Abstract
Background
Silencing EphA2 has been shown to result in anti-tumor efficacy. However, it is not known whether increasing EphA2 expression specifically results in increased tumor growth and progression. We examined the effects of stable EphA2 transfection into poorly invasive ovarian cancer cells with regard to in vitro invasive and in vivo metastatic potential.
Results
In low cell density, EphA2-overexpressing A2780 cells (A2780-EphA2) displayed less cell-cell contact, increased cell-extracellular matrix (ECM) attachment and anchorage-independent cell growth compared to empty vector controls. There was no significant effect on anchorage-dependent cell proliferation, migration or invasion. Increased expression of EphA2 promoted tumor growth and enhanced the metastatic potential in A2780-EphA2 human ovarian cancer xenografts. The overexpression of EphA2 resulted in enhanced microvessel density (MVD), but had no effect on tumor cell proliferation.
Methods
EphA2 gene was introduced into A2780 cells by retroviral infection. The effects of increased EphA2 expression were examined on cellular morphology, and anchorage-dependent and independent cell growth. Furthermore, the effect of EphA2 overexpression on metastatic ability was determined using an orthotopic nude mouse model of ovarian carcinoma.
Conclusions
EphA2 promotes tumor growth by enhancing cell-ECM adhesion, increasing anchorage-independent growth and promoting angiogenesis.
Keywords: EphA2, ovarian cancer
Introduction
EphA2 is a tyrosine kinase receptor in the ephrin family, originally known as epithelial cell kinase (Eck). Developmentally, EphA2 was found to play a functional role in neuronal migration, and it is largely absent in most normal adult tissues.1-4 It is expressed in its active phosphorylated form in organs with a high proportion of epithelial cells such as skin, intestine and lung.1,5,6 For oncology applications, interest in EphA2 has grown recently due to its increased expression in most cancers including lung, breast, ovary, prostate, colorectal, skin and esophagus.7-13 Interestingly, EphA2 is located on chromosome 1p36.1, which is a hotspot for rearrangements in many human cancers including ovarian cancer.14-16
We have previously demonstrated that EphA2 is overexpressed in 76% of epithelial ovarian cancers.17 This increased expression was associated with high tumor grade and stage as well as decreased overall survival.17 We have also demonstrated that increased EphA2 expression in ovarian cancer samples is strongly associated with critical factors involved in angiogenesis and invasion such as greater microvessel density and higher matrix metalloproteinase (MMP) expression.18 Based on the high frequency of EphA2 overexpression and its association with aggressive tumor features, we and others have employed either agonist antibodies or in vivo siRNA to decrease EphA2 levels. Indeed, these approaches demonstrated decreased tumor growth and improved survival in orthotopic ovarian cancer mouse models.7,19 The mechanisms underlying this therapeutic efficacy were related to reduced levels of VEGF following decreased EphA2 levels and tumor angiogenesis.7,19 While these data have provided an increasing understanding of the role that EphA2 plays in malignant tumor behavior, in the current study, we asked whether increased EphA2 expression in poorly invasive ovarian cancer cells could directly promote malignant behavior. To answer this question, we utilized a series of in vitro and in vivo experiments following introduction of EphA2 into a non-expressing cell line.
Results
EphA2 overexpression in A2780 cells
We have previously demonstrated that EphA2 is expressed at high levels in ovarian cancer cells with aggressive features such as SKOV3ip1 compared to the poorly invasive A2780 cells or the non-transformed HIO-180 cells.17 Here, we asked whether introduction of EphA2 into the A2780 cells would promote aggressive behavior. Therefore, human EphA2 cDNA or an empty vector control was transfected by means of retrovirus into the A2780 cells. Following G418 selection, EphA2 expression was confirmed by Western blot (Fig. 1A and B). We also determined the phosphorylation status of the overexpressed EphA2 in the A2780-EphA2 cells and found that it was not phosphorylated.
Figure 1.
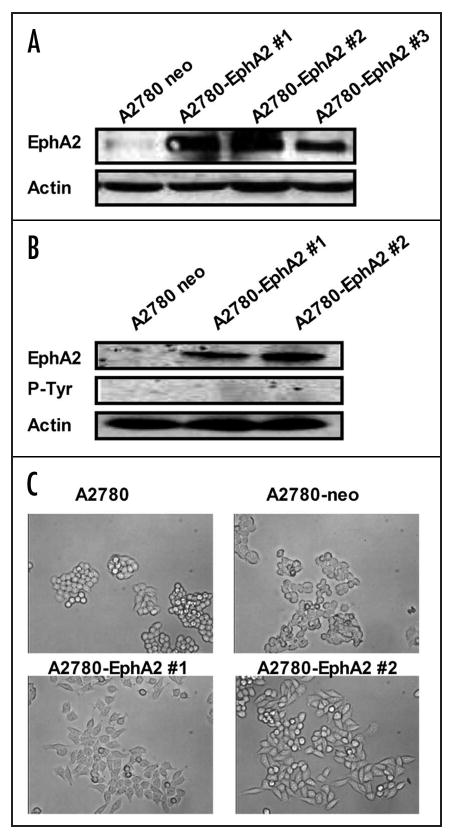
EphA2 overexpression in A2780 cells. (A) pNeoMSV-EphA2 or pNeoMSV vector was stably transfected into A2780 cells. Whole cell lysate was extracted, and Western blot was performed to detect EphA2 expression. (B) phospho-EphA2 status in EphA2 overexpressing A2780 cells. EphA2 expression was determined by Western blot, phospho-EphA2 expression was examined by immnoprecipitation (with EphA2 antibody) and Western blot (with phospho-tyrosine specific antibody). (C) Microscopic analyses of EphA2 transfected (A2780-EphA2) and empty vector (A2780-neo) transfected cells.
EphA2 overexpression promotes cell-ECM attachment
Microscopic analyses of EphA2 transfected (A2780-EphA2) and empty vector (A2780-neo) transfected cells revealed substantial morphological changes (Fig. 1C). While there were no obvious differences between A2780 and A2780-neo cells, the A2780-EphA2 cells displayed altered morphology with less cell-cell contact. Cell-cell contact and cell-ECM adhesions are inversely regulated in epithelial cells, and several investigators have demonstrated that ECM adhesion provides signals that promote cell growth, migration and survival.27-29 It has been suggested that EphA2 may facilitate cell-ECM interactions.30,31 Therefore, we next determined the effect of EphA2 on cell adhesion to a defined Matrix (collagen I/FBS/ and fibronectin). A2780-EphA2 cells (clone #1 and clone #2) showed significantly greater ability to adhere to collagen, FBS and fibronectin in comparison to A2780-neo cells (Fig. 2).
Figure 2.
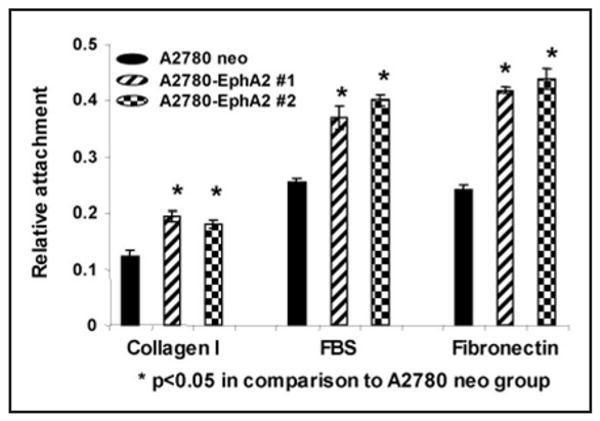
EphA2 overexpression promotes cell-ECM attachment. The A2780-neo and A2780-EphA2 cells were seeded on collagen I, FBS or fibronectin coated plates, and incubated for one hour, the amount of attached cells were evaluated by OD value.
EphA2 overexpression increases anchorage-independent cell growth in vitro
To determine the direct effect of EphA2 on A2780 cell growth and metastatic potential, we performed MTT assay to measure anchorage-dependent cell growth, and soft agar assay to analyze anchorage-independent cell growth. A2780-EphA2 cells significantly increased anchorage-independent cell growth; the number of colonies was increased 20% and 65% in A2780-EphA2 clone #1 and #2, respectively, in comparison to A2780-neo cells (Fig. 3A). Overexpression of EphA2 had no significant effect on anchorage-dependent cell growth (Fig. 3B), migration or invasion (data not shown).
Figure 3.
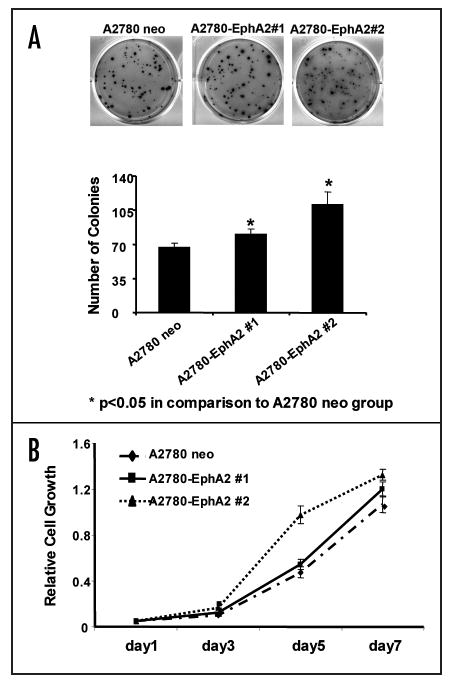
Effect of EphA2 on tumor cell growth. (A) EphA2 overexpression increases anchorage-independent cell growth. A2780-neo and A2780-EphA2 Cells were cultured in SeaPlaque agarose, and the number of colonies formed in soft agar were counted. (B) Effect of EphA2 overexpression on cell viability, as measured by MTT assay. There was no significant difference in cell viability between A2780-neo and A2780-EphA2 cells.
EphA2 overexpression promotes tumorigenesis in an orthotopic mouse model of ovarian carcinoma
The effect of EphA2 transfection into poorly-aggressive ovarian cancer cells with regard to metastatic ability was determined by using an orthotopic nude mouse model of ovarian carcinoma, which was previously described.7,17,32 Two clones of A2780-EphA2 cells, the non-transfected and vector-alone transfected A2780-neo cells were injected i.p. into female nude mice. After 23 days, mice were sacrificed and a necropsy was performed. The A2780-EphA2 cells resulted in significantly increased tumor growth. The tumor weight was 2.0 and 8.4 fold higher in A2780-EphA2 clones #1 and #2, respectively in comparison to the A2780-neo cells (p < 0.005; Fig. 4). The number of tumor nodules was significantly increased (4 fold) in the A2780-EphA2 clone #2 model (p < 0.005) in comparison to the A2780-neo model. In EphA2 clone #1 model, there was a 50% increase in the number of tumor nodules in comparison to A2780-neo, but this difference was not statistically different (p > 0.05). There was no significant difference in tumor growth between the non-transfected and vector-alone transfected A2780-neo cells. The metastatic spread was significantly greater in EphA2 overexpressing A2780 orthotopic mouse model compared to A2780-neo cells, indicating that EphA2 promoted the metastatic potential.
Figure 4.
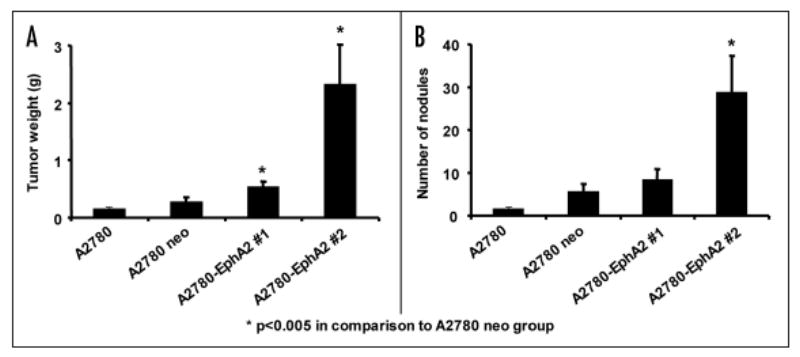
EphA2 overexpression promotes tumorigenesis in an orthotopic mouse model of ovarian carcinoma. Two clones of A2780-EphA2 cells, the non-transfected and vector-alone transfected A2780-neo cells were injected i.p. into female nude mice. The tumor weight (A) and number of nodules (B) were recorded 23 days after tumor cell injection.
EphA2 overexpression increases angiogenesis
Based on our previous work related to reduced angiogenesis with EphA2 silencing,7,33 we next asked whether the increased tumor growth with A2780-EphA2 cells was related to increased angiogenesis. To investigate this possibility, we examined microvessel density. First, we confirmed that the EphA2 expression level in A2780-EphA2 tumor tissue was indeed increased following transfection (Fig. 5). EphA2 was also detected at higher levels in tumors harvested from the A2780-EphA2 injected animals compared to the A2780-neo tumors. We also examined MVD using IHC for CD31; the MVD increased by 60% in both A2780-EphA2 (clone #1 and clone #2) models, in comparison to the A2780-neo model (p < 0.05).
Figure 5.
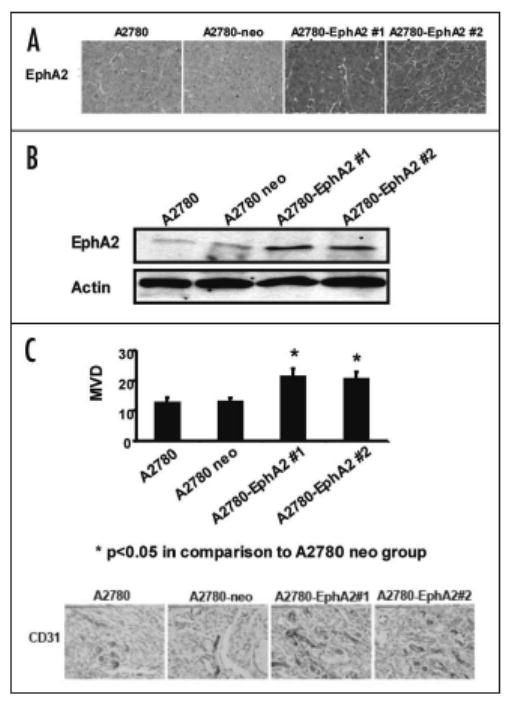
Overexpression of EphA2 increases MVD. EphA2 expression in tumor tissue collected from xenografts of A2780, A2780-neo and two clones of A2780-EphA2 cells were determined by IHC (A) and Western blot (B). Tumors were subjected to IHC for CD31(C) to allow identification of endothelial cells. A lumen with positive CD31 staining was counted as a single microvessel.
Discussion
In the present study, we demonstrated that EphA2 overexpression weakened cell-cell contacts, enhanced cell-ECM adhesion, and increased anchorage-independent cell growth. Furthermore, increased EphA2 expression promoted in vivo tumor growth by increasing angiogenesis.
We have previously demonstrated that even though EphA2 is overexpressed in highly invasive ovarian cancer cells, it is not phosphorylated.17 EphA2 overexpression in human ovarian cancers is associated with advanced tumor stage, grade and shorter overall survival.17 Similar findings have been reported in many other cancers including breast, cervix, colorectal and prostate cancers.33 The mechanisms underlying the increased expression of EphA2 in cancer cells is not fully understood, but may reflect altered ligand binding.34 Alternatively, the phosphotyrosine content of EphA2 may also be negatively regulated by associated phosphatases such as LMW-PTP.35,36 The effect of EphA2 overexpression was examined in breast cancer by Zelinski and colleagues9 who demonstrated that EphA2 overexpression was sufficient to confer malignant transformation and tumorigenic potential on non-transformed (MCF-10A) mammary epithelial cells. Nasreen and colleagues reported that EphA2 overexpression significantly enhanced the proliferation and haptotaxis of malignant mesothelioma cells.37 Results from this study strengthen our previous work by providing a new understanding of the effects of EphA2 on ovarian cancer growth.
Based on the overexpression of EphA2 in most tumors and its suspected role in tumor growth and progression, it is being pursued as a therapeutic target. For example, reducing EphA2 level using an agonist antibody or siRNA approaches can effectively decrease tumor growth in orthotopic ovarian cancer mouse models.7,33 The anti-tumor efficacy was reflective of anti-angiogenic effects mediated, in part, by reduced levels of vascular endothelial growth factor (VEGF) levels.7 Similar results with regard to the growth inhibitory effects of EphA2 suppression have been demonstrated in breast and prostate cancers, glioma and malignant mesothelioma.38-40
We then asked how does EphA2 promote tumorigenesis? In the present study, we demonstrated that EphA2 overexpression resulted in weakened cell-cell contacts and enhanced cell-ECM adhesion. It was known that interactions among tumor cells and their microenvironment provide structural and chemical cues that control many aspects of cancer cell behavior, including cell proliferation, survival, migration and invasion. Defects in cell-cell contacts are characteristics of aggressive cancer cells, and the ability to attach to ECM and remodel the local microenvironment is an important step in metastasis. Generally, cell-cell and cell-ECM adhesions are separate events and are inversely regulated in epithelial cells. Hu and colleagues showed that EphA2 selectively inhibits cell-cell adhesions by increasing cell attachment and upregulating the ECM protein fibronectin.41 We and others have shown evidence that EphA2 interacts with important adhesion and cytoskeletal proteins, including E-cadherin, Src, phosphatidylinositol 3′-kinase, Fak and p130Cas.7,9,42,43 A recent study has shown that overexpression of EphA2 destabilizes adherens junctions via a RhoA-dependent mechanism.44 EphA2 has been also shown to promote tumorigenesis by enhancing angiogenesis. The effects on angiogenesis may be direct (EphA2 overexpression has been noted in angiogenic blood vessels and may contribute to vasculogenic mimicry)18 or indirect (role in VEGF mediated angiogenesis).45,46 In the present study, we found that EphA2 overexpression resulted in significantly increased tumor growth and microvessel density, suggesting that increased angiogenesis likely contributed to the greater tumor growth.
In summary, this study provides further understanding of EphA2 function in tumor growth and progression. EphA2 overexpression weakened cell-cell contacts and enhanced cell-ECM adhesion. In vitro, it also increased anchorage-independent cell growth. EphA2 overexpression promoted tumor growth in A2780 human ovarian cancer xenografts, partially due to enhanced angiogenesis. Findings of this study provide further support for developing new therapeutic approaches targeted against EphA2 for ovarian cancer patients.
Materials and Methods
Cell culture and transfection
The A2780 ovarian cancer cell line was established from an untreated patient with epithelial ovarian carcinoma.20,21 It was selected for our work due to low baseline expression of EphA2.17 A2780 cells were maintained and propagated in vitro, as previously described.7,19 To establish stable cell lines with EphA2 overexpression, the pNeoMSV-EphA2 and pNeoMSV vectors were used for recombinant VSV-G pseudotyped retrovirus production by using 293GPG (kindly provided by Richard C. Mulligan22). 293 GPG stably expresses vesicular stomatitis virus glyconprotein (VSV-G) and MoMLV gal-pol coding sequences. pNeoMSV-EphA2 or pNeoMSV was introduced into 293GPG by a standard calcium phosphate precipitation method. Briefly, 30 μg of pNeoMSV-EphA2 or pNeoMSV were used for preparing the transfection mixture of one 10 cm plate of 293 GPG cells. After overnight transfection, the precipitate was taken off and VSV-G expression was induced by withdrawing doxycycline. Forty-eight hours after the induction, the medium was harvested and used for viral stock. The A2780 cells were then transfected with EphA2 or vector alone viral stock. Furthermore, the transfected cells were selected by adding G418 (800 μg/ml), while the EphA2 expression level was confirmed by Western blot. Four clones were isolated, and two with the highest EphA2 expression were used for subsequent in vitro and in vivo experiments.
Western blot and immunoprecipitation analysis
Western blot analysis was performed as described previously.7 Briefly, lysates from cultured cells and tumor tissue were prepared using modified RIPA buffer, the protein concentrations were determined using a BCA Protein Assay Reagent kit (Pierce Biotechnology, Rockford, IL). Lysates were loaded and separated on 10% sodium dodecyl sulfate–polyacrylamide gels. Proteins were transferred to a nitrocellulose membrane by semidry electrophoresis (Bio-Rad Laboratories, Hercules, CA), and then incubated overnight at 4°C with primary antibody [mouse antihuman/mouse EphA2 monoclonal antibody (clone D7, Upstate, Lake Placid, NY)], after washing with TBST, the membranes were incubated with 1 μg/mL horseradish peroxidase (HRP)-conjugated horse anti-mouse IgG (Amersham, Piscataway, NJ). HRP was visualized by use of an enhanced chemiluminescence detection kit (Pierce). To confirm equal sample loading, the blots were stripped and reprobed with an antibody specific for β-actin (0.1 μg/mL; Sigma). For immunoprecipitation studies, 500 μg of whole cell lysate was incubated with 6 μL of anti-EphA2 antibody for two hours at 4°C. Protein A sepharose beads (60 μL of a 1:1 dilution in PBS) were then added, and the mixture was incubated for two hours at 4°C. The beads were then separated and the supernatants were used for Western blot analysis with anti-pY primary antibody (Upstate, Lake Placid, NY), as described above.
Cell viability assay
Cells were plated in a 96-well plate, and four wells used for each experimental condition. At different time points, cell growth was assessed by adding 50 μL of 0.15% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma) to each well. Following incubation for two hours at 37°C, cells were reconstituted in 100 μL of dimethyl sulfoxide (DMSO, Sigma). The absorbance at 570 nm was recorded as relative cell growth parameter using a FALCON microplate reader (Becton Dickinson Labware, Franklin Lakes, NJ). Each data point was obtained by calculating the average of the four duplicate wells for each condition.
Cell attachment assays
Plates (96-well) were first coated with 100 μl/well of collagen I (20 μg/ml), 100% of Fetal Bovine Serum (FBS), fibronectin (20 μg/ml), or 2% denatured BSA for two hours at room temperature. Then, additional matrix was removed, and the plates were allowed to dry for two hours. Cells (5 × 104) were then seeded in coated 96-well plates in triplicate, and incubated for one hour at 37°C, washed with PBS, fixed with 3.7% formaldehyde for 30 minutes, and stained with 0.1% crystal violet and solubilized in 10% acetic acid. The amount of adherent cells was recorded by reading the OD value using spectrophotometry at 540 nm wavelength. The BSA coating was used as nonspecific binding control.
Colony formation in soft agar
Soft agar assay for anchorage-independent growth was performed as described previously.23 Briefly, Approximately 5 × 104 of cells were suspended in 4 mL of 0.35% SeaPlaque agarose (FMC BioProducts, Rockland, ME) supplemented with complete culture medium. This suspension was layered over 1.5 mL of 0.7% agar/medium base layer in one well of a 6-well plate. Colonies with a diameter larger than 0.05 mm were counted.
Animals, orthotopic in vivo model and tissue processing
A reproducible and reliable orthotopic model for ovarian cancer metastasis has been found to be useful for studying ovarian cancer biology.24 We and others have utilized intraperitoneal injection of ovarian cancer cells into nude mice to study metastatic ovarian cancer since this model mimics the pattern of tumor spread in human patients with advanced ovarian carcinoma.25,26 Female athymic nude mice (NCr-nu) were purchased from the National Cancer Institute, Frederick Cancer Research and Development Center (Frederick, MD) and maintained in specific pathogen-free conditions. The animals were cared for according to guidelines set forth by the American Association for Accreditation of Laboratory Animal Care and the US Public Health Service Policy on Human Care and Use of Laboratory Animals. All mouse studies were approved and supervised by the MD Anderson Cancer Center Institutional Animal Care and Use Committee. To produce orthotopic tumors, mice were injected with 1 × 106 tumor cells into the peritoneal cavity. The cells were trypsinized, washed and resuspended in Hanks' balanced salt solution (Gibco, Carlsbad, CA) at a concentration of 5 × 106 cells/ml. About three weeks after tumor cell injection, all mice in the experiment were sacrificed and a necropsy was performed. The individual tumor nodules were isolated from the supporting tissue and counted. The total tumor weight was also measured. Tissue samples were fixed in formalin for paraffin embedding, and frozen in optimal cutting temperature (OCT) media for preparation of frozen slides, or snap frozen for lysate preparation as described above.
Immunohistochemistry (IHC)
IHC was performed as described previously.7,23 Briefly, tumor tissues were fixed in formalin and embedded with paraffin, or fixed and frozen in OCT. Slides were deparaffinized sequentially in xylene, 100% ethanol, 95% ethanol, 80% ethanol and PBS. Antigen retrieval was then performed by heating slides in a steam cooker for 10 minutes in 0.2 M Tris buffer, pH 9.0. CD31 was stained using frozen slides. These slides were fixed in cold acetone for 20 minutes and did not require antigen retrieval. Endogenous peroxide was blocked by adding 3% H2O2 in methanol for five minutes, after washing, the nonspecific proteins were blocked using 5% normal horse serum and 1% normal goat serum in PBS for 15 minutes at room temperature. For EphA2 staining, slides were then incubated with 0.13 μg/mL mouse IgG Fc blocker (Jackson Laboratory, Bar Harbor, ME) for two hours. These slides were further incubated with primary antibody to EphA2 (EA5 clone, MedImmune, Inc.,) or CD31 (PECAM-1, rat IgG, Pharmingen, San Diego, CA) in blocking solution overnight at 4°C. After washing with PBS, the appropriate HRP-conjugated secondary antibody in blocking solution was added for one hour at room temperature. Slides were stained with DAB substrate (Phoenix Biotechnologies, Huntsville, AL) and counterstained with Gil No. 3 hematoxylin (Sigma). The intensity of protein expression was evaluated using OPTIMAS 6.5 software. To quantify MVD, the microvessels within five randomly selected 0.159-mm2 fields at ×100 were counted for each sample, a single microvessel was defined as a discrete cluster or at least three cells stained positive for CD31 (CD31+). The presence of a lumen was required for scoring as a microvessel.
Statistical analyses
Differences in continuous variables were analyzed using Student's t test or ANOVA as appropriate. A p value ≤0.05 was considered statistically significant. The Statistical Package for the Social Sciences (SPSS, SPSS Inc.,) was used for all statistical analyses.
Acknowledgments
We thank Dr. Richard C. Mulligan for his generosity in providing reagents; Drs. Rosemarie Schmandt, Jyotsna B. Halder, Selanere Mangala, Mirthala Moreno-Smith and Guillermo Armaiz-Pena for helpful discussions; Mary Catherine Rodgers for help in preparing the manuscript.
This research was funded in part by the Department of Defense grant #W81XWH-04-1-0227, the Marcus Foundation, and the UTMD Anderson Cancer Center SPORE in Ovarian Cancer (P50 CA083639).
Abbreviations
- ECM
extracellular matrix
- MVD
microvessel density
- MMP
matrix metalloproteinase
- FBS
fetal bovine serum
- VEGF
vascular endothelial growth factor
References
- 1.Lindberg RA, Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol Cell Biol. 1990;10:6316–24. doi: 10.1128/mcb.10.12.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gale NW, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–45. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, et al. Germ-line inactivation of the murine Eck receptor tyrosine kinase by gene trap retroviral insertion. Oncogene. 1996;12:979–88. [PubMed] [Google Scholar]
- 5.Lickliter JD, et al. Embryonic stem cells express multiple Eph-subfamily receptor tyrosine kinases. Proc Natl Acad Sci USA. 1996;93:145–50. doi: 10.1073/pnas.93.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Ruley HE. An enhancer element in the EphA2 (Eck) gene sufficient for rhombomere-specific expression is activated by HOXA1 and HOXB1 homeobox proteins. J Biol Chem. 1998;273:24670–5. doi: 10.1074/jbc.273.38.24670. [DOI] [PubMed] [Google Scholar]
- 7.Landen CN, Jr, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–70. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 8.Andres AC, et al. Expression of two novel eph-related receptor protein tyrosine kinases in mammary gland development and carcinogenesis. Oncogene. 1994;9:1461–7. [PubMed] [Google Scholar]
- 9.Zelinski DP, et al. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–6. [PubMed] [Google Scholar]
- 10.D'Amico TA, et al. Predicting the sites of metastases from lung cancer using molecular biologic markers. Ann Thorac Surg. 2001;72:1144–8. doi: 10.1016/s0003-4975(01)02979-4. [DOI] [PubMed] [Google Scholar]
- 11.Walker-Daniels J, et al. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–80. doi: 10.1002/(sici)1097-0045(19991201)41:4<275::aid-pros8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka H, et al. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004;95:136–41. doi: 10.1111/j.1349-7006.2004.tb03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duxbury MS, et al. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–56. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez AA, et al. Allele loss on chromosome 1p36 in epithelial ovarian cancers. Gynecol Oncol. 2001;82:94–8. doi: 10.1006/gyno.2001.6175. [DOI] [PubMed] [Google Scholar]
- 15.Sulman EP, et al. ECK, a human EPH-related gene, maps to 1p36.1, a common region of alteration in human cancers. Genomics. 1997;40:371–4. doi: 10.1006/geno.1996.4569. [DOI] [PubMed] [Google Scholar]
- 16.Pejovic T. Genetic changes in ovarian cancer. Ann Med. 1995;27:73–8. doi: 10.3109/07853899509031940. [DOI] [PubMed] [Google Scholar]
- 17.Thaker PH, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–50. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 18.Lin YG, et al. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer. 2007;109:332–40. doi: 10.1002/cncr.22415. [DOI] [PubMed] [Google Scholar]
- 19.Landen CN, Jr, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–8. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 20.Louie KGBB, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Radiation survival parameters of antineoplastic drug-sensitive and -resistant human ovarian cancer cell lines and their modification by buthionine sulfoximine. Cancer Research. 1985;45:2110–5. [PubMed] [Google Scholar]
- 21.Behrens BCHT, Masuda H, Grotzinger KR, Whang-Peng J, Louie KG, Knutsen T, McKoy WM, Young RC, Ozols RF. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Research. 1987;47:414–8. [PubMed] [Google Scholar]
- 22.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–6. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu C, et al. cFos is critical for MCF-7 breast cancer cell growth. Oncogene. 2005;24:6516–24. doi: 10.1038/sj.onc.1208905. [DOI] [PubMed] [Google Scholar]
- 24.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft and mouse allograft preclinical cancer models. Clinical Cancer Research. 2003;9:4227–39. [PubMed] [Google Scholar]
- 25.Tedjarati S, et al. Synergistic therapy of human ovarian carcinoma implanted orthotopically in nude mice by optimal biological dose of pegylated interferon alpha combined with paclitaxel. Clinical Cancer Research. 2002;8:2413–22. [PubMed] [Google Scholar]
- 26.Yoneda J, et al. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. Journal of the National Cancer Institute. 1998;90:447–54. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 27.Ruoslahti E. Cell adhesion and tumor metastasis. Princess Takamatsu Symp. 1994;24:99–105. [PubMed] [Google Scholar]
- 28.Keely P, Parise L, Juliano R. Integrins and GTPases in tumour cell growth, motility and invasion. Trends Cell Biol. 1998;8:101–6. doi: 10.1016/s0962-8924(97)01219-1. [DOI] [PubMed] [Google Scholar]
- 29.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–6. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 30.Domanska-Janik K, et al. Interrelations between nuclear-factor kappa B activation, glial response and neuronal apoptosis in gerbil hippocampus after ischemia. Acta Neurobiol Exp (Wars) 2001;61:45–51. doi: 10.55782/ane-2001-1383. [DOI] [PubMed] [Google Scholar]
- 31.Walker-Daniels J, et al. Differential regulation of EphA2 in normal and malignant cells. Am J Pathol. 2003;162:1037–42. doi: 10.1016/S0002-9440(10)63899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu C, et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res. 2007;67:1757–68. doi: 10.1158/0008-5472.CAN-06-3700. [DOI] [PubMed] [Google Scholar]
- 33.Landen CN, Kinch MS, Sood AK. EphA2 as a target for ovarian cancer therapy. Expert Opin Ther Targets. 2005;9:1179–87. doi: 10.1517/14728222.9.6.1179. [DOI] [PubMed] [Google Scholar]
- 34.Bartley TD, et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994;368:558–60. doi: 10.1038/368558a0. [DOI] [PubMed] [Google Scholar]
- 35.Kikawa KD, et al. Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J Biol Chem. 2002;277:39274–9. doi: 10.1074/jbc.M207127200. [DOI] [PubMed] [Google Scholar]
- 36.Chiarugi P, et al. LMW-PTP is a positive regulator of tumor onset and growth. Oncogene. 2004;23:3905–14. doi: 10.1038/sj.onc.1207508. [DOI] [PubMed] [Google Scholar]
- 37.Nasreen N, Mohammed KA, Antony VB. Silencing the receptor EphA2 suppresses the growth and haptotaxis of malignant mesothelioma cells. Cancer. 2006;107:2425–35. doi: 10.1002/cncr.22254. [DOI] [PubMed] [Google Scholar]
- 38.Wykosky J, Gibo DM, Debinski W. A novel, potent and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6:3208–18. doi: 10.1158/1535-7163.MCT-07-0200. [DOI] [PubMed] [Google Scholar]
- 39.Brantley-Sieders DM, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu DP, et al. Ephrin-A1 is a negative regulator in glioma through downregulation of EphA2 and FAK. Int J Oncol. 2007;30:865–71. [PubMed] [Google Scholar]
- 41.Hu M, et al. EphA2 induction of fibronectin creates a permissive microenvironment for malignant cells. Mol Cancer Res. 2004;2:533–40. [PubMed] [Google Scholar]
- 42.Miao H, et al. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–9. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 43.Carter N, et al. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas) Nat Cell Biol. 2002;4:565–73. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- 44.Fang WB, et al. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci. 2008;121:358–68. doi: 10.1242/jcs.017145. [DOI] [PubMed] [Google Scholar]
- 45.Brantley DM, et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–26. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- 46.Cheng N, et al. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol Cancer Res. 2002;1:2–11. [PubMed] [Google Scholar]


