Abstract
Bestrophins form Ca2+-activated Cl− channels when they are expressed heterologously. Here we report the functional characterization of murine bestrophin 1 (mBest1). We isolated mBest1 transcript from mouse heart and analyzed the biophysical properties and expression of this channel protein using a tetracycline inducible system. mBest1 expression is localized at the membrane of transfected HEK cells, in agreement with its role as a channel. Whole-cell patch clamp experiments revealed a calcium sensitive, time independent chloride current. mBest1 current displayed slight voltage dependence, exhibited an anion permeability sequence of SCN− > I− > Cl− and was sensitive to DIDS- and niflumic acid. Anion replacement studies were also performed on mBest2 and mBest3 and differences were observed in their relative permeability and slope conductance to SCN−. Our study provides the first characterization of the biophysical properties of mBest1 and a framework for the elucidation of the physiological role of bestrophins.
Keywords: Bestrophin, calcium-activated chloride channel
Introduction
Ca2+-activated Cl− channels (ClCa) are expressed in a variety of tissue types where they have important functions [1]. A complete understanding of these channels is limited by the fact that their molecular identity remains unresolved. Bestrophins are one family of proteins that have been described by a number of independent groups to be Cl− channels which are activated by cytosolic Ca2+ [2]. In each case Ca2+-sensitive Cl− currents (IClCa) were recorded using the patch clamp technique [3–10] and their current voltage (I-V) relationships displayed either ohmic, inwardly, or outwardly rectifying behavior [2]. Bestrophin Cl− channel function was corroborated with the identification of putative pore domain residues, which when mutated can alter the permeation and selectivity of Cl− [5; 6; 10–12].
In mouse, three bestrophin homologues (mBest1-3) have been identified in various tissue types using RT-PCR [12; 13]. Two independent groups have characterized the biophysical properties of mBest2 in detail [3; 5; 6; 11]. We and others have reported whole-cell patch clamp analysis for mBest3 [7; 8; 12]. To date there are no detailed reports on the electrophysiological properties of mBest1. hBest1, the ortholog of mBest1 sharing 62% amino acid identity, is the prototypic member of the bestrophin family [9; 10; 14; 15]. Mutations in this gene produce Best’s vitelliform macular dystrophy, an early onset form of macular degeneration [9]. hBest1 elicits IClCa when expressed heterologously [9; 10], however there are few comprehensive electrophysiological studies on hBest1 in terms of anion selectivity and pharmacology. Using a gene-silencing approach it was demonstrated that Best1 contributes to IClCa in epithelial tissues [15; 16]. More recently, mBest1 knockout (vmd2−/−) mice have been generated [14; 17]. One group suggested that mBest1, together with mBest2, plays a role in Ca2+-dependent Cl− secretion in mouse airways [14]. However, Marmorstein et al. reported that a IClCa was still present in retinal pigmented epithelial cells from vmd2−/− mice [17]. This suggests that either mBest1 plays no role in generating IClCa or that other bestrophins could be compensating for the absence of mBest1. Indeed, this group proposed that bestrophin 1 functions as a regulator of L-type Ca2+ channels [17; 18]. Since the electrophysiological properties of mBest1 in isolation are unknown, its contribution to IClCa could not be investigated further.
In this study, we used a tetracycline regulated expression system in combination with the patch clamp technique to provide a comprehensive report of the biophysical characteristics of recombinant mBest1. We demonstrate that mBest1 is expressed at the membrane of transfected cells, consistent with its role as a transmembrane chloride channel. This study provides evidence that mBest1 encodes a ClCa and it is the first examination of the biophysical and pharmacological properties of this channel. A preliminary report of these results has been presented [19].
Materials and Methods
Cloning of mBest1 from heart
Adult BALB/c mice were sedated by exposure to isoflurane prior to cervical dislocation and excision of the heart. This protocol was approved by the University of Nevada Institutional Animal Care and Use Committee. Total RNA was isolated using TRIzol (Invitrogen) and cDNA was prepared using Superscript II reverse transcriptase (Invitrogen). A 1658 bp fragment containing the coding sequence for mBest1 was amplified using AmpliTaq Gold® (Applied Biosystems) and mBest1specific primers. For expression studies, the stop codon was removed and mBest1 transcript was ligated into pcDNA4/TO/c-myc-HIS vector (Invitrogen) in frame with the C-terminal c-myc epitope tag. Recombinant plasmids were sequenced at the Nevada Genomics Center.
Tetracycline regulated expression of mBest1 in mammalian cells
Expression of mBest1 was carried out using a tetracycline regulated expression system (T-Rex™, Invitrogen) as described previously for mBest3 [12]. A stable line expressing mBest1-c-myc was generated in TRex-293 cells (express tetracycline repressor protein). Transfected and untransfected TRex-293 cells were seeded onto coverslips 24–48 h before recordings or immunocytochemistry. mBest1 channel expression was induced by tetracycline (1μg/ml) addition to the media. In some instances mBest1, mBest2 or mBest3 were transiently transfected into HEK TsA201 cells for patch clamp analysis. mBest3 in pcDNA4/TO/c-myc-HIS vector was generated as described [12]. mBest2 in pCMV-Sport6 (IMAGE clone ID 4989959) was obtained from Invitrogen. Each 35 mm culture dish was transfected with 2 μg plasmid DNA using Polyfect transfection reagent (Qiagen).
Analysis of mBest1-c-myc protein expression
Protein isolated from transfected and untransfected TRex-293 cells was examined by Western blotting in a manner similar to that described previously [12]. mBest1-c-myc reactivity was detected with a c-myc mouse monoclonal antibody (1:200 dilution; AbCam) followed by incubation with an alkaline phosphatase conjugated goat anti mouse antibody (1:7500 dilution; Promega). For immunofluorescence labeling, transfected and untransfected TRex-293 cells expressing mBest1-c-myc fusion protein were fixed in 4 % paraformaldehyde for 20 min and labeled with c-myc monoclonal antibody (1:2000 dilution) followed by secondary labeling with Alexa 488 conjugated chicken anti-mouse antibody (Molecular Probes), as described [12]. Propidium iodide was used as a nuclear stain. Confocal composites were constructed from Z-scans acquired using a Bio-Rad Radiance 2100 Laser scanning confocal microscope with LaserSharp 2000 software (Bio-Rad). Final images were prepared using Adobe Photoshop software.
Electrophysiological methods and recording solutions
Macroscopic currents were recorded at room temperature in the whole-cell configuration. The bath and pipette solutions were chosen to facilitate the recording of Cl− currents in isolation as described [12]. In all experiments, Cl− currents were studied with [Ca2+]i clamped at known concentrations. Pipette solutions contained 10 mM BAPTA, and free [Ca2+] was set to either <1, 250 or 500 nM by the addition of 0.84–8.7 mM CaCl2 as determined by the calcium chelator program EQCAL (Biosoft). For recording steady-state I-V relationships, cells were held at −50 mV and stepped from −100 to +100 mV in 10 mV increments for 1 s. The ionic nature of the charge carrier was determined from the reversal potential (Erev) values elicited by a ramp protocol. Cells were stepped from −50mV to +60 mV and then stepped to voltages between −100 mV and +100 mV (10 mV increments) followed by a repolarizing step to −60 mV. For external anion replacement experiments, 126 mM Cl− was replaced by an equimolar concentration of thiocyanate (SCN−), iodide (I−) or D-gluconate−. Changes in junction potential were minimized using a 3 M KCl agar-bridge. The relative permeability was determined by measuring the shift in the Erev upon changing the bath solution from one containing Cl− to another monovalent anion, where X is the substitute anion. The permeability ratios were estimated using the Goldman-Hodgkin-Katz (GHK ) equation as described [12]. All chemicals were obtained from Sigma-Aldrich. DIDS and niflumic acid were prepared in DMSO. Data are reported as the mean of n cells ± s.e.m.
Data analysis
Erev and pharmacological percentage block were determined by curve fitting of individual current traces using Clampfit (PClamp, version 9.2; Molecular Devices). All data were exported to Origin 7.5 (OriginLab) or Graphpad PRISM 3.0 for plotting and curve fitting. PRISM 3.0 was used to determine statistical significance between groups with one-way ANOVA followed by Dunnett’s Multiple Comparison test. p < 0.05 was considered statistically significant.
Results and Discussion
mBest1 is a membrane protein and induces IClCa when expressed heterologously
mBest1 cDNA cloned from heart was expressed in a tetracycline inducible expression system to characterize the channel properties. The mBest1 transcript we cloned corresponds to nucleotides 208-1869 of mBest1, Genbank accession # NM 011913, and is 100% identical. The mBest1 transcript was inserted into the pcDNA/TO/c-myc-His vector, in frame with a C-terminal c-myc epitope tag. This vector contains a tetracycline operon (TO) for tetracycline inducible expression. TRex-293 cells which express tetracycline repressor protein were transfected with mBest1-c-myc and a stable line was generated. This system enables rapid and efficient tetracycline inducible expression of ion channels in mammalian cell lines [20], as channel expression is under the control of a competition between the constitutively expressed tetracycline repressor protein and exogenously added tetracycline. Figure 1 shows how we adapted this system to investigate mBest1 expression by Western blotting, immunocytochemistry and whole cell patch clamp. For these experiments, mBest1 expression was induced by tetracycline addition to the cell media, and experiments were performed 3–6 hours later. mBest1/c-myc protein expression was examined by Western blotting using a c-myc antibody (Figure 1A). An immunoreactive signal (~64 kDa) is observed in the TRex-293 cells stably expressing mBest1/c-myc and induced with tetracycline. The predicted size of mBest1 protein is 63.8 kDA. For immunocytochemistry, mBest1-c-myc expressing cells were probed with a c-myc antibody. Figure 1B is a composite confocal image of a single TRex-293 cell expressing mBest1/c-myc protein. There is predominant membrane staining consistent with mBest1 being a transmembrane protein. The intracellular staining of mBest1 is likely to be the location of the over-expressed protein on intracellular membranous organelles. Control experiments included both untransfected cells and mBest1 transfected cells without tetracycline addition. In the absence of tetracycline, expression was not induced (data not shown).
Fig 1.
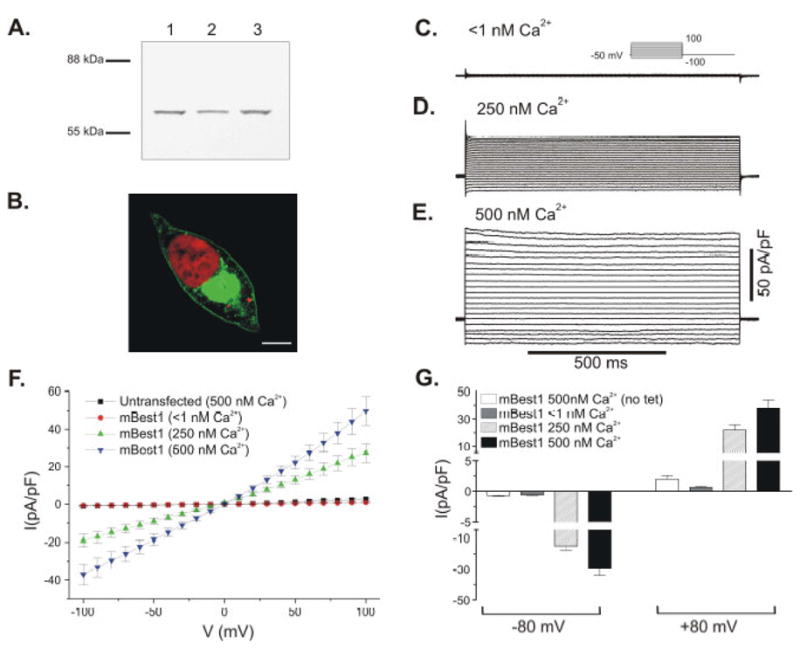
Tetracycline inducible expression of mBest1 channel in TRex-293 cells. (A) Western blotting of tetracycline induced mBest1/c-myc fusion protein expression in a TRex-293 stable cell line (Lanes 1–3). Membranes were probed with anti-c-myc. (B) Confocal image of mBest1/c-myc expression in a TRex-293 cell. c-myc (Alexa 488, green) and propidium iodide nuclear stain (red). The confocal image is composite of three Z sections taken from a Z stack (21 x 0.468μm steps) representing a region at the center of the cell as indicated by the presence of the nucleus. Scale bar is 5 uM. Panels C-E show families of currents recorded from TRex-293 cells transfected with mBest1 and expression induced with tetracycline. mBest1 currents were evoked with pipette solutions containing (C) <1 nM Ca2+, (D) 250 nM Ca2+ and (E) 500 nM Ca2+. The voltage protocol is shown as inset in C. (F) Mean I-V relationships for mBest1currents evoked in transfected TRex-293 cells by <1 nM Ca2+ (circles, n = 8), 250 nM Ca2+ (up triangles, n = 7), 500 nM Ca2+ (down triangles, n = 18) and 500 nM Ca2+ in untransfected TRex-293 cells (squares, n = 8). (G) Summary of mean current densities recorded at −80 and +80 mV. Error bars represent the s.e.m.
To confirm the role of mBest1 as a ClCa we used the whole-cell patch clamp technique. Under conditions designed to minimize the activity of endogenous K+ currents, Cl− currents were studied with free [Ca2+]i at fixed concentrations in the pipette solution, a technique used previously [12]. To investigate the requirement of intracellular Ca2+ for the activation of mBest1 currents, cells were treated with tetracycline and dialyzed with pipette solutions containing 10 mM BAPTA and either no added Ca2+ (free [Ca2+]i <1nM), 250 nM Ca2+ or 500 nM Ca2+. When [Ca2+]i was <1 nM the currents recorded were small (Figure 1C). Increasing [Ca2+]i to 250 nM (Figure 1D) or 500 nM (Figure 1E) led to increasingly larger mBest1 currents. Figure 1F shows the mean I-V relationships for currents evoked with <1 nM Ca2+ (n = 8), 250 nM Ca2+ (n = 7) and 500 Ca2+ nM (n = 18) from cells transfected with mBest1 and from untransfected cells with 500 nM Ca2+ (n = 8).
The mBest1 currents generated with both 250 and 500 nM Ca2+ displayed an almost linear I-V relationship and reversed close to zero as expected for a Cl− selective channel (Figure 1F). mBest1 Cl− currents recorded with 500 nM Ca2+ were largely time- and voltage-independent (Figure 1E), but exhibited small voltage dependence at the extremes of the voltage range (Figure 1F). These biophysical properties are similar to those of mBest2 and mBest3 shown previously in our studies, and in those of other groups [3; 6; 7; 12]. Control experiments were carried out in untransfected cells and in cells transfected with mBest1 but not treated with tetracycline. Neither of these control experiments displayed significant IClCa (Figure 1F and 1G respectively). The amplitude of the currents evoked by 500 nM Ca2+ in transfected and tetracycline treated cells was significantly larger at all potentials (p < 0.001) than currents evoked by the same pipette solution in these control cells. Figure 1G shows a summary of the mean current densities measured at −80 and +80 mV. This data shows that mBest1 currents elicited with 500 nM Ca2+ display slight outward rectification with current densities at −80 and +80 mV of −29.5 ± 4.6 pA/pF and 37.9 ± 5.9 pA/pF, respectively (n =18; p < 0.001).
There is heterogeneity in channel kinetics among the bestrophin homologues studied to date. Heterologously expressed hBest1, hBest2, dBest1 [9; 10], xBest2a and 2b [4], mBest2 [3; 6] and mBest3 [7; 8; 12] display little if any voltage-dependent activation/inactivation. However, expressed ceBest1 [9], hBest3 [10] and hBest4 [10; 21] currents display voltage-dependent kinetics. Bestrophin channels are opened by an increase in [Ca2+]i. This was demonstrated by the whole-cell patch clamp technique [3–7; 9–12; 21] and also using excised, inside-out patch recordings [21]. These studies reported a physiological KD value for Ca2+ in the nanomolar range, similar to the Ca2+ sensitivity that we observe for mBest1. A recent study has defined specific regions in the C-terminus of human Best1 that play an important role in the Ca2+ regulation of this channel [22]. These regions are highly conserved among the bestrophin family members including mBest1.
Anion selectivity of mBest1
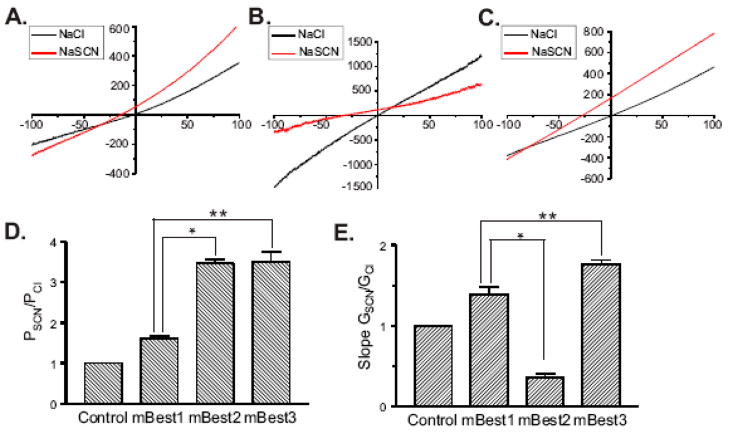
The measured reversal potential of the mBest1 currents (Erev = −1.3 ± 0.49, n = 8) agrees with the calculated Nernst equilibrium potential for Cl− with 126 mM Cl− on both sides of the membrane. In our experiments the major extracellular cation was Na+ and the major intracellular cation was Cs+. Therefore, if the mBest1 channel was permeable to these cations, the Erev would be significantly different from zero. To establish that mBest1 currents are indeed carried by chloride, ion replacement studies were performed. In these experiments, mBest1 currents were recorded with 500 nM Ca2+ in the pipette solution. Figure 2A is a representative trace showing the effect of replacing extracellular Cl− with an equimolar concentration of SCN− on mBest1 currents. The Erev shifted to the left suggesting that the mBest1 channel is more permeable to SCN− than Cl−. This was also the effect of replacing Cl− with I−. Replacing the extracellular Cl− with D-gluconate− shifted the reversal potential to more positive values, suggesting a substantial Cl− permeability. The relative permeability (Px/PCl) of external ions (X) with respect to Cl− was estimated by the shift in the Erev of the current under bionic conditions and calculated using the GHK equation. Figure 2B shows the relative permeability for Cl−, SCN− (n = 7), I− (n = 3) and D-Gluconate− (n = 4). The relative permeability ratio of SCN−:I−:Cl−:Gluconate− = 1.6:1.4:1:0.4. The anion selectivity sequence for mBest1, SCN− > I− > Cl−, is similar to other bestrophins [2] and to native Ca2+-activated Cl− channels [1]. However, the relative permeability ratio of SCN− relative to Cl− for mBest1 is quite different to what we and others have reported for mBest2 and mBest3 [5–7; 12]. Therefore, we examined and compared the effect of replacing extracellular Cl− with SCN− on currents recorded from either mBest1 (Figure 3A), mBest2 (Figure 3B) or mBest3 (Figure 3C) expressed transiently in HEK-TsA201cells in the presence of 500 nM Ca2+. For each mBest current the Erev shifted to the left suggesting that in all cases SCN− was more permeable than Cl−. However, we found the relative permeability (PSCN/PCl) for mBest1 to be significantly less than that for mBest2 and mBest3, in that PSCN/PCl of mBest1, 2 and 3 was 1.6 ± 0.06, 3.47 ± 0.09 and 3.51 ± 0.24 respectively (n = 3–8, p<0.001). This data is summarized in Figure 3D. Figure 3E depicts the average relative slope conductance ratios (GSCN/GCl) obtained from the measured slopes of the I-V relationships between −50 and +50 mV for each of the murine bestrophins. Substitution of extracellular Cl− with SCN− produced an increase in conductance for mBest1 (1.39 ± 0.09) and mBest3 (1.76 ± 0.05), whereas it resulted in a decrease in the conductance of mBest2 (0.36 ± 0.05). A decrease in GSCN/GCl for mBest2 is in agreement with that previously reported by Qu et al [6]. GSCN/GCl of mBest1 was significantly greater than that of mBest2 and significantly less than that of mBest3 (n = 3–8, p<0.001).
Fig 2.
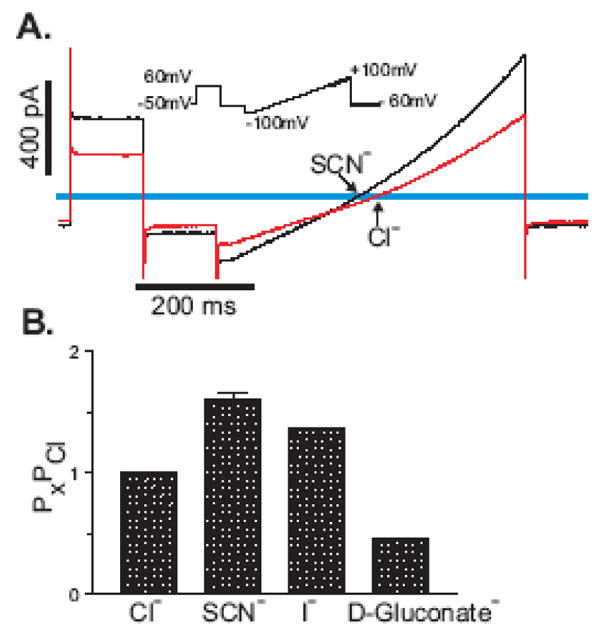
Ionic nature of the mBest1 current. Whole cell mBest1 currents, activated by 500 nM [Ca2+]i were measured with bath solutions containing either 126 mM NaCl, NaSCN, NaI, or Na-D-Gluconate. (A) Representative trace of mBest1 currents measured before and after external SCN− substitution. The I-V relationship shows an anion permeability of SCN− > Cl−. The inset shows the ramp protocol used to determine the voltage dependence of the activated current under different external anionic conditions. (B) Relative permeability ratios (Px/PCl) were calculated using the GHK equation from measured differences in the Erev between Cl− and other anions (SCN−, n = 7; I−, n = 3, D-Gluconate−, n = 4).
Fig 3.
SCN− selectivity and conductance of the murine bestrophins. Whole cell mBest currents, activated by 500 nM [Ca2+]i were measured with bath solutions containing 126mM Cl− (black) or bath solutions in which Cl− was substituted with 126 mM SCN− (red). The effect of external SCN− on the I-V relationship of (A) mBest1, (B) mBest2 and (C) mBest3 is shown. (D) PSCN/PCl were calculated using the GHK equation from measured differences in the Erev between Cl− and SCN−. (E) GSCN/GCl were obtained from the measurement of the slope of the I-V relationship between −50 and +50 mV from the Erev. Error bars represent s.e.m. PSCN/PCl and GSCN/GCl ratios of mBest1 are significantly different than mBest2 (*) and mBest3 (**) (p<0.001, n= 3–8).
While the PSCN/PCl for mBest1 is much lower than the other murine bestrophins, it is very similar to that demonstrated for dBest1 in HEK293 cells [23]. Chien et al attributed the PSCN/PCl difference between dBest1 and mBest2 to differences in certain amino acids within the second transmembrane domain, a region shown to be important in anion permeation [5; 11]. Since the Cl− channel properties of several bestrophins have not been studied in great detail, it is possible that sequence variability between bestrophins could result in important functional differences. The results of our study indicate that differences exist amongst murine bestrophins in terms of PSCN/PCl and GSCN/GCl, thus providing each bestrophin with a distinct characteristic which may prove useful during electrophysiological studies in tissues which express multiple bestrophin transcripts. Indeed, in mBest1 knockout mice IClCa was not abolished [17], and it was suggested that mBest2 and mBest3 might be compensating for the absence of mBest1, yet this could not be resolved since the electrophysiological properties of mBest1 were unknown.
Pharmacology of mBest1 current
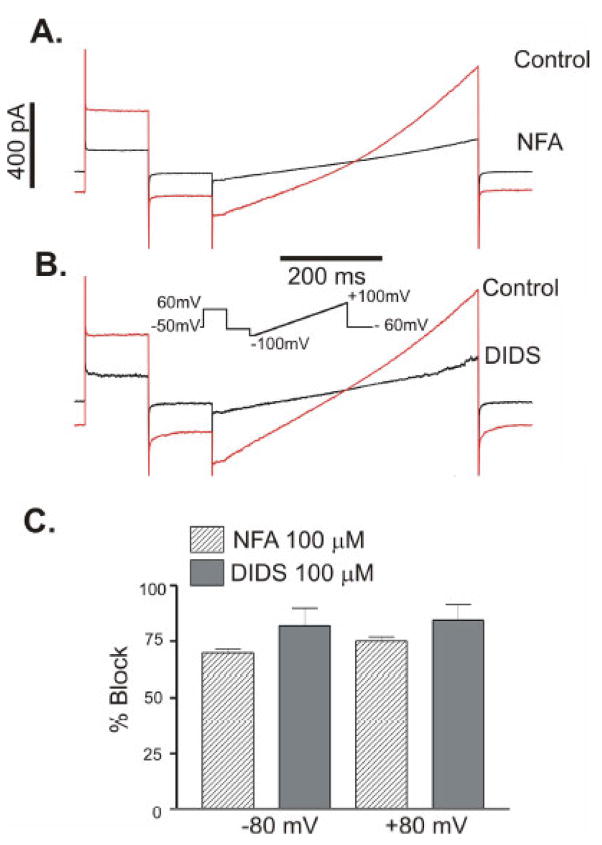
We used the Cl− channel blockers niflumic acid (NFA) and DIDS to investigate the pharmacological properties of mBest1. IClCa was evoked from TRex-293 cells expressing mBest1 with 500 nM [Ca2+]i using a ramp protocol to determine the voltage dependence of the inhibited current. Figure 4A and 4B are representative traces showing the effect of NFA and DIDS on mBest1 currents. Mean data for similar experiments are shown in Figure 4C. 100μM NFA blocked mBest1 currents by 69.8 ± 1.6 % at −80mV and 77.1 ± 1.7 % at +80 mV (n = 2). 100μM DIDS blocked mBest1 current by 81.9 ± 7.8 % at −80 mV and 84.4 ± 7.2 % at +80 mV (n = 4). Inhibition of the mBest1 currents by these drugs appears to be voltage independent.
Fig 4.
Niflumic acid and DIDS inhibit mBest1 currents. The effect of (A) NFA and (B) DIDS on currents recorded from TRex-293 cells stably expressing mBest1. Cells were voltage clamped with 500 nM [Ca2+]i and the I-V relationships were obtained with the protocol shown. (C). The mean percentage block of mBest1 current by 100 μM NFA (n = 2) and 100 μM DIDS (n = 4) at −80 and +80 mV. Error bars represent the s.e.m.
Conclusion
This is the first study to characterize the Ca2+-activated Cl− current elicited by the recombinant expression of mBest1. The biophysical properties of this channel indicate that mBest1 functions as a DIDS- and NFA-sensitive IClCa when expressed heterologously in HEK 293 cells. mBest1 current is anion selective with a permeability profile of SCN− > I− > Cl− and is activated by nanomolar concentrations of intracellular Ca2+. In addition, mBest1 protein is expressed at the membrane of mBest1-transfected HEK cells. This study completes the biophysical characterization of the known murine bestrophin members, mBest1-3. While each murine bestrophin has similar whole cell currents and Ca2+ sensitivity, this study highlights that each isoform has a distinct permeability and conductance to SCN−. Our data contributes to the characterization of the biophysical properties of mBest1 and helps further investigations to determine the physiological role of bestrophins.
Acknowledgments
The authors thank Honglin Tian, Martha Baring and Catherine Lachendro for excellent technical assistance. This publication was made possible by grant P20RR15581 (Britton) from the National Center for Research Resources, a component of the National Institutes of Health and National Heart, Lung and Blood Institute Grant R01 HL091238-01 (Britton) and R01 HL075477-01 (Leblanc).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 2.Hartzell HC, Qu Z, Yu K, Xiao Q, Chien LT. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev. 2008;88:639–672. doi: 10.1152/physrev.00022.2007. [DOI] [PubMed] [Google Scholar]
- 3.Pifferi S, Pascarella G, Boccaccio A, Mazzatenta A, Gustincich S, Menini A, Zucchelli S. Bestrophin-2 is a candidate calcium-activated chloride channel involved in olfactory transduction. Proc Natl Acad Sci USA. 2006;103:12929–12934. doi: 10.1073/pnas.0604505103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu Z, Wei RW, Mann W, Hartzell HC. Two bestrophins cloned from Xenopus laevis oocytes express Ca(2+)-activated Cl(−) currents. J Biol Chem. 2003;278:49563–49572. doi: 10.1074/jbc.M308414200. [DOI] [PubMed] [Google Scholar]
- 5.Qu Z, Hartzell C. Determinants of anion permeation in the second transmembrane domain of the mouse bestrophin-2 chloride channel. J Gen Physiol. 2004;124:371–382. doi: 10.1085/jgp.200409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu Z, Fischmeister R, Hartzell C. Mouse bestrophin-2 is a bona fide Cl(−) channel: identification of a residue important in anion binding and conduction. J Gen Physiol. 2004;123:327–340. doi: 10.1085/jgp.200409031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu Z, Cui Y, Hartzell C. A short motif in the C-terminus of mouse bestrophin 3 [corrected] inhibits its activation as a Cl channel. FEBS Lett. 2006;580:2141–2146. doi: 10.1016/j.febslet.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava A, Romanenko VG, Gonzalez-Begne M, Catalan MA, Melvin JE. A variant of the Ca2+-activated Cl channel Best3 is expressed in mouse exocrine glands. J Membr Biol. 2008;222:43–54. doi: 10.1007/s00232-008-9098-4. [DOI] [PubMed] [Google Scholar]
- 9.Sun H, Tsunenari T, Yau KW, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci USA. 2002;99:4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsunenari T, Sun H, Williams J, Cahill H, Smallwood P, Yau KW, Nathans J. Structure-function analysis of the bestrophin family of anion channels. J Biol Chem. 2003;278:41114–41125. doi: 10.1074/jbc.M306150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu Z, Chien LT, Cui Y, Hartzell HC. The anion-selective pore of the bestrophins, a family of chloride channels associated with retinal degeneration. J Neurosci. 2006;26:5411–5419. doi: 10.1523/JNEUROSCI.5500-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Driscoll KE, Hatton WJ, Burkin HR, Leblanc N, Britton FC. Expression, localization, and functional properties of Bestrophin 3 channel isolated from mouse heart. Am J Physiol Cell Physiol. 2008;295:C1610–C1624. doi: 10.1152/ajpcell.00461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer F, Stohr H, Weber BH. Cloning and characterization of the murine Vmd2 RFP-TM gene family Cytogenet. Genome Res. 2004;105:107–114. doi: 10.1159/000078016. [DOI] [PubMed] [Google Scholar]
- 14.Barro-Soria R, Schreiber R, Kunzelmann K. Bestrophin 1 and 2 are components of the Ca(2+) activated Cl(−) conductance in mouse airways. Biochim Biophys Acta. 2008;1783:1993–2000. doi: 10.1016/j.bbamcr.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Barro SR, Spitzner M, Schreiber R, Kunzelmann K. Bestrophin 1 enables Ca2+ activated Cl− conductance in epithelia. J Biol Chem. 2006 doi: 10.1074/jbc.M605716200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsey LL, Winpenny JP. Bestrophin expression and function in the human pancreatic duct cell line, CFPAC-1. J Physiol. 2009 doi: 10.1113/jphysiol.2008.159087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmorstein LY, Wu J, McLaughlin P, Yocom J, Karl MO, Neussert R, Wimmers S, Stanton JB, Gregg RG, Strauss O, Peachey NS, Marmorstein AD. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (best-1) J Gen Physiol. 2006;127:577–589. doi: 10.1085/jgp.200509473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal R, Bakall B, Kinnick T, Peachey N, Wimmers S, Wadelius C, Marmorstein A, Strauss O. Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J. 2006;20:178–180. doi: 10.1096/fj.05-4495fje. [DOI] [PubMed] [Google Scholar]
- 19.O'Driscoll KE, Leblanc N, Britton FC. Molecular and Functional Characterization of Murine Bestrophin 1 Cloned from Heart The FASEB. Journal. 2008;22:25. [Google Scholar]
- 20.Trapani JG, Korn SJ. Control of ion channel expression for patch clamp recordings using an inducible expression system in mammalian cell lines BMC. Neurosci. 2003;4:15. doi: 10.1186/1471-2202-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsunenari T, Nathans J, Yau KW. Ca2+-activated Cl− current from human bestrophin-4 in excised membrane patches. J Gen Physiol. 2006;127:749–754. doi: 10.1085/jgp.200609527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Q, Prussia A, Yu K, Cui YY, Hartzell HC. Regulation of bestrophin Cl channels by calcium: role of the C terminus. J Gen Physiol. 2008;132:681–692. doi: 10.1085/jgp.200810056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien LT, Zhang ZR, Hartzell HC. Single Cl− channels activated by Ca2+ in Drosophila S2 cells are mediated by bestrophins. J Gen Physiol. 2006;128:247–259. doi: 10.1085/jgp.200609581. [DOI] [PMC free article] [PubMed] [Google Scholar]