Abstract
The treatment of cancer using macromolecular therapeutics such as oligonucleotides or peptides requires efficient delivery systems capable of intracellular penetration and may also benefit from use of a combination of therapeutics with different mechanisms of action. With this possibility in mind, we constructed cationic liposome loaded with the proapoptotic peptide, D-(KLAKLAK)2 and the bcl-2 antisense oligodeoxynucleotide, G3139, and determined whether the combination of the proapoptotic macromolecules in a single cationic liposome can enhance antitumor efficacy. Advantage was taken of alternating charge interaction to entrap macromolecules of opposite charge. The polycationic pepetide D-(KLAKLAK)2 was first condensed with the polyanionic oligodeoxynucleotide G3139 to obtain overall negatively charged peptide/oligodeoxynucleotide complexes. The complexes were then entrapped into DOTAP/DOPE cationic liposomes (CL). This sequential charge interaction ensured efficient entrapment of D-(KLAKLAK)2 and G3139 with a high loading efficiency (50 %) and capacity (7.5 wt%). In vitro treatment of mouse melanoma B16(F10) with CL loaded with D-(KLAKLAK)2/G3139 led to significantly enhanced antitumor efficacy, mediated by stimulated induction of apoptotic (Caspase 3/7) activity, when compared to CL loaded with G3139 alone. Intratumoral injection of CL loaded with D-(KLAKLAK)2/G3139 in B16(F10) mice xenograft also led to suppressed tumor growth associated with enhanced apoptotic activity. Thus, the combination of proapoptotic peptide D-(KLAKLAK)2 and antisense oligonucleotide G3139 in a cationic liposome led to enhanced apoptotic/antitumor efficacy and may provide a promising tool for cancer treatment.
Keywords: Cancer Gene Therapy, Cationic Liposome (CL), Proapoptotic Peptide, D-(KLAKLAK)2, Proapoptotic bcl-2 oligodeoxynucleotide, G3139
Introduction
Mitochondria have been prime targets for therapeutic interventions of cell death by apoptosis. The induction of apoptosis can occur either by promotion of mitochondrial outer membrane permeability (MOMP) engaging pro-apoptotic members of the Bcl-2 family or by the induction of a mitochondrial permeability transition 1, 2. Thus, two separate strategies have been proposed that target mitochondria for induction of apoptosis and antitumor therapy 3: selective targeting of anti-apoptotic proteins with therapeutic agents, such as peptides, genetic materials, and small molecules, or disrupting of mitochondrial membranes more directly using the agents with high membrane disrupting capacity.
The 14-amino-acid amphipathic α-helical peptide D-(KLAKLAK)2 has been shown to induce apoptosis in cancer cells 4. This peptide does not disrupt the zwitterionic plasma membranes of eukaryotic cells but disrupts anionic prokaryotic cytoplasmic membranes and the eukaryotic mitochondrial membranes. When internalized, D-(KLAKLAK)2 can disrupt the negatively charged mitochondrial membrane, resulting in cell death by mitochondrial-dependent apoptosis 5. The use of this peptide for enhancing the apoptotic activity should involve targeting and internalization strategies. Chemical conjugates of the D-(KLAKLAK)2 with RGD peptide 5, protein transduction domain 6, and antibody 7 have been used for intracellular and extracellular targeting.
G3139 (Genasense, Genta Inc., NJ) is an 18-mer phosphorothioate antisense oligodeoxynecleotide (ODN) that selectively targets the initiation codon region of the bcl-2 mRNA for degradation by RNase H and thereby decreases Bcl-2 protein production. Bcl-2 protein plays a major role in preventing apoptosis 8 and overexpression of the protein was confirmed to be related to the tolerance of various tumors towards chemotherapy 9. The treatment of cancer cells with G3139 down-regulated Bcl-2 protein and increased the susceptibility towards the apoptosis, enhancing thus the tumor response to combined chemotherapy 10. This approach is now being evaluated in several clinical trials 11, 12. Despite the high efficacy, the use of G3139 has to overcome several extracellular and intracellular barriers inherent to gene therapeutics, and an efficient delivery system should assist in solving these problems.
The treatment of cancer using the macromolecular therapeutics, such as oligonucleotides or peptides, requires efficient delivery systems capable of intracellular penetration and may also benefit from use of a combination of therapeutics with different mechanisms of action. With this possibility in mind, we have prepared a cationic liposome-based formulation co-loaded with D-(KLAKLAK)2 and G3139 with the aim to enhance the apoptotic anti-tumor efficacy via (i) the combination of two apoptotic agents with different mechanisms of apoptosis induction, and (ii) by the enhanced intracellular delivery endowed by the cationic nature of the liposomal carrier. The formulation was constructed by pre-condensation of the cationic peptide with the anionic ODN and subsequent entrapment of these preformed overall negatively charged complexes in CL by lipid membrane rehydration/extrusion techniques. The CL loaded with the D-(KLAKLAK)2 and G3139 were evaluated for their antitumor efficacy following the cytotoxicity and pro-apoptotic activity toward the mouse melanoma B16(F10) cells.
Materials and Methods
Materials
Phosphorothioate bcl-2 antisense oligodeoxynucleotides (ODN) G3139 and control ODN G3622 were purchased from Calbiochem (San Diego, CA). Pro-apoptotic peptide D-(KLAKLAK)2 was purchased from Tufts Core Facility (Boston, MA). Fluorescently-labeled 5-FAM-D-(KLAKLAK)2 was purchased from AnaSpec, Inc (San Jose, CA). l,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), dioleoyl-1,2-diacyl-3-trimethylammonium-propane (DOTAP) were purchased from Avanti Polar Lipids (Alabaster, AL). All other chemicals were of the reagent grade.
Preparation of cationic liposomes loaded with D-(KLAKLAK)2 and G3139
D-(KLAKLAK)2 peptide and G3139 ODN were separately diluted in HEPES-buffered 5% glucose solution (pH 7.4) to a final volume of 250 μl. After 10 minutes incubation at room temperature, the solutions were rapidly mixed and vortexed immediately, resulting in 500 μl of peptide/ODN complexes at a +/- charge ratio of 1:2. The peptide/ODN complex solution was then added to a cationic lipid film of DOPE and DOTAP (1:1 mol/mol) at +/-/+ charge ratio of 1:2:6 and incubated for 4 hrs, followed by 11 times extrusion through a stack of two polycarbonate membranes with a 200 nm pore size using a hand-held extruder (Avanti Polar Lipid, AL). The amount of peptide and ODN and DOTAP were calculated to obtain the desired charge ratio by assuming that each peptide molecule contains six positive charges from lysine groups and that each ODN molecule contained 18 negative charges from phosphate groups, and that each DOTAP molecule contained one positive charge from an amine group. A formulation loaded with G3139 ODN alone was prepared as a control for in vitro and in vivo evaluation of antitumor efficacy. The attempt to prepare a similar formulation loaded with only peptide was unsuccessful, indication the strict requirement for the peptide/ODN complex formation for the efficient loading.
The resulting formulations were characterized with respect to complex formation, size distribution, zeta-potential, and loading efficiency. Complex formation was confirmed by the agarose gel electrophoresis. Electrophoresis was performed using the E-Gel elctrophoresis system (Invitrogen, Carlsbad, CA). A precast 0.8% E-Gel cartridge was pre-run for 2 min at 60 V and 500 mA followed by the loading of 2 μg of ODN. The size distribution and zeta-potential were determined by quasi-elastic light scattering technique using a Zeta Plus Analyzer (Brookhaven Instruments, Brookhaven, NY).
The peptide loading into CL was analyzed by the size exclusion chromatography (SEC) using the fluorescently-labeled peptide as a tracer. We attempted to prepare the formulations with 5-FAM-D-(KLAKLAK)2, and the fractions of the Sepharose CL4B column eluent were checked for the fluorescence intensity using a fluorescence plate reader (Synergy HT Multi-Mode Microplate Reader, BioTek Instrument, USA). The entrapment efficiency of the peptide was calculated as the percentage of total area found in the first peak. A formulation without ODN was used as a control to demonstrate the effect of complexation of peptide by ODN on the encapsulation efficiency.
Cytotoxicity assay
The mouse melanoma cell line B16(F10) was grown in DMEM supplemented with 10% fetal bovine serum (FBS) in 96-well plates to ∼ 50 % confluency. The cells were treated by replacement of the media with serum-free media (100 μl) containing a serial dilution of each formulation up to 1 μM of ODN (corresponding to 1.5 μM of D-(KLAKLAK)2 peptide and 50 μM of DOTAP). After 24 hrs incubation, the cells were washed twice with PBS and returned to fresh complete media. Then, 20 μl of CellTiter-Blue® Cell Viability Assay Reagent (Promega, Madison, WI) was added to each well and the plates were re-incubated for 2 hrs. The fluorescence at 490 nm was measured for each well using a 96-well fluorescence plate reader (Synergy HT Multi-Mode Microplate Reader, BioTek Instrument). Relative cell viability was calculated with cells treated with medium only as a control. The assay was carried out in triplicates.
Caspase-3/7 activity
The B16(F10) cells were grown and treated as above for 4 hrs. After washing the cells and adding fresh media, 20 μl of Apo-ONE Caspase-3/7 Reagent (Promega, Madison, WI) was added to each well and the plates were re-incubated for 2 hrs. The fluorescence at 490 nm was measured for each well using a 96-well plate reader (Synergy HT Multi-Mode Microplate Reader, BioTek Instrument). Relative Caspase-3/7 activity was calculated by comparing the results from the cells treated with medium only.
Antitumor activity in tumor-bearing mice
Male C57BL/6 mice (Charles River Laboratories) were inoculated subcutaneously in the left flank with 1 × 106 B16(F10) melanoma tumor cells 14 days before the treatment according to a protocol approved by the Institutional Animal Care and Use Committee at Northeastern University. When tumors reached a volume of approximately 200 mm3 ([length × width2]/2), a dose of 20 μl CL formulation loaded with peptide/G3139 or G3139 alone was administered in mice twice daily by the intra-tumoral injection (each dose corresponds to 4 μg of G3139 and 1.52 μg of D-(KLAKLAK)2 peptide). Saline-injected mice with similar-sized tumors were used as controls. Five days after the second injection when the control group's mean tumor volume reached approximately 1000 mm3, the mice were sacrificed by CO2, and tumors were excised. Percent tumor growth inhibition (%TGI) was calculated by comparing the treatment group's tumor volume (T) to the control group's tumor volume (C) at that time ([1-(T/C) × 100]).
TUNEL assay
Tumor-bearing mice were administered a single dose of 60 μl of the CL formulation loaded with peptide/G3139 when tumor volume reached approximately 600 mm3 by the intra-tumoral injection. Non-treated mice with similar-sized tumors were used as controls After 24 hours, the mice were sacrificed by CO2, and excised tumors were immediately frozen in Tissue-Tek OCT 4583 compound (Sakura Finetek, CA) without fixation, and 8 μm thick sections were prepared with a cryostat. A terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay was performed with a TdT-FragEL DNA fragmentation detection kit (Calbiochem, NJ), and the sections were examined by fluorescence microscopy (Olympus BX51).
Results
Cationic liposomes loaded with D-(KLAKLAK)2 peptide and G3139 ODN
Alternating charge interaction was employed to load the polycationic peptide in CL. The polycationic D-(KLAKLAK)2 peptide was first condensed with the polyanionic ODN to obtain overall negatively charged peptide/oligodeoxynucleotide complexes and then the complexes were entrapped into CL made of DOTAP/DOPE. The sequential charge interaction ensured efficient entrapment of D-(KLAKLAK)2 and G3139 with high loading efficiency (50 %) and capacity (7.5 wt%).
The 18-mer single stranded phosphorothioate ODN was first complexed with cationic peptide D-(KLAKLAK)2 and then entrapped into cationic liposome by hydrating the cationic lipid film with the aqueous buffer containing the negatively charged peptide/ODN complexes followed by the membrane extrusion. The charge ratio of (+) in the peptide to (-) in ODN to (+) in DOTAP was 1:2:6, i.e., positive charges were in excess.
The complexation of the polycationic peptide with polyanionic ODN led to nanoscale complexes with a narrow size distribution and negative zeta potential (235.7 ± 20.6 nm, -28.9 ± 3.5 mV, n = 3). The suspension obtained after the hydration of the cationic lipid film showed a multimodal size distribution with a mean diameter greater than 1300 nm and positive zeta potential (1436 ± 183.4 nm, 58.1 ± 3.8 mV, n = 3). After the extrusion, the suspension was formed with a narrow and unimodal size distribution (mean diameter of 194.7 ± 2.6 nm). Zeta potential measurement revealed that the negative charge of the peptide/ODN complexes (-28.9 ± 3.5 mV) was completely shielded by the cationic lipid membrane, resulting in positively charged particles (43.2 ± 3.6 mV) (Table 1). The liposome formulations remained colloidally stable in HBS (10mM HEPES, 150mM NaCl, pH 7.4) for at least 3 weeks.
Table 1.
Size distribution and zeta potential of peptide/ODN complexes and cationic liposome loaded with peptide/ODN complexes, CL(Peptide/ODN) were determined by QELS. Data represent mean ± s.e.m. (n ≥ 3).
| Size distribution (nm) |
Zeta potential (mV) |
|
|---|---|---|
| Peptide/ODN complex | 235.7 ± 20.6 | -28.9 ± 3.5 |
| CL(Peptide/ODN) | 194.7 ± 2.6 | 43.15 ± 3.6 |
The complex formation between the peptide and ODN was confirmed by the electrophoresis on a 0.8% agarose gel (Figure 1a). When complexed with the peptides, ODN could not enter the gel and retains in wells because of the size restriction. All ODN was retained in wells without the migration of free ODN (lane 1), indicating complete complexation. The ODN in the complex was released by the treatment with heparin (lane 2). The ODN in the CL formulation also cannot enter the gel, and ODN in the CL was also retained in wells (lane 3). The ODN was released from CL after the treatment with Triton X-100 followed by heparin and could enter the gel after that (lane 4).
Figure 1.
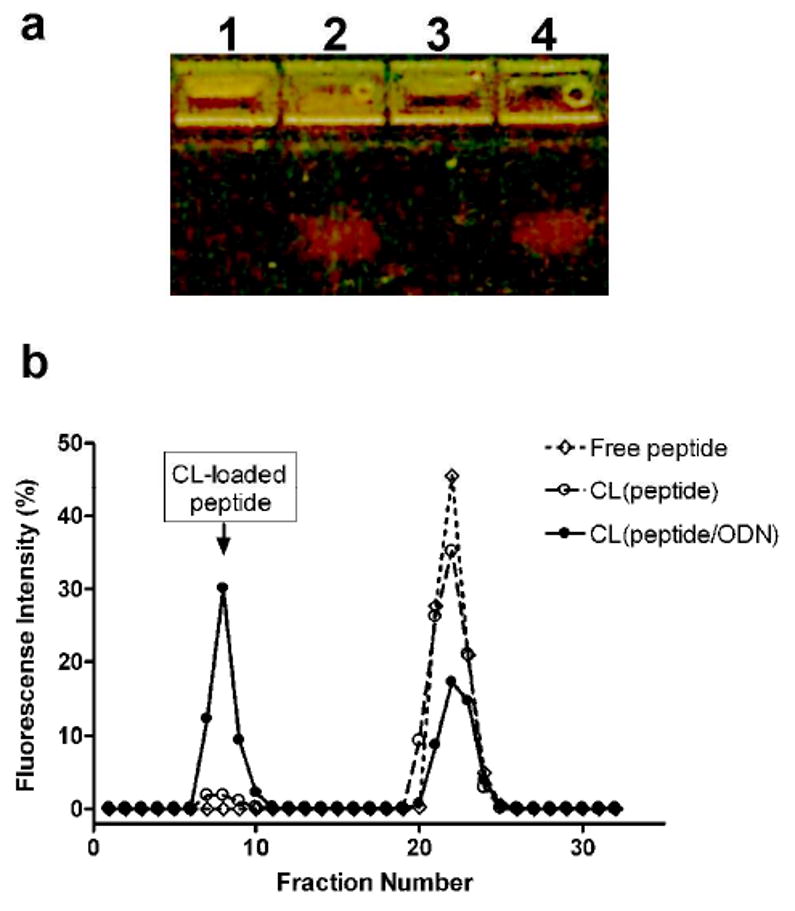
Preparation of cationic liposome (CL) loaded with D-(KLAKLAK)2 peptide and G3139 ODN. (a) Agarose gel electrophoresis confirmed the complex formation between peptide and ODN and subsequent entrapment of the complex with cationic liposome. Lane1: naked peptide/ODN complexes, nontreated; Lane2: naked peptide/ODN complexes, treated with heparin; Lane 3: CL loaded with peptide/ODN complexes, nontreated; Lane 4: CL loaded with peptide/ODN complexes, treated with heparin and Triton X-100; (b) Size exclusion chromatography (SEC) using FAM-D-(KLAKLAK)2 tracer revealed that about 54 % of peptide was loaded in CL. Free peptide was separated from CL-loaded peptide on a Sepharose CL4B column. Entrapped D-(KLAKLAK)2 eluted with CL at void volume. Without pre-complexation, the loading of peptide into CL was insignificant.
Size exclusion chromatography showed that about 54% of FAM-D-(KLAKLAK)2 peptide was entrapped into the CL (eluted in the void volume), but only when it was first complexed with the ODN (Figure 1b). Peptide alone cannot be entrapped into the CL (compare respective curves). Thus, the precomplexation of the peptide by ODN is an absolute request for the preparation of peptide/ODN-co-loaded CL.
In Vitro Anti-tumor Activity
The in vitro cytotoxicity of CL loaded with D-(KLAKLAK)2/G3139 complexes was assessed using the B16(F10) melanoma cells. The cell line and drug concentration ranges were chosen based on previous results 13-16. The formulations loaded with either G3139 alone or with the complex of the peptide with a reverse sequence ODN G3622 were used as controls. About 20% reduction in cell viability was observed with the liposome formulation loaded with both D-(KLAKLAK)2 peptide and G3139 ODN at 125 nM ODN concentration, while no cytotoxicity was observed with control formulations. Only ODN concentration in control preparation as high as 250 nM resulted in ca. 10% decrease in cell viability, while at the same ODN concentration the CL preparation loaded with both D-(KLAKLAK)2 and G3139 killed about 40% of all cells. At 1 μM ODN concentration, however, the control formulations led to cytotoxicity comparable to the formulation with D-(KLAKLAK)2 and G3139 indicating non-antisense mediated cell death (Figure 2).
Figure 2.
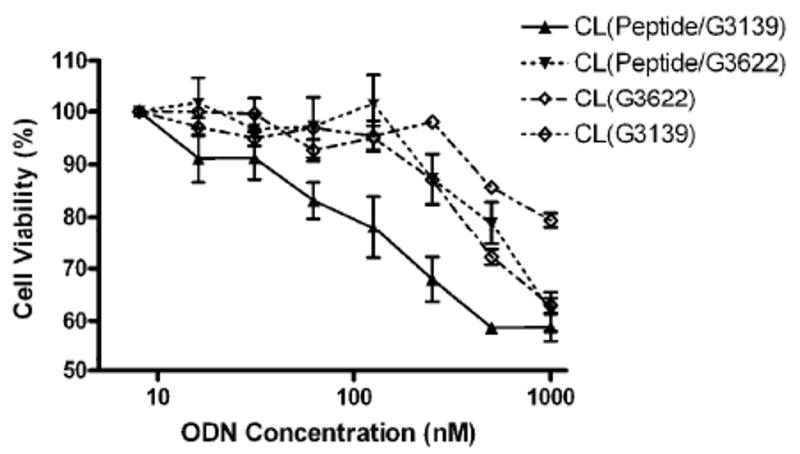
In vitro anti-tumor activity of CL loaded with D-(KLAKLAK)2/G3139 toward B16(F10) melanoma cells. The cells were treated for 24 hours before detection of redox enzymatic activity. CL loaded with G3139 only, complexes of the D-(KLAKLAK)2 peptide with a reverse sequence ODN G3622, and G3622 only were used as controls. Relative cell viability was expressed as a percentage of control cells treated with the medium. Reduced cell viability was observed in the cells treated with CL loaded with the D-(KLAKLAK)2 peptide and G3139 ODN at a relatively low ODN concentration, demonstrating the advantage of the combined apoptotic therapeutic.
In Vitro Apoptosis Assay (Caspase 3/7 Activity)
The CL loaded with D-(KLAKLAK)2/G3139 complexes were also assessed for the induction of apoptosis in B16(F10) melanoma cells. Caspase 3/7 activity was determined after 4 hrs treatment with different formulations. About a 40 - 50% increase in Caspase 3/7 activity was observed over 15 – 500 nM ODN concentration range in cells treated with formulations loaded with both, D-(KLAKLAK)2 and ODN. At the same range of ODN concentration, only about 15 – 25 % increase in Caspase 3/7 activity was observed in the cells treated with formulations loaded with antisense ODN G3139 alone. The formulation loaded only with control ODN G3622 alone led to 10 – 15 % increase only at high ODN concentration (125 and 500 nM) (Figure 3). Somewhat apparently reduced activity of CL co-loaded with the peptide and G3139 after the maximum reached at 30 nM concentration, could be explained by the fact that at the same time points we actually observe different stages of apoptosis for different concentrations of the drug: the caspase 3/7 activity shows maximum at the early stage of apoptosis and a higher concentrations of the drug provide the maximal apoptosis at earlier times, so we “miss” the highest caspase activity at the selected time point.
Figure 3.
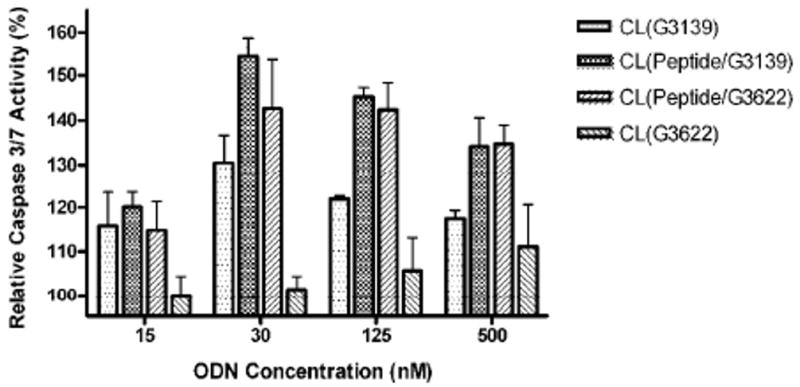
Caspase 3/7 activity in B16(F10) melanoma cells treated with CL loaded with D-(KLAKLAK)2/G3139. Cells were treated for 4 hours followed by 24 hours incubation before detection of the enzymatic activity. CL loaded with G3139 only, complexes of D-(KLAKLAK)2 peptide with a control ODN G3622, and G3622 only served as controls. Significantly increased Caspase 3/7 activity was observed in the cells treated with CL loaded with D-(KLAKLAK)2 and G3139, demonstrating the advantages of combining the apoptotic therapeutics for enhanced anti-tumor efficacy through increased apoptotic activity.
In Vivo Anti-tumor Efficacy
The enhanced anti-tumor efficacy in vivo was demonstrated in mice bearing the B16(F10) tumor. First, the inhibition of tumor growth was accessed by measuring tumor volumes after intra-tumoral administration of the CL preparation loaded with the peptide/G3139 complex (Fig. 4). The average mouse weight at the beginning of the treatment was 24.4 ± 0.35 g and increased by 7.6 % by the end of the study, i.e., day 4 post-injection. The average tumor volume at the beginning of the treatment was 218 ± 72 mm3. The percentage change in tumor volume by day 4 post-injection was 470 ± 95 % for the control group, whereas 308 ± 128 % and 186 ± 120 % of tumor volume increases were observed for the groups treated with the CL loaded with G3139 alone or with peptide/G3139 complex, respectively.
Figure 4.
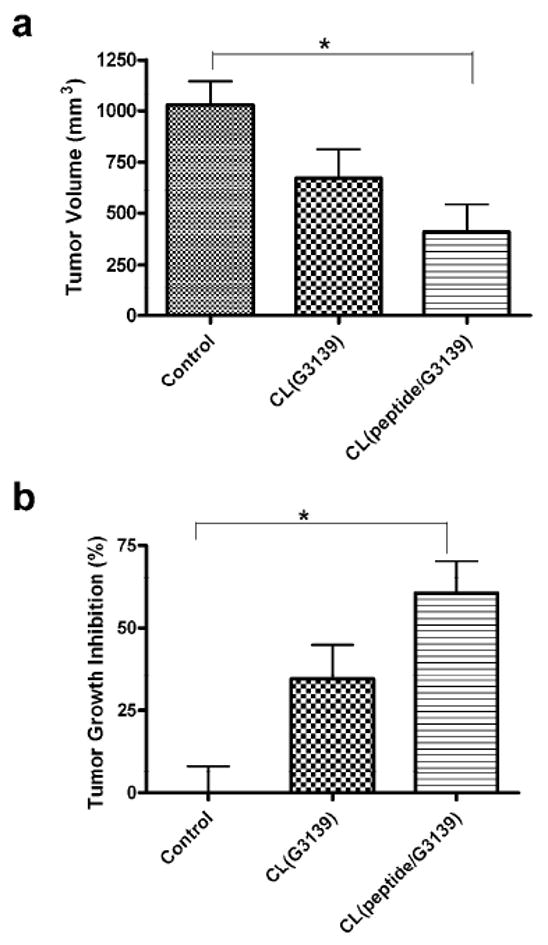
In vivo anti-tumor efficacy of CL loaded with D-(KLAKLAK)2/G3139 in the tumor-bearing mice. The mice bearing B16(F10) tumors were administered via intratumoral injection with CL loaded with D-(KLAKLAK)2/G3139 or G3139 alone, CL(peptide/G3139) and CL(G3139) respectively. After 4 days post-injection, (a) tumor volumes were measured and (b) relative inhibition of tumor growth was accessed by comparing tumor volumes of the treated animals to saline-treated controls. Intra-tumoral administration of CL(peptide/G3139) led to significantly decreased tumor volumes whereas CL(G3139) alone showed no significant decrease (n=4, one-way ANOVA followed by Tukey post-hoc test).
Thus, treatment of tumor-bearing mice with the CL loaded with the peptide/G3139 led to the significant tumor growth inhibition of 60 ± 25% as compared to the untreated control group (p<0.05, n=4). The treatment with the CL loaded with G3139 alone showed much smaller inhibition (34 ± 27 %, n=4). The apoptotic activity of the combined treatment was clearly confirmed by the TUNEL staining of the tumor sections from the animals treated with the CL loaded with the peptide/G3139 complex (Fig. 5). The brightly stained apoptotic nuclei were readily found in the sections from the tumor treated with this preparation.
Figure 5.
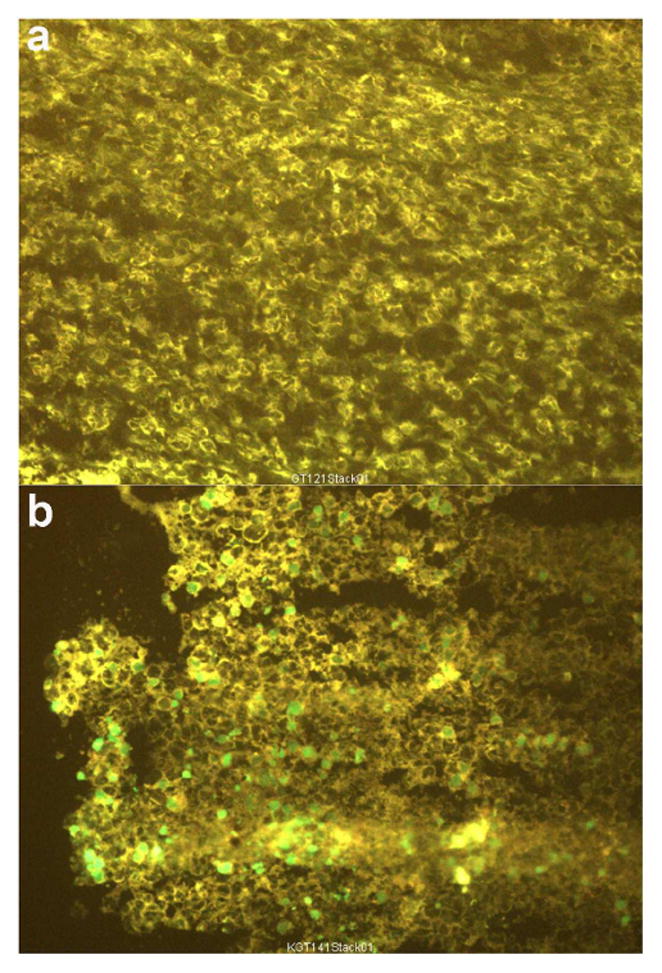
In vivo induction of apoptosis by CL loaded with D-(KLAKLAK)2/G3139 in the tumor-bearing mice. The mice bearing B16(F10) tumors were administered CL loaded with the peptide/G3139 via intra-tumoral injection. At 24 hrs post-injection, apoptotic activity in tumors was accessed by DNA fragmentation with TUNEL staining. Dual channel fluorescence microscopy of frozen tumor sections from in vivo grown-B16(F10) tumors is shown. (a) Tumor section from a non-treated animal (background pattern); (b) Tumor section from an animal injected with CL loaded with D-(KLAKLAK)2/G3139. Intra-tumoral injection of CL loaded with D-(KLAKLAK)2/G3139 led to bright green staining of apoptotic nuclei.
Discussion
The results of the current study are compatible with the following conclusions: first, the suggested method provides a promising way to formulate CL for intracellular delivery of cationic peptide therapeutics, while previously, the application of the CL limited to the delivery of anionic gene therapeutics; second, the CL loaded with pro-apoptotic peptide D-(KLAKLAK)2 and bcl-2 antisense ODN G3139 exerts enhanced anti-tumor efficacy in vitro and in vivo towards B16(F10) melanoma.
Earlier, it was shown that approx. 400 μM cationic peptide D-(KLAKLAK)2 is required to kill 50% of eukaryotic cells in the monolayer (LC50) 5. In a cell-free system, however, the peptide showed 0.44 μM and 0.4 μM of the half-maximal effect (ED50) in a mitochondrial swelling and mitochondrial membrane potential (ΔΨm) loss assay 3, i.e., only 0.1 % the LC50 for eukaryotic cells. This indicated that the peptide, with efficient means of intracellular delivery, could be used for anti-tumor treatment via the induction of apoptosis. As with other gene therapeutics of poor in vivo stability during extracellular phase, G3139 is distributed and eliminated rapidly after i.v. injection with 5 and 37 min of α and β half-lives, respectively, and demonstrates an elevated renal accumulation 17. In addition to the unfavorable pharmacokinetics, G3139 has to overcome intracellular barriers inherent to gene therapeutics to be effective. All said clearly indicates that an efficient delivery system for the intracellular delivery of the peptide and antisense ODN G3139 is required for the effective anti-tumor therapy.
In this study, we constructed and assessed CL co-loaded with the D-(KLAKLAK)2 peptide and antisense ODN G3139 with an aim of solving the delivery problems for both drug and producing an enhanced anti-tumor efficacy through the combination of two different strategies for induction of apoptosis. Cationic liposomes have provided efficient tool for enhanced intracellular delivery of antisense gene therapeutics. Complexation with cationic lipids, such as oligofectamine and DDAB/DOPE resulted in a 25- and 50-fold increase, respectively, in antisense cellular uptake 18. In general, the use of CL for the delivery of cationic peptide would not be practical due to the lack of charge interaction that provides the mechanism for an efficient loading and intracellular delivery of anionic gene therapeutics. However, we proposed that the CL can be used for delivery of cationic peptides by employing a two-step alternating charge interaction, i.e., by first pre-complexing a cationic peptide with anionic ODN into a complex with an overall negative charge, and then entrapping such complexes within CL with high efficacy due to charge interaction. This procedure would produce a CL formulation simultaneously loaded with cationic peptides and anionic ODN, and provide an efficient means of intracellular delivery of two apoptotic agents to reach a therapeutic intracellular level.
The combination of a simple lipid film hydration and extrusion procedure with pre-complexation of the oppositely charged polyelectrolytes (polycationic peptide and polyanionic ODN), is a modification of the previously described procedure for liposome encapsulation of PEI/ODN polyplexes 19, and can find analogs in procedures, which generate a “pre-condensed stable plasmid lipid particles” (pSPLP) 20 or a “multifunctional envelope-type nano device” (MEND) 21. Consistent with the previous reports, a high encapsulation efficiency of 54 % was achieved with pre-condensation while just negligible quantity of the positively charged peptide could be achieved without pre-condensation.
The promise of CL co-loaded with D-(KLAKLAK)2 and G3139 for anti-tumor therapy was clearly confirmed by the enhanced cytotoxicity in cell culture and inhibition of tumor growth in the mouse model. In the subsequent apoptotic assay, the enhanced anti-tumor efficacies were proven to be the results of increased apoptotic activity in the cultured tumor cells and in vivo grown tumor tissues. Thus, these in vitro and in vivo experiments clearly demonstrated a positive apoptotic effect obtained by loading two pro-apoptotic agents into CL. In our in vivo study, the formulation was directly injected into tumor tissues to ensure the maximal exposure of the tumor tissues to the formulation and thus demonstrate the feasibility of in vivo use of the formulation. However, although the intratumoral drug administration attracts an increasing attention 22-24, the use of the intratumoral injection could in some cases be limited to more easily accessible tumors. This is why in our future experiments we plan to investigate the in vivo therapeutic efficacy of the formulation on internal or disseminated tumors other than melanoma following i.v. administration.
Concluding, the CL co-loaded with both pro-apoptotic peptide and ODN led to significantly enhanced anti-tumor efficacy and suggests a promising approach to cancer combined pro-apoptotic/gene therapy.
Acknowledgments
This research was supported by the NIH grant 1R01CA128486 and 1R01CA121838 to Vladimir P. Torchilin.
References
- 1.Green DR, Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? J Clin Invest. 2005;115(10):2610–7. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchier-Hayes L, Lartigue L, Newmeyer DD. Mitochondria: pharmacological manipulation of cell death. J Clin Invest. 2005;115(10):2640–7. doi: 10.1172/JCI26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacotot E, Deniaud A, Borgne-Sanchez A, Touat Z, Briand JP, Le Bras M, Brenner C. Therapeutic peptides: Targeting the mitochondrion to modulate apoptosis. Biochim Biophys Acta. 2006;1757(910):1312–23. doi: 10.1016/j.bbabio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Javadpour MM, Juban MM, Lo WC, Bishop SM, Alberty JB, Cowell SM, Becker CL, McLaughlin ML. De novo antimicrobial peptides with low mammalian cell toxicity. J Med Chem. 1996;39(16):3107–13. doi: 10.1021/jm9509410. [DOI] [PubMed] [Google Scholar]
- 5.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, Bredesen DE, Pasqualini R. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5(9):1032–8. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 6.Mai JC, Mi Z, Kim SH, Ng B, Robbins PD. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001;61(21):7709–12. [PubMed] [Google Scholar]
- 7.Marks AJ, Cooper MS, Anderson RJ, Orchard KH, Hale G, North JM, Ganeshaguru K, Steele AJ, Mehta AB, Lowdell MW, Wickremasinghe RG. Selective apoptotic killing of malignant hemopoietic cells by antibody-targeted delivery of an amphipathic peptide. Cancer Res. 2005;65(6):2373–7. doi: 10.1158/0008-5472.CAN-04-2594. [DOI] [PubMed] [Google Scholar]
- 8.Klasa RJ, Gillum AM, Klem RE, Frankel SR. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002;12(3):193–213. doi: 10.1089/108729002760220798. [DOI] [PubMed] [Google Scholar]
- 9.Jansen B, Wacheck V, Heere-Ress E, Schlagbauer-Wadl H, Hoeller C, Lucas T, Hoermann M, Hollenstein U, Wolff K, Pehamberger H. Chemosensitisation of malignant melanoma by BCL2 antisense therapy. Lancet. 2000;356(9243):1728–33. doi: 10.1016/S0140-6736(00)03207-4. [DOI] [PubMed] [Google Scholar]
- 10.Tarhini AA, Kirkwood JM. Oblimersen in the treatment of metastatic melanoma. Future Oncol. 2007;3(3):263–71. doi: 10.2217/14796694.3.3.263. [DOI] [PubMed] [Google Scholar]
- 11.Oblimersen: Augmerosen, BCL-2 antisense oligonucleotide - Genta, G 3139, GC3139, oblimersen sodium. Drugs R D. 2007;8(5):321–34. doi: 10.2165/00126839-200708050-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kim R, Emi M, Matsuura K, Tanabe K. Antisense and nonantisense effects of antisense Bcl-2 on multiple roles of Bcl-2 as a chemosensitizer in cancer therapy. Cancer Gene Ther. 2007;14(1):1–11. doi: 10.1038/sj.cgt.7700986. [DOI] [PubMed] [Google Scholar]
- 13.Sou K, Endo T, Takeoka S, Tsuchida E. Poly(ethylene glycol)-modification of the phospholipid vesicles by using the spontaneous incorporation of poly(ethylene glycol)-lipid into the vesicles. Bioconjug Chem. 2000;11(3):372–9. doi: 10.1021/bc990135y. [DOI] [PubMed] [Google Scholar]
- 14.Lopes de Menezes DE, Hudon N, McIntosh N, Mayer LD. Molecular and pharmacokinetic properties associated with the therapeutics of bcl-2 antisense oligonucleotide G3139 combined with free and liposomal doxorubicin. Clin Cancer Res. 2000;6(7):2891–902. [PubMed] [Google Scholar]
- 15.Ueda H, Sawa Y, Matsumoto K, Kitagawa-Sakakida S, Kawahira Y, Nakamura T, Kaneda Y, Matsuda H. Gene transfection of hepatocyte growth factor attenuates reperfusion injury in the heart. Ann Thorac Surg. 1999;67(6):1726–31. doi: 10.1016/s0003-4975(99)00279-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Peng P, Longley C, Zhang Y, Borowski V, Mehlig M, Reddy P, Xia J, Borchard G, Lipman J, Benimetskaya L, Stein CA. Delivery of G3139 using releasable PEG-linkers: impact on pharmacokinetic profile and anti-tumor efficacy. J Control Release. 2007;119(2):143–52. doi: 10.1016/j.jconrel.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Raynaud FI, Orr RM, Goddard PM, Lacey HA, Lancashire H, Judson IR, Beck T, Bryan B, Cotter FE. Pharmacokinetics of G3139, a phosphorothioate oligodeoxynucleotide antisense to bcl-2, after intravenous administration or continuous subcutaneous infusion to mice. J Pharmacol Exp Ther. 1997;281(1):420–7. [PubMed] [Google Scholar]
- 18.Dai G, Chan KK, Liu S, Hoyt D, Whitman S, Klisovic M, Shen T, Caligiuri MA, Byrd J, Grever M, Marcucci G. Cellular uptake and intracellular levels of the bcl-2 antisense g3139 in cultured cells and treated patients with acute myeloid leukemia. Clin Cancer Res. 2005;11(8):2998–3008. doi: 10.1158/1078-0432.CCR-04-1505. [DOI] [PubMed] [Google Scholar]
- 19.Ko YT, Bhattacharya R, Bickel U. Liposome encapsulated polyethylenimine/ODN polyplexes for brain targeting. J Control Release. 2008 doi: 10.1016/j.jconrel.2008.10.013. In press. [DOI] [PubMed] [Google Scholar]
- 20.Heyes J, Palmer L, Chan K, Giesbrecht C, Jeffs L, MacLachlan I. Lipid encapsulation enables the effective systemic delivery of polyplex plasmid DNA. Mol Ther. 2007;15(4):713–20. doi: 10.1038/sj.mt.6300101. [DOI] [PubMed] [Google Scholar]
- 21.Kogure K, Moriguchi R, Sasaki K, Ueno M, Futaki S, Harashima H. Development of a non-viral multifunctional envelope-type nano device by a novel lipid film hydration method. J Control Release. 2004;98(2):317–23. doi: 10.1016/j.jconrel.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Chvapil M. Inhibition of breast adenocarcinoma growth by intratumoral injection of lipophilic long-acting lathyrogens. Anticancer Drugs. 2005;16(2):201–10. doi: 10.1097/00001813-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Duvillard C, Polycarpe E, Romanet P, Chauffert B. Intratumoral chemotherapy: experimental data and applications to head and neck tumors. Ann Otolaryngol Chir Cervicofac. 2007;124(2):53–60. doi: 10.1016/j.aorl.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Weinberg BD, Blanco E, Gao J. Polymer implants for intratumoral drug delivery and cancer therapy. J Pharm Sci. 2008;97(5):1681–702. doi: 10.1002/jps.21038. [DOI] [PubMed] [Google Scholar]


