Synopsis
Diastolic dysfunction is characterized by prolonged relaxation, increase filling pressure, decreased contraction velocity, and reduced cardiac output. Phenotypical features of diastolic dysfunction can be observed at the level of the isolated myocyte. A slower relaxation rate is one of the primary underlying causes of the manifestation of this disabling syndrome. We here review the cellular mechanism that control relaxation at the level of the myocyte in the healthy situation, and discuss the alterations that can impact on physiological function during disease. We will specifically focus on the mechanisms that regulate intracellular calcium handling, and the response of the myofilaments to calcium, including the changes in these components that can contribute to diastolic dysfunction.
Keywords: Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA), Na+/Ca2+ exchanger (NCX), Phospholamban (PLB), Ryanodine receptor(RyR2), Heart Failure, Calcium transport, Ca2+ sensitivity, extracellular matrix, Cytoskeleton, Ca2+ leak and contractile proteins
Introduction
Heart failure is a complex syndrome in which both systolic and diastolic functional abnormalities can be identified. In patients with systolic dysfunction the primary abnormality is a reduction in contractile reserve with poor pumping function of the left ventricle; whereas diastolic dysfunction is associated with impaired relaxation and inability of the ventricle to accept adequate volume of blood. Diastolic dysfunction is characterized by prolonged relaxation, increase filling pressure, decreased contraction velocity, decreased responsiveness to β-adrenergic stimulation, and reduced cardiac output. It has become increasingly clear that diastolic dysfunction is predominant in HF patients and to some extent it is present in patients with systolic HF. Often these two conditions co-exist and are not easy to separate as they may contribute to each other. Diastolic dysfunction can cause serious problems including progression into congestive heart failure as a result of fluid retention in the lung, kidney, and veins. Currently, other than treating symptoms of these patients there are no effective treatment options. Despite significant advances in our ability to diagnose functional abnormalities, there is only limited progress towards understanding the mechanisms that control muscle relaxation and there is much controversy over to what controls abnormal relaxation/diastolic dysfunction1–3. The focus of this review is to evaluate current knowledge and recent developments in our understanding of mechanisms that regulate cardiac muscle relaxation at the single cell level as well as derangements in these processes.
What causes abnormal muscle relaxation?
It has been long recognized that both structural and biochemical changes within the myocytes are responsible for impaired relaxation of the ventricle. However, it has been very challenging to pinpoint which of these cause diastolic dysfunction and which are actually compensatory factors that are adaptive in failing muscle. The heart is a complex organ both in terms of architecture and geometry. There are multitudes of differences both in architecture and composition between endocardium and epicardium, right vs. left ventricular muscle. Therefore, it is often difficult to state that changes in structure and or function occur in both chambers uniformly during cardiac remodeling. Most often diastolic dysfunction is associated with concentric cardiac hypertrophy and stiffening of ventricular muscle; inability of the muscle fibers to return to normal length and poor ventricular filling under low pressure4. Although impaired filling can result from any number of anatomical or organ-level abnormalities that delay filling, this review will focus on intrinsic abnormalities within the myocytes as a result of hypertrophy induced remodeling.
1. The role of sarcoplasmic reticulum Ca2+ transport in muscle relaxation
The sarcoplasmic reticulum (SR) Ca2+ transport plays a central role in regulating both cardiac muscle contraction and relaxation. The cardiac SR is an intracellular membrane network that surrounds the contractile machinery; it serves not only as a Ca2+ store for Ca2+ release but also actively maintains cytosolic Ca2+ concentration during contraction-relaxation of the muscle 5–7. SR function is coordinated by a set of Ca2+ handling proteins localized in the T–tubule and SR membrane. These proteins can be classified as involved in 1) Ca2+ release, 2) Ca2+ uptake, and 3) Ca2+ storage8–10. During excitation contraction-coupling, Ca2+ entry through the L-type Ca2+ channel activates Ca2+ release from the SR Ca2+ stores via the ryanodine receptor, RYR2 5, 6, 8, 10. This raises cytosolic Ca2+ and initiates muscle contraction, and the free cytosolic Ca2+ concentration co-determines the extent of muscle contraction and therefore force development. Subsequent removal of cytosolic Ca2+ by the sarco(endo)plasmic reticulum Ca2+ ATPase pump (SERCA2a) and Na+/Ca2+ exchanger (NCX) results in deactivation of the contractile apparatus, allowing for muscle relaxation. The rate of muscle relaxation is influenced by the rate of Ca2+ removal and or SERCA2a pump activity10–13. In the heart, SERCA pump activity is regulated by two small molecular weight proteins: Phospholamban 13–19 and sarcolipin 14, 16, 19, 20. In this review we will discuss how alterations in SERCA pump expression and activity affect muscle function in particular relaxation.
2. Dynamic regulation of SERCA pump expression and its role in muscle contractility
SERCA2a is the major isoform expressed in the adult atria and ventricles, while its alternate isoform, SERCA2b, is expressed at low levels in the heart at all stages9, 12, 21, 22. The SERCA pump is localized in the longitudinal SR and represents the most abundant protein in the SR membrane representing about 40% of total SR protein. The expression levels of SERCA2a are not uniform throughout the heart. There are chamber specific differences in the expression levels of SERCA pump. In rodents, SERCA protein level is ~ 2-fold higher in atria compared to the ventricle 23, and the higher SERCA pump levels may contribute at least in part to the shorter duration of contraction and faster rates of relaxation in atrial versus ventricular tissue 24. During cardiac development, the expression of SERCA2a pump gradually increases 25–27 and this correlates with a shortening of relaxation time in adult versus neonatal ventricle 28. It has been well documented that changes in thyroid hormone levels modify SERCA levels, Ca2+ transport and cardiac muscle contractility. Induction of hypothyroidism in animals causes a decrease in SERCA2a protein but in an increase in PLB, whereas hyperthyroidism increases SERCA2a levels but reduces PLB expression 27, 29–31. The increases in SERCA expression are consistent with increased velocity of Ca2+ uptake and enhanced rates of contraction and relaxation observed in hyperthyroidism in adult hearts 32, whereas hypothyroidism produced opposite changes. Interestingly, there is a very close correlation between the Ca2+ dependence of Ca2+ uptake and the ratio of PLB to SERCA in hypothyroid, euthyroid, and hyperthyroid hearts, and this determines the contractile parameters of the heart 12, 32. These data indicate that a change in PLB to SERCA ratio can affect 1) SR Ca2+ uptake rates, and 2) the affinity of SERCA pump for Ca2+, and thereby influencing to the speed of muscle relaxation. Therefore, an increase in PLB could adversely affect both contractility and relaxation. A decrease in content and activity of SERCA was reported with ageing both in animal models and in senescent human myocardium 26, 33, 34 and this decrease was associated with a prolonged contraction/relaxation time and depressed myocardial function.
3. Regulation of SERCA pump activity by the phosphoprotein Phospholamban
Phospholamban (PLB) is a 52 amino acid phosphoprotein and interacts with the SERCA pump in a dynamic manner during the contraction relaxation cycle of the heart. Importantly, PLB is responsible for mediating the β-adrenergic responses in the heart. It has been well documented that phosphorylation of PLB at Ser 16 and Thr17 by kinases PKA and CAMKII respectively can increase SERCA pump activity 13, 14, 17, 35–37, Ca2+ transport, and correlates with improved speed of muscle relaxation. Studies have shown that unphosphorylated PLB acts as an inhibitor (brake) on the SR Ca2+ ATPase and that phosphorylation releases inhibition and induces substantial increase in calcium transport to levels up to four fold or greater 13, 14, 35, 36. Thus, regulation of the SERCA pump by PLB is considered to be the primary mechanism for β-adrenergic mediated response of the heart, as well for enhanced Ca2+ transport by the SR. In addition, the mechanism of PLB action on SERCA and its relevance in cardiac muscle physiology has been studied extensively using genetically altered mouse models. By generating a PLB null mouse model 38 Dr. Kranias and colleagues provided the crucial evidence that PLB is an important regulator of the SERCA2a; absence of PLB enhanced SR calcium uptake and increased rates of contraction and relaxation. These studies discovered that PLB affects the pump affinity for Ca2+ and loss of PLB resulted in an increase in SERCA2a affinity for calcium 38. In contrast, over-expression of PLB in the heart resulted in a decrease in SR Ca2+ uptake and depressed cardiac contractile performance in vivo 39. These studies further demonstrated that an alteration in the PLB-to-SERCA ratio can affect SR Ca2+ transport and has profound effects on myocardial contractility.
4. Abnormal calcium homeostasis and diastolic dysfunction in failing hearts
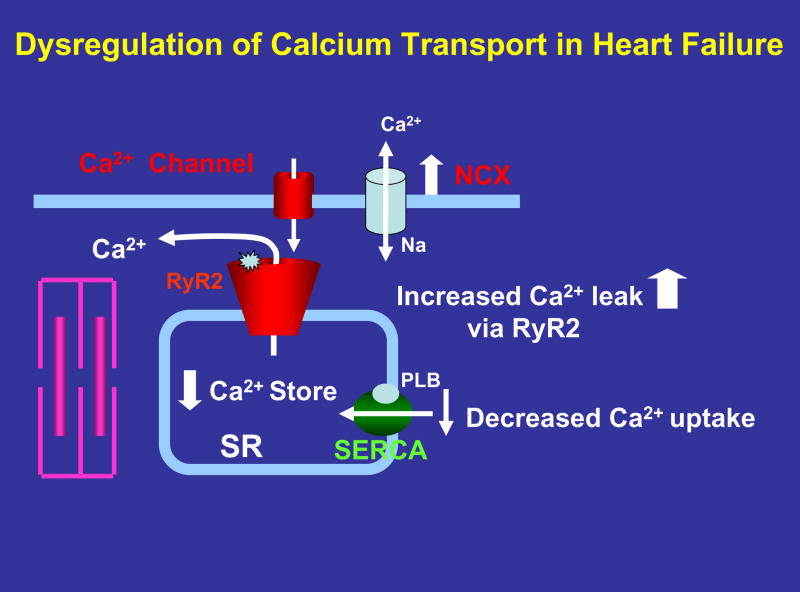
Contractile dysfunction in end-stage human heart failure has been attributed to both depressed myofilament Ca2+ sensitivity and as well altered calcium handling. In particular Ca2+ transients are decreased 40, 41. Smaller amplitude of Ca2+ transients could be due to either lower fractional SR Ca2+ release or lower SR Ca2+ content. A decreased Ca2+ store could be due to reduced SR Ca2+ uptake, or increased Ca2+ leak during diastole42 ( Figure. 1 ). Decreases in SR Ca2+-ATPase gene and protein expression levels have been described in animal models of heart failure and failing human myocardium 40–49. In addition, increased activity and gene expression of the sarcolemmal Na+/Ca2+ exchanger were reported in failing human myocardium and in animal models of heart failure 44, 45, 48–50. A reduction in SERCA and an increase in Na+/Ca2+ exchanger are expected to facilitate Ca2+ removal from the cell at the expense of its uptake into the SR, resulting in decreased SR Ca2+ levels ( Figure. 1 ). Studies document that a decrease in SERCA pump activity and Ca2+ uptake are seen in many forms of heart failure regardless of etiology of the disease. In addition, Pieske et al 51 demonstrated a direct correlation between depressed SR Ca2+-ATPase expression and depressed force-frequency response in failing myocardium by studying left ventricular trabeculae isolated from end stage failing heart. They further showed that in failing myocardium the increase in SR Ca2+ content at high frequency was only about 10% of that in non-failing myocardium and suggested that a decrease in SR Ca2+ transport at high frequency is likely responsible for a blunted or negative force-frequency relation.
Figure 1.
Potential contributors to Ca2+ dysregulation in failing heart muscle. A decreased expression and activity of SERCA pump could explain slowed rate of Ca2+ uptake and prolongation of muscle relaxation. Enhanced NCX expression and activity could increase Ca2+ efflux and compete with SERCA pump, reducing SR Ca2+ stores. RyR2 phosphorylation by PKA or CaMKII causes increased opening and Ca2+ leak, further contributing to loss of Ca2+. A net loss of Ca2+ could contribute to both systolic and diastolic dysfunction.
Another potential cause of reduced SR Ca2+ content is enhanced diastolic leak of Ca2+ via the ryanodine receptors (RyRs) 52, 53. Recent studies by Marks and coworkers 54, 55 have suggested that RyR phosphorylation by PKA results in dissociation of FKBP12.6 from RyRs, rendering the channels hyperactive and spontaneous release of calcium in failing hearts. This phenomena has been described as diastolic Ca2+ leak ( Figure. 1 ). Other laboratories have contradicted this finding and did not find any evidence for phosphorylation-induced dissociation of FKBP12.6 from RyRs in normal or diseased hearts56–58. Thus, further studies are needed to determine whether the abnormal RyR modulation by luminal Ca2+ is caused by an altered interaction with FKBP12.6. There are some recent studies which suggest Ca2+ leak might occur in heart failure but through entirely different mechanisms involving luminal Ca2+ ion concentration 59. In a recent study, Guo et al 60 suggested that CaMKII-dependent phosphorylation of RyR by endogenous CaMKII (but not PKA-dependent phosphorylation) increases resting SR Ca2+ release or leak and is likely responsible for enhanced SR diastolic Ca2+ leak and certain triggered arrhythmias seen in heart failure. In addition, the quantitative effect of SR Ca2+ leak remains incompletely understood. Each heart beat, the SR is triggered to release a substantial amount of Ca2+ via the RyR, which gets taken back up via the SERCA pumps. It remains unknown whether a diastolic Ca2+ leak that is typically much smaller than a triggered release can actually functionally deplete the SR, and thus a physiological relevance of a diastolic calcium leak has not yet been quantified. In summary, although there is some evidence that diastolic calcium leak can occur, and potentially contribute to impairment of relaxation, the underlying mechanisms responsible for RYR mediated SR Ca2+ leak and its functional relevance in heart failure remains to be fully understood.
5. SERCA pump level impacts both muscle contraction and relaxation rates
To understand the significance of alterations in SERCA pump expression on myocardial contractility, transgenic (TG) mouse and rat models that express higher levels of SERCA pump were developed. Transgenic overexpression of SERCA2a or SERCA1a in the heart demonstrated that SERCA pump can be overexpressed in the heart resulting in an increased Ca2+ transport and contractility 11, 12, 61–64. Overexpression of the skeletal muscle isoform, SERCA1a in the mouse heart results in a net increase of SERCA pump to 2.5 fold compared to non-transgenic (NTG) control hearts. However, the endogenous SERCA2a pump levels in these mice decreased to 50% suggesting that there is a limit how much the SR can accommodate. Mice with higher levels of SERCA pump show normal in vivo heart function, and do not exhibit cardiac pathology. Overexpression of SERCA pump led to increased SR calcium transport which in turn increased the rates of cardiac contraction and relaxation 11, 12, 61–64. These studies further demonstrated that increased SERCA expression results in higher rates of contraction and relaxation suggesting that SERCA is a dominant player in SR Ca2+ uptake and could potentially be used to treat conditions such as heart failure where SERCA levels and/or activity is decreased.
To examine the effect of decreased SERCA expression a SERCA2 gene knockout mouse model was developed 65–67. Disruption of both alleles is lethal and the homozygous null (SERCA2−/−) mice die early in development; there was no compensatory upregulation of SERCA1a. The heterozygous (SERCA2−/+) mice did not show any evidence of cardiac pathology, albeit they had reduced SERCA2 levels (65% of WT hearts). These mice did not show any deficit in cardiac function and the hearts responded well to β-adrenergic stimulation 65, 66. It is likely that SERCA2−/+ mice have adapted to this new state since they were born with reduced SERCA level. However, when stressed with pressure overload, SERCA2−/+ mice developed heart failure much more rapidly than WT controls 68. Thus, these studies employing transgenic and gene knock out animal models proved that SERCA pump level is a critical determinant of SR Ca2+ uptake function and decreased SERCA expression can lead to cardiac dysfunction when stress is imposed 11.
6. Role of myofilaments proteins in diastolic dysfunction
Myofilament properties play a central role in the governing of cardiac relaxation. Although it is undisputed that the intracellular calcium concentration needs to decline to facilitate and initiate relaxation, the actual rate at which healthy myocardium relaxes is predominantly regulated by myofilament properties. The peak of the calcium transient amplitude is generally reached long before the peak of force development, and once force development starts to decline, the calcium concentration is already near or below the concentration where myofilament can be activated. The exact governing mechanisms of myocardial relaxation are however still not well understood. This incomplete grasp of the normal physiological relaxation process likely has significantly hampered the understanding of impaired relaxation. Insight into impaired relaxation that, at its basis, stems from a myofilament malfunction has come from specific mutations that have been found in various myofilament proteins in familial cardiomyopathies. Specific single amino-acid mutations in the myosin heavy chain 69, myosin binding protein C 70, troponinT 71 TroponinC 72, Tropomyosin 73 and other proteins have been implicated to ultimately cause or contribute to impairment of myocardial relaxation. Still, the mechanism via which these myofilament mutations lead to the various cardiomyopathies is largely unknown, and is subject to ongoing studies in many labs. Not only genetic alterations, but also post-translational modification of myofilament proteins can impair cardiac relaxation. One of the most investigated post-translational modifications of the myofilaments is TnI phosphorylation. Protein Kinase A mediated phosphorylation of Troponin-I (TnI) is the major determinant of the acceleration of relaxation that occurs under β-adrenergic stimulation 74. In addition, when heart rate increases, TnI appears also to become increasingly phosphorylated, via yet unknown mechanisms 75. Phosphorylation of TnI desensitizes the myofilaments for activator calcium76 and thereby facilitates accelerates relaxation. This acceleration of relaxation is essential, especially at higher heart rates. However, if the mechanisms responsible for phosphorylation or are impaired, or fail, the lack of acceleration of relaxation in combination with the high heart rate will cause incomplete relaxation, and likely manifest as diastolic dysfunction. When these sites are no longer available, for instance in mice expressing slow skeletal TnI in the heart 77, relaxation is hampered. Specifically under conditions where PKA-mediated phosphorylation of TnI is needed to desensitize the myocardium to allow for the prevailing higher heart rates that occur under β-adrenerigc stimulation, lack of these PKA sites hampers relaxation. These findings are confirmed in murine model/experiments where the PKA-sites of TnI are mutated to prevent phosphorylation, impairment of relaxation occurs 78. Besides TnI, several other myofilaments can undergo post-translational modifications. MyBPC, TnT, and MLC-2 can be phosphorylated which then changes their interaction with other proteins, resulting in altered relaxation kinetics. Thus, given the multitude of pathways that can result in myofilament protein phosphorylation or other post-translational modification, when the processes that either induce or prevent these modifications are impaired, the resulting altered function of these proteins can contribute to impaired contractile performance.
7. The role of cytoskeleton and extracellular matrix
In addition to alterations in calcium handling and myofilament properties, there are multitude of changes occurring in the failing myocyte, it is often difficult to distinguish whether these changes are causative or compensatory adaptations of the failing heart. Among structural alterations, changes in cardiac myocyte cytoskeleton and extracellular matrix and have been implicated as potential underlying causes of diastolic dysfunction79–82. Cooper and colleagues 3, 83, 84 have observed significant changes in microtubule architecture and advanced the hypothesis that changes in the microtubule network (increased network density in hypertrophied/failing myocytes) could contribute to altered viscosity and increased stiffness seen in hypertrophied myocardium; he argues that in these hypertrophied myocytes normal microtubule transport function is disturbed due to alterations in microtubule architecture and could potentially cause heart failure by limiting transport of key macromolecules including RNA and protein. Others have pointed out that changes in collagen expression (ratio of Collagen type 1 vs. Type III) and cross-linking may potentially play a role in relaxation impairment 81, 82. Similarly, changes in titin (responsible for elasticity of muscle stiffness) expression can be correlated with diastolic stiffening 85–89. However, currently there is limited data in human samples with regard to its relevance.
8. Therapeutic strategies to treat diastolic dysfunction
There are only limited therapeutic approaches to treat diastolic dysfunction and most of these are already in use to treat heart failure patients, albeit with limited success. Here we will discuss non pharmacological approaches to restore contractility of the heart using gene transfer methods. Recent studies were focused on restoring SERCA pump activity, either by adenoviral mediated gene transfer of SERCA2a or inhibiting PLB. Adenoviral gene transfer into cardiac myocytes showed that overexpression of SERCA2a in failing human ventricular myocytes increased Ca2+ transport and restored contraction and relaxation velocity 90. The negative frequency-response was normalized in cardiomyocytes overexpressing SERCA2a 90. Encouraged by these in vitro studies, Hajjar and colleagues developed a catheter-based technique to introduce genes into the myocardium 91–94. SERCA2a gene transfer in a rat model of pressure-overload hypertrophy (where SERCA2a levels were decreased and severe contractile dysfunction was evident) restored both systolic and diastolic dysfunction to normal levels. SERCA2a overexpression decreased left ventricular size and restored the slope of the end-diastolic pressure-dimension relationship to control levels. Interestingly, overexpression of SERCA2a in failing heart restored and normalized the levels of PCr and ATP and suggested that normalizing Ca2+ transport would improve energetics 91, 93, 94. PLB ablation was shown to prevent structural and functional abnormalities in mouse models 14, 95. Therefore, strategies to suppress PLB inhibition and enhance SERCA activity have been tested. One possible approach to increase SERCA pump activity is to decrease the levels of phospholamban. Del monte et al 96 showed that adenoviral gene transfer of antisense phospholamban in failing human cardiomyocytes restored contractility, Ca2+ handling, and the force frequency response. In addition, a pseudo-phosphorylated mutant PLB peptide (S16EPLN) was tested in BIO14.6 CM hamsters (showing a progressive stage of dilated cardiomyopathy and heart failure). Expression of mutant PLB enhanced myocardial SR Ca2+ uptake and suppressed progressive impairment of left ventricular (LV) systolic function and contractility up to 28–30 weeks. In addition, parvalbumin gene transfer was shown to improve diastolic function in senescent and diseased myocytes 97, 98.
9. Summary and Conclusions
In conclusion, diastolic dysfunction is a complex disease and it is difficult to pinpoint single etiology or a causative mechanism to be targeted. Diastolic dysfunction is also a progressive disease and could be compounded by other pathological conditions. Despite significant advances in our understanding of basic cellular mechanisms regulating relaxation, there are still many hurdles with regard to the development of efficient therapeutic approaches to combat diastolic dysfunction.
Acknowledgments
We thank Ms Carolyn Rutter for editorial assistance and Dr Sandor Gyorke for providing the template for the Figure 1.
MP is supported by NIH R01 grants HL64140 and NIH HL 088555, PMLJ is supported by NIH R01 HL73816, NIH K02 HL83957, and by an Established Investigator Award from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borlaug BA, Kass DA. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med. 2006 Nov;16(8):273–279. doi: 10.1016/j.tcm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004 Jun 25;94(12):1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 3.Cooper Gt. Cytoskeletal networks and the regulation of cardiac contractility: microtubules, hypertrophy, and cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2006 Sep;291(3):H1003–1014. doi: 10.1152/ajpheart.00132.2006. [DOI] [PubMed] [Google Scholar]
- 4.Zile MR, Baicu CF, Bonnema DD. Diastolic heart failure: definitions and terminology. Prog Cardiovasc Dis. 2005 Mar–Apr;47(5):307–313. doi: 10.1016/j.pcad.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Fabiato A, Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- 6.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002 Jan 10;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 7.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 8.Lederer WJ, Berlin JR, Cohen NM, Hadley RW, Bers DM, Cannell MB. Excitation-contraction coupling in heart cells. Roles of the sodium-calcium exchange, the calcium current, and the sarcoplasmic reticulum. Ann N Y Acad Sci. 1990;588:190–206. doi: 10.1111/j.1749-6632.1990.tb13210.x. [DOI] [PubMed] [Google Scholar]
- 9.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993 Feb;72(2):463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 10.Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol. 2004 Aug;37(2):417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca(2+)homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001 Jun;33(6):1053–1063. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- 12.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve. 2007 Feb 7;35(4):430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 13.Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998 Oct;78(4):921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- 14.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003 Jul;4(7):566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 15.MacLennan DH, Asahi M, Tupling AR. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann N Y Acad Sci. 2003 Apr;986:472–480. doi: 10.1111/j.1749-6632.2003.tb07231.x. [DOI] [PubMed] [Google Scholar]
- 16.Bhupathy P, Babu GJ, Periasamy M. Sarcolipin and phospholamban as regulators of cardiac sarcoplasmic reticulum Ca(2+) ATPase. J Mol Cell Cardiol. 2007 Mar 12; doi: 10.1016/j.yjmcc.2007.03.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brittsan AG, Kranias EG. Phospholamban and cardiac contractile function. J Mol Cell Cardiol. 2000 Dec;32(12):2131–2139. doi: 10.1006/jmcc.2000.1270. [DOI] [PubMed] [Google Scholar]
- 18.Frank K, Kranias EG. Phospholamban and cardiac contractility. Ann Med. 2000 Nov;32(8):572–578. doi: 10.3109/07853890008998837. [DOI] [PubMed] [Google Scholar]
- 19.Vangheluwe P, Sipido KR, Raeymaekers L, Wuytack F. New perspectives on the role of SERCA2’s Ca2+ affinity in cardiac function. Biochim Biophys Acta. 2006 Nov;1763(11):1216–1228. doi: 10.1016/j.bbamcr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Asahi M, Nakayama H, Tada M, Otsu K. Regulation of sarco(endo)plasmic reticulum Ca2+ adenosine triphosphatase by phospholamban and sarcolipin: implication for cardiac hypertrophy and failure. Trends Cardiovasc Med. 2003 May;13(4):152–157. doi: 10.1016/s1050-1738(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 21.Zarain-Herzberg A, MacLennan DH, Periasamy M. Characterization of rabbit cardiac sarco(endo)plasmic reticulum Ca2(+)-ATPase gene. J Biol Chem. 1990 Mar 15;265(8):4670–4677. [PubMed] [Google Scholar]
- 22.Arai M, Otsu K, MacLennan DH, Periasamy M. Regulation of sarcoplasmic reticulum gene expression during cardiac and skeletal muscle development. Am J Physiol. 1992 Mar;262(3 Pt 1):C614–620. doi: 10.1152/ajpcell.1992.262.3.C614. [DOI] [PubMed] [Google Scholar]
- 23.Luss I, Boknik P, Jones LR, et al. Expression of cardiac calcium regulatory proteins in atrium v ventricle in different species. J Mol Cell Cardiol. 1999 Jun;31(6):1299–1314. doi: 10.1006/jmcc.1999.0962. [DOI] [PubMed] [Google Scholar]
- 24.Minajeva A, Kaasik A, Paju K, et al. Sarcoplasmic reticulum function in determining atrioventricular contractile differences in rat heart. Am J Physiol. 1997 Nov;273(5 Pt 2):H2498–2507. doi: 10.1152/ajpheart.1997.273.5.H2498. [DOI] [PubMed] [Google Scholar]
- 25.Lompre AM, Anger M, Levitsky D. Sarco(endo)plasmic reticulum calcium pumps in the cardiovascular system: function and gene expression. J Mol Cell Cardiol. 1994 Sep;26(9):1109–1121. doi: 10.1006/jmcc.1994.1130. [DOI] [PubMed] [Google Scholar]
- 26.Lompre AM, Lambert F, Lakatta EG, Schwartz K. Expression of sarcoplasmic reticulum Ca(2+)-ATPase and calsequestrin genes in rat heart during ontogenic development and aging. Circ Res. 1991 Nov;69(5):1380–1388. doi: 10.1161/01.res.69.5.1380. [DOI] [PubMed] [Google Scholar]
- 27.Reed TD, Babu GJ, Ji Y, et al. The expression of SR calcium transport ATPase and the Na(+)/Ca(2+)Exchanger are antithetically regulated during mouse cardiac development and in Hypo/hyperthyroidism. J Mol Cell Cardiol. 2000 Mar;32(3):453–464. doi: 10.1006/jmcc.1999.1095. [DOI] [PubMed] [Google Scholar]
- 28.Gombosova I, Boknik P, Kirchhefer U, et al. Postnatal changes in contractile time parameters, calcium regulatory proteins, and phosphatases. Am J Physiol. 1998 Jun;274(6 Pt 2):H2123–2132. doi: 10.1152/ajpheart.1998.274.6.H2123. [DOI] [PubMed] [Google Scholar]
- 29.Arai M, Matsui H, Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res. 1994 Apr;74(4):555–564. doi: 10.1161/01.res.74.4.555. [DOI] [PubMed] [Google Scholar]
- 30.Arai M, Otsu K, MacLennan DH, Alpert NR, Periasamy M. Effect of thyroid hormone on the expression of mRNA encoding sarcoplasmic reticulum proteins. Circ Res. 1991 Aug;69(2):266–276. doi: 10.1161/01.res.69.2.266. [DOI] [PubMed] [Google Scholar]
- 31.Nagai R, Zarain-Herzberg A, Brandl CJ, et al. Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2966–2970. doi: 10.1073/pnas.86.8.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiss E, Jakab G, Kranias EG, Edes I. Thyroid hormone-induced alterations in phospholamban protein expression. Regulatory effects on sarcoplasmic reticulum Ca2+ transport and myocardial relaxation. Circ Res. 1994 Aug;75(2):245–251. doi: 10.1161/01.res.75.2.245. [DOI] [PubMed] [Google Scholar]
- 33.Jiang MT, Moffat MP, Narayanan N. Age-related alterations in the phosphorylation of sarcoplasmic reticulum and myofibrillar proteins and diminished contractile response to isoproterenol in intact rat ventricle. Circ Res. 1993 Jan;72(1):102–111. doi: 10.1161/01.res.72.1.102. [DOI] [PubMed] [Google Scholar]
- 34.Lakatta EG. Myocardial adaptations in advanced age. Basic Res Cardiol. 1993;88( Suppl 2):125–133. [PubMed] [Google Scholar]
- 35.Tada M. Molecular structure and function of phospholamban in regulating the calcium pump from sarcoplasmic reticulum. Ann N Y Acad Sci. 1992 Nov 30;671:92–102. doi: 10.1111/j.1749-6632.1992.tb43787.x. discussion 102–103. [DOI] [PubMed] [Google Scholar]
- 36.Tada M, Inui M. Regulation of calcium transport by the ATPase-phospholamban system. J Mol Cell Cardiol. 1983 Sep;15(9):565–575. doi: 10.1016/0022-2828(83)90267-5. [DOI] [PubMed] [Google Scholar]
- 37.Mattiazzi A, Mundina-Weilenmann C, Guoxiang C, Vittone L, Kranias E. Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovasc Res. 2005 Dec 1;68(3):366–375. doi: 10.1016/j.cardiores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Luo W, Grupp IL, Harrer J, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994 Sep;75(3):401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 39.Brittsan AG, Ginsburg KS, Chu G, et al. Chronic SR Ca2+-ATPase inhibition causes adaptive changes in cellular Ca2+ transport. Circ Res. 2003 Apr 18;92(7):769–776. doi: 10.1161/01.RES.0000066661.49920.59. [DOI] [PubMed] [Google Scholar]
- 40.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000 Sep;32(9):1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 41.Piacentino V, 3rd, Weber CR, Chen X, et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003 Apr 4;92(6):651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 42.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006 Dec;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 43.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Rem. 1998 Feb;37(2):279–289. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 44.Hasenfuss G, Schillinger W, Lehnart SE, et al. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999 Feb 9;99(5):641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 45.Hobai IA, O’Rourke B. Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation-contraction coupling in canine heart failure. Circulation. 2001 Mar 20;103(11):1577–1584. doi: 10.1161/01.cir.103.11.1577. [DOI] [PubMed] [Google Scholar]
- 46.O’Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res. 1999 Mar 19;84(5):562–570. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 47.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999 Jul 9;85(1):38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- 48.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999 Nov 26;85(11):1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 49.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001 Jun 8;88(11):1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 50.Hobai IA, O’Rourke B. Enhanced Ca(2+)-activated Na(+)-Ca(2+) exchange activity in canine pacing-induced heart failure. Circ Res. 2000 Oct 13;87(8):690–698. doi: 10.1161/01.res.87.8.690. [DOI] [PubMed] [Google Scholar]
- 51.Pieske B, Sutterlin M, Schmidt-Schweda S, et al. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J Clin Invest. 1996 Aug 1;98(3):764–776. doi: 10.1172/JCI118849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marks AR. Ryanodine receptors/calcium release channels in heart failure and sudden cardiac death. J Mol Cell Cardiol. 2001 Apr;33(4):615–624. doi: 10.1006/jmcc.2000.1343. [DOI] [PubMed] [Google Scholar]
- 53.Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000 May 12;101(4):365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 54.Wehrens XH, Lehnart SE, Marks AR. Ryanodine receptor-targeted anti-arrhythmic therapy. Ann N Y Acad Sci. 2005 Jun;1047:366–375. doi: 10.1196/annals.1341.032. [DOI] [PubMed] [Google Scholar]
- 55.Wehrens XH, Marks AR. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem Sci. 2003 Dec;28(12):671–678. doi: 10.1016/j.tibs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res. 2002 Nov 29;91(11):1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 57.Xiao B, Jiang MT, Zhao M, et al. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005 Apr 29;96(8):847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 58.Xiao B, Sutherland C, Walsh MP, Chen SR. Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+-release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6) Circ Res. 2004 Mar 5;94(4):487–495. doi: 10.1161/01.RES.0000115945.89741.22. [DOI] [PubMed] [Google Scholar]
- 59.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, et al. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006 Aug 18;99(4):398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 61.Baker DL, Hashimoto K, Grupp IL, et al. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiac contractility in transgenic mouse hearts. Circ Res. 1998 Dec 14–28;83(12):1205–1214. doi: 10.1161/01.res.83.12.1205. [DOI] [PubMed] [Google Scholar]
- 62.Loukianov E, Ji Y, Baker DL, et al. Sarco(endo)plasmic reticulum Ca2+ ATPase isoforms and their role in muscle physiology and pathology. Ann N Y Acad Sci. 1998 Sep 16;853:251–259. doi: 10.1111/j.1749-6632.1998.tb08273.x. [DOI] [PubMed] [Google Scholar]
- 63.Vetter R, Rehfeld U, Reissfelder C, et al. Transgenic overexpression of the sarcoplasmic reticulum Ca2+ATPase improves reticular Ca2+ handling in normal and diabetic rat hearts. Faseb J. 2002 Oct;16(12):1657–1659. doi: 10.1096/fj.01-1019fje. [DOI] [PubMed] [Google Scholar]
- 64.He H, Giordano FJ, Hilal-Dandan R, et al. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J Clin Invest. 1997 Jul 15;100(2):380–389. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji Y, Lalli MJ, Babu GJ, et al. Disruption of a single copy of the SERCA2 gene results in altered Ca2+ homeostasis and cardiomyocyte function. J Biol Chem. 2000 Dec 1;275(48):38073–38080. doi: 10.1074/jbc.M004804200. [DOI] [PubMed] [Google Scholar]
- 66.Periasamy M, Reed TD, Liu LH, et al. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem. 1999 Jan 22;274(4):2556–2562. doi: 10.1074/jbc.274.4.2556. [DOI] [PubMed] [Google Scholar]
- 67.Shull GE, Okunade G, Liu LH, et al. Physiological functions of plasma membrane and intracellular Ca2+ pumps revealed by analysis of null mutants. Ann N Y Acad Sci. 2003 Apr;986:453–460. doi: 10.1111/j.1749-6632.2003.tb07229.x. [DOI] [PubMed] [Google Scholar]
- 68.Schultz Jel J, Glascock BJ, Witt SA, et al. Accelerated onset of heart failure in mice during pressure overload with chronically decreased SERCA2 calcium pump activity. Am J Physiol Heart Circ Physiol. 2004 Mar;286(3):H1146–1153. doi: 10.1152/ajpheart.00720.2003. [DOI] [PubMed] [Google Scholar]
- 69.Seidman CE, Seidman JG. Mutations in cardiac myosin heavy chain genes cause familial hypertrophic cardiomyopathy. Mol Biol Med. 1991 Apr;8(2):159–166. [PubMed] [Google Scholar]
- 70.Maron BJ, Niimura H, Casey SA, et al. Development of left ventricular hypertrophy in adults in hypertrophic cardiomyopathy caused by cardiac myosin-binding protein C gene mutations. J Am Coll Cardiol. 2001 Aug;38(2):315–321. doi: 10.1016/s0735-1097(01)01386-9. [DOI] [PubMed] [Google Scholar]
- 71.Tardiff JC, Hewett TE, Palmer BM, et al. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest. 1999 Aug;104(4):469–481. doi: 10.1172/JCI6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidtmann A, Lindow C, Villard S, et al. Cardiac troponin C-L29Q, related to hypertrophic cardiomyopathy, hinders the transduction of the protein kinase A dependent phosphorylation signal from cardiac troponin I to C. Febs J. 2005 Dec;272(23):6087–6097. doi: 10.1111/j.1742-4658.2005.05001.x. [DOI] [PubMed] [Google Scholar]
- 73.Prabhakar R, Boivin GP, Grupp IL, et al. A familial hypertrophic cardiomyopathy alpha-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol. 2001 Oct;33(10):1815–1828. doi: 10.1006/jmcc.2001.1445. [DOI] [PubMed] [Google Scholar]
- 74.Yasuda S, Coutu P, Sadayappan S, Robbins J, Metzger JM. Cardiac transgenic and gene transfer strategies converge to support an important role for troponin I in regulating relaxation in cardiac myocytes. Circ Res. 2007 Aug 17;101(4):377–386. doi: 10.1161/CIRCRESAHA.106.145557. [DOI] [PubMed] [Google Scholar]
- 75.Varian KD, Raman S, Janssen PM. Measurement of myofilament calcium sensitivity at physiological temperature in intact cardiac trabeculae. Am J Physiol Heart Circ Physiol. 2006 May;290(5):H2092–2097. doi: 10.1152/ajpheart.01241.2005. [DOI] [PubMed] [Google Scholar]
- 76.Kranias EG, Solaro RJ. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature. 1982 Jul 8;298(5870):182–184. doi: 10.1038/298182a0. [DOI] [PubMed] [Google Scholar]
- 77.Wolska BM, Arteaga GM, Pena JR, et al. Expression of slow skeletal troponin I in hearts of phospholamban knockout mice alters the relaxant effect of beta-adrenergic stimulation. Circ Res. 2002 May 3;90(8):882–888. doi: 10.1161/01.res.0000016962.36404.04. [DOI] [PubMed] [Google Scholar]
- 78.Bilchick KC, Duncan JG, Ravi R, et al. Heart failure-associated alterations in troponin I phosphorylation impair ventricular relaxation-afterload and force-frequency responses and systolic function. Am J Physiol Heart Circ Physiol. 2007 Jan;292(1):H318–325. doi: 10.1152/ajpheart.00283.2006. [DOI] [PubMed] [Google Scholar]
- 79.Bronzwaer JG, Paulus WJ. Matrix, cytoskeleton, or myofilaments: which one to blame for diastolic left ventricular dysfunction? . Prog Cardiovasc Dis. 2005 Jan–Feb;47(4):276–284. doi: 10.1016/j.pcad.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006 May 2;113(17):2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 81.Norton GR, Tsotetsi J, Trifunovic B, Hartford C, Candy GP, Woodiwiss AJ. Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation. 1997 Sep 16;96(6):1991–1998. doi: 10.1161/01.cir.96.6.1991. [DOI] [PubMed] [Google Scholar]
- 82.Matsubara LS, Matsubara BB, Okoshi MP, Cicogna AC, Janicki JS. Alterations in myocardial collagen content affect rat papillary muscle function. Am J Physiol Heart Circ Physiol. 2000 Oct;279(4):H1534–1539. doi: 10.1152/ajpheart.2000.279.4.H1534. [DOI] [PubMed] [Google Scholar]
- 83.Cooper Gt. Cardiocyte cytoskeleton in hypertrophied myocardium. Heart Fail Rev. 2000 Oct;5(3):187–201. doi: 10.1023/A:1009836918377. [DOI] [PubMed] [Google Scholar]
- 84.Zile MR, Green GR, Schuyler GT, Aurigemma GP, Miller DC, Cooper Gt. Cardiocyte cytoskeleton in patients with left ventricular pressure overload hypertrophy. J Am Coll Cardiol. 2001 Mar 15;37(4):1080–1084. doi: 10.1016/s0735-1097(00)01207-9. [DOI] [PubMed] [Google Scholar]
- 85.Granzier H, Wu Y, Siegfried L, LeWinter M. Titin: physiological function and role in cardiomyopathy and failure. Heart Fail Rev. 2005 Sep;10(3):211–223. doi: 10.1007/s10741-005-5251-7. [DOI] [PubMed] [Google Scholar]
- 86.Wu Y, Bell SP, Trombitas K, et al. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 2002 Sep 10;106(11):1384–1389. doi: 10.1161/01.cir.0000029804.61510.02. [DOI] [PubMed] [Google Scholar]
- 87.Wu Y, Cazorla O, Labeit D, Labeit S, Granzier H. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J Mol Cell Cardiol. 2000 Dec;32(12):2151–2162. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]
- 88.Wu Y, Labeit S, Lewinter MM, Granzier H. Titin: an endosarcomeric protein that modulates myocardial stiffness in DCM. J Card Fail. 2002 Dec;8(6 Suppl):S276–286. doi: 10.1054/jcaf.2002.129278. [DOI] [PubMed] [Google Scholar]
- 89.LeWinter MM. Titin isoforms in heart failure: are there benefits to supersizing? Circulation. 2004 Jul 13;110(2):109–111. doi: 10.1161/01.CIR.0000137284.17083.93. [DOI] [PubMed] [Google Scholar]
- 90.del Monte F, Harding SE, Schmidt U, et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999 Dec 7;100(23):2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyamoto MI, del Monte F, Schmidt U, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000 Jan 18;97(2):793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hajjar RJ, del Monte F, Matsui T, Rosenzweig A. Prospects for gene therapy for heart failure. Circ Res. 2000 Mar 31;86(6):616–621. doi: 10.1161/01.res.86.6.616. [DOI] [PubMed] [Google Scholar]
- 93.del Monte F, Hajjar RJ, Harding SE. Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure. Circ Res. 2001 Jun 8;88(11):E66–67. doi: 10.1161/hh1101.092004. [DOI] [PubMed] [Google Scholar]
- 94.del Monte F, Williams E, Lebeche D, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001 Sep 18;104(12):1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Minamisawa S, Hoshijima M, Chu G, et al. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999 Oct 29;99(3):313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 96.del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002 Feb 26;105(8):904–907. doi: 10.1161/hc0802.105564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Michele DE, Szatkowski ML, Albayya FP, Metzger JM. Parvalbumin gene delivery improves diastolic function in the aged myocardium in vivo. Mol Ther. 2004 Aug;10(2):399–403. doi: 10.1016/j.ymthe.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 98.Wahr PA, Michele DE, Metzger JM. Parvalbumin gene transfer corrects diastolic dysfunction in diseased cardiac myocytes. Proc Natl Acad Sci U S A. 1999 Oct 12;96(21):11982–11985. doi: 10.1073/pnas.96.21.11982. [DOI] [PMC free article] [PubMed] [Google Scholar]