Abstract
The discriminative stimulus effects of cocaine are typically attributed to its ability to increase dopaminergic transmission, although drugs that have different mechanisms of action can substitute for cocaine and modulation of the GABAA receptor system has been reported to alter its discriminative effects. Therefore, a discrimination procedure was used to extend the characterization of cocaine’s discriminative effects and to examine the interaction between cocaine and pregnanolone, a drug that can modulate the GABAA receptor complex. Rats (n=15) were trained to discriminate saline from 5.6 or 10 mg/kg of cocaine under a fixed-ratio (FR) 20 schedule of food presentation. The dopamine releaser d-amphetamine and two monoamine uptake inhibitors bupropion and desipramine substituted for cocaine. In contrast, the positive GABAA modulators pregnanolone and lorazepam and the opioid agonist morphine did not substitute for cocaine. When administered prior to cocaine, the D2 receptor antagonist haloperidol and pregnanolone, but not lorazepam, produced a small rightward shift of the cocaine dose-effect curve. The results of the present studies suggest that the discriminative stimulus effects of cocaine are not solely mediated by increases in dopaminergic transmission and that positive modulation of GABAA receptors by pregnanolone can alter these effects, albeit at doses that also decrease overall response rate.
Keywords: pregnanolone, benzodiazepines, cocaine, dopamine, drug discrimination, positive GABAA modulators
Introduction
There have been an increasing number of studies in the literature suggesting an important interaction in the central nervous system between drugs that increase monoamine activity and drugs that positively modulate the GABAA receptor (e.g., Barrett et al., 2005). For example, positive modulators of the GABAA receptor such as the benzodiazepines have been shown to decrease endogenous release of dopamine in different areas of the brain (Dewey et al. 1997; Dewey et al. 1992; Finlay et al. 1992). Behaviorally, the positive GABAA modulators triazolam and pentobarbital were shown to decrease cocaine-lever responding in monkeys trained to discriminate cocaine from saline (Negus et al., 2000). In rats, midazolam and pentobarbital attenuated the discriminative effects of cocaine, which would suggest that positive GABAA modulators have the capacity to inhibit or attenuate certain cocaine-mediated behaviors.
Recently, novel substances such as pregnanolone (5β-pregnan-3α-ol-20-one) have also been shown to act as positive GABAA modulators in certain behavioral assays (Vanover, 2000; Gerak et al., 2004), and similar to the benzodiazepines, Smolders et al. (1995) showed that intrastriatal infusions of pregnanolone could decrease endogenous dopamine release. However, another study by Rouge-Pont et al. (2002) found that allopregnanolone (5α-pregnan-3β-ol-20-one) increased dopamine release in the nucleus accumbens of rats, suggesting differential effects of pregnanolone and allopregnanolone in rats depending on the brain area. Another possible explanation for these differential effects is differences in the pharmacology between pregnanolone and allopregnanolone in rats. In humans, endogenous pregnanolone and its stereoisomer allopregnanolone are putatively known as neuroactive steroids, and monoamine reuptake inhibitors (e.g., desipramine) as well as selective serotonin reuptake inhibitors (e.g., fluoxetine) have been shown to increase low levels of endogenous pregnanolone and allopregnanolone into the normal range in humans (Romeo et al. 1998; Uzunova et al. 1998). Neuroactive steroids can also be found in the brain in pharmacologically relevant concentrations following acute stress (Barbaccia et al. 1996; Purdy et al. 1991) and the dopamine reuptake inhibitor cocaine has been shown to act as a stressor (Yang et al. 1992; Knuepfer et al. 2001). Thus, there is the possibility that monoamine uptake inhibitors and pregnanolone may share certain effects in humans. However, pregnanolone has not been demonstrated to be a neuroactive steroid in rats and its positive GABAergic activity may predominate in rats, which would suggest effects for cocaine and pregnanolone that are mutually antagonistic.
One way to compare and contrast the behavioral effects of pregnanolone in rats with the monoamine uptake inhibitors and positive GABAA modulators directly is with a discrimination procedure. If pregnanolone acts predominately as a positive GABAA modulator, then it might attenuate cocaine’s discriminative effects as has been shown for other positive GABA modulators such as the benzodiazepines in rats (Barrett et al., 2005) and monkeys (Negus et al., 2000). If pregnanolone has effects more like allopregnanolone in the rat, then it might enhance cocaine’s discriminative effects by further enhancing dopamine transmission. Therefore, in this study, the discriminative stimulus effects of pregnanolone were compared with a wide range of compounds, including those for d-amphetamine, bupropion, desipramine and lorazepam in rats discriminating cocaine. The potentially opposing behavioral effects of pregnanolone and cocaine were compared with haloperidol and lorazepam by administering these drugs prior to cocaine in the same rats discriminating cocaine. The effects of morphine were also evaluated because μ-opioid agonists should not share discriminative stimulus effects with cocaine (Bowen et al. 2003; Broadbent et al. 1995).
Material and methods
Subjects
Drug-naive male Long-Evans hooded rats were housed individually in opaque plastic cages containing hardwood-chip bedding and maintained on a 14-hr light/10-hr dark cycle (lights on at 6:00 am) to mimic a natural long-day photoperiod. The housing room was maintained at 21 ± 1ºC with 50 ± 10 % humidity. Testing occurred during the light phase of the cycle. Subjects were fed a diet consisting of nutritionally balanced 45-mg food pellets (Research Diets, Inc. New Brunswick, NJ, USA) earned during experimental sessions and supplemental rat chow (PMI Nutrition, Brentwood, MO, USA) provided after session in order to maintain them at 85% of their free-feeding weight, which was determined at the beginning of the experiment. Rats had unlimited access to water in their home cage. These studies were carried out in accordance with the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center, and guidelines of the Committee on Care and Use of Laboratory Animal Resources, as adopted and promulgated by the U.S. National Institute of Health.
Apparatus
Experimental sessions were performed in six ventilated, sound-attenuated chambers (BRS/LVE, Laurel, MD) equipped with two response levers, stimulus lights above each lever, house lights and a food hopper. An interface (MedAssociates, St. Albans, VT) connected the chambers and cumulative recorders to a microprocessor, which controlled experimental events and collected data.
Procedure
Subjects were trained to discriminate cocaine from saline while responding under a FR-20 schedule of food presentation. During the initial training sessions, the house light was illuminated and every response on either lever resulted in food presentation. After rats reliably pressed the levers under this continuous reinforcement schedule (CRF), the number of responses required for food presentation was progressively increased from 1 to 20. In addition, a 1-min timeout component was added to the beginning of the session, and the duration of this component was increased over succeeding sessions until the terminal timeout duration of 10 min was reached. During the timeout, the chambers were dark and responses had no programmed consequence. Experimental sessions ended after a single timeout and a single 20-min FR component. When responding stabilized under the terminal schedule and rats routinely received at least 100 food pellets during each session, discrimination training began. In this phase of training, reinforcement under the FR-20 schedule was contingent on an injection of either saline or cocaine administered immediately before each timeout. For half of the rats in each group, responding on the left lever resulted in food presentation following administration of cocaine, and responding on the right lever resulted in food presentation following administration of saline; the lever designations were reversed for the other half of the rats. Responding on the incorrect lever reset the response requirement on the correct lever. In order to establish the lowest discriminable dose of cocaine in all the rats (and therefore, an equivalent effect in each rat), discrimination training began with a small dose of cocaine (e.g., 1 mg/kg). The dose was then increased by 0.25 log units every other week until rate-decreasing effects were observed or until the following criteria were satisfied for 9 of 10 sessions: ≥95% of the total responses emitted on the correct lever and fewer than 20 responses emitted on the incorrect lever prior to receiving the first food pellet. If rate-decreasing effects (80% of control rates or less) were observed before these criteria were met, the training dose was decreased by 0.25 log unit. When the criteria were satisfied, control of responding by the respective stimuli (i.e., cocaine or saline) was considered adequate for testing the effects of different doses or drugs. Test sessions were identical to training sessions under the terminal schedule except that responding on either lever resulted in food presentation, and the session was preceded by the administration of different doses or drugs. Following test sessions, at least three training sessions were conducted and subjects had to meet the training criteria during three consecutive days for test sessions to continue. After dose-effect curves for cocaine were established during test conditions, dose-effect curves for the other drugs (i.e., d-amphetamine, morphine, bupropion, desipramine, pregnanolone or lorazepam) were determined, or a pretreatment of haloperidol, pregnanolone or lorazepam was administered prior to cocaine in order to determine if these drugs could alter the discriminative effects of cocaine. All the drugs were not administered to every subject and this helped eliminate drug history as a variable for the substitution studies. The number of subjects reported for each figure represents every data point collected for that particular treatment.
Drugs
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Research Technical Branch, Rockville, MD) and dissolved in saline (0.9%). D-amphetamine sulfate (Sigma-Aldrich, St Louis, MO), desipramine hydrochloride (Sigma-Aldrich, St Louis, MO), bupropion hydrochloride (Sigma-Aldrich, St Louis, MO) and morphine sulfate (Merck and Co. Inc, Rahway, NJ) were also dissolved in saline. Haloperidol (McNeil laboratories Inc., Fort Washington, PA) was dissolved in 60% propylene glycol, 20% ethanol and 20 % saline. Pregnanolone (5β-Pregnan-3α-ol-20-one; Steraloids Inc, Newport, R.I., USA) was dissolved in 45% 2-hydroxypropyl γ-cyclodextrin in saline. Lorazepam (Wyeth Laboratories Inc, Marietta, PA, USA) was dissolved in 18% polyethylene glycol, 2% benzyl alcohol and 80% propylene glycol. All the drugs were administered intraperitoneally (i.p) and the injection volume was 1 ml/kg. In each subject, the doses for each particular drug ranged from an ineffective dose to a dose that produced significant rate-decreasing effects. In all cases, cocaine was administered immediately prior to the start of the experimental session. Amphetamine and morphine were administered immediately prior to the beginning of the session, whereas desipramine and bupropion were administered 20 and 30 min before the experimental session, respectively. Saline (control) injections were administered at the same pretreatment times as the respective drugs. Pregnanolone and lorazepam were always administered 5 minutes before the start of the session as were their respective vehicles. The doses of pregnanolone and lorazepam selected for the interaction experiments with cocaine (10 and 0.32 mg/kg, respectively) were chosen because these doses produced comparable rate-decreasing and error-increasing effects under another operant baseline (Quinton et al., 2005). Haloperidol (0.032 mg/kg and 0.1 mg/kg.) was administered 25 minutes before cocaine or saline injections. These pretreatment times were based on the onset of action of the various drugs as determined preliminarily by unpublished observations.
Data analysis
The data for each session were expressed as the percentage of responses on the lever for which cocaine was serving as a discriminative stimulus (i.e., “cocaine-lever” responding) and overall response rate (responses/sec). Within-session changes in responding were monitored by a cumulative recorder and a computer. Group data for each variable were expressed as the mean and standard error of the mean (SEM); however, due to individual differences in the sensitivity not all doses were studied in all rats, which led to differences in the number of subjects for each data point. Because no differences in the results of the drug substitution or interaction studies were observed between the two cocaine training doses (5.6 and 10 mg/kg), the data for both training doses were combined. Full substitution was defined as 80% or more of cocaine-lever responding. The overall response rate was considered different from saline when the mean was not within 20% of the rate measured under saline conditions. The percentage of cocaine-lever responses was not included in the analyses when the response rate was lower than 0.08 responses/sec. Doses that generated 50% cocaine-lever responding (ED50s) were estimated using a non-linear sigmoidal regression model (GraphPad Prism, GraphPad Software, Inc.). Shifts in the ED50 values of the dose–effect curves were considered to be significant when the ED50 values obtained following pretreatments were outside of the 95% confidence intervals of the mean ED50 value (95% CI) for cocaine alone.
Results
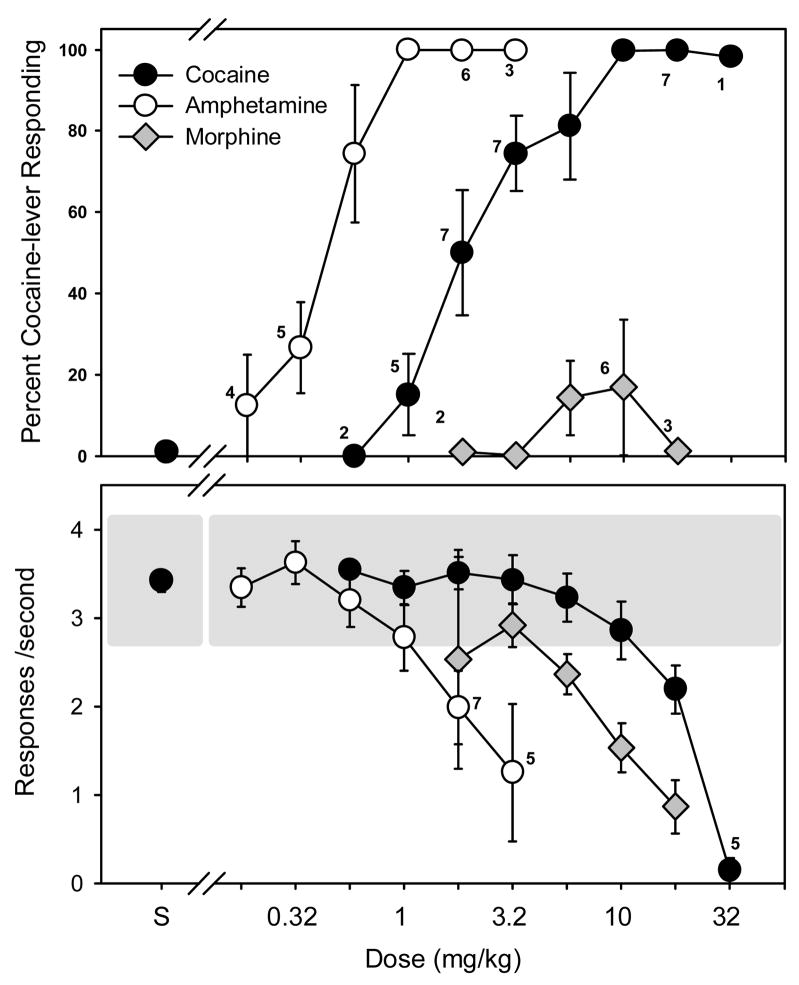
Figure 1 shows the percentage of cocaine-lever responses and the overall response rate for the group following the administration of cocaine, d-amphetamine or morphine. The average number of sessions required for the subjects to satisfy the testing criteria was 44.9 ± 3. 4 and the mean response rate was 3.42 ± 0.12 responses/sec (data above S, Figs. 1 and 2). Regardless of the training dose, cocaine produced dose-dependent increases in cocaine-lever responding, with doses larger than 5.6 mg/kg generating greater than 80% cocaine-lever responding in all the subjects. The mean ED50 values for cocaine lever–responding in the rats trained with the 5.6 and the 10 mg/kg dose of cocaine were 1.843 ± 0.347 and 3.11 ± 0.357 mg/kg, respectively. When doses of d-amphetamine were administered prior to test sessions, there was also a graded increase in cocaine-lever responding in all subjects with substitution occurring at doses larger than 0.56 mg/kg. The ED50 values for amphetamine-induced cocaine-lever responding were 0.972 ± 0.455 and 0.597 ± 0.239 mg/kg for the 5.6 and the 10 mg/kg training doses of cocaine, respectively. Because these ED50 values represented less than a twofold shift in the dose-effect curve, the data for the two training doses were averaged together. Both cocaine and d-amphetamine produced dose-dependent decreases in response rates, but decreases in rate occurred at doses larger than those required to produce greater than 80% cocaine-lever responding. Unlike d-amphetamine, morphine did not substitute for cocaine in any rat, up to doses that substantially decreased rates of responding.
Figure 1.
Percentage of cocaine-lever responding (upper panel) and overall rate of responding (lower panel) in subjects administered either cocaine (n=8, filled circles), amphetamine (n=7, unfilled circles) or morphine (n=7, grey diamonds). All three drugs were administered i.p. immediately before the start of the session. Values adjacent to a data point indicate the number of subjects represented by that point, if less than 8 for cocaine, or 7 for amphetamine and morphine. Percentages of cocaine-lever responding and response rates are expressed as mean ± S.E.M. The shaded area on the bottom panel represents the mean response rate following saline administration ± 20 %.
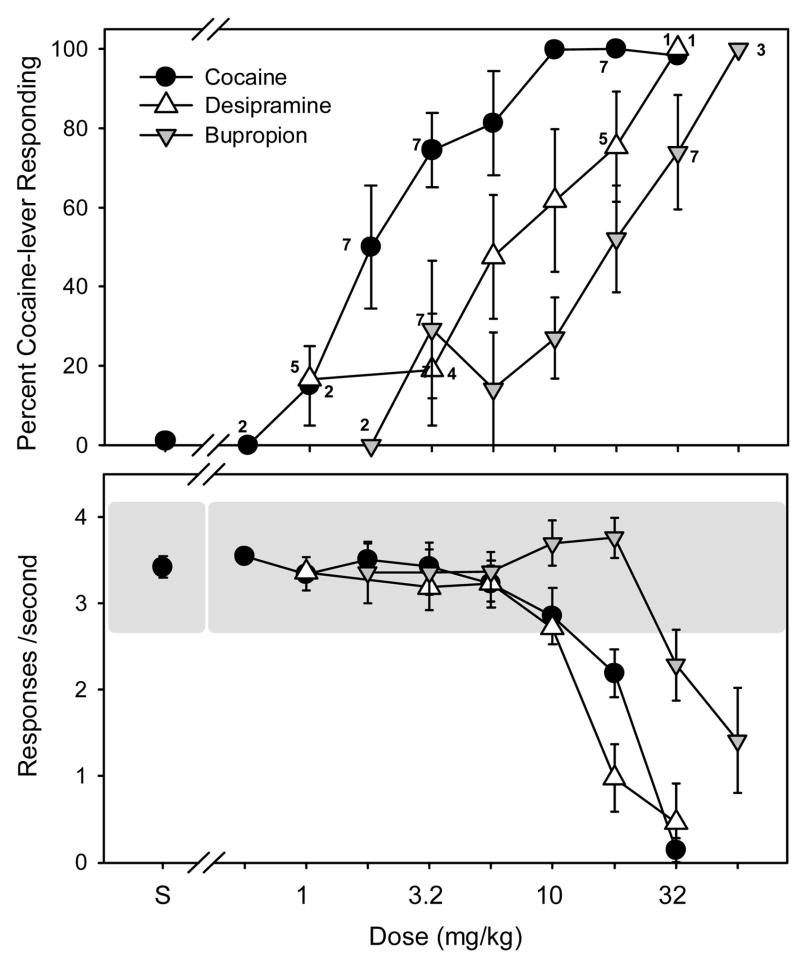
Figure 2.
Percentage of cocaine-lever responding (upper panel) and overall rate of responding (lower panel) in subjects administered either cocaine (n=8, filled circles), bupropion (n=8, grey triangles) or desipramine (n=8, unfilled triangles). Bupropion and desipramine were administered i.p. 30 and 20 min before the start of the session, respectively. Values adjacent to a data point indicate the number of subjects represented by that point, if less than 8. Percentages of cocaine-lever responding and response rates are expressed as mean ± S.E.M. The shaded area on the bottom panel represents the mean response rate following saline administration ± 20 %.
As shown in Figure 2, both bupropion and desipramine substituted for cocaine. In the 7 of 8 subjects for which bupropion and desipramine administration led to greater than 80% cocaine-lever responding, substitution was observed at doses smaller than those that produced decreases in rates of responding. However, in one subject for which desipramine only partially substituted for the effects of cocaine (i.e., 33.9% cocaine-lever responding), responding on the cocaine-lever occurred at doses that produced rate-decreasing effects (e.g., 18 mg/kg). Similar to amphetamine, the training dose of cocaine did not affect the ED50 values for the percentage of cocaine-lever responding following bupropion and desipramine (bupropion: 15.381 ± 4.644 and 22.607 ± 4.697 mg/kg; desipramine: 7.932 ± 3.043 and 5.545 ± 0.0175 mg/kg for rats trained with 5.6 and 10 mg/kg of cocaine, respectively). The relative potencies of substitution for the discriminative stimulus effects of cocaine were amphetamine> cocaine> desipramine> bupropion (Table 1).
Table 1.
Mean ED50 values and their 95% CIs for the percentage of cocaine-lever responding following cocaine, amphetamine, desipramine, and bupropion administration.
| Cocaine | d-amphetamine | Desipramine | Bupropion | |
|---|---|---|---|---|
| ED50 (mg/kg) | 3.067 | 0.865 | 7.851 | 18.665 |
| 95%CI | 1.055 to 5.079 | 0.068 to 0.1.662 | 3.236 to 12.466 | 10.075 to 27.255 |
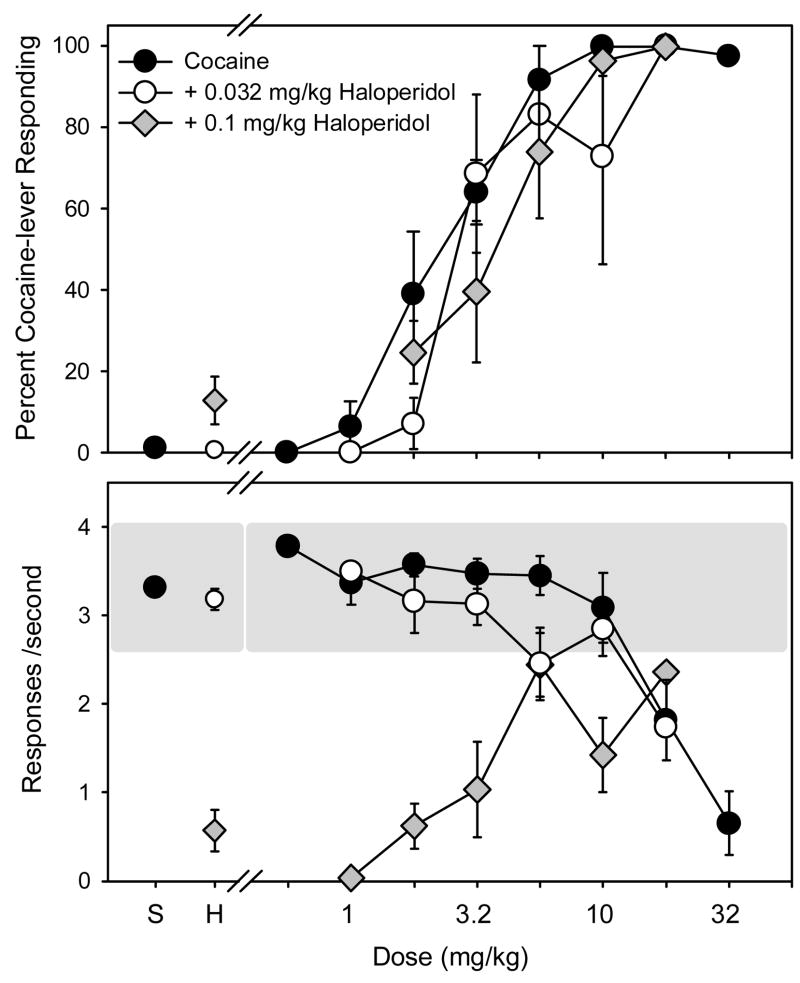
As indicated by the significant increase in ED50 value (Table 2), the dose-effect curve for cocaine-lever responding was shifted to the right in most subjects after 0.1 mg/kg of haloperidol, although these shifts were not evident in the grouped data depicted in Figure 3. More specifically, haloperidol produced a 2-fold increase in ED50 values in 4 of the 6 subjects following pretreatment with 0.032 mg/kg and a 2- to 3-fold increase in 4 of the 6 subjects following pretreatment with 0.1 mg/kg. Neither dose of haloperidol alone (points above H, Fig. 3) substituted for cocaine. Unlike the 0.032-mg/kg dose of haloperidol, which did not decrease response rates compared to control rates (i.e., 3.32 ± 0.11 responses/sec), the 0.1-mg/kg dose markedly decreased response rates in all the subjects when administered alone (Figure 3, bottom panel) and when administered in combination with doses of cocaine smaller than 3.2 mg/kg. Interestingly, the rate-decreasing effects of 0.1 mg/kg of haloperidol were attenuated by cocaine doses of 3.2 mg/kg or larger.
Table 2.
ED50 values and their 95% CI for the percentage of cocaine-lever responding produced by cocaine alone, or cocaine following a pretreatment with 0.032 or 0.1 mg/kg of haloperidol.
| 215 | 216 | 217 | 348 | 442 | 444 | Mean | |
|---|---|---|---|---|---|---|---|
| Control | 1.873 | 1.418 | 1.848 | 5.585 | 3.212 | 2.936 | 2.812 |
| 95% CI | 1.212–4.412 | ||||||
|
| |||||||
| Haloperidol | |||||||
| 0.032 mg/kg | 2.030 | 2.577 | 3.706 | 5.585 | 2.504 | 5.625 | 3.671 |
| 0.1 mg/kg | 3.895 | 2.459 | ND | 5.590 | 4.398 | 9.242 | 5.116* |
The asterisk represents an ED50 value that falls outside of the control confidence interval.
Figure 3.
Percentage of cocaine-lever responding (upper panel) and overall rate of responding (lower panel) in subjects administered either cocaine alone (filled circles) or cocaine following pretreatment with haloperidol [0.032 (unfilled circles) and 0.1 (grey diamonds) mg/kg] (n=6). Cocaine and haloperidol were administered i.p. immediately or 25 minutes before the start of the session, respectively. Percentages of cocaine-lever responding and response rates are expressed as mean ± S.E.M. The shaded area on the bottom panel represents the mean response rate following saline administration ± 20 %.
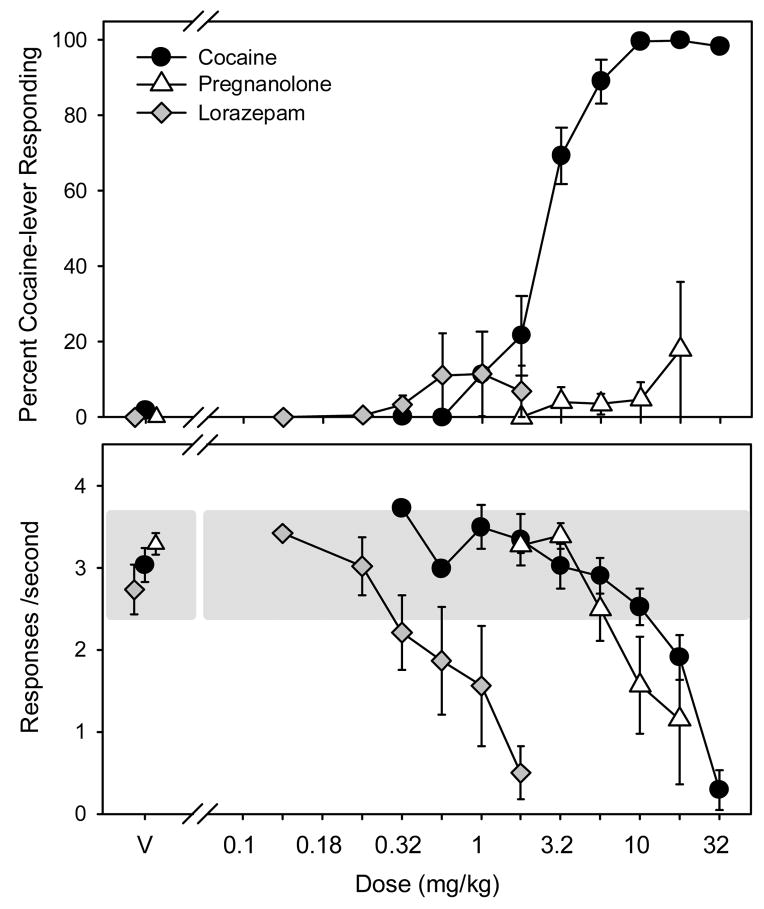
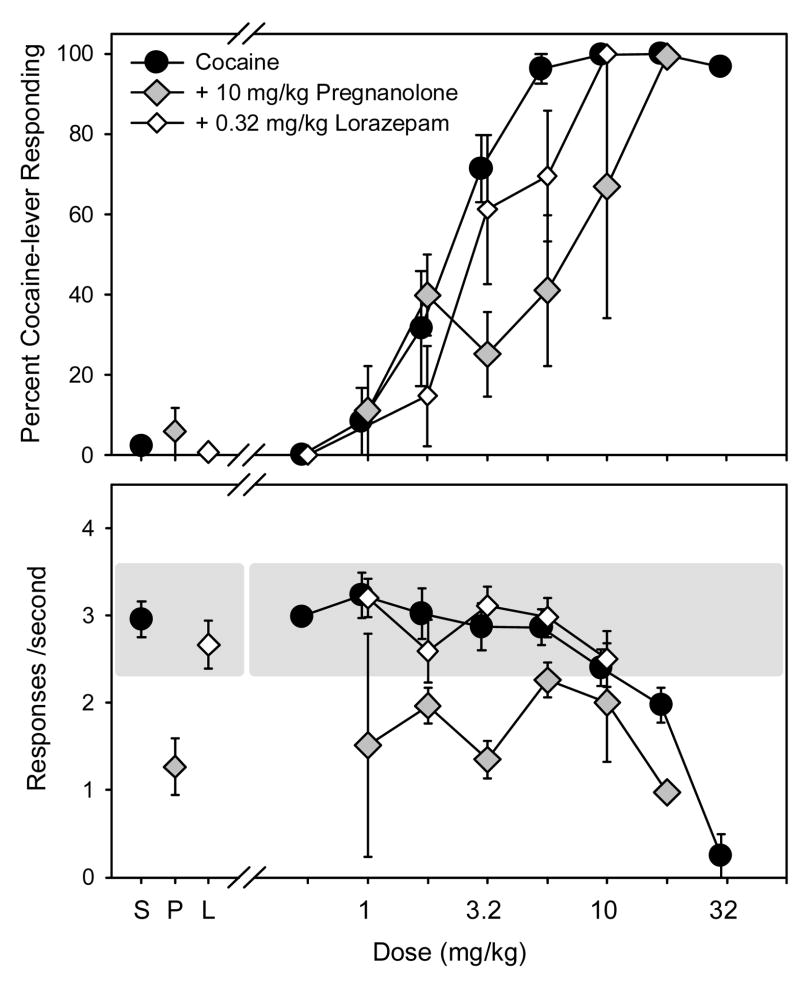
The ability of pregnanolone and lorazepam to substitute for cocaine is depicted in Figure 4. Pregnanolone administration did not result in more than 20% cocaine-lever responding in any rat. Doses larger than 5.6 mg/kg of pregnanolone produced significant rate-decreasing effects in most subjects. Similar to pregnanolone, lorazepam did not produce more than 20% responding on the cocaine lever. Rate-decreasing effects were observed in most subjects at doses larger than 0.56 mg/kg of lorazepam.
Figure 4.
Percentage of cocaine-lever responding (upper panel) and overall rate of responding (lower panel) in subjects administered either cocaine (n=10, filled circles), pregnanolone (n=6, unfilled triangles), or lorazepam (n=6, grey diamonds). Cocaine was administered i.p. immediately before the start of the session and pregnanolone and lorazepam were administered i.p. 5 minutes before the start of the session. Percentages of cocaine-lever responding and response rates are expressed as mean ± S.E.M. The shaded area on the bottom panel represents the mean response rate following saline administration ± 20 %.
As shown in Figure 5, 10 mg/kg of pregnanolone prior to the session produced a rightward shift in the mean dose-response curve for cocaine-lever responding. This dose of pregnanolone also produced rate-decreasing effects when compared to control rates (2.96 ±0.21 responses/sec), but they were attenuated by cocaine. This was most evident at the 5.6-mg/kg dose of cocaine where the response rate obtained following the pregnanolone pretreatment was similar to the saline control rate. In contrast to pregnanolone, pretreatment with 0.32 mg/kg of lorazepam did not noticeably alter the discriminative stimulus or rate-decreasing effects of cocaine. The ED50 values for cocaine following the pregnanolone and lorazepam pretreatments are shown in Table 3. Although the mean ED50 values following both pretreatments fell outside of the confidence interval established for the control data, the mean ED50 value for the combination of cocaine and 10 mg/kg of pregnanolone was approximately 2.5-fold higher than the ED50 value for cocaine alone, whereas the lorazepam pretreatment produced a more modest (less than 2-fold) shift. Furthermore, the pregnanolone pretreatment produced a rightward shift of the cocaine dose-effect curve in every subject tested, whereas the lorazepam pretreatment only produced a comparable shift in 2 out of 6 subjects, and actually produced a leftward shift of the cocaine dose-effect curve in subject PR-443.
Figure 5.
Percentage of cocaine-lever responding (upper panel) and overall rate of responding (lower panel) in subjects administered either cocaine alone (n=9, filled circles) or cocaine following a pretreatment with 10 mg/kg of pregnanolone (n=5, grey diamonds), or 0.32 mg/kg of lorazepam (n=6, unfilled diamonds). Cocaine was administered i.p. immediately before the start of the session and pregnanolone and lorazepam were administered i.p. 5 minutes before the start of the session Percentages of cocaine-lever responding and response rates are expressed as mean ± S.E.M. The shaded area on the bottom panel represents the mean response rate following saline administration ± 20 %.
Table 3.
ED50 values and their 95% CI for the percentage of cocaine-lever responding produced by cocaine administered alone, or cocaine following a pretreatment with 10 mg/kg of pregnanolone or 0.32 mg/kg of lorazepam.
| 216 | 297 | 444 | 473 | 442 | 474 | 331 | 299 | 443 | Mean | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1.414 | 2.398 | 2.936 | 2.503 | 2.979 | 2.446 | ||||
| 95% CI | 1.662–3.23 | |||||||||
| Pregnanolone | 2.05 | 7.83 | 3.61 | 11.87 | 5.53 | ND | ND | ND | ND | 6.177* |
|
| ||||||||||
| Control | 2.503 | 2.979 | 3.573 | 3.522 | 2.504 | 2.504 | 2.931 | |||
| 95% CI | 2.394–3.468 | |||||||||
| Lorazepam | ND | ND | ND | 2.504 | 7.8 | 3.037 | 5.288 | 2.504 | 0.8959 | 3.661* |
The asterisk represents an ED50 value that falls outside of the control confidence interval.
Discussion
In the present study, rats were successfully trained to discriminate cocaine from saline. D-amphetamine, bupropion and desipramine substituted for cocaine. In contrast, morphine and two positive GABAA modulators, pregnanolone and lorazepam, engendered predominantly saline-lever responding, indicating that these compounds do not share discriminative stimulus effects with cocaine. When pregnanolone was administered prior to cocaine, there was a significant attenuation of the discriminative stimulus effects of cocaine. Attenuation of the discriminative stimulus effects of cocaine only occurred in 2 out of 6 rats when the benzodiazepine lorazepam was administered as the pretreatment. The D2 receptor antagonist haloperidol produced a small shift to the right in the cocaine dose-effect curve in most subjects, but the effect was not statistically significant for the group. The differences in the training dose of cocaine did not appear to change the outcome of the substitution or interaction studies. This is probably due to the training conditions that were designed to ensure an equivalent effect of the training dose of cocaine in all subjects.
The substitution of d-amphetamine, bupropion and desipramine for cocaine was consistent with the idea that discriminative stimulus effects of cocaine comprise more than an increase in dopaminergic transmission. The CNS stimulant d-amphetamine, which has the ability to release dopamine and norepinephrine, substituted for cocaine and this was replicated from several previous studies (Colpaert et al. 1978; de la Garza R. et al. 1985; Garza et al. 1983). Both the typical (desipramine) and the atypical (bupropion) antidepressants substituted for cocaine despite large differences in dopamine transporter binding affinities. These effects with desipramine were somewhat surprising because Jones et al. (1980) reported that cocaine produced full substitution in rats trained to discriminate 20 mg/kg of bupropion from saline, and desipramine resulted in saline-lever responding. In another study by Baker et al. (1993), desipramine produced only partial substitution in rats trained to discriminate 10 mg/kg of cocaine from saline, suggesting differences between the discriminative stimulus effects of cocaine and those of desipramine. Given that desipramine is more selective for the norepinephrine transporter than the serotonin transporter (Richelson et al. 1984; Koe 1976), and that bupropion has some affinity for the norepinephrine transporter, norepinephrine seems to play an integral role in the discriminative stimulus effects of cocaine. This has been supported by other studies where norepinephrine reuptake inhibitors have produced partial substitution for cocaine or enhanced the discriminative stimulus effects of cocaine in rats (Kleven et al. 1998; Baker et al. 1993) and monkeys (Spealman 1995), although alpha and beta adrenergic receptor agonists do not substitute for cocaine. The alpha1 receptor antagonist prazosin was shown to antagonize the discriminative stimulus effects of cocaine in monkeys (Spealman 1995). Consistent with the idea that other monoamines play a role in the discriminative stimulus effects of cocaine, was the fact that haloperidol only produced a small rightward shift of the cocaine dose-effect curve.
Unlike bupropion and desipramine, which substituted for cocaine in the present study, pregnanolone was similar to morphine and lorazepam in that it did not produce cocaine-lever responding. Morphine, in particular, was chosen as a comparison drug because of previous studies indicating a lack of interaction between cocaine and mu opioids in drug discrimination (Broadbent et al. 1995), whereas lorazepam was chosen because it is another positive GABAA modulator (Galpern et al. 1990). The assumption that both pregnanolone and lorazepam are working via positive modulation of the GABAA receptor is based largely on previous findings indicating that pregnanolone can act as a positive GABAA modulator (Gerak et al. 2004) and on data from drug discrimination studies indicating that lorazepam can share discriminative stimulus effects with pregnanolone (Vanover 2000).
Positive GABAA modulators have been reported to reduce basal or cocaine-induced dopamine release (Dewey et al. 1997; Dewey et al. 1992; Schiffer et al. 2003). Accordingly, high efficacy benzodiazepines as well as pentobarbital have been shown to attenuate the discriminative stimulus effects of cocaine in primates (Negus et al. 2000) and rats (Barrett et al. 2005). In the present studies, although both pregnanolone and lorazepam in combination with cocaine produced a significant rightward shift in the ED50 values when compared to the ED50 value for the dose-effect curve for cocaine alone, the individual data clearly indicate that pregnanolone was more effective than lorazepam at antagonizing the discriminative stimulus effects of cocaine. In contrast to lorazepam, which only produced a shift to the right in 2 subjects, pregnanolone produced a shift to the right in every subject. Unfortunately, this difference cannot be attributed to differences in the drugs capacity to interact with GABAA receptor because the doses were not as equivalent under this discrimination procedure as they had been in rats responding under a multiple schedule of repeated acquisition and performance (Quinton et al. 2005). In that study, both pregnanolone (10 mg/kg, i.p.) and lorazepam (0.32 mg/kg, i.p.) produced very similar error-increasing effects when administered alone or in combination with cocaine, suggesting that the effects of these doses of pregnanolone and lorazepam might be somewhat task dependent.
Of course, the attenuation of cocaine’s discriminative effects by pregnanolone might be attributable, in part, to its capacity for producing rate-decreasing effects at higher doses. Similar to pregnanolone’s effects in another discrimination study (Vanover, 2000), 10 mg/kg of pregnanolone decreased responding from baseline levels by approximately 50%. This, however, is not an entirely likely explanation for the observed attenuation of cocaine’s discriminative effects as the two dependent measures, response rate and drug appropriate-lever responding, should be considered independently. That is, rate-decreasing effects are not synonymous with either a greater or lesser magnitude of drug-appropriate responding as evidenced by a host of drug discrimination studies and by the haloperidol in the present study. For example, similar to the pregnanolone interaction, the response rate after the combination of 0.1 mg/kg of haloperidol and 10 mg/kg of cocaine was about 50% of baseline levels; however, cocaine-lever responding was still over 90% and approximated that for cocaine alone.
In summary, these data suggest that the discriminative stimulus effects of pregnanolone in rats are not similar to monoamine inhibitors such as cocaine. This was most evident from the substitution of other drugs that inhibit monoamine uptake and the lack of substitution of drugs that have completely different mechanisms of action such as morphine and lorazepam. Furthermore, pregnanolone attenuated the discriminative stimulus effects of cocaine to a greater extent than lorazepam, providing additional evidence that pregnanolone has effects that are similar to many positive GABAA modulators and that there may also be important differences between pregnanolone and other positive modulators of the GABAA receptor.
Acknowledgments
This study was supported in part by Grant AA09803S1 from the National Institute on Alcohol Abuse and Alcoholism and Grants DA11417 and DA12427 from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Baker LE, Riddle EE, Saunders RB, Appel JB. The role of monoamine uptake in the discriminative stimulus effects of cocaine and related compounds. Behav Pharmacol. 1993;4:69–79. [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Bolacchi F, Concas A, Mostallino MC, Purdy RH, Biggio G. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol Biochem Behav. 1996;54:205–210. doi: 10.1016/0091-3057(95)02133-7. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Negus SS, Mello NK, Caine SB. Effect of GABA agonists and GABA-A receptor modulators on cocaine- and food-maintained responding and cocaine discrimination in rats. J Pharmacol Exp Ther. 2005;315:858–871. doi: 10.1124/jpet.105.086033. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK. Effects of mixed-action kappa/mu opioids on cocaine self-administration and cocaine discrimination by rhesus monkeys. Neuropsychopharmacology. 2003;28:1125–1139. doi: 10.1038/sj.npp.1300105. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Gaspard TM, Dworkin SI. Assessment of the discriminative stimulus effects of cocaine in the rat: lack of interaction with opioids. Pharmacol Biochem Behav. 1995;51:379–385. doi: 10.1016/0091-3057(94)00408-b. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJ, Janssen PA. Discriminative stimulus properties of cocaine and d-amphetamine, and antagonism by haloperidol: a comparative study. Neuropharmacology. 1978;17:937–942. doi: 10.1016/0028-3908(78)90135-1. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. Discriminative stimulus properties of cocaine in pigeons. Psychopharmacology (Berl) 1985;85:23–30. doi: 10.1007/BF00427317. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Chaurasia CS, Chen CE, Volkow ND, Clarkson FA, Porter SP, Straughter-Moore RM, Alexoff DL, Tedeschi D, Russo NB, Fowler JS, Brodie JD. GABAergic attenuation of cocaine-induced dopamine release and locomotor activity. Synapse. 1997;25:393–398. doi: 10.1002/(SICI)1098-2396(199704)25:4<393::AID-SYN11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Yu DW, Ferrieri RA, King PT, MacGregor RR, Martin TP, Wolf AP. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J Neurosci. 1992;12:3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JM, Damsma G, Fibiger HC. Benzodiazepine-induced decreases in extracellular concentrations of dopamine in the nucleus accumbens after acute and repeated administration. Psychopharmacology (Berl) 1992;106:202–208. doi: 10.1007/BF02801973. [DOI] [PubMed] [Google Scholar]
- Galpern WR, Miller LG, Greenblatt DJ, Shader RI. Differential effects of chronic lorazepam and alprazolam on benzodiazepine binding and GABAA-receptor function. Br J Pharmacol. 1990;101:839–842. doi: 10.1111/j.1476-5381.1990.tb14167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza RD, Johanson CE. The discriminative stimulus properties of cocaine in the rhesus monkey. Pharmacol Biochem Behav. 1983;19:145–148. doi: 10.1016/0091-3057(83)90323-4. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Stevenson MW, Winsauer PJ, Moerschbaecher JM. Effects of pregnanolone alone and in combination with other positive GABA(A) modulators on complex behavior in rats. Psychopharmacology (Berl) 2004 doi: 10.1007/s00213-003-1717-2. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Discriminative stimulus properties of cocaine: enhancement by monoamine reuptake blockers. J Pharmacol Exp Ther. 1998;284:1015–1025. [PubMed] [Google Scholar]
- Knuepfer MM, Purcell RM, Gan Q, Le KM. Hemodynamic response patterns to acute behavioral stressors resemble those to cocaine. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1778–R1786. doi: 10.1152/ajpregu.2001.281.6.R1778. [DOI] [PubMed] [Google Scholar]
- Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976;199:649–661. [PubMed] [Google Scholar]
- Lambert JJ, Peters JA, Sturgess NC, Hales TG. Steroid modulation of the GABAA receptor complex: electrophysiological studies. Ciba Found Symp. 1990;153:56–71. doi: 10.1002/9780470513989.ch4. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Fivel PA. Effects of GABA agonists and GABA-A receptor modulators on cocaine discrimination in rhesus monkeys. Psychopharmacology (Berl) 2000;152:398–407. doi: 10.1007/s002130000543. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton MS, Gerak LR, Moerschbaecher JM, Winsauer PJ. Interaction of cocaine with positive GABAA modulators on the repeated acquisition and performance of response sequences in rats. Psychopharmacology (Berl) 2005;181:217–226. doi: 10.1007/s00213-005-2241-3. [DOI] [PubMed] [Google Scholar]
- Richelson E, Pfenning M. Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: most antidepressants selectively block norepinephrine uptake. Eur J Pharmacol. 1984;104:277–286. doi: 10.1016/0014-2999(84)90403-5. [DOI] [PubMed] [Google Scholar]
- Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Marsteller D, Dewey SL. Sub-chronic low dose gamma-vinyl GABA (vigabatrin) inhibits cocaine-induced increases in nucleus accumbens dopamine. Psychopharmacology (Berl) 2003;168:339–343. doi: 10.1007/s00213-003-1446-6. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1995;275:53–62. [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE. Effects of benzodiazepine receptor ligands and ethanol in rats trained to discriminate pregnanolone. Pharmacol Biochem Behav. 2000;67:483–487. doi: 10.1016/s0091-3057(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]