Summary
Seasonal changes in day length enhance or suppress aspects of immune function in mammals. Following adaptation to short, winter-like short photoperiods, cytokine and behavioral responses to lipopolysaccharide (LPS)-induced simulated infections are attenuated in LPS-naive Siberian hamsters. This experiment examined whether diminished initial responses to LPS in short days are accompanied by decrements in the development of innate immunological memory that leads to endotoxin tolerance. Male hamsters exposed to short days (9h-light/day; SD) or kept in their natal long-day photoperiod (15h-light/day; LD) for 12–13 weeks were injected with bacterial LPS (625 µg/kg, i.p.) or sterile saline. Ten days later all hamsters were challenged with LPS (625 ug/kg, i.p.), and behavioral sickness responses (anorexia and reductions in nest building) were assessed. In LD hamsters, behavioral responses to the second LPS injection were markedly attenuated but still evident, indicative of partial tolerance. SD hamsters, in contrast, failed to exhibit anorexic or thermoregulatory responses to the second LPS injection, indicative of complete behavioral tolerance to LPS. Thus despite engaging greater naive responses to LPS, LD hamsters exhibited incomplete LPS tolerance relative to SD hamsters. The expression of behavioral tolerance to endotoxin is relatively diminished during the breeding season, a time of year when naive responses to endotoxin are at their greatest. During winter, enhancements in behavioral endotoxin tolerance may conserve energy and facilitate survival in the face of energetically-challenging conditions.
Keywords: seasonality, sickness behavior, endotoxin tolerance, photoperiodism, Siberian hamster
Introduction
Sick animals exhibit profound, transient changes in behavior and motivational state, collectively termed ‘sickness behaviors’ (Hart, 1988). In the case of bacterial infections, sickness behaviors are initiated by the activity of cells in the innate immune system, principally macrophages. Following recognition of bacterial cell wall lipopolysaccharide (LPS), macrophages synthesize and secrete pro- (IL-1β, IL-6, and TNF-α) and anti- (IL-10, IL-1ra) inflammatory cytokines, which act on central and peripheral targets to trigger changes in physiology and behavior (Dantzer, 2001). The ensuing motivational reorganization includes reductions in ingestive, social, and sexual behaviors, induction of an anhedonic state, lethargy, and thermoregulatory changes. Sickness behaviors generated during the acute phase response (APR) to infection forestall bacterial replication, facilitate leukocytogenesis, and are critical to survival (Kluger, 1975; Hart, 1988).
Innate inflammatory responses to microbes are not immodifiable. Rather, environmental factors can exacerbate or attenuate infection-induced cytokine production and the generation of sickness behaviors (e.g., Aubert et al., 1997). Among the environmental cues capable of robust modulation of the response to LPS are changes in day length (photoperiod). After exposure to short, winter-like photoperiods, male Siberian hamsters challenged with a simulated gram-negative bacterial infection (systemic LPS) exhibit lower IL-1β, IL-6, and TNF-α production, and marked decreases in the magnitude and persistence of anorexic and thermoregulatory responses, relative to hamsters exposed to longer, summer-like photoperiods (Bilbo et al., 2002; Prendergast et al., 2003). Several other components of the hamster immune system have also been shown to be enhanced under winter relative to summer photoperiods, including skin inflammatory responses and lymphocyte subsets (Bilbo et al., 2002). These and other data (see Nelson, 2004, for review) have formed the empirical foundations of the ‘winter immunoenhancement hypothesis’, which proposes that photoperiodic adjustments in immune function may reflect an adaptive reallocation of metabolic resources away from reproductive physiology and towards host defense and survival at times of year when reproduction is unlikely to be successful (Nelson & Demas, 1996). ‘Immunoenhancement’, here, refers to survival and fitness, and does not imply quantitative increases in all immunological responses following adaptation to short days. Because acute-phase sickness responses are extremely energetically expensive (Bilbo et al., 2002), the attenuation of LPS-induced sickness behaviors during winter accelerates behavioral recovery from severe infections. Earlier termination of fever and resumption of foraging may be adaptive in winter environments where ambient temperatures are relatively lower and food is scarce (Nelson, 2004).
If engaged repeatedly or sustained for prolonged intervals, inflammatory responses can lead to any of several pathophysiological conditions, including metabolic syndrome, somatic wasting, and septic shock (Nathan, 2002). Constraints on the extent of LPS-induced inflammation are afforded by cellular negative-feedback mechanisms that are engaged in parallel with the initial inflammatory response to LPS (Sly et al., 2004; Foster et al., 2007). This negative regulation induces a state of ‘LPS tolerance’, characterized by organismal and macrophage hyporesponsiveness to restimulation by LPS, thereby decreasing the likelihood of sepsis. Following an initial (naive) treatment with LPS, subsequent LPS treatments elicit attenuated cytokine and behavioral (fever, food intake) responses in tolerant animals (Langhans et al., 1991; Nava & Carta, 2000). The degree of tolerance varies in a dose-dependent manner with the amount of the initial LPS treatment (Beeson, 1947; Labeta et al., 1993). In addition to protecting against sepsis, LPS tolerance also substantially decreases the energetic consequences of reexposure to LPS (e.g., diminished anorexia and fever). Once instated, LPS tolerance can endure for weeks, reflecting a form of short-term memory in the innate immune system (Valles et al., 2005; Gantner & Singh, 2007).
Whether photoperiod affects the development of LPS tolerance remains unresolved. On one hand, the relatively greater proinflammatory response to LPS under long, relative to short days may be accompanied by a relatively greater concurrent activation of negative regulators of inflammation (i.e., mechanisms that induce tolerance), and therefore greater behavioral tolerance might be predicted in long days. Alternatively, however, if winter adaptations in the immune system are also manifest in mechanisms that control the development of LPS tolerance, then one would predict LPS tolerance to be facilitated in hamsters adapted to short photoperiods, consistent with the winter immunoenhancement hypothesis. A modest attenuation of LPS tolerance under long, relative to short, days has been reported in a population of female meadow voles (Engeland et al., 2003); however, asymmetries in the proportion of voles that were concurrently pregnant in long-days (40%) versus short-days (0%) preclude a definitive interpretation of the data. Moreover, in meadow voles photoperiod does not affect the magnitude of the initial, acute response to LPS (Engeland et al., 2003). To directly address this issue, this experiment tested whether adaptation to a short photoperiod facilitates or impairs the development of behavioral tolerance to LPS. LPS-induced anorexia and suppression of nest building behavior were measured, as these are the two most robustly photoperiodic sickness behaviors yet described in rodents (Wen et al., 2007), and each bears critically on winter energy balance.
Methods
Animals and photoperiod manipulations
Procedures in this experiment conformed to the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Chicago Institutional Animal Care and Use Committee. Siberian hamsters (Phodopus sungorus; n=45) from our laboratory breeding colony were raised 2–4 per cage (28×17×12 cm) under a 15L:9D photoperiod (LD; lights-off: 1800h) with ad libitum access to food and filtered water. Ambient temperature was 20 ± 0.5°C and relative humidity was 53 ± 2% throughout the experiment. At 3–4 months of age (week 0), hamsters were either transferred into 9L:15D (SD; lights-off: 1800h; n=25) or remained in LD (n=20). On weeks 0 and 12, testis volumes were determined under light isoflurane anesthesia to assess gonadal responses to the photoperiod manipulations. SD hamsters that failed to exhibit >40% decrease in testis size by week 12 (n=4) were regarded as nonresponsive to SD and excluded from all analyses (Wen et al., 2007).
Induction of LPS tolerance
On week 12, naive responses to LPS were evaluated according to established behavioral methods in our laboratory (Wen et al., 2007). To elicit naive responses to LPS, hamsters were injected i.p. with either bacterial LPS (E. coli 026:B6; 625 µg/kg; Sigma; LD, n=10; SD, n=10) or 0.1 ml of sterile 0.9% saline (LD, n=10; SD, n=11) 30 minutes before the onset of darkness. Food in the cage hopper was weighed (±0.1 g) at the time of injection and at 24 h intervals thereafter to determine daily food intake. On the night following injection, coincident with lights-off, each hamster was also provided with access to a small piece of cotton batting (~3 g) which was weighed before, and again 3 hours after, presentation. This provided a measure of thermoregulatory behavior: energetic challenges increase the use of nesting material (Puchalski et al., 1988; Kauffman et al., 2003), and the availability of a nest reduces food intake in this species (Kauffman et al., 2003; Wen et al., 2007). Ten days later (week 13.5), behavioral tolerance was assessed by injecting all hamsters with LPS (625 µg/kg, i.p.) and measuring food intake and nesting material use over the next 72 h using procedures identical to those described for week 12. Statistics. ANOVA was used to compare behavioral responses to LPS among hamsters that had previously been treated with LPS (LPS-LPS) and those that had previously been treated with saline (SAL-LPS). Behavioral responses among saline-injected controls (SAL) provided a baseline against which to assess suppression of LPS-induced sickness behaviors by prior LPS exposure (i.e., LPS tolerance). Pairwise comparisons of mean food intake values were conducted using Fisher’s PLSD tests. Nesting material use was compared between groups using a Kruskal-Wallis test followed by Mann-Whitney U tests.
Results
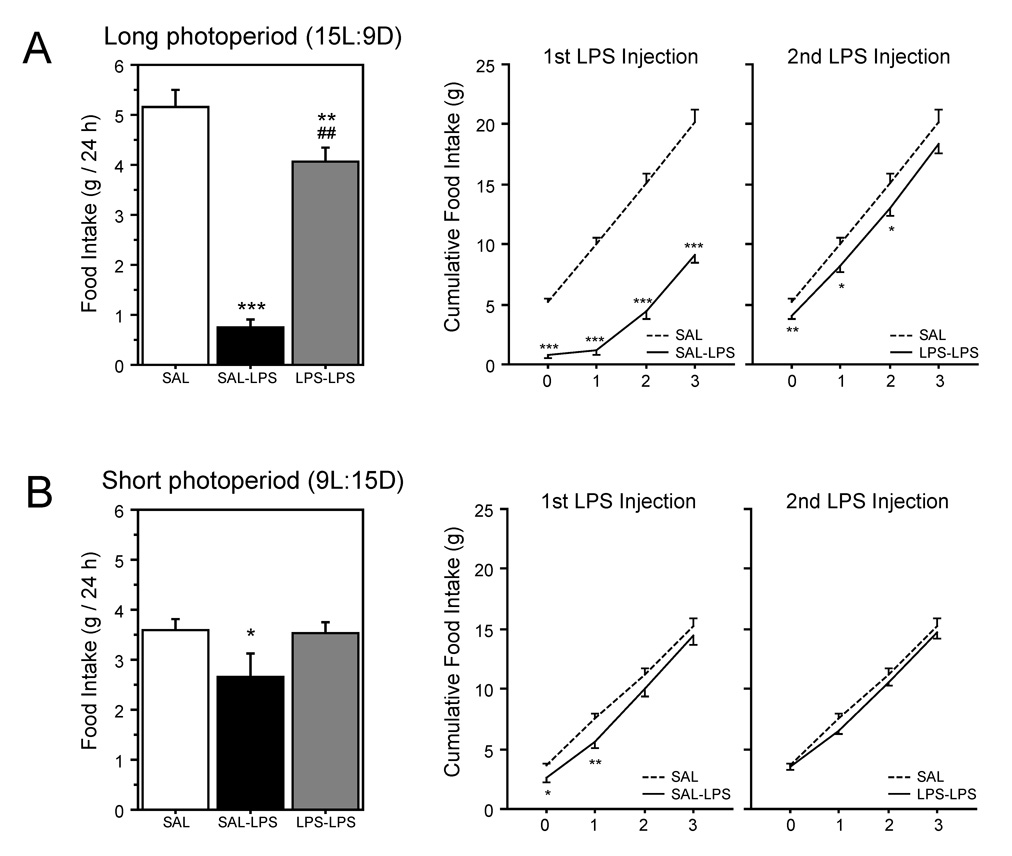
A single LPS-treatment caused anorexia in both LD (P<0.0001) and SD (P<0.05) hamsters (Fig. 1A, 1B). Although diminished in magnitude, anorexic responses following the second LPS treatment (on week 13.5) were evident in LD (P<0.01), but were completely absent in SD (P>0.8) (Fig. 1A, 1B). The hypophagic effect of endotoxin treatment among LPS-LPS hamsters in housed in LD endured for ≥72 h after the second endotoxin treatment (Fig 1B).
Figure 1.
Mean (±SEM) 24 h food intake (left panel) and cumulative 72 h food intake (right panels) of male Siberian hamsters housed in long days (A) or short days (B) for 12–13 weeks, then treated with sterile 0.9% saline (SAL), SAL followed 10 days later by 625 µg/kg bacterial lipopolysaccharide (SAL-LPS), or 625 µg/kg lipopolysaccharide followed 10 days later by a second treatment with 625 µg/kg of bacterial lipopolysaccharide (LPS-LPS). All injection treatments were delivered i.p. Within each panel: *P<0.05, **P<0.01, and ***P<0.001 vs. SAL value; # P<0.05 and ## P<0.01 vs. SAL-LPS value.
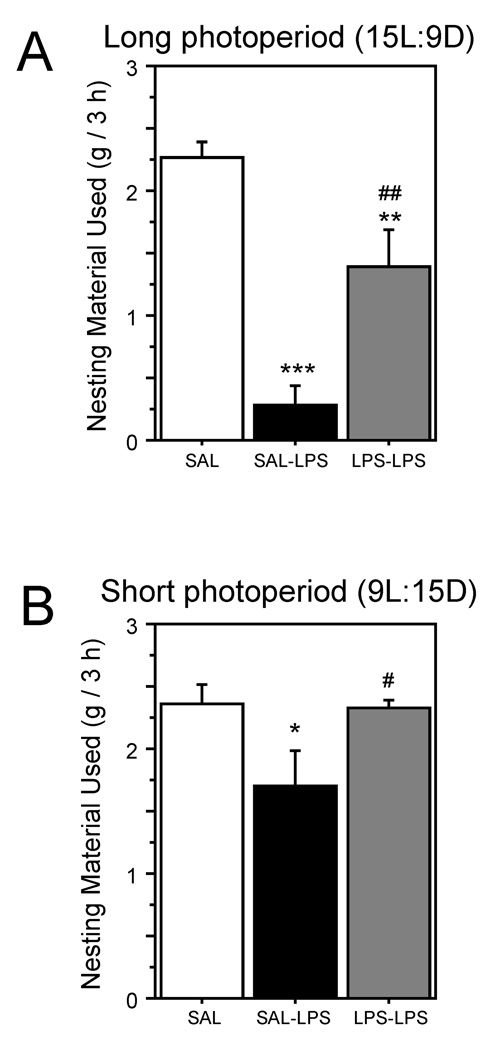
LPS likewise suppressed nest-building among naive animals in both photoperiods (LD: P<0.0001; SD: P<0.05; Fig. 2). However, a second treatment with LPS suppressed nest-building behavior in LD hamsters (P<0.005; Fig. 2A), but failed to do so in SD hamsters (P>0.9; Fig. 2B).
Figure 2.
Mean (±SEM) 3 h nesting material use of male Siberian hamsters housed in long days (A) or short days (B), then treated with SAL, SAL-LPS, or LPS-LPS. Photoperiod and injection treatments as described in Figure 1. Within each panel: *P<0.05, **P<0.01, and ***P<0.001 vs. SAL value; #P<0.001 vs. SAL-LPS value.
Discussion
The present results demonstrate that the effects of LPS from E. coli. on Siberian hamster food intake and nest-building behavior disappear with repeated injection. This work confirms in Siberian hamsters data from several other mammals (humans, rats, mice, rabbits) indicating that a single exposure to LPS results in the eventual development of behavioral tolerance (Beeson, 1947; Langhans et al., 1991; Nava & Carta, 2000; Nathan, 2002). In LD hamsters, tolerance to the effects of LPS on food intake and nest building were partially evident, but significant anorexia and suppression of nest building still occurred after the 2nd LPS treatment, indicating that behavioral tolerance to LPS was not absolute. In contrast, behavioral symptoms of simulated infection were entirely absent in SD hamsters following repeated LPS treatment. Thus, adaptation to short photoperiods markedly enhanced the expression of behavioral tolerance to endotoxin. Taken together, these data extend the scope of photoperiodic adaptations in the immune system of Siberian hamsters to include mechanisms that govern the manifestation of behavioral endotoxin tolerance.
Sickness behaviors are critical to survival, but they are energetically costly behavioral adaptations (Hart, 1988). Fever, suppression of food intake, and deficits in nest construction, however transient, constitute an extreme energetic challenge. The immediate energetic costs of sickness may be amplified during winter, when food is scarce and ambient temperatures are relatively lower (Bilbo et al., 2002). Adaptation to short photoperiods attenuates the magnitude of LPS-induced sickness behaviors in hamsters: proinflammatory cytokine production is decreased in SD relative to LD, as are anorexia, anhedonia, and suppression of nest building (Bilbo et al., 2002; Wen et al., 2007; present data). Collectively, these adaptations conserve energy during winter, and are broadly consistent with the winter immunoenhancement hypothesis (Nelson & Demas, 1996) which proposes that selection may favor individuals that suppress energetically-expensive immune responses during the winter. Enhanced behavioral tolerance to LPS in SD provides further evidence in support of the winter immunoenhancement hypothesis. Ten days after the initial LPS treatment, a second injection with LPS elicited a ~20% reduction in food intake and a 40% decrease in nesting material use in LD hamsters, but failed to affect these behaviors in SD hamsters. Short photoperiods thus appear to modulate behavioral responses to infection in at least two capacities: the naive response to endotoxin is suppressed and behavioral tolerance to endotoxin is enhanced. Collectively, these processes likely facilitate the conservation of energy in the face of recurrent infections in nature.
LPS tolerance is mediated by the selective silencing of “tolerizable” proinflammatory genes via chromatin modifications (Foster et al., 2007). The inhibition of acute-phase proinflammatory cytokine responses, together with enhanced antimicrobial responses to LPS in tolerant individuals (Foster et al., 2007), has led to the view that tolerance is an adaptive process (West & Heagy, 2002). Other work, documenting impaired prognosis in tolerant patients, has challenged aspects of this argument, however, at least as it regards septic patients in clinical settings (Munoz et al., 1991; Heagy et al., 2000). This transient suppression provides a ligand-specific short-term memory in the innate immune system (Gantner & Singh, 2007). The present data are somewhat surprising, in light of data from other rodent model systems which have established that the degree of endotoxin tolerance correlates positively with the magnitude of the initial response to LPS (Beeson, 1947; Labeta et al., 1993). A full understanding of the molecular mechanisms by which behavioral tolerance becomes impaired in LD relative to SD was beyond the scope of the present study. Exposure to LD may be associated with relative decrements in any of the multiple mechanisms that contribute to negative regulation of the proinflammatory response during the window of LPS tolerance (Sly et al., 2004; Foster et al., 2007). Alternatively, suppression of proinflammatory gene expression following initial LPS treatment may occur to a comparable degree in both LD and SD hamsters, but, because naive proinflammatory cytokine responses to LPS are already suppressed in SD (Bilbo et al., 2002; Prendergast et al., 2003), the additional suppression afforded by tolerance may be sufficient to completely inhibit cytokine production in SD hamsters, but only accomplish a partial silencing in LD hamsters. In this latter scenario, mechanisms that constrain the naive proinflammatory response to LPS in SD hamsters would largely account for the observed photoperiodic differences in behavioral tolerance.
To summarize, the present results indicate that Siberian hamsters exhibit tolerance to the hypophagic and behavioral thermoregulatory effects of LPS, but that tolerance is markedly enhanced following adaptation to SD photoperiods. The net effect of enhanced LPS tolerance in SD is a complete abrogation of energetically-expensive behavioral responses to infection. This adaptation may facilitate overwinter survival in nature.
Acknowledgements
I thank Jarvi Wen and Scott Baillie, who assisted in data collection, and Jerome Galang and Nicole Sikora, who provided expert technical assistance.
Contributors
Brian Prendergast designed the study, assisted in data collection, undertook the statistical analyses, and wrote the manuscript.
Role of Funding Source
Funding for this study was providerd by NIH Grant AI-67406 from the National Institute of Allergy and Infectious Diseases. The NIAID had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
The Author declares that he has no conflicts of interest.
References
- Aubert A, Goodall G, Dantzer R, Gheusi G. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav. Immun. 1997;11:107–118. doi: 10.1006/brbi.1997.0485. [DOI] [PubMed] [Google Scholar]
- Beeson PB. Tolerance to bacterial pyrogens I: Factors influencing its development. J. Exp. Med. 1947;86:39–44. doi: 10.1084/jem.86.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Drazen DL, Quan N, He L, Nelson RJ. Short day lengths attenuate the symptoms of infection in Siberian hamsters. Proc. Biol. Sci. 2002;269:447–454. doi: 10.1098/rspb.2001.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann. N. Y. Acad. Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Kavaliers M, Ossenkopp KP. The influence of photoperiod and sex on lipopolysaccharide-induced hypoactivity and behavioral tolerance development in meadow voles (Microtus pennsylvanicus) Psychoneuroendocrinology. 2003;28:970–991. doi: 10.1016/s0306-4530(02)00118-x. [DOI] [PubMed] [Google Scholar]
- Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Gantner BN, Singh H. Immunology: Short-term memory. Nature. 2007;447:916–917. doi: 10.1038/447916a. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Heagy W, Hansen C, Nieman K, Cohen M, Richardson C, Rodriguez JL, West MA. Impaired ex vivo lipopolysaccharide-stimulated whole blood tumor necrosis factor production may identify "septic" intensive care unit patients. Shock. 2000;14:271–276. doi: 10.1097/00024382-200014030-00005. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188:166–168. [PubMed] [Google Scholar]
- Labeta MO, Durieux JJ, Spagnoli G, Fernandez N, Wijdenes J, Herrmann R. CD14 and tolerance to lipopolysaccharide: biochemical and functional analysis. Immunology. 1993;80:415–423. [PMC free article] [PubMed] [Google Scholar]
- Langhans W, Balkowski G, Savoldelli D. Differential feeding responses to bacterial lipopolysaccharide and muramyl dipeptide. Am. J. Physiol. 1991;261:R659–R664. doi: 10.1152/ajpregu.1991.261.3.R659. [DOI] [PubMed] [Google Scholar]
- Munoz C, Carlet J, Fitting C, Misset B, Blériot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J. Clin. Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Nava F, Carta G. Repeated lipopolysaccharide administration produces tolerance to anorexia and fever but not to inhibition of thirst in rat. Int. J. Immunopharmacol. 2000;22:943–953. doi: 10.1016/s0192-0561(00)00058-8. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE. Seasonal changes in immune function. Q. Rev. Biol. 1996;71:511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. Seasonal immune function and sickness responses. Trend Immunol. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Hotchkiss AK, Bilbo SD, Kinsey SG, Nelson RJ. Photoperiodic adjustments in immune function protect Siberian hamsters from lethal endotoxemia. J. Biol. Rhythms. 2003;18:51–62. doi: 10.1177/0748730402239676. [DOI] [PubMed] [Google Scholar]
- Sly LM, Rauh MJ, Kalesnikoff J, Song CH, Krystal G. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity. 2004;21:227–239. doi: 10.1016/j.immuni.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Valles A, Marti O, Armario A. Mapping the areas sensitive to long-term endotoxin tolerance in the rat brain: a c-fos mRNA study. J. Neurochem. 2005;93:1177–1188. doi: 10.1111/j.1471-4159.2005.03100.x. [DOI] [PubMed] [Google Scholar]
- Wen JC, Dhabhar FS, Prendergast BJ. Pineal-dependent and -independent effects of photoperiod on immune function in Siberian hamsters. Horm. Behav. 2007;51:31–39. doi: 10.1016/j.yhbeh.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MA, Heagy W. Endotoxin tolerance: a review. Crit. Care Med. 2002;30:S64–S73. [PubMed] [Google Scholar]