Abstract
Longitudinal research indicates that approximately 50% of women treated for gynecologic cancer have sexual dysfunctions as they recover and become cancer survivors. This outcome occurs in the context of satisfactory quality of life in other domains. This study, comparing gynecologic cancer survivors (n = 61) and gynecologically healthy women (n = 74), documents the reliability of the latter observations with measures of quality of life (general, depressive symptoms, social contacts, and stress), sexual functioning, and health. Of added importance are analyses focused on variables that may predict risk for sexual morbidity. Specifically, sexual self-schema is tested as an important, sexually relevant individual difference. In regression analyses that controlled for estimates of precancer sexual behavior (intercourse frequency), extent of disease–treatment, and menopausal symptoms, sexual self-schema accounted for significant variance in predicting current sexual behavior and responsiveness.
The role of individual difference factors as predictors for psychological and behavioral morbidity has been an important focus of research in health psychology (see review by Adler & Matthews, 1994). Ganz and her collaborators (Coscarelli Schag et al., 1993; Ganz et al., 1993) have noted the importance of such an effort for cancer research, as there has been a shift from description of adjustment processes to the identification of risk factors for psychosocial distress. With a disease such as cancer, there are medical contributors to risk, such as the extent of disease or treatment, as indicated in studies of breast cancer patients (e.g. Bloom et al., 1987; Maunsell, Brisson, & Deschenes, 1992), but psychological variables appear important too. Specifically, individual differences appear relevant, and furthermore, we have suggested that predictive power may be enhanced by choosing ones that are outcome specific (Andersen, 1994a). For example, if emotional distress is the outcome to be predicted, Carver’s data (Carver et al., 1993) suggest that optimism is important. In their longitudinal study of women with breast cancer, they found that optimism was inversely related to later distress and, moreover, that this effect appeared to be mediated by the tendency of optimists to cope differently. In coping, optimists used acceptance and positive refraining rather than denial or behavioral disengagement.
Our research has focused on one area of distress and behavioral dysfunction—sexual morbidity—for female survivors. Several controlled studies, retrospective as well as longitudinal, document that sexuality is the life area that undergoes major change for women with cancer (see review by Andersen, 1985). For those with gynecologic cancer, there have been many studies describing the sexual problems and they suggest that approximately 50% of women have continuing, difficult sexual problems or dysfunctions (e.g., see Andersen & van der Does, 1994, or Weimar Schultz, van de Weil, Hahn, & Bouma, 1992, for reviews). None of these studies, however, have specified models for predicting which women would be at risk for the development of sexual problems. During times of limited resources for psychosocial services, it is increasingly important to identify individuals most in need of preventive or rehabilitative care. Our prior descriptive research with women with cancer provides the ground work for this study: a test of a specific individual difference factor—sexual self-schema—for predicting risk for sexual morbidity.
Self-schema and the use of a schema to process information in one’s environment are central concepts in social cognition research on the self (e.g. Markus & Wurf, 1987). This theoretical view posits that individuals have important self-views, and, moreover, these self-views or self-schemas are thought to be dynamic and multifaceted. There may be some aspects of the self that may be more central than other ones and, we suggest, there are aspects specifically relevant to sexuality. We have formulated the construct of sexual self-schema and operationalized it with a 24-item trait adjective measure (Andersen & Cyranowski, 1994). Specifically, a sexual self-schema (or sexual self-concept) is a cognitive view about sexual aspects of oneself; it is derived from past experience, it is manifest in current experience, and it guides the processing of domain-relevant social information. When well articulated, it functions not only as a quick referent of one’s sexual history but also as a point of origin for information—judgments, decisions, inferences, predictions, and behaviors—about the current and future sexual self. In addition to regulating interpersonal processes, sexual schema also appears to mediate interpersonal processes, the most obvious being sexuality within relationships. Women who differ in the valence of their sexual self-views have very different sexual lives. Women with a positive sexual schema enter sexual relationships more willingly, have a more extensive behavioral repertoire, evidence more positive emotions when in sexual relationships, and anticipate having positive sexual relationships in the future. Also, the affects and behaviors indicative of loving, intimate attachments are central to women with a positive sexual schema. In contrast, women with a negative sexual schema tend to describe themselves as emotionally cold or unromantic and, as well, they are behaviorally inhibited in their sexual and romantic relationships. They may describe themselves as self-conscious, embarrassed, or inexperienced in sexual matters. Importantly, our longitudinal data indicate that these are stable self-views, impervious, for example, to the passage of time or the waxing and waning of specific sexual or romantic relationships.
The present research is an analysis of the construct with cancer survivors. Because this was an initial test, we chose an efficient strategy (a cross-sectional design) and a cancer group (women treated for gynecologic cancer) with a high incidence (approximately 50%) of sexual morbidity. Consistent with the conceptualization, we predicted that women with negative sexual self-schemas would be at greater risk for sexual difficulties during the survivor period. Specifically, women with negative self-views of their sexuality would be predicted as having lower levels of general sexual responsiveness and, in fact, engage in lower rates of sexual behavior. Conversely, women with a positive sexual schema were predicted to have higher levels of sexual responsiveness and higher rates of sexual behavior. Our previous longitudinal research documented that for approximately 50% of gynecologic cancer survivors, posttreatment sexual difficulties do not resolve. Instead, sexual difficulties are an “island” of disruption in an otherwise positive scenario for major life areas, including mood and social adjustment (Andersen, Anderson, & deProsse, 1989a, 1989b). Although the purpose of this study was to be an initial field test of the schema construct, we felt it important to again document the reliability of this dichotomy. Therefore, we included data from an age-matched sample of healthy women to provide the comparison of the groups on both sexual and nonsexual quality of life (QoL; e.g., mood, social integration, stress) domains before proceeding to the test of the schema construct.
In formulating this question, we were mindful of at least three other variables that might be important correlates of the level of sexual morbidity (Andersen, 1993; 1994a). First, sexual status before the onset of cancer is important. This would include consideration of whether a woman was sexually active or not (i.e., sexually active–inactive), and, if active, a quantification of important sexual activities, such as intercourse frequency. The predictive usefulness of sexual status variables for adult sexual behavior extends beyond tests with healthy adults (see Kinsey, Pomeroy, Martin, & Gebhard, 1953, as well as contemporary demonstrations, Lanmann, Gagnon, Michael, & Michaels, 1994; Wyatt, Peters, & Guthrie, 1988a, 1988b) to studies of sexuality for adults with chronic conditions and illnesses (e.g. Curry, Levine, Jones, & Kurit, 1993). Second, cancer and its treatments may directly change the sexual body and sexual responses. Distinctions among the extent of disease and treatments can be made using disease stage and treatment information, including data about toxicities and short- and long-term side effects. For women with gynecologic cancer, studies have shown differential levels of morbidity from limited surgeries, for example, in contrast to radical surgeries or combination therapy (see Andersen & van der Does, 1994 for a review). Third, health status in general, and hormonal status in particular, are important correlates of sexual functioning for women. For example, menopausal changes can produce significant gynecologic and psychologic effects (see Pearce, Hawton, & Blake, 1995, or Walling, Andersen, & Johnson, 1990, for discussions). Thus, in considering these variables, we formulated the difficult test for the schema construct: Would sexual self-schema predict posttreatment sexual functioning beyond known correlates, specifically prior sexual status, extent of disease and treatment, and menopausal symptoms?
Method
Participants
Cancer Survivors
Sixty-one women initially diagnosed with Stage I or II gynecologic cancer participated. Disease sites and stages included the cervix (n = 22, Stage I; n = 3, Stage II), endometrium (n = 21, Stage I; n = 4, Stage II), ovary (n = 7, Stage I), vulva (n = 2, Stage I; n = 1, Stage II) and vagina (n = 1, Stage I), and reflected the expected distribution of sites among localized gynecologic tumors (American Cancer Society, 1996). Cancer treatments received included surgery only or combinations of surgery, radiotherapy, and chemotherapy. Mean time since diagnosis was 21.4 months (range, 8 to 60 months; SD = 12.4 months), and mean time since treatment was 18.8 months (range, 6 to 57 months; SD = 12.3 months). A demographic analysis revealed that the mean age for the participants was 49 years (range, 24 to 73; SD = 13.4), the mean level of education was 13 years (vocational school), the average income ranged from $20,000 to $35,000, and the racial distribution of the group was 2 African American and 59 Caucasian women. A majority (79%) of the sample was postmenopausal, either naturally (46%) or prematurely (33%) as a result of cancer treatment, and 66% were married or living with a partner.
Healthy Women
Seventy-four women without a history of cancer served as comparison participants to estimate the base rate of sexual activity and responsiveness and general QoL among similarly aged healthy women. Participants included women seeking routine gynecologic care (n = 42) or women who were “older” undergraduate students (n = 32) at a large midwestern university. A demographic analysis revealed that the mean age was 42 years (range, 25 to 75 years; SD = 11.2), the mean level of education was 14 years (associate’s degree), the average income ranged from $35,000 to $50,000, and the racial distribution included 6 African American, 1 Hispanic, 2 “other,” and 65 Caucasian women. Furthermore, 20% of the sample was postmenopansal, 16% perimenopausal, and 64% premenopausal; 66% were married or living with a partner.
Measures
Individual Difference in Sexuality
The Sexual Self-Schema Scale for Women was used (Andersen & Cyranowski, 1994). This scale contains 26 trait adjectives (e.g., cautious, loving, open-minded, experienced) plus 24 adjective fillers (e.g., generous, shallow, kind, practical) that are self-rated from 0 (not at all descriptive of me) to 6 (very descriptive of me). Factor-analytic studies reveal that the items tap three dimensions: (a) loving–romantic, (b) direct–open, and (c) embarrassment–conservatism. Items from Factors 1 and 2 were summed, and items from Factor 3 were subtracted so that a schema score can range from −42 to 102, with numerically lower scores representing a more negative sexual self-view and higher scores reflecting a more positive self view. Comparison of “younger” and “older” female samples reveals that there are no generational differences, and, importantly, process studies indicate that respondents are unaware that a sexual construct is being assessed. Internal consistency for the scale is .75. Test–retest reliability indicates stability, with 2- week estimates of .89 and 2-month reliability of .88. The measure is uncontaminated with either social desirability (r = −.11 with the Marlow–Crowne) or negative affect (r =−.13) biases.
Quality of Life (QoL)
General evaluation
The SF-36 Health Status Survey (Ware, 1993) is a commonly used measure of QoL. The measure includes eight areas: (a) physical functioning, (b) social functioning, (c) role limitations caused by physical problems, (d) role limitations caused by emotional problems, (e) bodily pain, (f) general mental health, (g) vitality, and (h) general health perceptions. Studies have reported reliability coefficients between .81 and .88.
Social network
The Berkman and Syme Social Integration Scale (Berkman & Syme, 1979) was used to quantify level of social contacts with spouse, friends, family, and church and social group memberships and contacts. Validity data link the index to mortality among middle-aged women (Berkman & Syme, 1979).
Depression
A short form (11-items: Kohout, Berkman, Evans, & Cornoni-Huntley, 1993) of the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977) was used to assess current depressive symptomatology. Each of the 11 items was rated on a 3-point frequency scale: 0 = hardly ever or never, 1 = some of the time, and 2 = much or most of the time, with total scores ranging from 0 to 22. Internal consistency is .80.
Stress
The recent occurrence and rated distress for five negative events (e.g., death or serious illness of a close friend or relative, major financial difficulty, divorce or breakup) were considered. For each event, women indicated whether the event had occurred during the previous 12 months and, if so, how much they had been upset by the experience (3 = very much, 2 = moderately, 1 = not much, and 0 = not endorsed). Total number of events and the sum of distress ratings were calculated.
Sexuality and Sexual Functioning
Sexual behavior
Three types of data were obtained. The sexually active status of each woman was determined. Women were defined as sexually active if they indicated that they had experienced intercourse at least once a month for two specific 6-munth periods: (a) the 6-month period immediately preceding participation in the study and (b) either the 6-month period ending 2 years before participation for the healthy women or the 6-month period immediately before the onset of cancer symptoms, for the women with cancer. For the healthy women, we asked them to make estimates for the period 2 years previously because that interval was roughly equivalent to the same interval recalled for the cancer sample. For the cancer survivors, the 6-month period before symptom onset was chosen because previous research had documented that the appearance of cancer symptoms is disruptive to sexual functioning for women with gynecologic disease but that their level of activity before symptom onset is equivalent to that of healthy women (Andersen, Lachenbruch, Anderson, & deProsse, 1986). Using these two time periods for both samples, we determined that a subset of the women in both groups were not sexually active (inactive), and these women completed nine additional items to clarify the reason (or reasons) for sexual inactivity during the period.
Estimates of current and 2 years previous (past) frequency of intercourse were obtained, using a 7-point rating scale, ranging from 0 (this activity did not occur) to 7 (this activity occurred once a day). Data from cancer patients and healthy participants indicate 4-month test–retest reliability of .75 for these estimates (Andersen & Broffitt, 1988).
Current frequencies of three nonintercourse sexual behaviors (i.e., “friendly”–affectionate kissing of partner, passionate–sexual arousing kissing of partner, sexual fantasies–daydreams; Derogatis & Melisaratos, 1979) were obtained using a 9-point rating scale, ranging from 0 (this activity did not occur) to 9 (this activity occurred more than four times a day) for each behavior.
Sexual response cycle
A 25-item questionnaire measure assessing the sexual response cycle (Kaplan, 1979; Masters & Johnson, 1966) was used. Items were drawn from a structured interview format that had been used successfully in similar research with cancer and healthy samples (Andersen et al., 1989a). Each phase of the response cycle—desire, excitement, orgasm, and resolution—was assessed using four to eight items assessing the signs–symptoms of the phase. For example, the eight items for the excitement phase assessed such judgments as the woman’s general sexual excitement, awareness of vaginal lubrication, and pain–discomfort. Four additional items provided a general evaluation–satisfaction with sexuality (e.g., satisfaction with the frequency of sexual activity). Reliability (internal consistency) estimates for the sub-areas were .84 (desire), .84 (excitement), .70 (orgasm), .79 (resolution), and .70 (general).
Global evaluation
A 9-point scale ranging from 0 (could not be worse) to 9 (could not be better), with 4 = average as the midpoint was used for women to rate their view of their sexual life for the past and current time points as defined above. This item is drawn from the Derogatis Sexual Functioning Inventory (Derogatis & Melisaratos, 1979).
Health Status
Systems evaluation
The disease and associated therapy for 12 body systems (e.g,, cardiovascular, pulmonary, gastrointestinal) were quantified to document the general health of the women. Each system was rated on a 5-point scale from 0 (no symptoms) to 4 (most severe, life threatening). Four-month test–retest reliability is .71 (Andersen et al., 1989a).
Menopausal status and symptoms
Interview, medical chart, and physician evaluation data were used to determine current menopausal status and any recent changes in menopausal status. Women were considered premenopausal if they had monthly menses for the 6 consecutive months immediately before study entry. Women were assigned to one of the following three categories: (a) no change in menopausal status; (b) change from premenopausal to postmenopausal with subsequent estrogen replacement therapy (the cause of change, including surgical or radiation-induced menopause or natural processes was also noted) and (c) change from premenopausal to postmenopausal without estrogen replacement therapy. The cause of any change (i.e., surgical or radiation induced menopause, natural processes) was noted.
Participants indicated the occurrence and level of annoyance on a 6-point scale (0 = symptom did not occur, 1 = symptom occurred but not at all bothersome, and 5 = symptom occurred and was extremely bothersome) for each of 48 physical symptoms–signs of estrogen deficiency and menopause (Blatt, Wiesbader, & Kupperman, 1953; Matthews, Wing, Kuller, Meilahn, & Plantinga, 1994). This measure can differentiate symptom experiences of women who are postmenopansal without estrogen replacement therapy, postmenopausal with therapy, or premenopausal (e.g., Matthews et al., 1990).
Physical activity
Women were provided with brief definitions of physical inactivity, and light, moderate, and heavy levels of physical activity. Women then rated on a 4-point scale (1 = inactive, 2 = light, 3 = moderate, and 4 = heavy activity level) three items: the level of activity at work, at home, and during leisure time. Two additional items asked if women had significantly changed to a more strenuous or less strenuous lifestyle during the past 12 months. These data were included because women who exercise tend to be less troubled by menopausal symptoms.
Extent of disease–treatment
For the women with cancer, disease stage at diagnosis and cancer treatment history were documented from medical chart review and, when necessary, physician consultation. From this information, the extent of disease–treatment was operationalized by distinguishing “limited” vs. “extensive” disease–treatment groups. For women who had had pelvic tumors (i.e., cervix, endometrium, ovary, or vagina), the limited group represented surgery only, whereas the extensive group included women who received combination therapy— radiation or chemotherapy, with or without surgery, or high-dose radiotherapy alone. Women with vulvar disease were classified on the extent of surgery to the external genitals, as sexual outcomes vary with the magnitude of surgery (Andersen & Hacker, 1983; Andersen, Turnquist, LaPolla, & Turner, 1988); women who received a modified vulvectomy were assigned to the limited group, and the woman who received a radical vulvectomy was assigned to the extensive group. Other data included time since diagnosis and time since last treatment in months.
Procedures
Cancer patients were recruited by an experimenter (Xichel A. Woods) or the assisting gynecologic oncology staff during a 9-month period from consecutive outpatients seeking routine, follow-up examinations in a Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, at a large university medical center. Women were excluded from participation for any of the following reasons: age <20 or >75 years; history of advanced disease (Stage III or IV) at diagnosis or recurrent disease; time since diagnosis–treatment <6 months or >5 years.
Healthy participants were recruited in the same manner during the same period from two locations at the same university. Women returning for routine annual gynecologic exams in the same obstetrics–gynecology department served as the primary site for recruitment. Second, healthy women aged between 25 and 75 years were also recruited from the pool of research participants enrolled in introductory psychology. Women were preliminarily screened by their age and invited to participate if they had no history of significant disease (e.g., cancer) or gynecologic condition (e.g., pregnancy, benign hysterectomy, in situ dysplasia, urinary incontinence, prolapsed uterus, sexually transmitted disease) within the previous 2 years. Comparison participants were selected to match the age distribution of the cancer group by decades (e.g., 40s, 50s, 60s).
All women were approached for participation in a study entitled “Women’s Health,” described as an inquiry into the relationship between women’s health and self-concept in which participants would complete several questionnaires regarding health habits, moods, aspects of their social life, health conditions, and sexuality. After informed consent, the medical systems evaluation was completed with the woman. She was then provided with a questionnaire packet for return by mail. Women contacted through the clinics received $10 for participation, whereas students received 2 hr of experiment participation credit.
Results
Part 1: Group Comparison Analyses on the Pattern of Adjustment
Nonsexuality QoL Outcomes
Of the 78 cancer patients eligible for the study, 10 declined to participate. Reasons for nonparticipation from the women included being too busy or not interested (n = 5) or viewing the study topics as too personal (n = 5). In addition, 7 women agreed to participate but failed to return their questionnaires. One-way analyses of variance (ANOVAs) were conducted between participants (n = 61) and nonparticipants (n = 17) on all available medical status measures, including site of disease, stage at diagnosis, treatments received, change in menopausal status, time since diagnosis, and time since past treatment. There were no significant differences on any medical or follow up variable, with the exception of percentage of women receiving estrogen replacement therapy, F(1, 76) = 5.2, p < .02; fewer of the participants (30%) than nonparticipants (60%) were receiving estrogen replacement therapy.
Comparisons were made between the cancer survivors (n = 61) and the healthy women (n = 74) on demographic variables. There were no significant differences (p > .10) between the samples on employment status, occupation, or race, although the groups differed significantly (p < .05) in age (49 years for the cancer patients and 41 for the healthy group) and education Regarding the latter, the cancer patients were characterized as having “some vocational training” after high school graduation, whereas the healthy sample was characterized as having “some college.” This pattern of the two samples is consistent with the higher incidence of gynecologic tumors in lower educational groups (Baquet, Horm, Gibbs, & Greenwald, 1991) and our strategy of selecting some of the healthy women from “ older” undergraduate women. However, we wanted to rule out potential biases in the data that were due to these factors, and we examined correlations between age and education and the QoL variables. All correlations were not significant and of small magnitude (e.g., r = .03 for age and the SF-36 total score).
A one-way (Group: Cancer vs. Healthy) multivariate analysis of variance (MANOVA) was conducted for the nonsexuality QoL measures (including general, social network, depression, and stress). There was an overall main effect for group F(5, 93) = 2.3, p < .04; however follow-up ANOVAs for the individual measures found no significant group differences. The only individual measure that approached significance was the general measure (SF-36), F(3, 86) = 3.3, p = .08; healthy women scored slightly higher (M = 116.3, SD = 12.9) than the cancer patients (M = 111.3, SD = 16.2).
Health
A MANOVA was conducted for the measures (systems evaluation, menopausal symptoms, and physical activity). There was no overall main effect for group (p > .20).
Sexual Functioning
Preliminary analyses and overview
The healthy sample and cancer patient sample were compared on the women’s estimates of prior (i.e. 2 years previous) sexual activity. Women were regarded as previously sexually active if they reported having had intercourse at least once a month during the time in question. The data indicated that 34% of the women eventually diagnosed with gynecologic cancer were sexually inactive and 20% of the healthy women were inactive. The most common reason for inactivity in both groups was the lack of a partner (71% of the inactive cancer patients and 53 % of the inactive healthy women). Considering the remaining reasons, there was a similar pattern of responding between groups. For example, among the sexually inactive women, 14% of the inactive cancer survivors and 20% of the inactive healthy women reported that they had been disinterested in sexual activity during the prior period. As the focus of the investigation was the impact of gynecologic cancer on sexual functioning, women not previously engaged in sexual activity were not considered in any further analyses (we note that the QoL and health analyses previously discussed were repeated with the sexually active women from each group and the results were comparable with only the mean values changing slightly).
Comparisons were made between the previously sexually active cancer survivors (n = 40) and the previously active healthy women (n = 59) on demographic variables. There were no significant differences (p > .10) between the samples on age (cancer M = 45 vs. healthy M = 41), occupation, employment status, marital status, or race, although the groups differed significantly (p < .05) in education. As previously, the cancer sample was characterized as having “some vocational training” after high school graduation, whereas the healthy sample was characterized as having “some college.” Although this group difference was expected and small (i.e., equivalent to 1 year of postsecondary school), we nevertheless examined correlations between education and sexual outcomes for the group comparisons, and they were found to be insignificant (p > .10) and of low magnitude (e.g., r = .05 for education and previous frequency of intercourse).
An assumption of the design used here is that 2 years previously the two groups (40 cancer survivors and 59 healthy women) had comparable levels of sexual functioning. Therefore, the groups were compared on measures of previous sexual activity. There were no significant differences (p > .10) in previous frequency of intercourse (M = 3.0, SD = 1.6 for cancer patients; M = 3.5, SD = 1.6 for healthy sample), indicating that the women in both groups had recalled having intercourse, on average, of once per week. There was also no significant difference (p > .70) in their global evaluation of their previous sexual life (M = 5.1, SD 1.6 for cancer patients; M = 4.9, SD = 1.8 for healthy sample), indicating that all women recalled their prior sexual life as above average in quality, suggesting comparable baselines of sexual activity for the groups.
Finally, a one-way ANOVA was conducted between the groups (cancer vs. healthy) on the schema scores to test the comparability of the distributions. As expected, there were no group differences (cancer vs. healthy) for sexual self-schema, F(1, 97) = .01, p < .91. As the median scores for the two groups were similar (Mdns = 57 and 59 for the cancer patients and healthy sample, respectively), almost identical to that of the validation sample (Andersen & Cyranowski, 1994), a cutoff score of 59 was used to create negative and positive schema subgroups (i.e., schema score <60 vs. schema score >59, respectively). For the group comparisons (group: cancer vs. healthy) below, we include schema as an individual difference factor (schema: negative vs. positive). Our previous research would lead us to predict a main effect for schema and the absence of an interaction between group and schema.
Sexual behavior
For the current sexual behavior MANOVA, there was a significant effect for Group, F(2, 93) = 4.34, p < .01, but no significant effects for schema or the interaction. Follow-up ANOVAs were significant, indicating that cancer survivors reported less frequent intercourse, F(3, 94) = 4.79, p < .05, and nonintercourse sexual activity, F(3, 94) = 8.66, p < .01. For example, women with cancer reported intercourse as occurring, on average, one to two times per month, whereas the healthy women reported intercourse occurring once a week.
Sexual response cycle
For this MANOVA, there were main effects for Group, F(5, 78) = 5.67, p < .001; and for Schema, F(5, 78) = 6.00, p < .0001; and no significant interaction effect. Follow-up ANOVAs revealed that cancer survivors reported lower levels of sexual desire, F(3, 82) = 10.7, p < .001; lower levels of sexual excitement, F(3, 82) = 24.0, p < .0001; disrupted resolution, F(3, 82) = 5.2, p < .05; and, in general, lowered responsiveness, F(3, 82) = 8.1, p < .01, than did healthy women. The main effect for schema indicated that women whose sexual self-schema was negative reported lower levels of sexual desire, F(3, 82) = 15.7, p < .001; lower levels of excitement, F(3, 82) = 11.8, p < .001; disrupted orgasm, F(3, 82) = 10.0, p < .01; disrupted resolution, F(3, 82) = 4.0, p < .05, and, in general, lowered responsiveness, F(3, 82) = 16.6, p < .0001, than did the women with a positive sexual schema. This difference between self-views is consistent with our prior research (see Part I, Study 4, in Andersen & Cyranowski, 1994).
Global evaluation
For this analysis, women’s reports of their (2 years) prior and current evaluations of their sexual life were used. A 2 × 2 (Group × Time) ANOVA was conducted. There was a main effect for Time, F(1, 96) = 14.1, p < .001, such that global evaluations decreased from the prior time to the current time. More importantly, the significant interaction, F(1, 96) = 6.25, p < .01, indicates that the cancer survivors reported a significantly greater decline in their sexual life (see Figure 1) when the two periods were considered.
Figure 1.
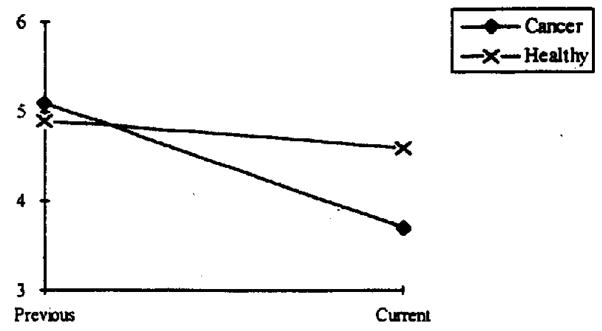
The significant Group × Time interaction suggesting significant declines in cancer survivors’ evaluations of their sexual life. Possible responses ranged from 0 to 9; 3 = somewhat inadequate; 4 = average; and 5 = above average.
Part 2: Tests of the Relationship Between Sexual Self-Schema and Sexual Morbidity
The above analyses suggest that sexual functioning is significantly disrupted after the diagnosis and treatment of gynecologic cancer. Next, we tested variables as correlates of risk for sexual morbidity. Using regression analyses, we entered the following variables: sexual functioning before diagnosis, extent of disease–treatment, current menopausal symptoms, and, finally, sexual self-schema. Prior sexual functioning was operationalized by the inclusion of only women who had been sexually active before their diagnosis and, in turn, their estimates of intercourse frequency. The extent of disease–treatment was quantified by distinguishing the limited versus extensive disease–treatment groups as discussed above (see Measures). The limited group consisted of 23 (58%) of the 40 women who were treated with surgery only for pelvic tumors. The extensive group consisted of 16 women with pelvic tumors who received combination therapy—radiation or chemotherapy, with or without surgery, and 1 woman who received a radical vulvectomy. The total score on the menopause symptom measure was used to quantify menopausal symptom disruption.
Two hierarchical multiple regression analyses were conducted to predict current sexual functioning. For both, variables were entered as hypothesized: prior intercourse frequency (Step 1), extent of treatment (Step 2), menopausal symptoms (Step 3), and schema (Step 4). Table 1 displays the results of both analyses (see Table 1). For the first analysis, each control variable added significant incremental variance to the outcome for current sexual behavior (sum of the items assessing frequency of intercourse and nonintercourse activities). Following these, schema added an additional, significant, 6% of the variance, with a total of 48% of the variance accounted for by the predictors. A second analysis was conducted to predict current sexual responsiveness, operationalized by the total sum of the scores for the phases of desire, excitement, orgasm, resolution, and general. For this outcome, only sexual schema was a significant predictor, adding an increment of 28% of the variance. The other variables (prior levels of sexual activity, extent of medical treatment, and symptoms) played a lesser role; the entire model accounted for 34% of the variance.
Table 1.
Test o f Sexual Self-Schema: Hierarchical Regression Analyses Predicting Sexual Functioning
| Step and predictor | β | Multiple R | R2 | t | df s |
|---|---|---|---|---|---|
| 1. Predicted outcome: Current frequency of sexual behavior | |||||
| 1. Previous frequency of intercourse | 1.88 | .49 | .24 | 3.43** | 1, 37 |
| 2. Extent of disease–treatment | −3.65 | .58 | .33 | −2.22* | 2, 36 |
| 3. Total menopausal symptoms | −0.06 | .65 | .42 | −2.36* | 3, 35 |
| 4. Sexual self-schema | 0.09 | .69 | .48 | 1.97* | 4, 34 |
|
| |||||
| 2. Predicted outcome: Total sexual responsiveness | |||||
| 1. Previous frequency of intercourse | 0.33 | .03 | .00 | 0.18 | 1, 34 |
| 2. Extent of disease–treatment | −6.01 | .19 | .04 | −1.10 | 2, 33 |
| 3. Total menopausal symptoms | −0.07 | .24 | .06 | −0.82 | 3, 32 |
| 4. Sexual self-schema | 0.50 | .58 | .34 | 3.67** | 4, 31 |
p < .05.
p < .001.
Discussion
Women treated for gynecologic tumors represent approximately 40% of the female cancer survivor population (Byrne, Kessler, & DeVessa, 1992), and these data suggest that, in large measure, the cancer experience for the majority yields no generalized decrements in quality of life following a lengthy recovery. However, these women appear to undergo significant sexuality disruption; this is a familiar outcome (e.g., Andersen, 1994b). More importantly, the present article proposes, tests, and provides support for specific mechanisms, both medical and psychological, which may lead to differential levels of sexual morbidity.
Before discussing the findings, it is appropriate to discuss the strengths and weaknesses of the present methodology. Retrospective data collection does provide an efficient strategy for preliminary tests of novel concepts. We can not make cause–effect conclusions, but we have attempted to reduce the likelihood of plausible rival hypotheses for this pattern of data. This specific method—assessing previous sexual functioning (i.e., precancer responses and functioning of 2 years previously for the healthy women), as well as current functioning in a cancer group and a comparison group—yielded data that replicated retrospective findings using the same paradigm (e.g. Andersen & Jochimsen, 1985) and the later prospective study (Andersen et al., 1989a). Some might hypothesize that if the cancer experience had had a particularly adverse effect on one’s sexual functioning, then women might be overly positive in recalling their previous rates of activity or their global evaluations of their sex life. The fact that, again, women provide evaluations equivalent to the healthy women would suggest that this might not have been the case. Also, we juxtaposed, the sexuality data with that of the QoL assessment—measures of depressive symptomatology, social adjustment, and stressors—where no group differences were found. This pattern is the same as a prior longitudinal comparison showing a return to prior levels (precancer) of general adjustment as women recovered, yet with residual disruption in the domain of sexuality (Andersen, Anderson, & deProsse, 1989a, 1989b).
Turning to the focus of the article, the data in Part II provide an empirical test of a model discussed elsewhere (Andersen, 1993; 1994a) for predicting differential sexual morbidity. Although not every construct in the model was tested, the major variables were operationalized. Models and theories are important to advance our understanding of the psychological processes of adjustment to the cancer stressor. Additionally, they may have clinical usefulness, such as being used to identify high-risk groups and channel limited resources to those most in need. Furthermore, if models are to have practical utility usefulness, it is essential to include the most predictive variables but also ones that can be easily determined and readily quantified. In addition to schema, we have chosen ones that could be easily identified (such as extent of disease–treatment; frequency of intercourse before symptom appearance) and available at or near the time of diagnosis and initial treatment. Some areas within the field of psychological and behavioral aspects of cancer have made sufficient progress in the recent 20 years to move from description to the testing of models that clarify the mechanisms of individual differences in psychological and behavioral responses to cancer (Burish, 1991), and this, too, may be one area ready for model testing.
We tested the schema construct with the cancer sample in the prediction of two different sexual outcomes—sexual responsiveness (e.g., desire, excitement, orgasm, and resolution) and sexual behavior. The size of the sexually active cancer sample (n = 40) constrained the number of variables that could be tested; however, we chose three of the most relevant ones. A marker of pretreatment sexuality (frequency of intercourse), extent of disease–treatment, and menopausal symptomatology were selected in view of their general relevance to sexuality and their specific relevance to data on the sexual outcomes after cancer (Weimar Schultz, van de Weil, Hahn, & Bouma, 1992). As indicated, sexual self-schema accounted for a significant and large portion of the variance (28%) in the prediction of current sexual responsiveness. In the prediction of sexual behavior, other components of the model were singly and, in combination, also powerful contributors, accounting for 42% of the variance. Still, sexual schema contributed an additional, significant 6% for a final total of 48% of the variance.
The regression data provide basic information on the psychological processes of female sexuality. The predictive value of sexual schema differed in the two regression analyses, which is consistent with two literatures. First, they underscore the dual, independent, importance of sexual responsiveness and sexual behavior in understanding female sexuality (see Andersen & Cyranowski, 1995, for a discussion). Second, that different psychological–behavioral processes would govern the two outcomes is consistent with data suggesting that frequency of intercourse for a woman in a heterosexual relationship is governed more by a male’s preference than the female’s (e.g. see Kinsey, Pomeroy, Martin, & Gebhard, 1953, for the earliest demonstration and Laumann, Gagnon, Michael, & Michaels, 1994, for a more recent one). Thus, if sexual schema has a role in female sexuality, one might anticipate that its effect would be clearer in those aspects for which a woman has greater control, such as her own responsiveness, and less predominant when others also have influence, such as the frequency of partnered sex.
Regarding the latter, these data do not speak to the direct impact of sexual partners on women’s sexuality after gynecologic cancer. However, our reasons for including only previously sexually active women and entering the prior frequency of intercourse into the regression analyses reflected our effort to address, in part, the importance of having any sexual partner and further, the level of the couple’s intimacy before the cancer stressor. As indicated in the regression analysis for current sexual behavior, these are powerful variables. Our longitudinal studies (e.g., Andersen et al., 1989a, 1989b) provided suggestive data that during the women’ s recovery the partners may, in turn, begin to experience their own sexual difficulties (e.g., erectile problems) when confronted with a female partner with treatment-related sequellae (e.g., vaginal dyspareunia). Future research will need to examine the differential importance, if any, of more specific partner variables or one’s own sexual self-concept in sexual morbidity.
The findings are supportive for the consideration of sexual schema construct in understanding sexual outcomes after cancer. Consistent with the schema definition, we anticipated that women with a more negative sexual self-concept, in contrast to women with a more positive view, would have greater sexual morbidity. Women with a more negative sexual schema were expected to have more difficulties because they are, in general, less romantic or passionate in their emotions, less open to sexual experiences, and more likely to have negative feelings about their sexuality. Thus, in the context of cancer, with disease or treatment factors causing direct changes to the sexual body or sexual responses and symptoms of premature menopause, we anticipated that women with negative sexual self-schemas would evidence lower rates of activity and less responsiveness. We suggest that women with negative self-views of their sexuality might find, for example, that their sexual arousability may have lessened further, that they are less apt to try new sexual activities as a way to cope with their sexual difficulties, or they may be prone to negative cognitions or feelings, such as embarrassment, about any body changes. In earlier efforts (e.g. Andersen & Elliot, 1993), we have detailed a process model of the occurrence and maintenance of dysfunctional and nondysfunctional sexual response patterns in women with cancer. On the basis of our empirical findings, the dysfunctional pattern is characterized by low arousal, behavioral inhibition, and negativity—a constellation of responses relevant to sexual schema.
Finally, these data suggest that preventive or rehabilitative interventions are particularly important for the women with a less positive (more negative) view of her sexuality, and, moreover, the schema construct could give theoretical guidance to such efforts. A schema-guided intervention could, for example, challenge the women’s typical self-view. Techniques could be designed to enhance sexual self-concept, providing a woman with strategies for enhancing arousal, increasing the sexual behavioral repertoire, and lowering negative affects, such as embarrassment. Such possibilities, along with traditional behavioral strategies (Wincze & Carey, 1991) might provide the needed, important assistance to the woman who survives gynecologic cancer but must cope with the resulting sexual difficulties.
Acknowledgments
This research was supported by Grant PBR-89 from the American Cancer Society, Grant DAMD17-94-J-4165 from the U.S. Army Medical Research and Development Command, and awards from the Department of Psychology, College of Social and Behavioral Sciences, and the Sidney Pressey Honors Endowment Fund, Ohio State University.
We acknowledge the assistance of the following individuals: the female participants, Nicole Chaput in data collection, Jill Cyranowski for data analysis, Linda Havenar in identifying participants, and the assisting physicians George Lewandowski and Louis Vaccarello.
Contributor Information
Barbara L. Andersen, Department of Psychology, Ohio State University
Xichel A. Woods, Department of Psychology, Ohio State University
Larry J. Copeland, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Ohio State University
References
- Adler N, Matthews K. Health psychology: Why do some people get sick and some stay well? Annual Review of Psychology. 1994;45:229–259. doi: 10.1146/annurev.ps.45.020194.001305. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer facts & figures. Atlanta, GA: Author; 1996. [Google Scholar]
- Andersen BL. Sexual functioning morbidity among cancer survivors: Present status and future research directions. Cancer. 1985;55:1835–1842. doi: 10.1002/1097-0142(19850415)55:8<1835::aid-cncr2820550832>3.0.co;2-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL. Predicting sexual and psychologic morbidity and improving the quality of life for women with gynecologic cancer. Cancer. 1993;71(Suppl):1678–1690. doi: 10.1002/cncr.2820710437. [DOI] [PubMed] [Google Scholar]
- Andersen BL. Surviving cancer. Cancer. 1994a;74:1484–1495. doi: 10.1002/1097-0142(19940815)74:4+<1484::aid-cncr2820741614>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Andersen BL. Yes, there are sexual problems. Now, what can we do about them? Gynecologic Oncology. 1994b;52:10–13. doi: 10.1006/gyno.1994.1003. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Anderson B, deProsse C. Controlled prospective longitudinal study of women with cancer: I. Sexual functioning outcomes. Journal of Consulting and Clinical Psychology. 1989a;57:683–691. doi: 10.1037//0022-006x.57.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Anderson B, deProsse C. Controlled prospective longitudinal study of women with cancer: II. Psychological outcomes. Journal of Consulting and Clinical Psychology. 1989b;57:692–697. doi: 10.1037//0022-006x.57.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Broffitt B. Is there a reliable and valid measure of sexual behavior? Archives of Sexual Behavior. 1988;17:509–525. doi: 10.1007/BF01542339. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Cyranowski JM. Women’s sexual self schema. Journal of Personality and Social Psychology. 1994;67:1079–1100. doi: 10.1037//0022-3514.76.4.645. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Cyranowski JM. Women’s sexuality: Behavior, responses, and individual differences. Journal of Consulting and Clinical Psychology. 1995;63:891–906. doi: 10.1037//0022-006x.63.6.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Elliot ML. Sexuality for women with cancer: Assessment, theory and treatment. Sexuality and Disability. 1993;11:7–37. [Google Scholar]
- Andersen BL, Hacker NE. Psychosexual adjustment after vulvar surgery. Obstetrics and Gynecology. 1983;62:457–462. [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Jochimsen PR. Sexual functioning among breast cancer, gynecologic cancer, and healthy women. Journal of Consulting and Clinical Psychology. 1985;53:25–32. doi: 10.1037//0022-006x.53.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Lachenbruch PA, Anderson B, deProsse C. Sexual dysfunction and signs of gynecologic cancer. Cancer. 1986;57:1880–1886. doi: 10.1002/1097-0142(19860501)57:9<1880::aid-cncr2820570930>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Tumquist D, LaPolla J, Turner D. Sexual functioning after treatment of in-situ vulvar cancer: Preliminary report. Obstetrics and Gynecology. 1988;71:15–19. [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, van der Does J. Surviving gynecologic cancer and coping with sexual morbidity: An international problem. International Journal of Gynecologic Cancer. 1994;4:225–240. doi: 10.1046/j.1525-1438.1994.04040225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquet CR, Horm JW, Gibbs T, Greenwald P. Socioeconomic factors and cancer incidence among blacks and whites. Journal of the National Cancer Institute. 1991;83:551–557. doi: 10.1093/jnci/83.8.551. [DOI] [PubMed] [Google Scholar]
- Berkman LK, Syme SL. Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda County residents. American Journal of Epidemiology. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Blatt MHG, Wiesbader H, Kupperman HS. Vitamin E and climacteric syndrome. AMA Archives of Internal Medicine. 1953;91:792–799. doi: 10.1001/archinte.1953.00240180101012. [DOI] [PubMed] [Google Scholar]
- Bloom JR, Cook M, Fotopoulos S, Flamer D, Gates C, Holland JC, Muenz LR, Murawski B, Penman D, Ross RD. Psychological response to mastectomy: A prospective comparison study. Cancer. 1987;59:189–196. [Google Scholar]
- Burish TG. Behavioral and psychosocial cancer research: Building on the past, preparing for the future. Cancer. 1991;67:865–867. doi: 10.1002/1097-0142(19910201)67:3+<865::aid-cncr2820671420>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Byrne J, Kessler LG, DeVessa SS. The prevalence of cancer among adults in the United States: 1987. Cancer. 1992;69:2154–2159. doi: 10.1002/1097-0142(19920415)69:8<2154::aid-cncr2820690823>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Carver CS, Pozo C, Harris SD, Noriega V, Scheier MF, Robinson DS, Ketcham AS, Moffat FL, Jr, Clark KC. How coping mediates the effect of optimism on distress: A study of women with early stage breast cancer. Journal of Personality and Social Psychology. 1993;65:375–90. doi: 10.1037//0022-3514.65.2.375. [DOI] [PubMed] [Google Scholar]
- Coscarelli Schag CA, Ganz PA, Polinsky ML, Fred C, Hirji K, Petersen L. Characteristics of women at risk for psychosocial distress in the year after breast cancer. Journal of Clinical Oncology. 1993;11:783–793. doi: 10.1200/JCO.1993.11.4.783. [DOI] [PubMed] [Google Scholar]
- Curry SL, Levine SB, Jones PK, Kurit DM. Medical and psychosocial predictors of sexual outcome among women with systemic lupus erythematosus. Arthritis Care and Research. 1993;6:23–30. doi: 10.1002/art.1790060106. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The DFSI: A multidimensional measure of sexual functioning. Journal of Sex and Marital Therapy. 1979;5:244–281. doi: 10.1080/00926237908403732. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Hirji K, Sim MS, Coscarelli Schag CA, Fred C, Polinsky ML. Predicting psychosocial risk in patients with breast cancer. Medical Care. 1993;31:419–431. doi: 10.1097/00005650-199305000-00004. [DOI] [PubMed] [Google Scholar]
- Kaplan HS. Disorders of sexual desire. New York: Brunner/Mazel; 1979. [Google Scholar]
- Kinsey AE, Pomeroy WB, Martin CE, Gebhard PH. Sexual behavior in the human female. Philadelphia: W. B. Saunders; 1953. [Google Scholar]
- Kohout EJ, Berkman LE, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D Depression Symptoms Index. Journal of Aging and Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Laumann EO, Gagnon JH, Michael RT, Michaels S. The social organization of sexuality: Sexual practices in the United States. Chicago: The University of Chicago Press; 1994. [Google Scholar]
- Markus H, Wurf E. The dynamic self-concept: A social psychological perspective. Annual Review of Psychology. 1987;38:299–337. [Google Scholar]
- Masters WH, Johnson VE. Human sexual response. Boston: Little, Brown; 1966. [Google Scholar]
- Matthews KM, Wing RR, Kuller LH, Meilahn EN, Kelsey SE, Costello EJ, Caggiula AW. Influences of natural menopause on psychological characteristics and symptoms of middle-aged healthy women. Journal of Consulting and Clinical Psychology. 1990;58:345–351. doi: 10.1037//0022-006x.58.3.345. [DOI] [PubMed] [Google Scholar]
- Matthews KM, Wing RR, Kuller LH, Meilahn EN, Plantinga P. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Archives of Internal Medicine. 1994;154:2349–2355. [PubMed] [Google Scholar]
- Maunsell E, Brisson J, Descbenes L. Psychological distress after initial treatment of breast cancer. Cancer. 1992;70:120–125. doi: 10.1002/1097-0142(19920701)70:1<120::aid-cncr2820700120>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Pearce J, Hawton K, Blake F. Psychological and sexual symptoms associated with the menopause and the effects of hormone replacement therapy. British Journal of Psychiatry. 1995;167:163–173. doi: 10.1192/bjp.167.2.163. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Walling M, Andersen BL, Johnson SR. Hormonal replacement therapy for postmenopausal women: A review of sexual outcomes and related gynecologic effects. Archives of Sexual Behavior. 1990;19:119–137. doi: 10.1007/BF01542227. [DOI] [PubMed] [Google Scholar]
- Ware JE. SF-36 Health Survey: Manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- Weimar Schultz WCM, van de Weil HBM, Hahn DEE, Bouma J. Psychosexual functioning after treatment for gynecological cancer: An integrative model, review of determinant factors and clinical guidelines. International Journal of Gynecologic Cancer. 1992;2:281–290. doi: 10.1046/j.1525-1438.1992.02060281.x. [DOI] [PubMed] [Google Scholar]
- Wincze JP, Carey MP. Sexual dysfunction: A guide for assessment and treatment. New York: Guilford Press; 1991. [Google Scholar]
- Wyatt GE, Peters SD, Guthrie D. Kinsey revisited, Part I: Comparisons of the sexual socialization and sexual behavior of white women over 33 years. Archives of Sexual Behavior. 1988a;17:201–239. doi: 10.1007/BF01541741. [DOI] [PubMed] [Google Scholar]
- Wyatt GE, Peters SD, Guthrie D. Kinsey revisited, Part II: Comparisons of the sexual socialization and sexual behavior of black women over 33 years. Archives of Sexual Behavior. 1988b;17:289–332. doi: 10.1007/BF01541810. [DOI] [PubMed] [Google Scholar]


