Abstract
Ablation studies are used to elucidate cell lineage relationships, developmental roles for specific cells during embryogenesis and mechanisms of tissue regeneration. Previous chemical and genetic approaches to directed cell ablation have been hampered by poor specificity, limited efficacy, irreversibility, hypersensitivity to promoter leakiness, restriction to proliferating cells, slow inducibility or complex genetics. Here, we provide a step-by-step protocol for a hybrid chemical-genetic cell ablation method in zebrafish that, by combining spatial and temporal control, is cell-type specific, inducible, reversible, rapid and scaleable. Bacterial Nitroreductase (NTR) is used to catalyze the reduction of the innocuous prodrug metrodinazole (Mtz), thereby producing a cytotoxic product that induces cell death. Based on this principle, NTR is expressed in transgenic zebrafish using a tissue-specific promoter. Subsequent exposure to Mtz by adding it to the media induces cell death exclusively within NTR+ cells. This approach can be applied to regeneration studies, as removing Mtz by washing permits tissue recovery. Using this protocol, cell ablation can be achieved in 12–72 h, depending on the transgenic line used, and recovery initiates within the following 24 h.
INTRODUCTION
Analysis of cell-ablated organisms can uncover the roles of specific tissues, or tissue interactions, during development and homeostasis. In addition, analysis of recovery after ablation may reveal novel cellular and molecular mechanisms underlying the regeneration process, thus bringing new insights to the field of regenerative medicine.
Danio rerio (zebrafish) is a vertebrate model organism increasingly being used to study a variety of developmental mechanisms and disease pathologies. It combines genetic tractability (both forward and reverse genetics), optical transparency, accessibility during embryogenesis and a well-documented capacity to regenerate several tissues after injury, including fin, retinal axons, spinal cord, heart, hair cells, melanocytes and liver1–7. Additionally, drugs are easily administered to zebrafish via water, and the ability to generate large numbers of embryos allows large-scale analyses. The ability to precisely ablate cells and tissues in this model organism opens new avenues into developmental, disease and regeneration studies.
Existing cell and tissue ablation methods
Previous genetic methods designed to specifically ablate cells in different model organisms exhibit various limitations, as discussed below. Unless specified, these methods have not been tried in zebrafish.
Diphtheria toxin A. Expression of diphtheria toxin A-chain (DTA) in a tissue-specific manner can lead to the effective ablation of the target cell population, but it can also result in the undesired ablation of other cells, or even death of the organism, due to its potency8,9. This problem has prevented the establishment of stable zebrafish lines carrying the DTA transgene, so this method has only been used in transient transgenesis9. This technique could be used in an inducible manner if a suitable inducible promoter that is completely silent in the off-state could be identified.
Kid/Kis. This strategy consists of expressing the bacterial toxin Kid in the targeted cell population while, simultaneously, expressing its antidote (Kis) in the rest of the organism to protect it from the cell death induced by Kid10. This method has been used in zebrafish, but only in embryos that transiently express Kid/Kis.
HSV tymidine kinase/ganciclovir. This approach uses promoter-based expression of HSV tymidine kinase together with a chemical (ganciclovir) to inhibit cell proliferation and induce cell death. This method is routinely used ex vivo and has been successfully used in rodents11,12 but only kills proliferating cells13. Thus, quiescent or slow-cycling cell populations are more refractory to this method.
Tamoxifen-inducible c-Myc. Overexpression of tamoxifen-inducible c-MycER results in elevated apoptosis rates, but significant ablation in mouse takes a long time (6–10 d; see ref. 14), probably because this transgene also causes a persistently elevated proliferation rate in the targeted cells.
Toxic viral protein M2(H37A). Cell death is induced by expressing the M2(H37A) toxic ion channel of the influenza-A virus in the target cell population15,16. This toxic effect can be inhibited by the addition of the antiviral drug rimantadine in mammalian cell cultures and Xenopus embryos but not transgenic mice. Furthermore, this suggests that the establishment and maintenance of stable transgenic lines in many cases would require long-term exposure to this inhibitor.
The NTR-mediated technique described here can be used to genetically ablate cells in zebrafish in a specific and inducible manner17,18. It is germline transmissible and kills cells without regard to their cell-cycle status19.
Overview of NTR-mediated ablation
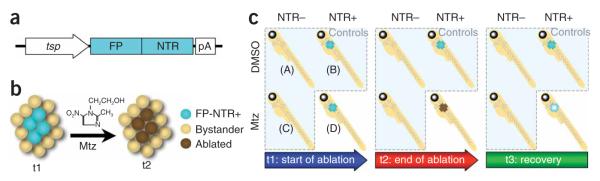
This technique is based on the ability of the Escherichia coli enzyme NTR to convert the non-toxic prodrug Mtz into a cytotoxic metabolite. Initially, NTR is reduced by NADH or NADPH. Then, Mtz binds to NTR and is electrochemically reduced and converted into a potent DNA interstrand cross-linking agent, which subsequently causes the death of the NTR-expressing cell20–22. The ectopic expression of a fluorescent protein—NTR fusion protein (FP-NTR) via a tissue-specific promoter (active in the cell population to be ablated) combined with exposure of the organism to Mtz can induce rapid destruction of the targeted cell population (see Fig. 1). Because the toxic form of Mtz remains confined to the NTR-expressing cell, no neighboring cells are affected, leading to the exclusive ablation of the NTR+ cells. This characteristic makes Mtz a better NTR substrate than others previously described, such as CB1954 (5-(aziridin-1-yl)-2,4-dinitrobenzamide), which exhibits a ‘bystander effect’, damaging neighboring cells together with the targeted NTR-expressing cells23. Moreover, the fusion of a fluorescent tag with NTR allows the fate of the NTR+ cells to be monitored throughout the course of ablation (see Fig. 2).
Figure 1.
Experimental design for Mtz/NTR tissue-specific ablation. (a) Components of the toxigene cassette: a defined tissue-specific promoter (tsp) is cloned upstream of a cassette comprised of a fluorescent protein (FP) fused to Nitroreductase (NTR) in a transgenesis vector. (b) FP-NTR fusion protein is expressed in a defined population of cells (blue) at the start of ablation (t1). Metronidazole (Mtz) added to the water is converted to a cytotoxin by FP-NTR, which causes apoptosis of the FP-NTR+ cells (brown), but not neighboring cells (tan; t2). (c) Samples are divided into four groups for each ablation experiment: DMSO treatment of (A) NTR- and (B) NTR+ embryos/larvae and Mtz treatment of (C) NTR- embryos/larvae to control for nonspecific effects of Mtz or the transgene, and Mtz treatment of (D) NTR+ embryos for tissue ablation. At t1 (start of ablation), the FP-NTR is visible in the tissue to be ablated (blue); at t2 (end of ablation), FP-NTR+ cells will be depleted or absent (brown); and at t3 (during recovery after Mtz washout), new FP-NTR+ cells may be produced.
Figure 2.
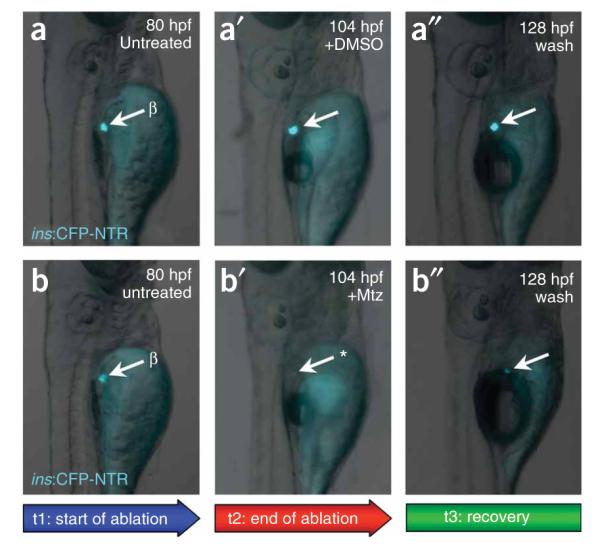
Analysis of ablation. Epifluorescent microscopy was used to monitor the expression of CFP (and therefore the progression of ablation) in a target group of cells (β cells, arrows) in individual Tg(ins:CFP-NTR)s892 larvae throughout their treatment with DMSO or Mtz. Larvae at 80 hpf before treatment (t1; a,b), at 104 hpf, after treatment for 24 h (t2) with DMSO (a’)or 10 mMMtz (b’), and at 128 hpf, after 24 h recovery (t3) from DMSO (a’’) or Mtz (b’’). Loss of CFP during t2 in treated individuals (asterisk in b’) indicates that cells have been successfully ablated; recovery of fluorescence during t3 (b’’) indicates cell recovery. CFP fluorescence is constant in untreated individuals (a,a’ and a’’), indicating that no ablation occurs.
Applications of Mtz/NTR-mediated ablation
Because the technique described here offers rigorous spatial specificity as well as temporal control, it has multiple applications. The examination of the effects of the absence of a tissue or cell population can, for instance, provide new insights into the understanding of the role of a tissue, or cell population, for morphogenesis, patterning or cell survival, during development or homeostasis. Furthermore, the NTR/Mtz system can be used as a versatile tool for regeneration studies: analysis of tissue recovery after ablation will enable the elucidation of cellular and molecular mechanisms underlying tissue regeneration. This approach can be used in a wild-type context as well as in mutant or genetically modified backgrounds. This versatility is particularly advantageous when investigating how certain signaling pathways or molecular players are involved during the process of regeneration (by coexpression of dominant negative factors, mutant alleles or Morpholino antisense injection). Moreover, this system can be combined with the use of other chemicals that modulate specific signaling pathways to investigate their role in tissue regeneration.
This technique has been successfully used to ablate cardiomyocytes, hepatocytes and pancreatic β-cells in zebrafish embryos and larvae by using promoters specific for each of these cell populations17,18. These studies also showed that the NTR/Mtz cell ablation technique is reversible, as tissue recovery could be observed after ablation. After damage of the larval heart was induced, which resulted in failure of the heart to contract and pump blood to the rest of the organism, cardiomyocytes regenerated, recovering morphology and function of the heart and reestablishing blood circulation. Similarly, following ablation of β-cells, the larval pancreas exhibited substantial recovery, generating new Insulin-producing cells in the islet.
There is no fundamental difference in applying this method to embryonic, larval, juvenile or adult zebrafish except the scale of the experiment (see Box 1). Thus, as with any other drug treatment, it is expected that this ablation technique will work in adults, and, in fact, we have observed effective β-cell ablation at all developmental stages tested (R.M.A. and D.Y.R.S., unpublished data).
BOX 1. JUVENILES OR ADULTS.
The following adaptation of our method should be applicable to fish older than 5 d post-fertilization (dpf):
Maintain juvenile or adult fish in the aquaculture system until needed.
Prepare enough Mtz solution (as described in REAGENT SETUP but by using aquarium water in place of egg water, 5 mM maximum concentration) for each pair of adult fish to be bathed in 500 ml of solution. Store protected from light at room temperature (23–25 °C) until use.
At the desired stage for ablation, remove FP-NTR+ transgenic fish from the aquaculture system and transfer to 1-liter chambers each containing two fish and 500 ml of solution. The same experiment and control groups (A—D) apply as in Step 4 of the main PROCEDURE. Fish are netted directly into the Mtz or control solution.
Keep adult fish in 1-liter tanks in a heated space (28 °C) in an opaque box to prevent photoinactivation of Mtz.
During the course of treatment, feed fish once per day with a normal diet. Approximately 10 min after feeding, replace the water with fresh Mtz or control solution.
Monitor the effects of treatment; these may be more difficult to observe in adult fish, and some fish may have to be sacrificed throughout a course of treatment to establish the baseline of ablation efficacy.
(Optional) Following treatment, wash adults in several changes of fresh system water over the course of 1 h, and then return them to the system for recovery.
Limitations of NTR-mediated ablation
Some limitations may, however, be associated with this technique, which depend on the tissue being investigated.
Availability of suitable promoter
Although the method offers great flexibility regarding the target tissue to be ablated, as it can be adapted by selecting an appropriate promoter to drive NTR expression, the ability to ablate certain cell populations, or tissues, will depend on the availability of such a promoter.
Some tissues resist complete ablation
A second limitation can be the inability to ablate the target cell population completely, depending on which tissue is being ablated. It is possible that some tissues, or cells within a tissue, will be more accessible to the prodrug than others. It is also possible that some tissues respond rapidly to the loss of cells and are replaced as quickly as they are destroyed. This scenario may explain why a few residual cells may still be detected after ablation. However, this mosaic effect may be advantageous when partial ablation is preferred. In addition, hepatocytes might metabolize Mtz and thus limit the efficacy of this method for their ablation17.
Some tissues require longer treatment for ablation
Another possible limitation is the time required to induce ablation, as it includes diffusion and accumulation of Mtz into the FP-NTR-expressing cells and induction of cell death. We have observed that some FP-NTR zebrafish transgenic lines require only a few hours of Mtz treatment, whereas others require longer than 24 h for an observable effect to be induced. The ablation time is variable and likely depends not only on the promoter used to drive FP-NTR expression, but also on each particular transgenic line. Different lines using the same promoter may show variable levels of FP-NTR expression, possibly due to positional effects of the transgene integration site. Optimization of the technique may thus require the generation of multiple transgenic lines and empirical determination of the necessary period of Mtz exposure.
Experimental design
Transgene constructs
The use of this method requires generation of a transgenic line (stable or transient) carrying a cassette in which FP fused to NTR is driven by a tissue-specific promoter (tsp) (Fig. 1a). The tsp defines the tissue to be ablated, NTR itself is the conditional toxigene and the FP enables monitoring of NTR-expressing cells during the procedure. Additionally, the FP facilitates the identification of transgenic carriers.
To generate the Tg(tsp:FP-NTR) zebrafish transgenic line, the promoter of interest can be cloned into the multiple cloning site of the tol2_CFP_NTR plasmid17 and co-injected with Tol2 transposase mRNA into 1-cell stage embryos24. Alternatively, the promoter and FP-NTR cassette can be flanked by I-SceI restriction sites and co-injected with I-SceI meganuclease25.
Within each stable transgenic line, extent of ablation may be affected by the following: (i) expression level of the transgene; (ii) mosaicism of promoter activity in the transgenic line; (iii) accessibility of the targeted cells to Mtz; (iv) turnover rate of the cells that are being ablated; and (v) ability of the cells to metabolize Mtz. Thus, each transgenic line will require thorough characterization and precise determination of the optimal conditions for cell ablation.
For some studies, ablation of only a subset of a promoter’s expression domain will be desired; in this case, it is preferable to use transient transgenic lines. Bear in mind that with this implementation, expression will vary greatly among the embryos.
Optimization of Mtz concentration and exposure
Critical variables in this ablation protocol are the concentration of Mtz used and the time of exposure to this prodrug. Each transgenic line will require optimization of the exact conditions for cell ablation/regeneration. Thus, when characterizing a new transgenic line, a range of different Mtz concentrations (such as 1, 2.5, 5, 7.5 and 10 mM) should be used, and embryos/larvae should be checked regularly for signs of cell ablation. We have observed ablation at concentrations ranging from 1 to 10 mM. See Table 1 for examples of time required for damage/regeneration for three established transgenic lines using promoters specific for cardiomyocytes (cardiac myosin light chain 2—cmlc226), pancreatic β-cells (insulin—ins)17 and hepatocytes (fatty acid binding protein 10, liver basic—fabp1027).
TABLE 1.
Time points for Mtz treatment (examples)
| Tg(tsp:CFP-NTR) | t1 | t2 | t3 |
|---|---|---|---|
| Tg(cmlc2:CFP-NTR)s890 | 48 hpf | 72 hpf | 96 hpf |
| Tg(fabp10:CFP-NTR)s891 | 96 hpf | 168 hpf | |
| Tg(ins:CFP-NTR)s892 | 84 hpf | 110 hpf | 145 hpf |
| Tg(ins:CFP-NTR)s892 | 90 dpf | 93 dpf |
t1, addition of Mtz and control (DMSO) solutions; t2, phenotype/tissue damage detected (wash out Mtz for regeneration studies); t3, recovery of damaged tissue observed; hpf, hours post-fertilization; dpf, days post-fertilization.
Controls
Three negative control conditions are appropriate for these ablation experiments, all of which should demonstrate no effect on developing larvae (Fig. 1c). First, 0.2% dimethyl sulfoxide (DMSO) (vol/vol) treatment of wild-type larvae provides a baseline for comparison with the ablated embryos and the other controls. Second, treatment of wild-type larvae with Mtz solution reveals any off-target effects of the drug alone. Finally, treatment of NTR+ larvae with DMSO alone reveals any drug-independent effects of the transgene.
It is critical to thoroughly analyze the FP-NTR expression pattern in each transgenic line for a correct interpretation of the results. And, of course, this system can be used to ablate different cell populations simultaneously, in the same animal, by combining transgenes driving expression of FP-NTR in different tissues.
Although the protocol described here applies to zebrafish (embryos, larvae and adults), the NTR/Mtz system may also be applied to other aquatic animals, to mammals and also possibly to insects such as fruitflies. The NTR/CB1954 combination has been previously used in mouse, to ablate neurons28, astrocytes29 and adipocytes30, and in several ‘gene suicide’ cancer therapy studies31. The use of Mtz instead of CB1954 as the NTR substrate should, however, avoid any undesired ‘bystander effect’. Zebrafish can be easily exposed to chemicals added to the water, whereas for other organisms, such as mouse or fruitfly, this may require peritoneal or subcutaneous administration or dissolving chemicals in the food. For this reason, zebrafish and other aquatic animals are ideal for this system, as the prodrug can be quickly and easily added or removed from the surrounding media.
The protocol below assumes that suitable transgenic lines have been previously generated (according to the guidelines above). Progeny are collected from these FP-NTR carriers and controls and are sorted and treated with freshly prepared Mtz or control solution. The progress of ablation is monitored until the desired effect is reached. For regeneration purposes, the drug is washed out and the recovery monitored.
MATERIALS
REAGENTS
Wild-type and Tg(tsp:FP-NTR) zebrafish lines, generated according to guidelines provided in the INTRODUCTION. Note that a plasmid containing CFP-NTR and a detailed map are available from the authors upon request
Egg water(0.075 g CaSO4, 0.3 g ‘Instant Ocean’ Sea Salt (Instant Ocean, http://www.instantocean.com), 1 liter of water)
Mtz (Sigma, cat. no. M1547)
DMSO (J.T. Baker, cat. no. 9224-01)
Mtz solution: 1–10 mM Mtz in 0.2% DMSO (vol/vol) in egg water (see REAGENT SETUP)
Control solution: 0.2% DMSO (vol/vol) in egg water
EQUIPMENT
Air incubator set at 28 °C
Epifluorescence-equipped dissecting stereo microscope
6-well culture plates or plastic Petri dishes
Glass pipettes and pipetter
Disposable 50-ml polypropylene conical tubes
Divided zebrafish-breeding tanks
REAGENT SETUP
Mtz solution
Approximately 15–30 min before the chosen time for starting ablation (t1), prepare the appropriate Mtz solution by first adding DMSO to the egg water, then the required amount of Mtz powder. Shake vigorously until Mtz is dissolved. ! CAUTION Mtz may be toxic at high concentrations or after prolonged exposure. Use of gloves when preparing the Mtz solution is advised. ▲ CRITICAL If unpigmented embryos are needed, add 0.2 mM phenylthiourea to the Mtz and control solutions. ▲ CRITICAL The required concentration of Mtz should be determined for each transgenic line and for the rate/extent of damage desired. However, the concentration of Mtz should not exceed 10 mM because nonspecific teratogenesis will occur. DMSO is not essential, but it helps solubilize Mtz and may facilitate permeation of the drug into the animals. See Troubleshooting section.
PROCEDURE
Preparation of embryos/larvae for treatment
1| Collect eggs from timed pair matings (using divided tanks) of Tg(tsp:FP-NTR) (heterozygous) and wild-type fish to ensure synchronized development of progeny.
2| Transfer fertilized eggs to 100-mm Petri dishes with egg water at a density no greater than 60 embryos per 25 ml of egg water.
3| Incubate eggs at 28 °C in egg water until they reach the desired stage for start of ablation (t1). The incubation period varies depending on the promoter used and the purpose of the experiment (examples are provided in Table 1).
▲ CRITICAL STEP If necessary, impede the development of pigmentation by adding 0.2 mM phenylthiourea to the media after gastrulation is complete.
4| Distribute samples into experimental and control treatment groups. Before the desired time to start ablation (t1), sort FP-NTR+ and FP-NTR- embryos/larvae (as determined by the presence or lack of the appropriate fluorescent signal, respectively) into four chambers (e.g., Petri-dishes or four wells of a multiwell plate) as follows: (A) FP-NTR-/DMSO (control); (B) FP-NTR+/DMSO (control); (C) FP-NTR-/Mtz (control); (D) FP-NTR+/Mtz (experiment) (Fig. 1c). Use a minimum of 20 embryos/larvae per group to account for any in vivo assay variability—a maximum density of 6–7 embryos per ml minimizes crowding effects.
▲ CRITICAL STEP Unhatched embryos should be manually or enzymatically dechorionated32 before Mtz treatment to ensure effective drug penetration.
Mtz treatment
5| Approximately 15–30 min before the chosen time for ablation (t1), prepare the appropriate Mtz solution as described in REAGENT SETUP.
6| Replace the water in each chamber with the freshly prepared Mtz solution in chambers C and D, and DMSO control solution in chambers A and B.
! CAUTION Mtz may be toxic at high concentrations or after prolonged exposure. Use of gloves when preparing the Mtz solution is advised.
7| Incubate with prodrug to induce cell ablation. Place the Petri dishes/multiwell plate in the incubator at 28 °C, in the dark, covered with aluminum foil or inside an opaque box, to prevent photoinactivation of Mtz.
Analysis of ablation
8| At t2, end of ablation, check under the microscope for any indication of cell death, such as decrease in the levels of FP, or obvious phenotype that may result from death of target tissue. If necessary, add 0.02 mg ml-1 tricaine to immobilize embryos/larvae for observation (after observation, replace medium with new Mtz and control solutions). Two examples of phenotypes that we have observed include the following: 18–24 h after 48 h post-fertilization (hpf) Tg(cmlc2:CFP-NTR)s890 embryos have been exposed to 10 mM Mtz, cardiomyocytes die, the heart stops contracting, and pooling of the blood can be observed using a bright field microscope; and 12–24 h after 84 hpf Tg(ins:CFP-NTR)s892 larvae have been exposed to 5–10 mM Mtz, strong reduction or complete ablation of fluorescence in the Insulin-producing β-cells can be observed (Fig. 2). Note that no significant cell death should be detected in any of the control samples: (A) FP-NTR-/DMSO; (B) FP-NTR+/DMSO; (C) FP-NTR-/Mtz. In some cell populations/tissues, treatment may not result in an obvious defect or decrease in FP-NTR fluorescence. To assess subtle effects, embryos/larvae are fixed in 3% formaldehyde (wt/vol) and evaluated for apoptosis using the TUNEL assay or activated Caspase-3 immunostaining17 (Fig. 3) or other analyses.
Figure 3.
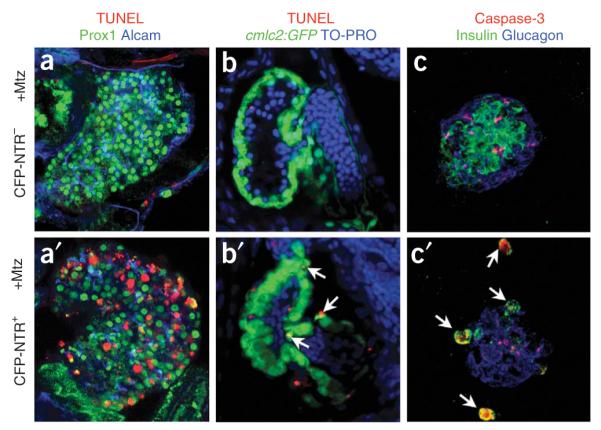
Cell death detection. Detection of apoptosis using (a,a’,b,b’) TUNEL assay or (c,c’) activated Caspase-3 immunostaining in (a,b,c)CFP-NTR- or (a’,b’,c’) CFP-NTR+ larvae after treatment with 10 mM Mtz. Mtz-treated CFP-NTR- control larvae show no substantial apoptosis in (a)liver, (b) heart or (c) pancreatic islet (activated Caspase-3-stained samples exhibit some nonspecific background staining). In contrast, significant apoptosis was observed in hepatocytes of (a’) Tg(fabp10:CFP-NTR)s891 larvae, cardiomyocytes of (b’) Tg(cmlc2:CFP-NTR)s890 larvae (arrows) and (c’) pancreatic β-cells of Tg(ins:CFP-NTR)s892 larvae (arrows).
? TROUBLESHOOTING
Post-ablation regeneration analysis (optional)
9| After damage has been satisfactorily completed (t2), wash samples with 3–5 changes of fresh egg water using a pipette; this procedure will remove all traces of the Mtz solution. Place the embryos/larvae back into the 28 °C incubator.
▲ CRITICAL STEP Ablation of certain tissues may cause secondary effects, which may lead to death of the organism or its distress to a point beyond recovery independent from any intrinsic ability of the ablated tissue to recover. For example, extensive ablation of cardiomyocytes will eliminate blood circulation; thus, it may be crucial to replace the Mtz solution with Mtz-free solution within a certain time window after damage has been observed.
10| At t3, following washout of Mtz, check regularly for recovery of ablated cells/tissue under microscope (for recovery of function or morphology of affected tissue or increase in levels of FP expression) or process sample for immunostaining, microarray, biochemical or other analyses.
▲ CRITICAL STEP The required period for regeneration, if it occurs, will vary significantly among NTR-expressing lines using different promoters, and even possibly among lines with the same transgene inserted in different loci.
• TIMING
The time required to perform this assay will vary significantly depending on the FP-NTR expressing transgenic line used. Here, we provide an estimate of the time required for sample manipulation during this assay.
Steps 1–4, preparation of embryos/larvae for treatment: 1–5 d
Steps 5–8, Mtz treatment and analysis of ablation: 12–72 h
Steps 9 and 10, post-ablation regeneration analysis (optional): 24–72 h
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
TABLE 2.
Troubleshooting table
| Step | Problem | Solution |
|---|---|---|
| REAGENT SETUP | Mtz does not fully dissolve | Make sure DMSO was added to the egg water (to a final concentration of 0.2%, vol/vol) |
| Warm the solution to 37 °C and continue vigorous shaking | ||
| 8 | No defect/cell death is detected in the experimental embryos/larvae (FP-NTR+/Mtz) |
As an internal positive control for apoptosis, cell death should be detectable by TUNEL or activated Caspase-3 immunostaining in specific wild-type tissues, for example, the lens at 20–48 hpf and medial fin fold at 60 hpf (ref. 33) |
| Cell death is detected in the positive control and not in the experimental sample |
Check cell death at multiple time points, as dying cells may be rapidly cleared |
|
| Increase the length of Mtz exposure | ||
| Increase the concentration of Mtz (be aware that concentrations of Mtz greater than 10 mM may induce nonspecific cell death in embryos/larvae) |
||
| Repeat experiment using another zebrafish line carrying the same transgene (due to positional effects, different transgenic lines, even if containing the same promoter driving FP-NTR expression, may show different levels of expression). In addition, increased expression of FP-NTR with two (or more) transgene insertions may increase the efficiency of ablation |
||
| Increase transgene dosage by homozygosing the FP-NTR allele, which should increase the expression level |
||
| Try embryos/larvae of different stages (some stages may be more suscep- tible than others, due to drug infiltration or expression level of FP-NTR) |
||
| FP-NTR-/Mtz control sample shows severe defects/cell death |
Such an observation indicates nonspecific Mtz toxicity. Reduce Mtz concentration |
ANTICIPATED RESULTS
Cell death should be induced in all transgenic animals carrying the tsp:FP-NTR transgene after exposure to a sufficient dose of Mtz, whereas no effect should be detected in any of the control embryos/larvae used: FP-NTR- or FP-NTR+ exposed to DMSO alone, or FP-NTR- exposed to Mtz. In our hands, all transgenic CFP-NTR embryos showed significant cell damage in the targeted tissue after exposure to 10 mM Mtz, although the extent of ablation varied depending on the tissue examined, the promoter used to drive FP-NTR and the transgenic line itself. Some examples of our results are shown in Figures 2 and 3.
The period of time required to induce the death of cells expressing FP-NTR may vary from a few hours to several days (see Table 1) and will depend greatly on the stage, length of Mtz treatment, Mtz concentration, targeted tissue and FP-NTR transgenic line used. Using this system to ablate cells, it is possible to reverse damage as some injured tissues may regenerate after removing Mtz by washing. However, the rate of recovery will depend both on the biology of the targeted tissue and the extent of damage. It is possible that the percentage of animals exhibiting recovery will never reach 100%; this situation may be advantageous for studying enhancement versus impairment of regeneration in modified backgrounds—opening new possibilities to discover factors that may inhibit or enhance cell and tissue regeneration.
ACKNOWLEDGMENTS
We thank Justin Bosch for comments on the manuscript and help in testing the protocol for adult ablations, Stephen J. Johnson (Washington University) for his suggestion to use the NTR/Mtz system in zebrafish, Jeff Mumm and Eric Schroeter (Washington University) for providing the CFP-NTR construct and Ana Ayala and Koroboshka Brand for expert help maintaining the fish. R.M.A. was supported by a postdoctoral fellowship from the JDRF. This work was supported in part by grants from the NIH (NHLBI and NIDDK) and the Packard Foundation to D.Y.R.S.
Footnotes
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Johnson SL, Weston JA. Temperature-sensitive mutations that cause stage-specific defects in Zebrafish fin regeneration. Genetics. 1995;141:1583–1595. doi: 10.1093/genetics/141.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernhardt RR, Tongiorgi E, Anzini P, Schachner M. Increased expression of specific recognition molecules by retinal ganglion cells and by optic pathway glia accompanies the successful regeneration of retinal axons in adult zebrafish. J. Comp. Neurol. 1996;376:253–264. doi: 10.1002/(SICI)1096-9861(19961209)376:2<253::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J. Comp. Neurol. 1997;377:577–595. doi: 10.1002/(sici)1096-9861(19970127)377:4<577::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 5.Harris JA, et al. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J. Assoc. Res. Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CT, Sengelmann RD, Johnson SL. Larval melanocyte regeneration following laser ablation in zebrafish. J. Invest. Dermatol. 2004;123:924–929. doi: 10.1111/j.0022-202X.2004.23475.x. [DOI] [PubMed] [Google Scholar]
- 7.Sadler KC, Krahn KN, Gaur NA, Ukomadu C. Liver growth in the embryo and during liver regeneration in zebrafish requires the cell cycle regulator, uhrf1. Proc. Natl. Acad. Sci. USA. 2007;104:1570–1575. doi: 10.1073/pnas.0610774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurita R, et al. Suppression of lens growth by alphaA-crystallin promoter-driven expression of diphtheria toxin results in disruption of retinal cell organization in zebrafish. Dev. Biol. 2003;255:113–127. doi: 10.1016/s0012-1606(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 9.Wan H, et al. Analyses of pancreas development by generation of gfp transgenic zebrafish using an exocrine pancreas-specific elastaseA gene promoter. Exp. Cell Res. 2006;312:1526–1539. doi: 10.1016/j.yexcr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Slanchev K, Stebler J, de la Cueva-Mendez G, Raz E. Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc. Natl. Acad. Sci. USA. 2005;102:4074–4079. doi: 10.1073/pnas.0407475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung J, et al. Ablation of tumor-derived stem cells transplanted to the central nervous system by genetic modification of embryonic stem cells with a suicide gene. Hum. Gene Ther. 2007;18:1182–1192. doi: 10.1089/hum.2007.078. [DOI] [PubMed] [Google Scholar]
- 12.Chu QD, et al. Rat adenocarcinoma cell line infected with an adenovirus carrying a novel herpes-simplex virus-thymidine kinase suicide gene construct dies by apoptosis upon treatment with ganciclovir. J. Surg. Res. 2007;143:189–194. doi: 10.1016/j.jss.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Springer CJ, Niculescu-Duvaz I. Approaches to gene-directed enzyme prodrug therapy (GDEPT) Adv. Exp. Med. Biol. 2000;465:403–409. doi: 10.1007/0-306-46817-4_35. [DOI] [PubMed] [Google Scholar]
- 14.Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 15.Smith CA, et al. Conditional ablation of T-cell development by a novel viral ion channel transgene. Immunology. 2002;105:306–313. doi: 10.1046/j.0019-2805.2002.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SJ, Kotecha S, Towers N, Mohun TJ. Targeted cell-ablation in Xenopus embryos using the conditional, toxic viral protein M2(H37A) Dev. Dyn. 2007;236:2159–2171. doi: 10.1002/dvdy.21233. [DOI] [PubMed] [Google Scholar]
- 17.Curado S, et al. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev. Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- 18.Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech. Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridgewater JA, et al. Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954. Eur. J. Cancer. 1995;31A:2362–2370. doi: 10.1016/0959-8049(95)00436-x. [DOI] [PubMed] [Google Scholar]
- 20.Lindmark DG, Muller M. Antitrichomonad action, mutagenicity, and reduction of metronidazole and other nitroimidazoles. Antimicrob. Agents Chemother. 1976;10:476–482. doi: 10.1128/aac.10.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anlezark GM, et al. The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954)—I. Purification and properties of a nitroreductase enzyme from Escherichia coli—a potential enzyme for antibody-directed enzyme prodrug therapy (ADEPT) Biochem. Pharmacol. 1992;44:2289–2295. doi: 10.1016/0006-2952(92)90671-5. [DOI] [PubMed] [Google Scholar]
- 22.Edwards DI. Nitroimidazole drugs—action and resistance mechanisms. II. Mechanisms of resistance. J. Antimicrob. Chemother. 1993;31:201–210. doi: 10.1093/jac/31.2.201. [DOI] [PubMed] [Google Scholar]
- 23.Bridgewater JA, Knox RJ, Pitts JD, Collins MK, Springer CJ. The bystander effect of the nitroreductase/CB1954 enzyme/prodrug system is due to a cell-permeable metabolite. Hum. Gene Ther. 1997;8:709–717. doi: 10.1089/hum.1997.8.6-709. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami K, et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell. 2004;7:133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Grabher C, Joly JS, Wittbrodt J. Highly efficient zebrafish transgenesis mediated by the meganuclease I-SceI. Methods Cell Biol. 2004;77:381–401. doi: 10.1016/s0091-679x(04)77021-1. [DOI] [PubMed] [Google Scholar]
- 26.Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 2003;228:30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- 27.Her GM, Chiang CC, Chen WY, Wu JL. In vivo studies of liver-type fatty acid binding protein (L-FABP) gene expression in liver of transgenic zebrafish (Danio rerio) FEBS Lett. 2003;538:125–133. doi: 10.1016/s0014-5793(03)00157-1. [DOI] [PubMed] [Google Scholar]
- 28.Isles AR, et al. Conditional ablation of neurones in transgenic mice. J. Neurobiol. 2001;47:183–193. doi: 10.1002/neu.1026. [DOI] [PubMed] [Google Scholar]
- 29.Cui W, Allen ND, Skynner M, Gusterson B, Clark AJ. Inducible ablation of astrocytes shows that these cells are required for neuronal survival in the adult brain. Glia. 2001;34:272–282. doi: 10.1002/glia.1061. [DOI] [PubMed] [Google Scholar]
- 30.Felmer R, Cui W, Clark AJ. Inducible ablation of adipocytes in adult transgenic mice expressing the E. coli nitroreductase gene. J. Endocrinol. 2002;175:487–498. doi: 10.1677/joe.0.1750487. [DOI] [PubMed] [Google Scholar]
- 31.Searle PF, et al. Nitroreductase: a prodrug-activating enzyme for cancer gene therapy. Clin. Exp. Pharmacol. Physiol. 2004;31:811–816. doi: 10.1111/j.1440-1681.2004.04085.x. [DOI] [PubMed] [Google Scholar]
- 32.Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) 4th edn University of Oregon Press; Eugene, Oregon: 2000. [Google Scholar]
- 33.Cole LK, Ross LS. Apoptosis in the developing zebrafish embryo. Dev. Biol. 2001;240:123–142. doi: 10.1006/dbio.2001.0432. [DOI] [PubMed] [Google Scholar]