Abstract
Psychomotor effects elicited by systemic administration of the noncompetitive NMDA (N-methyl-D-aspartate) receptor antagonist MK-801 (dizocilpine maleate) represent perturbation of glutamatergic pathways, providing an animal model for psychotic symptoms of schizophrenia. Hyperlocomotion and stereotypy are the two main psychomotor behaviors induced by MK-801. This study compared MK-801-induced hyperlocomotion and stereotypy in young (1-month old) and aged mice (12-month old), in order to determine how the aging process may influence these behaviors. The tested MK-801 doses ranged from 0.015 to 1 mg/kg. The data indicated that MK-801 impacted the aged mice more pronouncedly than the young mice, as both hyperlocomotion and stereotypy were increased significantly more in the aged mice relative to the young mice. These results suggest an age-related increase in MK-801 sensitivity in mice.
Keywords: Noncompetitive NMDA receptor antagonist, Dizocilpine maleate, Locomotion, Stereotypy, Aging, Differential drug sensitivity
1. Introduction
The noncompetitive NMDA (N-methyl-D-aspartate) receptor antagonist MK-801 (dizocilpine maleate) is well-known for its psychomotor effects in both experimental animals and humans. Systemic administration of MK-801 induced elevated levels of locomotor activities in rodents, as well as stereotypic behaviors at higher doses (Clineschmidt et al., 1982; Deutsch et al., 1997). Ataxia can also occur with high doses of MK-801 (Tricklebank et al., 1989; Loscher and Honack, 1992; Haggerty and Brown, 1996). Although these psychomotor effects show some similarities to those caused dopaminergic reagents, increasing evidence indicates that MK-801 effects are mechanistically distinct from that by the dopamine system (Carlsson and Carlsson, 1989; Tiedtke et al., 1990; Adriani et al., 1998; O’Neill and Shaw, 1999; Carlsson et al., 1999). The interest in behavioral effects of MK-801 stems in part from the increasing use of NMDA antagonists as animal models of psychosis (Thornberg and Saklad, 1996; Rung et al., 2005; Rujescu et al., 2006), based on observations that in humans NMDA antagonists mimic some of the psychotic symptoms of schizophrenia more completely than does amphetamine, as well as on animal experiments indicating that both typical and atypical antipsychotics antagonize the behavioral effects of NMDA antagonists (Carlsson et al., 1999). It has therefore been proposed that some aspects of schizophrenia may reflect a relative deficit in glutamatergic pathways (Kim et al., 1980; Javitt, 1987; Tiedtke et al., 1990; Carlsson and Carlsson, 1990; Moghaddam, 2003). The ability of various drugs to modulate MK-801-induced locomotion and stereotypy has become a preclinical test for screening new antipsychotic compounds.
NMDA receptors, as well as MK-801 effects mediated by these receptors, are known to undergo changes during the developmental and aging processes. The NMDA receptor site has been shown to be vulnerable to the effects of aging. In the mouse brain, aging has heterogeneous effects on different brain sites on the NMDA receptor, and [3H]MK801 binding exhibited moderate declines during mouse aging (C57BL/6 mice at 3-, 10-, and 28–30 month (Magnusson, 1995)). The level of protein expression representative of the NMDA receptor NR1, NR2A and NR2B was decreased in older mice (10- and 30-month) in the cerebral cortex (Magnusson et al., 2002). [3H]MK-801 binding was reduced in several brain areas in aged rats compared with younger rats (Fischer 344 rats at 6-, 12-, and 24-month), including the inner frontal cortex, entorhinal cortex and the lateral striatum (Mitchell and Anderson, 1998). Using autoradiographic images obtained in postmortem sections of human normal brain tissues, it was shown that there existed significant correlation between age and [(3)H]MK-801 binding sites in the basal ganglia — with advanced age, MK-801 binding sites were lower, suggesting hypoactive activity of the NMDA receptor complex system with advancing age (Villares and Stavale, 2001).
At the physiological level, aging impacts the effectiveness of NMDA receptor-mediated functions. Using the middle cerebral artery occlusion model in young and aged rats, it was shown that MK-801 administered immediately after the occlusion significantly reduced the extent of ischemic cerebral damage only in young rats, but not in aged rats (Sprague-Dawley rats at 8-weeks and 24-months (Suzuki et al., 2003)). With regard to the psychomotor effects of MK-801, young rats showed more pronounced increases of both locomotor and stereotypic activities induced by MK-801 than in older rats (Mill Hill hooded rats with ages from 1 month to 24 months)(Vasilev et al., 2003). When the motor and learning functions were measured in rats of different age groups (Wistar rats as 3–5, 20–21, and 29–31 months old), a decline in motor function and T-maze performance during aging was correlated with a loss of MK-801 binding sites, suggesting a crucial role for NMDA receptors in age-dependent motor and cognitive disturbances (Ossowska et al., 2001). Perinatal MK-801 treatment affects age-related changes in locomotor activity in rats (several age groups from 1 month to 6 months) (Schiffelholz et al., 2004), and locomotion elicited by s.c. injection of MK801 differed between young (10, 20, and 30 days) and adult rats (54–68 days) (Frantz and Van Hartesveldt, 1999).
Even though a number of studies examined age influence for MK-801-induced locomotion and stereotypy in rodents, few studies have examined both behaviors simultaneously, and with respect to the age-related differential effects, particularly in mice of different ages. As our earlier studies showed (Wu et al., 2005), it is important to analyze both locomotion and stereotypy in parallel and over an extended time span, in order to adequately delineate the complex behaviors elicited by MK-801. In the present study, we examined two age groups of C57BL/6 mice (young mice at 1-month old, and aged mice at 12-month old), and present a thorough comparison of the locomotor and stereotypy effects of MK-801.
2. Materials and methods
2.1. Materials
MK-801 (dizocilpine maleate, a noncompetitive NMDA receptor antagonist) was obtained from Tocris Cookson Inc., Ellisville, Missouri, USA. The compound was dissolved in saline (0.9% NaCl solution) as master stock solution (1 mg/ml), and aliquots were kept frozen at −70°C till use. Before each experiment, aliquots of master stock were thawed and diluted with saline to the final concentrations for systemic administration by i.p. injection. Dilutions were designed to give a final injection volume of 100 μl per 20 grams of animal weight for each dose of MK-801 administered. This study used MK-801 doses of 0.015, 0.05, 0.15, 0.3, 0.6, and 1 mg/kg.
2.2. Animals
Young (1-month old) and aged (12-month old) male C57BL/6 mice were obtained from Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China. Animals were housed under standard housing conditions, with plastic cages with sawdust bedding, free access to food and water, and on a 12-h light/dark cycle. Before experiments were conducted, animals were acclimated for 3–8 days, with the exception of 2 day acclimation for the saline group. All animals were used only once. Principles of laboratory animal care were followed in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996), the PRC national standards for laboratory animal quality, and guidelines for the use of experimental animals.
2.3. Automated locomotor activity measurements
Locomotion measurements were obtained by automatic detection of beam crossings, essentially the same as previously reported (Wu et al., 2005), using an automatic beam crossing detection system purchased commercially (San Diego Instruments, San Diego, California, USA). Animals were placed in a 45×25×20 cm chamber (L×W×H). Horizontal motion was detected by photobeam detectors spaced at 5 cm in the length direction and 4.5 cm in the width direction, at a height of 2 cm above the ground.
Animals were injected i.p. with saline or the appropriate dose of MK-801, then placed immediately into the recording chamber. Hence, animals were not habituated to the novel testing environment, and the recordings include the initial period of adaptation to the novel environment. The recording period was 280 min. Data are presented as beam breaks per 10-min period. n = 10 for all groups, except n = 8 for aged groups at 0.015 and 0.05 mg/kg MK-801.
For calculation of ½ peak duration and ½ peak area, the corresponding values for each animal were first calculated, and then the average of these values were calculated and presented.
2.4. Videotape analysis of stereotypic behaviors
For stereotypy measurements, the animals in the locomotion measurement chambers were videotaped for the initial 240 min. Hence, for each animal, stereotypy and locomotion data were obtained simultaneously, but independently of each other. Stereotypy measurement was scored for the first 2 min out of each 10-min time segment. Stereotypy measurements are presented as the percentage of time spent in stereotypic behaviors. Stereotypy was broadly defined similar to previously described (Wu et al., 2005), and included a number of abnormal movements (including head weaving, turning, rotation, and other abnormally repetitive actions). Specifically, head weaving: repeated twice or more of head weaving left-right or bobbing up-down; turning: complete turning of half a circle or more within a quadrant of the mouse activity chamber (45×25 cm, L×W); rotation: body unsteady and rolling partially from time to time when moving; other abnormally repetitive actions: body bending over and repeated licking hind paws/genital area, holding a posture (such as head pointing to a corner) for extended time that was never observed in control mice. It should be pointed out that in a previous study, we scored ataxia separately on a 1–5 scale (Wu et al., 2005), because at the high MK-801 doses used in that study (2 mg/kg and 5 mg/kg), severe ataxia was readily observed. In the current study, the highest dose of MK-801 used was 1 mg/kg, for which we did not observe severe, definitive ataxia. Rather, mild ataxia-like behaviors were seen, equivalent to categories 1–2 on our 1–5 ataxia scale (Wu et al., 2005); however, such mild ataxia-like behaviors were also similar to certain stereotypy behaviors (body unsteady and rolling partially from time to time when moving). Because of this resemblance, these mild ataxia-like behaviors were rather indistinguishable from stereotypy. For this reason, when we scored the behavior we did not attempt to separate mild ataxia-like behavior from stereotypy.
To assure adequate rating reliability, we utilized a set of reference training tapes that had been maintained since a previous study (Wu et al., 2005), containing videotaped mouse behavior at several MK-801 doses. A new rater was required to learn and practice until the stereotypy scoring results were within 25% of the reference values which were previously obtained by a group of experienced raters. If a rater had not performed behavioral scoring for 2 weeks or longer, this rater would need to go through retraining before collecting behavioral data. For the current study, inter-rater reliability was tested before data tabulation scoring took place, and a correlation of approximately 90% was obtained (first inter-rater reliability test: 11.8% variance; second inter-rater reliability test: 9.1% variance). Due to the relatively high reliability (considerable better than the 25% variance we set as the threshold), the video tapes were scored by one rater.
2.5. Data analysis
For all the data, statistical analyses were performed using the software GraphPad Prism 4.0 (San Diego, CA). Data are shown as mean ± S.E.M. The data were analyzed with two-way ANOVAs and, if significance was detected, by the Bonferroni post hoc test. Significance is ascribed for P < 0.05.
3. Results
3.1. MK-801-induced hyperlocomotion in young vs. aged mice
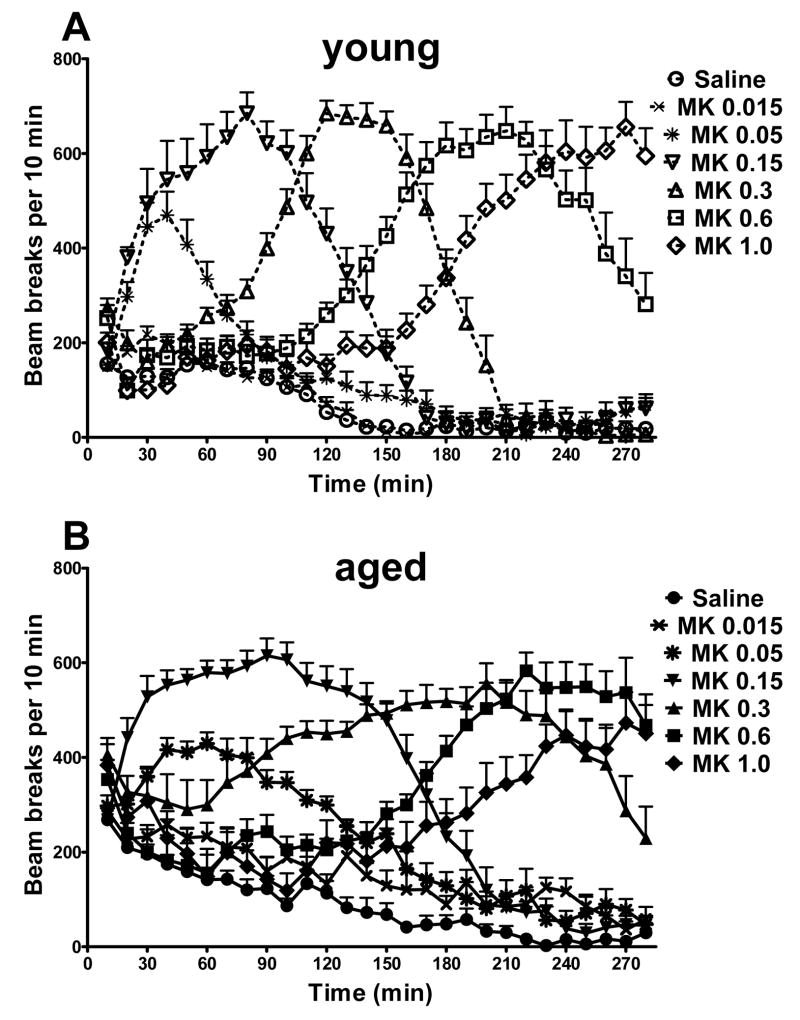
MK-801 is a noncompetitive NMDA receptor antagonist. To characterize the physiological effect of age difference on mobility and drug sensitivity, we examined young (1-month old) vs. aged (12-month old) C57BL/6 mice for their respective responsiveness to MK-801-induced hyperlocomotion, as a way to compare these characteristics in aged mice with those in younger mice. Upon MK-801 administration, animals were placed immediately into the recorder chambers; thus mice were not habituated to the novel testing environment. We recorded locomotor activities continuously for 280 min after systemic administration of either MK-801 or saline. As shown in Fig. 1, in both the young (Fig. 1A) and aged mice (Fig. 1B), MK-801 was able to elicit appreciable hyperlocomotion above the locomotion levels of the saline controls. The kinetic characteristics of drug-induced hyperlocomotion appeared to change in both age groups as the dosing of MK-801 was increased.
Fig. 1.
Locomotor activities of the young and aged mice at various doses of MK-801. Time course of MK-801-induced locomotor activities in the young (A) and aged mice (B) are shown as beam breaks per 10-minute intervals. The dose of MK-801 (mg/kg) or saline is indicated for each symbol, with open symbols and dotted lines for the young mice (A), and corresponding filled symbols and solid lines for the aged mice (B). n = 10 for all groups, except n = 8 for aged groups at 0.015 and 0.05 mg/kg of MK-801.
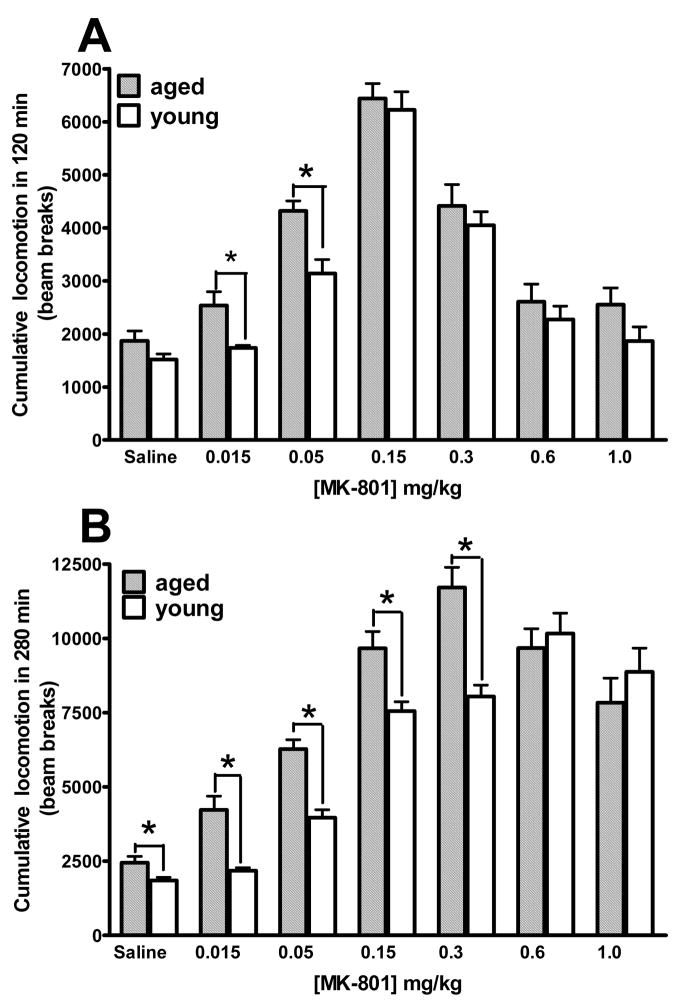
When the cumulative locomotion is displayed relative to MK-801 dosing, an inverted U-shaped dose-response relation is observed in both young and aged mice (Fig. 2). Similar observation has been reported before in developing and adult rats with an observation duration of 120 min (Frantz and Van Hartesveldt, 1999). When we examined our results for the first 120 min, the inverted U-shape was rather pronounced in both young and aged mice (Fig. 2A), similar to that reported in rats (Frantz and Van Hartesveldt, 1999). Since enhanced locomotion at higher MK-801 doses tend to be manifested at relatively later times (see Fig. 1), we also examined the cumulative locomotion for the entire 280 min observation duration (Fig. 2B). With such a more extended observation duration, the inverted U-shape was readily seen at the lower MK-801 dose range (from 0.015 mg/kg to 0.3 mg/kg), but less apparent at higher MK-801 doses (Fig. 2B). Further, cumulative locomotion highlighted age-related differential sensitivity to MK-801 effect, with the aged mice showing significantly enhanced locomotion than the young mice at several MK-801 doses of the lower range (Fig. 2).
Fig. 2.
Dose response relation for cumulative locomotion vs. MK-801 doses in the young and aged mice. Hatched bars, aged mice; open bars, young mice. (A) Cumulative locomotion in 120 min. (B) Cumulative locomotion in 280 min. * P < 0.05, significant difference for the indicated MK-801 dose.
3.2. Kinetic properties of MK-801-induced hyperlocomotion
To better compare the kinetic properties of MK-801-induced hyperlocomotion in the young and aged mice, the hyperlocomotion peak (the increased beam break curve above that of the saline level in Fig. 1) were analyzed to assess MK-801’s impact on specific peak parameters. First, we examined the rising phase of the hyperlocomotion peak. For rising time to 50% peak height on the rising phase, both age groups appeared to follow a similar pattern, with no significant difference between the two groups (Table 1, top rows). On the other hand, when the ratio of half-peak height over half-peak rising duration was calculated to assess the peak height vs. width relationship for the peak rising phase, the aged mice showed lower values, with a gradual decline of the height-to-duration ratio as the MK-801 doses increased (Table 1, bottom rows). The general tendency of both age groups to exhibit a decline in the height-to-duration peak ratio suggests that, for the rising phase of MK-801-induced hyperlocomotion peak, the response time (half-peak rising duration) was affected more substantially relative to the response amplitude (half-peak height). The lower ratios for the aged mice are indicative of a more pronounced widening of the peak, suggesting that the aged mice were more responsive to increasing dose effect of MK-801.
Table 1.
Rising phase properties of the MK-801 induced hyperlocomotion peak in the young and aged mice
| MK-801 (mg/kg) | 0.015 | 0.05 | 0.15 | 0.3 | 0.6 | 1 | |
|---|---|---|---|---|---|---|---|
| Rising time to half-peak height (min) | young | 6.5±1.0 | 15.0±1.4 | 30.0±6.4 | 85.7±4.6 | 139.6±5.0 | 185.4±7.0 |
| aged | 7.0±2.0 | 10.3±2.1 | 16.2±2.4 | 63.5±14.5 | 157.6±14.1 | 198.0±2.0 | |
| Half-peak height/half-peak rising duration | young | 4.3±0.7 | 15.2±3.8 | 10.2±2.1 | 8.8±0.8 | 6.3±0.8 | 5.3±0.7 |
| aged | 10.5±5.5 | 6.1±1.0a | 6.7±2.1 | 4.2±1.1a | 4.5±0.5a | 3.3±1.2 |
Rising time to half-peak height is the duration from MK-801 administration to when the locomotion reaches 50% of the peak value. Half-peak height/half-peak rising duration is the ratio of half-peak height over rising time to half-peak height. Results are expressed as mean ± S.E.M.
P < 0.05, significantly different from the young mice.
We also examined the declining phase of the hyperlocomotion peak. Both age groups showed a lengthening of declining time when MK-801 doses increased; however, the aged mice displayed longer times than the young mice across most of the doses (Table 2, top rows). When the ratio of half-peak height over half-peak declining duration was calculated, the aged mice showed lower ratios than the young mice across most of the MK-801 dosing range (Table 2, bottom rows), again suggesting that the aged mice were more responsive to increasing dose effect of MK-801.
Table 2.
Declining phase properties of the MK-801 induced hyperlocomotion peak in the young and aged mice
| MK-801 (mg/kg) | 0.015 | 0.05 | 0.15 | 0.3 | |
|---|---|---|---|---|---|
| Declining time to half-peak height (min) | young | 84.2±8.0 | 74.6±5.7 | 122.2±6.9 | 177.6±4.2 |
| aged | 95.8±7.2 | 125.2±8.1a | 161.5±7.2a | 257.5±5.9a | |
| Half-peak height/half-peak declining duration | young | 5.4±1.6 | 7.0±1.0 | 9.7±0.9 | 8.3±0.9 |
| aged | 3.5±1.2 | 3.8±0.9a | 5.2±0.4a | 5.1±1.1a |
Declining time to half-peak height is the duration from the MK-801 induced hyperlocomotion peak to when the locomotion declines by 50%. Half-peak height/half-peak declining duration is the ratio of half-peak height over declining time to half-peak height. Results are expressed as mean ± S.E.M.
P < 0.05, significantly different from the young mice.
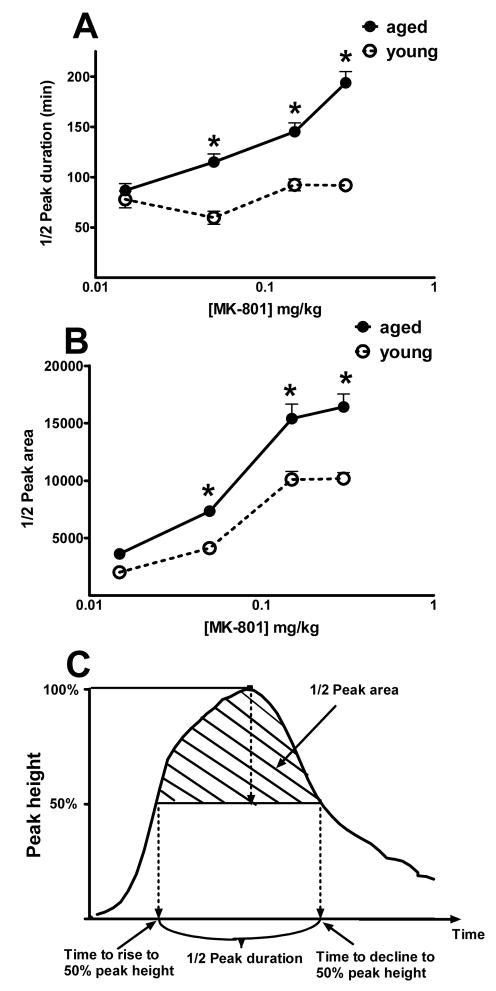
Composite parameters were calculated to integrate the dynamic properties of both the rising and the declining phases of MK-801-induced hyperlocomotion peak (Fig. 3C). Half-peak duration, a time measurement to quantify the width of the peak, showed different trends between the young and aged mice (Fig. 3A). Half-peak duration stayed relatively unchanged for the young animals (Fig. 3A, dotted line), indicating relatively consistent peak time across the MK-801 dose range tested. In contract, the aged animals showed a steady increase in the half-peak duration (Fig. 3A, solid line), indicating a gradual widening of the hyperlocomotion peak as the MK-801 doses became higher, suggesting an increased sensitivity to acute effect of MK-801. Another parameter for assessing the hyperlocomotion peak is half-peak area (Fig. 3B). Both the young and the aged mice showed a dose-dependent increase of the half-peak area, with the values for the aged mice significantly more than that for the young mice (Fig. 3B), again reflecting a more pronounced hyperlocomotion peak in aged animals.
Fig. 3.
Properties of the MK-801-induced hyperlocomotion peak in the aged and the young mice. MK-801 dosing is shown in log scale. Filled symbols with solid lines: aged mice; open symbols with dotted lines: young mice. (A) Half-peak duration is the time span between the rising phase half-peak point and the declining phase half-peak point. * P < 0.05, significant difference (F(1,86)=48.05) (B) Half-peak area is the area for the top half of the hyperlocomotion peak. * P < 0.05, significant difference (F(1,68)=58.75). (C) Diagram showing how the two parameters in (A) and (B) were calculated.
3.3. MK-801-induced stereotypy in young vs. aged mice
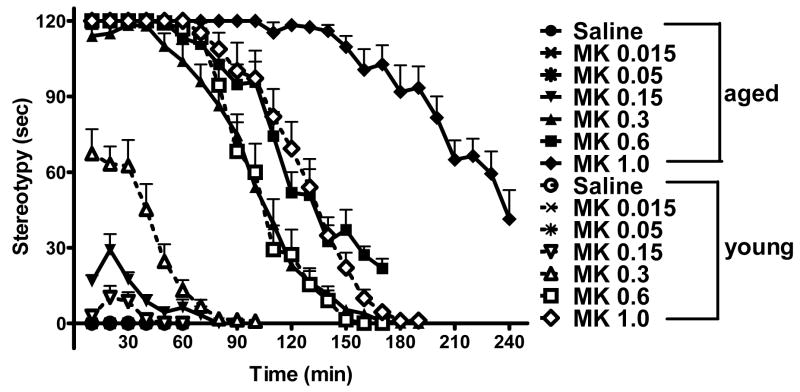
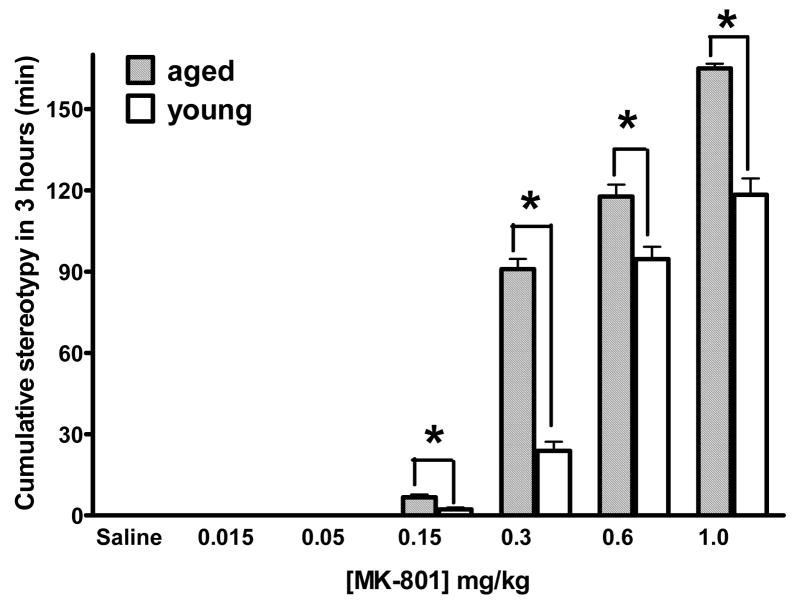
Drug-induced stereotypy represents certain types of abnormal and repetitive behaviors, often at the expense of other natural behaviors of rodents. Stereotypy in rodents is also considered a model for certain acute psychotic symptoms in human, and is frequently used in preclinical studies of psychoactive drugs. In order to determine whether there are differences in MK-801-induced stereotypic behavior between the young and the aged mice, we video-taped mouse activities during locomotion recording period, and analyzed the video tapes off-line to quantify the amount of stereotypic behavior. In saline groups as well as MK-801 doses of 0.015 mg/kg and 0.05 mg/kg, there was no stereotypy. At MK-801 doses of 0.15 mg/kg and higher, stereotypy in both young and aged mice was observed (Fig. 4). At higher MK-801 doses, the aged mice appeared to display stereotypic activities to a greater extent than the young mice.
Fig. 4.
MK-801-induced stereotypy in the aged and the young mice. MK-801-induced stereotypy was scored for the first 2 min out of each 10-min time segment, and is shown as the cumulative time (in sec). The dose of MK-801 (mg/kg) is indicated for each symbol, with filled symbols and solid lines for the aged mice, and corresponding open symbols and dotted lines for the young mice.
Quantitative analysis of the amount of stereotypy during the 3-h period confirmed the more pronounced stereotypic behavior in the aged mice relative to the young mice. Fig. 5 shows a pair-wise comparison for each MK-801 dose was made between the two age groups. In saline groups as well as MK-801 doses of 0.015 mg/kg and 0.05 mg/kg, there was no stereotypy. At MK-801 doses from 0.15 mg/kg and higher, the aged mice showed significantly more stereotypic behaviors than the young mice. These results indicate that the amount of time that mice were in stereotypy was closely correlated with the dose of MK-801, and that the aged mice displayed more pronounced stereotypy than the young mice, suggesting an enhanced sensitivity to MK-801 effect.
Fig. 5.
Cumulative stereotypy activity. Bar graph showing the cumulative time in stereotypy during the first 3 h for the aged (hatched bars) and the young mice (open bars) for each of the MK-801 doses tested. * P < 0.05, significant difference (F(1,69)=171.89).
3.4. Kinetic properties of MK-801-induced stereotypy
The kinetic properties of the stereotypy dose-response results were analyzed, to assess age-related difference in MK-801-induced stereotypy parameters. The time for stereotypy to decline to 50% was examined in order to characterize the wear-off kinetics of MK-801 effect, and this time became longer for both age groups as the MK-801 dose was increased (Table 3). The aged animals were impacted more significantly than the young mice at the higher dose range, with significant difference from the young mice for MK-801 doses at 0.3 mg/kg and higher (Table 3). These data indicate that the enhanced sensitivity to MK-801 in the aged mice was reflected in the kinetic properties of stereotypic behavior.
Table 3.
Stereotypy kinetic properties in the young and aged mice
| MK-801 (mg/kg) | 0 | 0.015 | 0.05 | 0.15 | 0.3 | 0.6 | 1 | |
|---|---|---|---|---|---|---|---|---|
| Time for stereotypy to decline to 50% (min) | Young | 0 | 0 | 0 | 24.8±5.2 | 39.3±3.2 | 97.1±4.9 | 123.3±5.9 |
| aged | 0 | 0 | 0 | 33.7±4.1 | 96.8±3.7a | 120.0±5.8a | 206.2±10.1a |
Stereotypy half-peak area is the area for the top half of the stereotypy peak.
P < 0.05, significantly different from the young mice.
4. Discussion
In the present study, we examined MK-801-induced locomotor and stereotypic behaviors in the young and aged C57BL/6 male mice, to characterize the age effect on mobility and drug sensitivity. The results show that the young and aged mice displayed differential sensitivity to MK-801 effects on both locomotion and stereotypy.
The differential effect between the young vs. the aged mice was reflected in a number of aspects of MK-801-induced hyperlocomotion. Using the cumulative locomotion as an overall indication of MK-801-induced hyperlocomotion, aged mice displayed higher values than the young mice (Fig. 2), suggesting of increased responsiveness with aging. This age-related increase in MK-801 sensitivity is reflected in a more pronounced widening of the hyperlocomotion peak, with the aged mice showing lower values of the half peak height vs. half peak duration (indicative of wider peak) for both the rising phase (Table 1) and the declining phase (Table 2) of the locomotion peak, as well as significantly more increases in the half peak duration and area under the curve (Fig. 3). These data are consistent with the notion of increased responsiveness to MK-801 effect on locomotion in the aged animals than the young animals.
Stereotypy in rodents is considered a model for certain acute psychotic symptoms in human, and is often used in preclinical studies of psychoactive drugs (Costall and Naylor, 1976). The present study showed age-related differential effects of MK-801-induced stereotypic behavior, with the aged mice displaying more pronounced stereotypic activities than the young mice (Fig. 4), across much of the tested MK-801 doses where stereotypy could be detected (Fig. 5). At the quantitative level, the age-related differences in stereotypy were rather profound. For example, the wearing off of stereotypy effect (Table 3: time for stereotypy to decline to 50%) was significantly lengthened in the aged mice over the young mice for several MK-801 doses, nearing doubling for some doses. These results indicate that for stereotypy, the aged mice responded to MK-801 faster, to a greater extent, and lasted longer. Thus, with respect to stereotypy, aging also enhanced animals’ sensitivity to MK-801.
It should be pointed out that the method of stereotypy is based on time duration, rather than a rating scale. Thus, our results do not present a direct quantification for stereotypy intensity afforded by a rating scale approach. In considering the behavioral scoring method of choice, studies by Segal, Kuczenski and colleagues on sensitization of drug-induced stereotypy provided a reference viewpoint. These investigators initially utilized a rating scale to measure stereotypy (Nishith et al., 2002); subsequently, they scored stereotypy both with a rating scale and by a time duration method, and chose to present the bulk of their data in the form of % time in stereotypy (Segal and Kuczenski, 1987). More recently, the time duration method became their main approach (Kuczenski and Segal, 1999), reflecting the view that the time duration method could adequately reflect the extent of stereotypy behaviors, and that data from the time duration method would allow more rigorous quantitative analysis for the extent of behavioral sensitization. In our study, we also used this approach for stereotypy quantification. However, it should be noted that since we did not compare a rating scale approach with our time duration method, stereotypy intensity was not directly measured — a potential caveat for interpreting the extent of stereotypy behavior.
Developmental processes, including aging, have been shown to affect not only NMDA receptors, but also behavioral effects mediated by MK-801 and other psychomotor drugs (Costall et al., 1975; Costall and Naylor, 1975; Kelly et al., 1975; Costall and Naylor, 1976; Magnusson, 1995; Mitchell and Anderson, 1998; Frantz and Van Hartesveldt, 1999; Villares and Stavale, 2001; Ossowska et al., 2001; Vasilev et al., 2003). With regard to NMDA receptors, the overall tendency appears to be an age-related decrease, both for MK-801 binding sites in mice (C57BL/6 mice at 3-, 10-, and 28–30 month (Magnusson, 1995)) and in rats (Fischer 344 rats at 6-, 12-, and 24-month (Mitchell and Anderson, 1998)) and at the receptor protein level (C57BL/6 mice at 3-, 10-, and 30-month (Magnusson et al., 2002)). Also, Age-related decline in motor functions has been correlated to reduced NMDA receptors (Wistar rats as 3–5, 20–21, and 29–31 months old) (Ossowska et al., 2001). At the behavioral level, aging appeared to reduce MK-801 hyperlocomotion in rats (Frantz and Van Hartesveldt, 1999; Ossowska et al., 2001; Vasilev et al., 2003). From this point of view, our results of aged mice showing increased sensitivity to the hyperlocomotor stimulation by MK-801 provided a contrasting observation to that in rats, possibly reflecting differences between rats and mice in their neural circuitries subserving drug-induced motor activity.
With regard to the age-related differential effect on stereotypy by MK-801, the common belief is that this behavior is also impacted by aging. In a study using Mill Hill hooded rats of various age groups (28–30 days, 48–50 days, 3 months, 12 months, and 24 months), Vasilev and colleagues showed more pronounced increases of both locomotor and stereotypic activities in younger rats than in older rats, for both MK-801 and amphetamine-induced effects (Vasilev et al., 2003). In this respect, it was also interesting that our data showed a clear heightened sensitivity for stereotypy in aged mice (Fig. 5, Table 3), again highlighting species differences between rats and mice in age effect on drug sensitivity.
In considering potential factors that may contribute to age-related differential sensitivity to MK-801 effects, pharmacokinetic difference between the young and the aged mice is a likely source. In this aspect, our analysis of the kinetic properties of locomotion peak provided some interesting points to consider. Age did not appear to affect the onset of MK-801 effect on locomotion, as there was no significant difference between the young and the aged mice for the time from MK-801 administration to half peak height (Table 1, top rows). Once MK-801 took effect from this point onward (marked by the rising phase half peak height), however, kinetics started to differentiate between the young vs. the aged animals — in almost every parameter of the locomotion peak. Thus, aged mice showed widening of the peak, as reflected collectively in half peak duration (Fig. 3A) and area under the curve (Fig. 3B), as well as specifically for the height-to-width ratios for both the rising phase half peak (Table 1, bottom rows) and the declining phase half peak (Table 2, bottom rows), in addition to the declining time from the peak top to half peak height (Table 2, top rows). For MK-801-induced stereotypy, although the almost immediate onset of stereotypy did not permit ready analysis of the rising phase, time for stereotypy to decline to 50% showed a clear lengthening for aged mice (Table 3). Taken together, these age-related kinetic differences are likely the consequence of pharmacokinetic difference due to aging.
Acknowledgments
This work was supported in part by National Natural Science Foundation of China (30270219), and the National Institutes of Health of USA (DA013471 and DA020555). We would like to thank the help from Shanghai Laboratory Animal Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Felici A, Sargolini F, Roullet P, Usiello A, Oliverio A, Mele A. N-methyl-D-aspartate and dopamine receptor involvement in the modulation of locomotor activity and memory processes. Exp Brain Res. 1998;123:52–59. doi: 10.1007/s002210050544. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Carlsson ML. Neurotransmitter interactions in schizophrenia--therapeutic implications. Biol Psychiatry. 1999;46:1388–1395. doi: 10.1016/s0006-3223(99)00117-1. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. The NMDA antagonist MK-801 causes marked locomotor stimulation in monoamine-depleted mice. J Neural Transm. 1989;75:221–226. doi: 10.1007/BF01258633. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia--implications for schizophrenia and Parkinson’s disease. Trends NeuroSci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Clineschmidt BV, Martin GE, Bunting PR, Papp NL. Central sympathomimetic activity of (+)-5-methyl-10,11-dihydro- 5H-dibenzo [D]cyclohepten-5, 10-immine(MK-801), a substance with potent anticonvulsant, central sympathomimetic, and apparent anxiolytic properties. Drug Dev Res. 1982;2:135–145. [Google Scholar]
- Costall B, Naylor RJ. The behavioural effects of dopamine applied intracerebrally to areas of the mesolimbic system. Eur J Pharmacol. 1975;32:87–92. doi: 10.1016/0014-2999(75)90326-x. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. Dissociation of stereotyped biting responses and oro-bucco-lingual dyskinesias. Eur J Pharmacol. 1976;36:423–429. doi: 10.1016/0014-2999(76)90096-0. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ, Neumeyer JL. Differences in the nature of the stereotyped behaviour induced by aporphine derivatives in the rat and in their actions in extrapyramidal and mesolimbic brain areas. Eur J Pharmacol. 1975;31:1–16. doi: 10.1016/0014-2999(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Mastropaolo J. Behavioral approaches to the functional assessment of NMDA-mediated neural transmission in intact mice. Clin Neuropharmacol. 1997;20:375–384. doi: 10.1097/00002826-199710000-00001. [DOI] [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C. Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects. Eur J Pharmacol. 1999;369:145–157. doi: 10.1016/s0014-2999(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Haggerty GC, Brown G. Neurobehavioral profile of subcutaneously administered MK-801 in the rat. Neurotoxicology. 1996;17:913–921. [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J Clin Psychiatry. 1987;9:12–35. [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kornhuber HH, Schmid-Burgk W, Holzmuller B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci Lett. 1980;20:379–382. doi: 10.1016/0304-3940(80)90178-0. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Sensitization of amphetamine-induced stereotyped behaviors during the acute response. J Pharmacol Exp Ther. 1999;288:699–709. [PubMed] [Google Scholar]
- Loscher W, Honack D. The behavioural effects of MK-801 in rats: involvement of dopaminergic, serotonergic and noradrenergic systems. Eur J Pharmacol. 1992;215:199–208. doi: 10.1016/0014-2999(92)90029-4. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Differential effects of aging on binding sites of the activated NMDA receptor complex in mice. Mech Ageing Dev. 1995;84:227–243. doi: 10.1016/0047-6374(95)01658-9. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Brain Res Mol Brain Res. 2002;99:40–45. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- Mitchell JJ, Anderson KJ. Age-related changes in [3H]MK-801 binding in the Fischer 344 rat brain. Neurobiol Aging. 1998;19:259–265. doi: 10.1016/s0197-4580(98)00058-x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Nishith P, Griffin MG, Poth TL. Stress-induced analgesia: prediction of posttraumatic stress symptoms in battered versus nonbattered women. Biol Psychiatry. 2002;51:867–874. doi: 10.1016/s0006-3223(01)01346-4. [DOI] [PubMed] [Google Scholar]
- O’Neill MF, Shaw G. Comparison of dopamine receptor antagonists on hyperlocomotion induced by cocaine, amphetamine, MK-801 and the dopamine D1 agonist C-APB in mice. Psychopharmacol. 1999;145:237–250. doi: 10.1007/s002130051055. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Wolfarth S, Schulze G, Wardas J, Pietraszek M, Lorenc-Koci E, Smialowska M, Coper H. Decline in motor functions in aging is related to the loss of NMDA receptors. Brain Res. 2001;907:71–83. doi: 10.1016/s0006-8993(01)02601-4. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, Giegling I, Genius J, McCarley RW, Moller HJ, Grunze H. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Rung JP, Carlsson A, Ryden MK, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:827–832. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Schiffelholz T, Hinze-Selch D, Aldenhoff JB. Perinatal MK-801 treatment affects age-related changes in locomotor activity from childhood to later adulthood in rats. Neurosci Lett. 2004;360:157–160. doi: 10.1016/j.neulet.2004.02.064. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- Suzuki Y, Takagi Y, Nakamura R, Hashimoto K, Umemura K. Ability of NMDA and non-NMDA receptor antagonists to inhibit cerebral ischemic damage in aged rats. Brain Res. 2003;964:116–120. doi: 10.1016/s0006-8993(02)04088-x. [DOI] [PubMed] [Google Scholar]
- Thornberg SA, Saklad SR. A review of NMDA receptors and the phencyclidine model of schizophrenia. Pharmacotherapy. 1996;16:82–93. [PubMed] [Google Scholar]
- Tiedtke PI, Bischoff C, Schmidt WJ. MK-801-induced stereotypy and its antagonism by neuroleptic drugs. J Neural Transm Gen Sect. 1990;81:173–182. doi: 10.1007/BF01245040. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Singh L, Oles RJ, Preston C, Iversen SD. The behavioural effects of MK-801: a comparison with antagonists acting non-competitively and competitively at the NMDA receptor. Eur J Pharmacol. 1989;167:127–135. doi: 10.1016/0014-2999(89)90754-1. [DOI] [PubMed] [Google Scholar]
- Vasilev V, Veskov R, Janac B, Rakic L, Stojiljkovic M. Age-related differences in MK-801- and amphetamine-induced locomotor and stereotypic activities of rats. Neurobiol Aging. 2003;24:715–723. doi: 10.1016/s0197-4580(02)00232-4. [DOI] [PubMed] [Google Scholar]
- Villares JC, Stavale JN. Age-related changes in the N-methyl-D-aspartate receptor binding sites within the human basal ganglia. Exp Neurol. 2001;171:391–404. doi: 10.1006/exnr.2001.7737. [DOI] [PubMed] [Google Scholar]
- Wu J, Zou H, Strong JA, Yu J, Zhou X, Xie Q, Zhao G, Jin M, Yu L. Bimodal effects of MK-801 on locomotion and stereotypy in C57BL/6 mice. Psychopharmacol. 2005;177:256–263. doi: 10.1007/s00213-004-1944-1. [DOI] [PubMed] [Google Scholar]