291–295. doi: 10.1111/j.1746-1561.2005.00040.x. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD. Cognitive and behavioral responses to acute exercise in youth: a review. Pediatric Exercise Science. 2003a;15:348–359. [Google Scholar]
- Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychologica. 2003b;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD. Physical activity, cognition, and aging: A review of reviews. In: Poon LW, Chodzko-Zajko WJ, Tomporowski PD, editors. Active living, cognitive functioning, and aging. Champaign, IL: Human Kinetics; 2006. pp. 15–32. [Google Scholar]
- Tomporowski PD, Davis CL, Miller PH, Naglieri JA. Exercise and children’s intelligence, cognition, and academic achievement. Educational Psychology Review. 2007 doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomporowski PD, Ganio MS. Short-term effects of aerobic exercise on executive processing, memory, and emotional reactivity. International Journal of Sport and Exercise Psychology. 2006;4:57–72. [Google Scholar]
- Trost S, Kerr L, Ward DS, Pate RR. Physical activity and deteminants of pshyical activity in obese and non-obese children. International Journal of Obesity. 2001;25(6):822–829. doi: 10.1038/sj.ijo.0801621. [DOI] [PubMed] [Google Scholar]
- Vandierendonck A. Executive functions and task switching. Psychologica Belgica. 2000;40(4):211–226. [Google Scholar]
- Zervas Y, Apostolos D, Klissouras V. Influence of physical exertion on mental performance with reference to training. Perceptual and Motor Skills. 1991;72:1215–1221. doi: 10.2466/pms.1991.72.3c.1215. [DOI] [PubMed] [Google Scholar]