Abstract
Adolescent alcohol use may produce long-term changes in the receptors and neurosteroids that putatively mediate alcohol's effects and consequently contribute to alcohol abuse and dependence as an adult. To test this possibility, ethanol (0.18-1.8 g/kg) and two neurosteroids, pregnanolone (1-10 mg/kg) and dehydroepiandrosterone (DHEA, 1-100 mg/kg), were administered alone and in combination to adult, male Long-Evans rats discriminating 1 g/kg ethanol (15% v/v) under a fixed ratio (FR) 20 schedule of food presentation after adolescent treatment with 15 injections of ethanol (n=9, 2 g/kg, 20% v/v) or saline (n=7). When compared as adults, ethanol-treated adolescents (as opposed to saline-treated adolescents) had higher percentages of ethanol-lever responding at doses smaller than the training dose, and higher response rates after both control and ethanol injections. Neither pregnanolone nor DHEA substituted for ethanol in either adolescent-treated group up to doses that substantially decreased response rates. When administered with ethanol, 1 and 3.2 mg/kg of pregnanolone enhanced the discriminative stimulus effects of small ethanol doses more in saline-treated adolescents than ethanol-treated adolescents. Unlike pregnanolone, 32 and 100 mg/kg of DHEA attenuated the discriminative stimulus effects of ethanol modestly in both adolescent-treated groups. These results in adult rats suggest that adolescent ethanol administration can enhance the discriminative stimulus effects of small ethanol doses and affect the capacity of pregnanolone, but not DHEA, to interact with ethanol's discriminative stimulus effects.
Keywords: ethanol, pregnanolone, dehydroepiandrosterone, drug discrimination, adolescence, rats
1. Introduction
One possible behavioral mechanism by which adolescent alcohol use in humans might contribute to the reported increase in alcohol abuse and dependence in adults (Hawkins et al., 1997; Grant and Dawson, 1997) is by permanently altering the perception or composition of ethanol's subjective effects. As stated by Holtzman (1990), “the qualitative nature of the subjective effects that a drug produces is a principal determinant of the abuse potential of that drug,” p. 193. Therefore, there is the possibility that experience with the effects of alcohol as an adolescent might alter the makeup of the interoceptive stimuli that comprise the subjective effects. For example, several studies have suggested that adolescent ethanol administration can produce long-lasting tolerance (Silvers et al., 2003; Silvers et al., 2006) or sensitivity (White et al., 2000; White et al., 2002) to some of the physiological and behavioral effects of alcohol. If such changes in the effects of ethanol persist, then there is also the likelihood that adolescent ethanol administration could also produce long-lasting changes to the subjective effects (or balance of subjective effects), essentially making the effects produced by ethanol different in ethanol-experienced and ethanol-naïve individuals.
One method for investigating the subjective effects of a drug experimentally is to have the subjective effects of a specific dose of that drug serve as discriminative stimuli for responding in a drug discrimination procedure. A powerful aspect of this methodology is that once the subjective (interoceptive) effects of a drug are conditioned to serve as discriminative stimuli reliably, the experimenter has a pharmacologically specific behavioral model for studying the components of a drug's actions and these actions generally reflect events at the neuronal level (Holtzman, 1990). Alcohol, in particular, has been shown to have several pharmacological components using this methodology (Grant and Colombo, 1993; Green and Grant, 1998; Stolerman and Olufsen, 2001), and this has been corroborated by a variety of in vitro studies (e.g., Crews et al., 1996). Recently, demonstrated similarities in the mechanisms of action of the 3α-hydroxy neurosteroids and ethanol have raised the possibility that endogenous neurosteroids might play a direct role in the effects of ethanol. For example, Morrow and colleagues (1999, 2001) as well as others (Sanna et al., 2004) have suggested that ethanol-induced changes in levels of endogenous neurosteroids, such as allopregnanolone, may mediate some of the behavioral and subjective effects of ethanol. These suggestions have also been supported by data from drug discrimination studies showing that specific neurosteroids including allopregnanolone and its epimer pregnanolone can substitute for ethanol (Ator et al., 1993; Bienkowski and Kostowski, 1997; Bowen et al., 1999a; Hodge et al., 2001; Ginsburg and Lamb, 2005); however, in terms of their discriminative stimulus effects, Gerak et al. (2008) recently found evidence that there may be asymmetrical generalization between ethanol and pregnanolone, because ethanol failed to substitute for pregnanolone in a large number of individual subjects. This example of asymmetrical generalization was similar to that frequently shown between the barbiturates, benzodiazepines and ethanol (Kostowski and Bienkowski, 1999). More specifically, ethanol generalizes reliably in subjects trained to discriminate barbiturates or benzodiazepines, but neither the barbiturates nor benzodiazepines generalize fully in subjects trained to discriminate ethanol.
The purpose of the present study was first to establish an ethanol discrimination in adult rats after administration of either saline or ethanol during adolescence, and second, to determine if the neurosteroid pregnanolone could reliably substitute in subjects trained to discriminate ethanol. Given that chronic intermittent ethanol (CIE) administration has been shown to produce long-lasting reductions in ethanol-induced increases in allopregnanolone in specific brain areas such as the hippocampus (Silvers et al., 2006), and that ethanol can interfere with allopregnanolone's positive modulatory effects at GABAA receptors (Majewska, 1988), there is the possibility that adolescent ethanol administration might also reduce the capacity of pregnanolone to substitute for ethanol, particularly if ethanol is releasing neurosteroids and they are mediating some of ethanol's effects. For comparison purposes, dehydroepiandrosterone (DHEA), a neurosteroid with negative GABAA modulatory effects (Corpechot et al., 1981; Demirgoren et al., 1991; Park-Chung et al., 1999) was also administered both alone and in combination with ethanol to the two adolescent-treated groups of subjects. Theoretically, a negative allosteric modulator of the GABAA receptor complex should have the capacity to attenuate the effects of a positive allosteric modulator of the GABAA receptor complex like ethanol; however, neither DHEA nor its sulfated form, DHEAS, has to date been shown to be effective in altering the discriminative stimulus effects of ethanol in adult subjects (Bienkowski and Kostowski, 1997; Bowen et al., 1999a).
2. Materials and Methods
2.1 Subjects
Sixteen male Long–Evans hooded rats were purchased from a commercial vendor (Harlan, Indianapolis, IN) at 25 days of age and served as subjects. Upon arrival, these subjects were housed 4 per cage and provided a standard diet of rodent chow ad libitum (Rodent Diet 5001, PMI Inc., St. Louis, MO, USA) until postnatal day (PD) 70. From PD 71 forward, subjects were housed individually and maintained at 95% of their free-feeding weight. Water was provided ad libitum in the homecage except during the experimental sessions. The colony room was maintained at 21 ± 2 C° with 50 ± 10% relative humidity on a 14L:10D light/dark cycle (lights on 06:00 h; lights off 20:00 h). Ethanol discrimination training and subsequent test sessions were conducted daily during the light cycle between the hours of 12:00 h and 14:00 h. Animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, Louisiana State University Health Sciences Center, and in compliance with the recommendations of the National Research Council in the Guide for the Care and Use of Laboratory Animals (1996).
2.2 Adolescent ethanol treatment
While still housed 4 per cage, subjects were randomly divided into two groups, a group that received ethanol between PD 35 and 63 (adolescent ethanol group) and a group that received saline during the same postnatal period (adolescent saline group). The adolescent ethanol group received 2 g/kg (20% v/v) of ethanol intraperitoneally (i.p.) every other day, while the adolescent saline group received an equal volume of saline every other day, for a total of 15 injections. This pattern of chronic intermittent injections (one injection every 48 h) has been shown to increase ethanol self-administration as compared to continuous ethanol administration in rats (O'Dell et al., 2004b), and is considered to be a representative model of human alcohol consumption (Olsen et al., 2005). At the beginning of adolescent saline or ethanol administration (PD 35), the mean weights and standard error for the means (SEM) for the groups were 155 ± 4.4 g and 163.7 ± 3.6 g, respectively. At the end of adolescent saline or ethanol administration (PD 63), the mean weights ± SEM for the groups were 312.3 ± 4.5 g and 310.1 ± 5.6 g, respectively.
2.3 Apparatus
Twelve operant test chambers (6 from MED Associates, Inc., St. Albans, VT, and 6 from BRS/Foringer, Beltsville, MD) enclosed within sound-attenuating cubicles were used to conduct the experiments. Each chamber was equipped with a houselight, pellet trough, pellet dispenser, and two response levers with stimulus lights located above each lever. White noise was present in each chamber to mask extraneous noise and a fan provided ventilation. Data were collected using MED-PC for Windows, Version IV (MED Associates, Inc., St. Albans, VT).
2.4 Training procedure
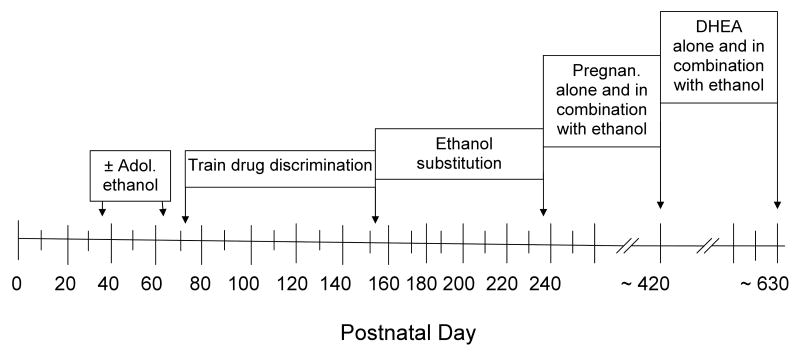
Beginning on PD 71 (see Figure 1), subjects in both adolescent treatment groups were trained to discriminate ethanol from saline while responding under a fixed-ratio (FR) 20 schedule of food presentation. During the initial training sessions, the house light was illuminated and every response on the lever resulted in delivery of a 45-mg food pellet. After rats reliably pressed both levers under this continuous reinforcement schedule (CRF), the number of responses required for food presentation on each lever was progressively increased from 1 to 20. During the final phase of training, reinforcement under the FR-20 schedule was contingent on an injection of either saline or 0.56 g/kg of ethanol administered before the session. As training progressed, the dose of ethanol was increased to the final training dose of 1 g/kg. For half of the rats in each adolescent-treated group, responding on the left lever resulted in food presentation following the administration of ethanol, and responding on the right lever resulted in food presentation following the administration of saline; the lever designations were reversed for the other half of the subjects. Responding on the incorrect lever reset the response requirement on the correct lever.
Fig. 1.
Diagram showing the timeline of manipulations for the subjects in both adolescent-treated groups.
All experimental sessions during the later stages of training began with a 10-min timeout period during which the house light was off, and responding on the levers had no programmed consequences. The timeout period was followed by a 30-min period during which the houselight was illuminated, and responding under the FR-20 schedule resulted in food presentation. Ethanol and saline were administered in a fixed daily sequence (E,S,S,E,S,E,E,S,E,S) that was repeated throughout the experiments. Training continued until the subjects met two criteria on 9 out of 10 consecutive days: 95% responding on the appropriate lever, and less than 20 responses on the incorrect lever prior to the first reinforcement. Substitution tests and tests involving pretreatments with the neuroactive steroids occurred thereafter, with the subjects required to meet these same criteria for 3 consecutive days between test sessions.
2.5 Test sessions
Test sessions were conducted similarly to daily training sessions; however, 20 responses on either lever resulted in presentation of a food pellet. Substitution tests with different doses of ethanol were conducted first to determine a dose-effect curve for ethanol. After completing an ethanol dose-effect curve for each subject (0.1-1.8 g/kg), doses of pregnanolone (1-18 mg/kg) and DHEA (10-100 mg/kg) were tested alone and in combination with ethanol. By the time testing occurred with DHEA, however, 3 of the 7 subjects treated with saline as adolescents had died of unknown causes. Vehicle was also administered prior to ethanol as a control for pregnanolone and DHEA pretreatments throughout the study and to make sure the ethanol dose-effect curves were not shifted as a result of testing with each neurosteroid.
2.6 Drugs
Ethanol (Pharmco Products Inc., Brookfield, CT) was diluted with saline (15% v/v), and injected i.p. 10 min before training or test sessions. Ethanol solutions were prepared each day of administration. Pregnanolone (5β-pregnan-3α-ol-20-one, Steraloids, Inc., Newport, RI) and dehydroepiandrosterone (DHEA; 5-androstene-3β-ol-17-one, Sigma-Aldrich, Inc, St. Louis, MO) were dissolved in a 45% solution of 2-hydroxypropyl-γ-cyclodextrin (Sigma-Aldrich, St. Louis, MO) in saline. The same vehicle was used for control injections, which consisted of injections of either vehicle alone or vehicle and saline. Pregnanolone, DHEA or vehicle were injected i.p. 15 min prior to session, and the injection volume for these drugs was always 0.1 ml/100 g body weight.
2.7 Data analyses
The data for each session were expressed as the percentage of responses on the lever for which ethanol was serving as a discriminative stimulus (i.e., “ethanol-lever” responding) and overall response rate in responses per second. Group data for each variable were tabulated by averaging the mean data for each subject and then expressed as a grand mean and SEM; however, due to individual differences in sensitivity (particularly at the larger doses) not all doses were studied in all subjects, which led to occasional differences in the number of subjects represented by each data point. For this study, full substitution was defined as 80% or more of ethanol-lever responding. Dosages of ethanol, pregnanolone and DHEA were considered to have an effect when the mean for ethanol-lever responding or overall response rate was not within 20% of the mean established for control conditions. Combinations of ethanol with either pregnanolone or DHEA were compared to the effects of each drug alone using the same criteria. The percentage of ethanol-lever responses was not included in the analyses when the response rate was less than 0.08 responses/sec. In order to characterize and compare the dose-effect curves that were determined, doses that generated 50% ethanol-lever responding (ED50s) were estimated using a non-linear sigmoidal regression model (SigmaPlot Software, SYSTAT Software, Inc. Point Richmond, CA, USA).
3. Results
All of the subjects successfully acquired the ethanol discrimination in 43-81 days, with a mean of 55.44 days until they met the criteria indicating that stimulus control was established. With respect to the two adolescent-treated groups, the mean number of days to establish stimulus control in the subjects treated with saline during adolescence was 54 with a range of 43-69 days. Similarly, the mean number of days to establish stimulus control in the subjects treated with ethanol during adolescence was 57 with a range of 45-81 days.
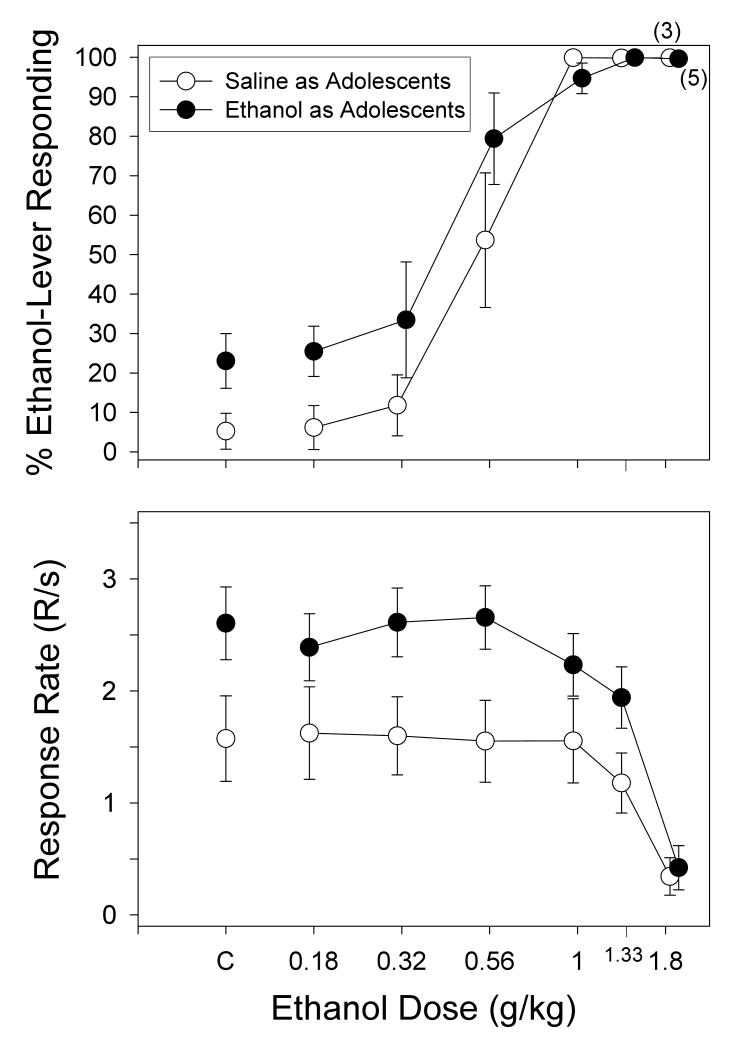
As shown in Figure 2, increasing doses of ethanol (0.18–1.8 g/kg) produced dose-dependent increases in ethanol-lever responding in both adolescent-treated groups; however, there were clear differences between the two groups in ethanol-lever responding and the overall response rate. More specifically, the dose-effect curve for the subjects that received ethanol as adolescents was shifted to the left of the curve for the subjects that received saline as adolescents, indicating that the ethanol-treated adolescent group had higher percentages of ethanol-lever responding as an adult at doses smaller than the training dose. The leftward shift in the ethanol dose-effect curves was also reflected in the ED50s for each group; the ED50 for the group that received saline as adolescents was 0.55 mg/kg, whereas the ED50 for the group that received ethanol as adolescents was 0.41 mg/kg. In addition, subjects that received ethanol as an adolescent also had a greater tendency to respond on the ethanol lever when saline was administered instead of the training dose (data above C). Specifically, ethanol-lever responding after a saline injection in the group that received saline as adolescents was 5.25%, whereas ethanol-lever responding after a saline injection in the group that received ethanol as adolescents was 23.07%.
Fig. 2.
Percentage of ethanol-lever responding (upper panel) and overall response rate (lower panel) after substitution of different doses of ethanol in adult rats that received either saline (n=7, unfilled points) or ethanol (n=9, filled points) as adolescents. The subjects in each group were discriminating a training dose of 1 g/kg (15% v/v) ethanol while responding under a FR-20 schedule of food presentation. Ethanol was administered i.p. 10 min before the start of the session, which began with a 10-min timeout. Data points and vertical lines above “C” in each panel represent the grand mean and SEM for each group, which was comprised of the means for 2 to 6 injections of saline for each subject in that group. The data points and vertical lines in the dose-effect curves for each group in each panel also represent a grand mean and SEM. The grand mean and SEM for each dose was comprised of the means for 1 to 3 determinations of that dose in each subject. Numerical values in parentheses and adjacent to a data point indicate the number of subjects represented by that point when it differed from the total number of subjects for that group.
In terms of the overall response rate, the group that received ethanol as adolescents had higher overall response rates after control injections and after the substitution of ethanol doses smaller than the training dose (i.e., 0.18-0.56 g/kg) when compared to the group that received saline as adolescents. Doses of ethanol larger than the training dose (i.e., 1.33-1.8 g/kg) decreased the response rates below 20% of the respective means for both groups. For example, 1.33 g/kg of ethanol decreased response rate from 2.6 to 1.94 (ethanol treated) and from 1.57 to 1.18 (saline-treated) responses per second, whereas 1.8 g/kg decreased response rates further to 0.42 (ethanol treated) and 0.34 (saline treated) responses per second.
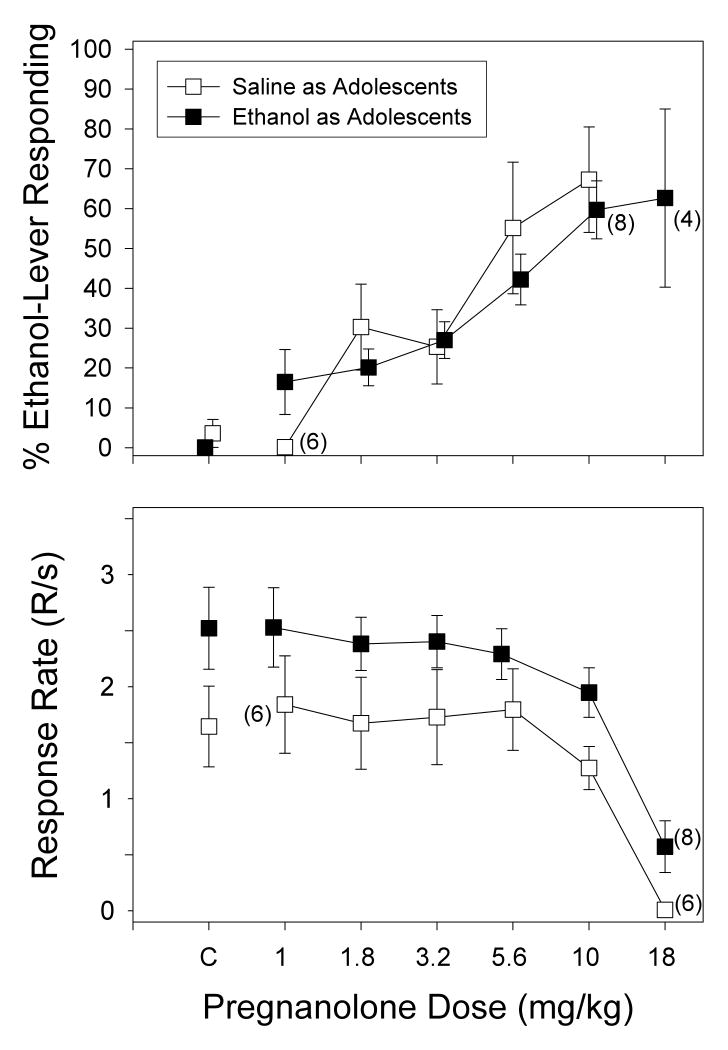
Figure 3 shows that when subjects in both adolescent-treated groups received the vehicle for pregnanolone prior to testing sessions, they responded on the saline-appropriate lever and response rates were similar to those obtained for the respective groups when saline was administered (compare Figs. 2 and 3). Substitution of pregnanolone (1-18 mg/kg) for the training dose of ethanol dose-dependently increased ethanol-lever responding in both adolescent-treated groups similarly when compared to their respective vehicle administrations. However, the mean percentage of ethanol-lever responding never exceeded 80% for either group and the slopes of the dose-effect curves were quite shallow over an almost 20-fold increase in dose. For example, after substituting 10 mg/kg of pregnanolone, ethanol-lever responding for subjects from the saline-treated adolescent group was 67.27%, whereas the mean percentage for the ethanol-treated adolescent group was 62.64%.
Fig. 3.
Percentage of ethanol-lever responding (upper panel) and overall response rate (lower panel) after substitution of different doses of pregnanolone in adult rats that received either saline (n=7) or ethanol (n=9) as adolescents. The subjects in each group were discriminating 1 g/kg (15% v/v) of ethanol while responding under a FR-20 schedule of food presentation. Doses of pregnanolone were administered i.p. 15 min before the start of the session, which began with a 10-min timeout. Data points and vertical lines above “C” in each panel represent the grand mean and SEM for each group, which was comprised of the means for 1 to 5 injections of vehicle for each subject in that group. The data points and vertical lines in the dose-effect curves for each group in each panel also represent the grand mean and SEM. The grand mean and SEM for each dose was comprised of the means for 1 to 5 determinations of that dose in each subject.
Similar to the effects of ethanol on response rate, increasing doses of pregnanolone dose-dependently decreased response rate in both adolescent-treated groups. Specifically, 10 mg/kg of pregnanolone decreased the response rate from a mean of 2.52 to 1.95 responses per second in the ethanol-treated group, whereas the same dose decreased response rate from a mean of 1.65 to 1.27 responses per second in the saline-treated group, which was less than 20% of the mean for both groups. Although 18 mg/kg of pregnanolone produced much larger decreases in the overall response rate of both groups, the rate-decreasing effects in the ethanol-treated group were somewhat smaller than those for the saline-treated group (i.e., 4 subjects in the ethanol-treated group had response rates large enough to plot their percentage of ethanol-lever responding).
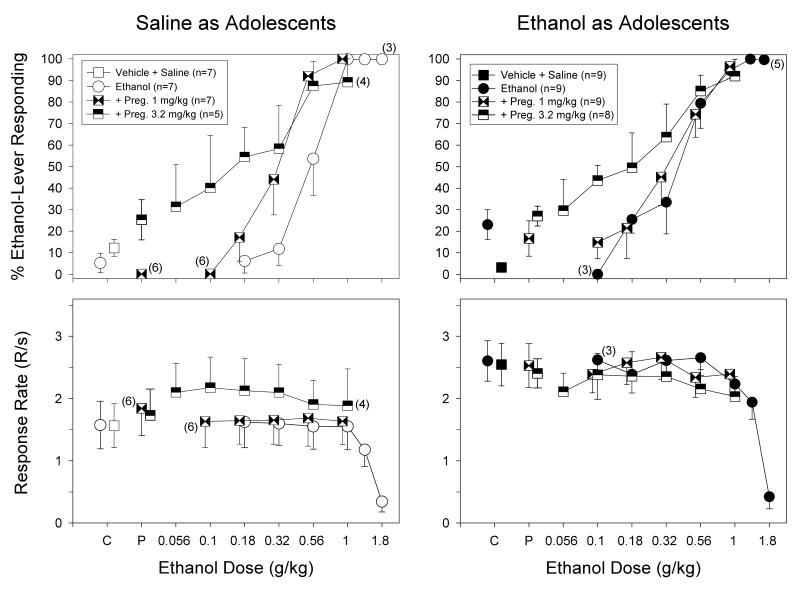
The top panels of Figure 4 show the effects of 1 and 3.2 mg/kg of pregnanolone administered in combination with ethanol on ethanol-lever responding in the saline- and ethanol-treated groups. As shown, pregnanolone dose-dependently shifted the dose-effects for ethanol in a complex manner that was dependent on the adolescent treatment. More specifically, 1 mg/kg of pregnanolone shifted the ethanol dose-effect curve to the left approximately two fold in the saline-treated group, whereas it did not shift the ethanol dose-effect curve in the ethanol-treated group. Unlike l mg/kg of pregnanolone, 3.2 mg/kg shifted the ethanol dose-effect curves for both groups similarly; that is, this dose increased ethanol-lever responding when it was administered with small doses of ethanol, but it did not uniformly increase ethanol-lever responding when it was administered with large doses of ethanol. For example, in the group treated with ethanol as adolescents, 3.2 mg/kg of pregnanolone in combination with 0.56 g/kg of ethanol did not produce greater ethanol-lever responding than either 0.56 g/kg of ethanol alone or 0.56 g/kg of ethanol in combination with 1 mg/kg of pregnanolone.
Fig. 4.
Interaction of two doses of pregnanolone with increasing doses of ethanol on the percentage of ethanol-lever responding (upper panels) and overall response rate (lower panels) in adult rats that received either saline (left-hand panels) or ethanol (right-hand panels) as adolescents. The effects of pregnanolone and ethanol alone on each dependent measure are also shown for comparison purposes. The subjects in each group were discriminating 1 g/kg of ethanol while responding under a FR-20 schedule of food presentation. Pregnanolone and ethanol were administered i.p. 15 and 10 min before the start of the session, respectively. Data points and vertical lines above “C” in each panel represent the grand mean and SEM for the respective control injections in each group, which were comprised of the means for 2 to 8 injections of saline, or vehicle in combination with saline. The data points above “P” in each panel represent the grand mean and SEM for at least two determinations of pregnanolone alone or pregnanolone and saline in every subject. The data points and vertical lines in the dose-effect curves for each group in each panel also represent a grand mean and SEM. The grand mean and SEM for each dose was comprised of the means for 1 to 4 determinations of that dose in each subject.
The effects of pregnanolone in combination with ethanol on response rate are shown in the bottom panels of Figure 4. Taking into account the aforementioned differences in the control rates of responding between the two groups, neither the 1- nor 3.2-mg/kg dose of pregnanolone had an effect when administered alone (data above C). However, 3.2 mg/kg of pregnanolone in combination with ethanol produced small, but consistent, rate-increasing effects in the saline-treated group compared to ethanol alone or ethanol in combination with 1 mg/kg of pregnanolone. For example, the response rate obtained after 3.2 mg/kg of pregnanolone combined with 0.18 g/kg of ethanol was 2.13 responses per second, whereas the response rate obtained after 0.18 g/kg of ethanol alone was 1.62 responses per second. In contrast, in the group treated with ethanol as adolescents, no rate-increasing effects were evident when 3.2 mg/kg of pregnanolone was administered in combination with ethanol.
Figure 5 depicts the effects obtained when DHEA (10-180 mg/kg) was substituted for the training dose of ethanol in a group of 13 subjects, 9 of which were treated with ethanol as adolescents and 4 of which were treated with saline as adolescents. For comparison purposes, ethanol-lever responding after ethanol (180-1800 mg/kg) and pregnanolone (1-18 mg/kg) administration are also shown for the same 13 subjects. The data for the two adolescent-treated groups were combined in this figure because DHEA did not substitute for ethanol in either of the groups. In fact, mean ethanol-lever responding did not exceed 15% for the subjects in either adolescent-treated group and only one dose (i.e., 10 mg/kg) for one of the thirteen subjects produced mean ethanol-lever responding greater than 80% (data not shown). Similar to the individual subject data, the combined data for the subjects administered DHEA show that it did not produce more than 10% ethanol-lever responding up to doses that substantially decreased the overall rates of responding. For example, ethanol-lever responding after 100 mg/kg of DHEA was 3.93% while decreasing response rate to 63% of the control rate, whereas ethanol-lever responding after 180 mg/kg was 1.48% while decreasing response rate to 25% of the control rate.
Fig. 5.
Effects of substituting 180 – 1800 mg/kg of ethanol, 1 – 18 mg/kg of pregnanolone or 10 – 180 mg/kg of DHEA on the percentage of ethanol-lever responding (upper panel) and overall response rate (lower panel) in a group (n=13) of rats discriminating 1000 mg/kg of ethanol while responding under a FR-20 schedule of food presentation. The group was comprised of rats that received saline or ethanol as adolescents. Data points and vertical lines above “C” in each panel represent the grand mean and SEM for each control condition (i.e., injections of saline or vehicle). The data points and vertical lines in the dose-effect curves for this group also represent a grand mean and SEM for each dose of each drug.
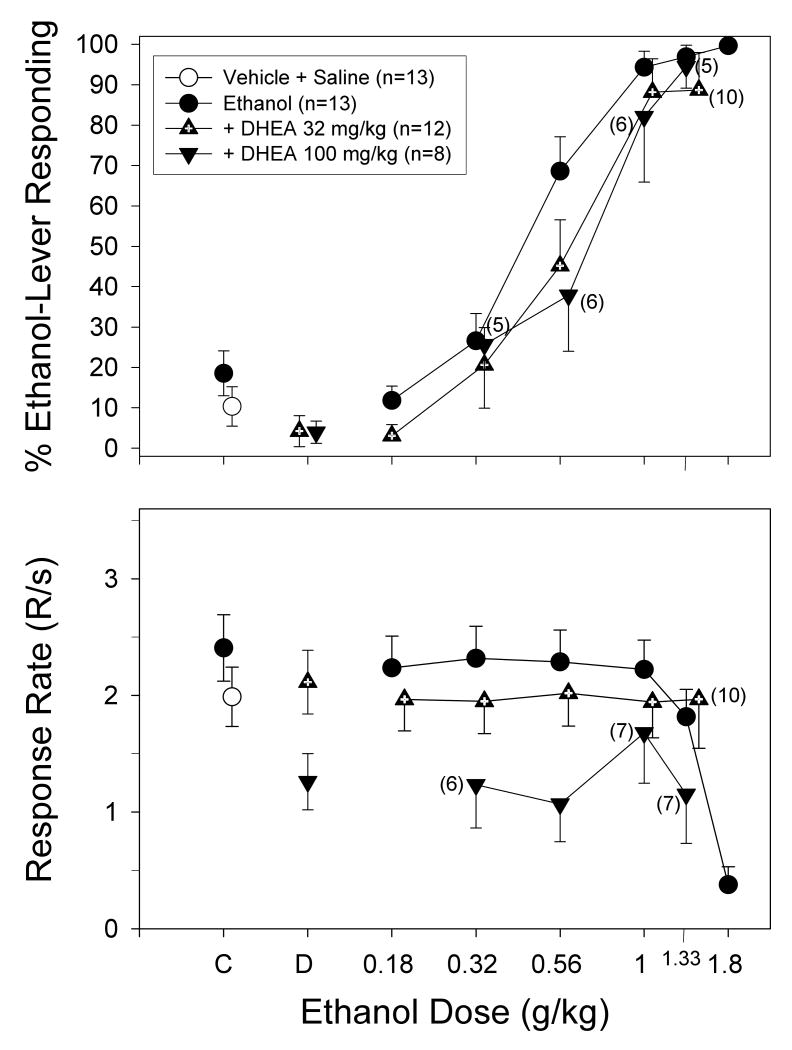
The effects of DHEA in combination with ethanol in subjects trained to discriminate ethanol from saline are shown in Figure 6. In contrast to pregnanolone, the combination of DHEA and ethanol shifted the dose-effect curves for the discriminative stimulus effects of ethanol to the right modestly (i.e., less than two fold). This rightward shift was largely due to the decrease in ethanol-lever responding when 32 and 100 mg/kg of DHEA were administered in combination with 0.56 g/kg of ethanol. For example, the ED50 for the dose-effect curve for ethanol alone was 0.41 mg/kg, whereas the ED50s for the curves for ethanol in combination with 32 and 100 mg/kg of DHEA were 0.48 and 0.62 mg/kg, respectively.
Fig. 6.
Interaction of two doses of DHEA with increasing doses of ethanol on the percentage of ethanol-lever responding (upper panel) and overall response rate (lower panel) in a group of adult rats. The group was comprised of rats that received saline or ethanol as adolescents. All of the subjects were discriminating 1 g/kg of ethanol while responding under a FR-20 schedule of food presentation. DHEA and ethanol were administered i.p. 15 and 10 min before the start of the session, respectively. Data points and vertical lines above “C” in each panel represent the grand mean and SEM for the group, which was comprised of the means for 1-8 injections of saline or vehicle and saline for each subject in this group. The data points above “D” in each panel represent the grand mean and SEM for at least two determinations of DHEA alone or DHEA and saline in every subject. The data points and vertical lines in the dose-effect curves for each group in each panel also represent a grand mean and SEM. The grand mean and SEM for each dose was comprised of the means for 1 to 4 determinations of that dose in each subject.
The effects of DHEA on the overall rate of responding were also different from those of pregnanolone in that the high dose of DHEA (100 mg/kg) produced rate-decreasing effects both alone (above “D”) and in combination with ethanol. More specifically, the only dose combination that did not produce a marked rate-decreasing effect was 100 mg/kg of DHEA and 1 g/kg of ethanol, which was the training dose of ethanol. The effects of 32 mg/kg of DHEA on the overall rate of responding were not substantially different than those for ethanol alone.
4. Discussion
The first aim of the present study was to administer ethanol or saline during adolescence to determine if early experience with the effects of alcohol could affect the establishment of an ethanol discrimination in adult rats. Based on the results obtained, chronic intermittent administration of 2 g/kg of ethanol to adolescent rats did not affect the establishment of the discrimination, but it did enhance the sensitivity of adult subjects to the discriminative stimulus effects of ethanol without affecting the rate-decreasing effects of ethanol when compared to adolescent saline administration. In addition, 2 g/kg during adolescence also increased responding under control conditions in adulthood, suggesting that ethanol-treated adolescents were not only differentially sensitive to the effects of ethanol as adults, but that their early experience with these effects altered conditioned responding in general. For example, ethanol-treated adolescents as a group had consistently higher percentages of ethanol-lever responding and higher rates of responding after saline injections than saline-treated adolescents. Moreover, these differences in rate under the FR-20 schedule occurred even though both groups were kept at constant weights as adults and the mean weight for the groups was comparable before and after adolescent treatment. With respect to ethanol-lever responding, ethanol-treated adolescents had a much higher percentage of false alarms (i.e., responding on the ethanol-appropriate lever after a saline injection) as adults than the saline-treated adolescents as adults, which is difficult to explain given that both groups were trained identically using the same operant chambers, and both groups acquired the discrimination over a comparable number of sessions.
One possible explanation for these differences might be that adolescents administered CIE develop persistent or perseverative response patterns (Santucci et al., 2004) in the absence of strong stimulus control. Theoretically, such persistent response patterns could contribute to the repeated selection of one of two levers in a two-lever discrimination procedure, and to higher rates of responding under FR schedules where the repetition of a similar response (i.e., lever press) results in reinforcement. In one study, for example, Walker et al. (1981) found that rats administered an ethanol containing diet for 5 months responded poorly in a spontaneous alternation task in a T-maze when tested after a 2-month ethanol-free period. More specifically, ethanol-treated rats alternated correctly at only chance levels (48%), whereas rats in a control group alternated correctly on 83% of the trials. File and Mabbutt (1990) also identified perseverative responding after chronic alcohol administration in a habituation task and a passive avoidance task. Interestingly, this same type of perseveration and resistance to extinction in laboratory animals has been associated with damage to the orbital frontal cortex (Volkow and Fowler, 2000).
Although the development of perseverative response patterns after CIE can help explain the differences obtained under control conditions in the ethanol-treated subjects, such patterns would not entirely explain the increased sensitivity to doses of ethanol smaller than the training dose. This type of dose dependency in drug discrimination procedures suggests that the dose of ethanol was a critical variable even though responding is usually quantal in nature (Colpaert et al., 1976). That is, as was the case in the present study, medial levels of responding in these procedures usually reflect differences among subjects rather than medial levels of drug-lever responding by individual subjects. Thus, the data from the two adolescent-treated groups suggests that 2 g/kg of ethanol during adolescence produced adult subjects with perseverative response patterns in the absence of ethanol, increased response rates under a FR schedule, and an increased sensitivity to the discriminative stimulus effects of small ethanol doses.
The second aim of this study was to determine if pregnanolone would substitute for ethanol, and thereby demonstrate an asymmetrical generalization between pregnanolone and ethanol that is similar to the one that occurs when benzodiazepines and barbiturates are administered to subjects trained to discriminate ethanol (Kostowski and Bienkowski, 1999). In a previous study from this laboratory, Gerak et al. (2008) found that ethanol did not produce greater than 80% pregnanolone-lever responding despite these drugs' similarly complex discriminative stimulus effects, and the fact that some of the same receptors (e.g., GABAA and NMDA receptors) have been shown to play prominent roles in mediating the discriminative stimulus effects of these drugs (Kostowski and Bienkowski, 1999). Therefore, Gerak et al. (2008) concluded that the capacity of ethanol to substitute for pregnanolone likely varied among individuals because the discriminative stimulus effects substantially overlapped, but were not identical under the experimental conditions established. The medial levels of ethanol-lever responding in each adolescent group after pregnanolone substitution in this study would seem to support a similar conclusion and would be consistent with discrimination data from Vanover et al. (1999). In addition, even in those studies where pregnanolone and other positive GABAA modulators at the neurosteroid site have been reported to substitute for ethanol (Ator et al., 1993; Bienkowski and Kostowski, 1997; Bowen et al., 1999b), the results were not entirely different from those reported in this study for pregnanolone. For example, Bienkowski and Kostowski (1997) found that 1.3 – 12 mg/kg of tetrahydrodeoxycorticosterone (5β-THDOC) produced less than 80% ethanol-lever responding at doses that had no effect on responding and only 87% ethanol-lever responding at the highest dose, which markedly decreased responding.
Another implication of the partial substitution of pregnanolone for ethanol in both adolescent-treated groups is that prior experience with the effects of ethanol, or a history of ethanol administration, does not increase or decrease the likelihood that complete substitution of pregnanolone will occur. Because ethanol has been shown to increase the release of specific neurosteroids (O'Dell et al., 2004a; Sanna et al., 2004) and change the sensitivity of GABAA receptors to other allosteric modulators after chronic treatment (Negro et al., 1993; Mehta and Ticku, 1998; Kang et al., 1998; Mehta and Ticku, 2001), there was reason to suspect that adolescent administration of ethanol might potentially have long-term consequences or effects on neurosteroid sensitivity. However, this was not the case in terms of pregnanolone's capacity to substitute for ethanol's discriminative stimulus effects in either adolescent-treated group, or in terms of pregnanolone's rate-decreasing effects, although the rate-decreasing effects of the highest dose of pregnanolone (18 mg/kg) were smaller in the group that was treated with ethanol as adolescents than the group that was treated with saline as adolescents. One study has shown that CIE reduced the sleep time produced by the neuroactive steroid alphaxolone (Cagetti et al., 2003), but to date, there has been little or no data to indicate that CIE has the capacity to attenuate pregnanolone's rate-decreasing effects in rats. One reason for the absence of such data may be that most studies showing an interaction between protracted ethanol administration and the neuroactive steroids examined the interaction either during withdrawal (e.g., Devaud et al., 1996; Mehta and Ticku, 1998) or shortly after the last injections of ethanol (e.g., Negro et al., 1993; Mehta and Ticku, 1998). At these time points, ethanol was found to enhance the effects of certain neuroactive steroids.
One effect of pregnanolone that was clearly evident in the present study was its capacity for potentiating the discriminative stimulus effects of low doses of ethanol when the two drugs were administered in combination. In addition, pregnanolone produced a leftward shift in the curve for ethanol-lever responding at a smaller dose in saline-treated adolescents than in ethanol-treated adolescents (Figure 4). If pregnanolone administration was serving to enhance a component or components of ethanol's discriminative stimulus effects, then this would indicate that adolescent ethanol administration reduced the capacity of pregnanolone to enhance that component or components when the subject was an adult. Such an effect would be consistent with the notion that neuroactive steroids have many overlapping effects with ethanol and suggests that adolescent ethanol administration not only produces long-lasting tolerance as suggested by some investigators (Silvers et al., 2003; Tokunaga et al., 2006; Silvers et al., 2006), but may also produce cross tolerance with pregnanolone as well because CIE reduced pregnanolone's capacity to enhance a potentially shared effect of the two drugs.
Another possible explanation for the positive interaction data obtained between pregnanolone and ethanol is that the baseline sensitivity of the ethanol-treated subjects to the discriminative stimulus effects of ethanol was already enhanced compared to saline-treated subjects (Figure 2). In many ways, these data conform to the law of initial values (Wilder, 1967), which would predict that an increase in sensitivity to a drug is more readily observable when the sensitivity under control conditions is low. Thus, 1 and 3.2 mg/kg of pregnanolone produced both leftward and upward shifts of the ethanol dose-effect curves, respectively, for the adult group treated with saline as adolescents, whereas 3.2 mg/kg only produced an upward shift in the ethanol dose-effect curve for the adult group treated with ethanol as adolescents (Figure 4). This type of differential effect for pregnanolone in rats discriminating ethanol would seem to indicate that drug history, along with training dose (Bowen et al., 1999a), can be an important variable in the magnitude of this drug interaction. These interaction data would also seem to suggest that there is a limit to which pregnanolone can potentiate ethanol's discriminative stimulus effects, just as there was limited substitution of pregnanolone for ethanol and vice versa (Gerak et al., 2008).
Although CIE administration has been shown to produce long-term changes in ethanol-induced impairments of motor coordination (White et al., 2002), and acute ethanol administration has been shown to increase levels of the endogenous 3α-hydroxy neurosteroid allopregnanolone (Barbaccia et al., 1999; O'Dell et al., 2004a; Silvers et al., 2006), administration of pregnanolone in combination with ethanol did not potentiate the rate-decreasing effects of ethanol. To the contrary, 3.2 mg/kg of pregnanolone in combination with several doses of ethanol actually increased response rate under the FR-20 schedule above control levels in subjects that received saline as adolescents. This finding is interesting because it indicates that the potentiation of ethanol's discriminative stimulus effects by pregnanolone was largely independent of either drug's rate-decreasing effects, and supports the notion that certain combinations of these drugs are as capable of increasing response rate as decreasing it. Melchior and Allen (1992) reported that pregnanolone could increase activity in mice and suggested that it might have biphasic effects on locomotor activity similar to those for ethanol and pentobarbital; however, they also stated that many of the studies already in the literature at that time involving the neurosteroids and ethanol may not have had the appropriate conditions for observing increases in activity.
In accordance with the literature, DHEA did not substitute for ethanol at any dose tested (Bienkowski and Kostowski, 1997; Bowen et al., 1999b), and pretreatment with DHEA prior to ethanol administration did not shift the ethanol dose-effect curve for the discriminative stimulus effects more than two fold (Bienkowski and Kostowski, 1997; Bowen et al., 1999a). The rationale for examining this interaction is that, in theory, negative modulators of the GABAA receptor complex may have the capacity to antagonize the effects of positive GABAA modulators like ethanol, even though the allosteric sites on the GABAA receptor complex are different. For example, RO 15-4513, which is a negative allosteric modulator that binds to the benzodiazepine binding site on the GABAA receptor complex, has been shown to block a variety of the effects of ethanol (e.g., Suzdak et al., 1986; Samson et al., 1987; Rassnick et al., 1993). Similarly, O'Dell et al. (2005) found that the neuroactive steroids epipregnanolone and PCA ([3α,5α]-20-oxo-pregnane-3-carboxylic acid), which are considered to be negative modulators of the GABAA receptor complex, can decrease ethanol self administration. With respect to the neuroactive steroids, however, there remains a good deal of confusion regarding the exact nature of their interaction because both can interact with GABAA receptors directly (Grobin et al., 1998; Losel et al., 2003), both have effects at other ion channels, such as the NMDA receptor (Crews et al., 1996; Rupprecht and Holsboer, 1999), and ethanol could affect the GABAA and NMDA receptor complexes indirectly by increasing plasma and brain concentrations of specific neuroactive steroids such as allopregnanolone (Barbaccia et al., 1999; VanDoren et al., 2000; Sanna et al., 2004). With regard the discriminative stimulus effects of ethanol though, the findings from the present study along with those from Bowen et al. (1999a) indicate that DHEA is rather ineffective at antagonizing these particular effects, just as RO 15-4513 was shown to be ineffective at blocking the discriminative stimulus effects of ethanol (Hiltunen and Jarbe, 1988; 1989).
In summary, CIE administration of a behaviorally disruptive dose (i.e., 2 g/kg) during adolescence produced long-term effects that were manifest in adult subjects as perseverative response patterns in the absence of ethanol, increased response rates under a FR schedule, and an increased sensitivity to the discriminative stimulus effects of small doses of ethanol. CIE administration of the same dose, however, did not increase the capacity of pregnanolone to substitute for ethanol, suggesting that neither the training drug nor the experience of ethanol's effects as an adolescent can alter this outcome readily. CIE of 2 g/kg also did not alter the rate-decreasing effects of pregnanolone in adult subjects except at the largest dose (18 mg/kg) in some subjects suggesting that CIE during adolescence can produce some changes in the sensitivity to certain effects of the neurosteroids independent of their discriminative stimulus effects. In terms of the interaction of ethanol and pregnanolone, a small dose of pregnanolone (1 mg/kg) produced a larger potentiation of the discriminative stimulus effects of ethanol in saline-treated adolescents than in ethanol-treated adolescents, but this effect may be attributable to initial differences in sensitivity between the two adolescent-treated groups as suggested by the limited interactive effects obtained with a larger dose of pregnanolone (3.2 mg/kg) in both groups. Finally, DHEA did not substitute for ethanol in adult subjects that had been treated with either saline or ethanol as adolescents and its rate-decreasing effects were similar in both groups of subjects. Administered in combination with ethanol, however, a dose of DHEA that substantially decreased overall rates of responding shifted the ethanol dose-effects curves for ethanol-lever responding modestly to the right by attenuating the discriminative effects of a dose smaller than the training dose (i.e., 0.56 g/kg).
When considered together these results show that adolescent ethanol administration can have long-term effects on conditioned behavior, both in the presence and absence of ethanol, and affect the interaction between ethanol and neuroactive steroids such as pregnanolone with overlapping effects. To what extent these effects serve to facilitate abuse of, or dependence on, alcohol as an adult remains to be determined. However, what seems to be clear from this and other research is that adolescent administration may set the occasion for adult abuse and dependence, but adolescent ethanol administration alone does not appear to be sufficient for producing this response (i.e., abuse or dependence). Future research will also need to focus on achieving a greater understanding of ethanol's interaction with the neuroactive steroids, especially if this understanding could lead to additional advances in the alcohol field and the development of novel pharmacotherapies for alcoholism (Morrow et al., 2001; Ford et al., 2007). Certainly, data from the present study would suggest that the androstane steroids are more likely to block the discriminative effects of alcohol than the pregnane steroids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ator NA, Grant KA, Purdy RH, Paul SM, Griffiths RR. Drug discrimination analysis of endogenous neuroactive steroids in rats. Eur J Pharmacol. 1993;241:237–243. doi: 10.1016/0014-2999(93)90208-y. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur J Pharmacol. 1999;384:R1–R2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kostowski W. Discriminative stimulus properties of ethanol in the rat: effects of neurosteroids and picrotoxin. Brain Res. 1997;753:348–352. doi: 10.1016/s0006-8993(97)00165-0. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. An investigation of endogenous neuroactive steroid-induced modulation of ethanol's discriminative stimulus effects. Behav Pharmacol. 1999a;10:297–311. doi: 10.1097/00008877-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. Ethanol-like discriminative stimulus effects of endogenous neuroactive steroids: effect of ethanol training dose and dosing procedure. J Pharmacol Exp Ther. 1999b;289:405–411. [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJ, Janssen PA. Theoretical and methodological considerations on drug discrimination learning. Psychopharmacologia. 1976;46:169–177. doi: 10.1007/BF00421388. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Morrow AL, Criswell H, Breese G. Effects of ethanol on ion channels. Int Rev Neurobiol. 1996;39:283–367. doi: 10.1016/s0074-7742(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Demirgoren S, Majewska MD, Spivak CE, London ED. Receptor binding and electrophysiological effects of dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience. 1991;45:127–135. doi: 10.1016/0306-4522(91)90109-2. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of gamma-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- File SE, Mabbutt PS. Long-lasting effects on habituation and passive avoidance performance of a period of chronic ethanol administration in the rat. Behav Brain Res. 1990;36:171–178. doi: 10.1016/0166-4328(90)90171-a. [DOI] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res. 2007;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Moerschbaecher JM, Winsauer PJ. Overlapping, but not identical, discriminative stimulus effects of the neuroactive steroid pregnanolone and ethanol. Pharmacol Biochem Behav. 2008;89:473–479. doi: 10.1016/j.pbb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Lamb RJ. Alphaxalone and epiallopregnanolone in rats trained to discriminate ethanol. Alcohol Clin Exp Res. 2005;29:1621–1629. doi: 10.1097/01.alc.0000179374.39554.04. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther. 1993;264:1241–1247. [PubMed] [Google Scholar]
- Green KL, Grant KA. Evidence for overshadowing by components of the heterogeneous discriminative stimulus effects of ethanol. Drug Alcohol Depend. 1998;52:149–159. doi: 10.1016/s0376-8716(98)00086-6. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen AJ, Jarbe TU. Ro 15-4513 does not antagonize the discriminative stimulus-or rate-depressant effects of ethanol in rats. Alcohol. 1988;5:203–207. doi: 10.1016/0741-8329(88)90053-5. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ, Jarbe TU. Discriminative stimulus properties of ethanol: effects of cumulative dosing and Ro 15-4513. Behav Pharmacol. 1989;1:133–140. [PubMed] [Google Scholar]
- Hodge CW, Nannini MA, Olive MF, Kelley SP, Mehmert KK. Allopregnanolone and pentobarbital infused into the nucleus accumbens substitute for the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2001;25:1441–1447. doi: 10.1097/00000374-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus effects of drugs: Relationship to potential for abuse. In: Adler MW, Cowan A, editors. Testing and Evaluation of Drugs of Abuse. John Wiley & Sons; New York: 1990. pp. 193–210. [Google Scholar]
- Kang MH, Spigelman I, Olsen RW. Alteration in the sensitivity of GABA(A) receptors to allosteric modulatory drugs in rat hippocampus after chronic intermittent ethanol treatment. Alcohol Clin Exp Res. 1998;22:2165–2173. [PubMed] [Google Scholar]
- Kostowski W, Bienkowski P. Discriminative stimulus effects of ethanol: neuropharmacological characterization. Alcohol. 1999;17:63–80. doi: 10.1016/s0741-8329(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Interaction of ethanol with the GABAA receptor in the rat brain: possible involvement of endogenous steroids. Alcohol. 1988;5:269–273. doi: 10.1016/0741-8329(88)90064-x. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Chronic ethanol administration alters the modulatory effect of 5alpha-pregnan-3alpha-ol-20-one on the binding characteristics of various radioligands of GABAA receptors. Brain Res. 1998;805:88–94. doi: 10.1016/s0006-8993(98)00649-0. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Unsulfated and sulfated neurosteroids differentially modulate the binding characteristics of various radioligands of GABA(A) receptors following chronic ethanol administration. Neuropharmacology. 2001;40:668–675. doi: 10.1016/s0028-3908(00)00200-8. [DOI] [PubMed] [Google Scholar]
- Melchior CL, Allen PM. Interaction of pregnanolone and pregnenolone sulfate with ethanol and pentobarbital. Pharmacol Biochem Behav. 1992;42:605–611. doi: 10.1016/0091-3057(92)90005-z. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Negro M, Casanova E, Chinchetru MA, Fernandez-Lopez A, Calvo P. Differential effect of chronic ethanol treatment on barbiturate and steroid modulation of muscimol-binding to rat brain cortex. Neurosci Lett. 1993;158:83–86. doi: 10.1016/0304-3940(93)90618-u. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Purdy RH, Covey DF, Richardson HN, Roberto M, Koob GF. Epipregnanolone and a novel synthetic neuroactive steroid reduce alcohol self-administration in rats. Pharmacol Biochem Behav. 2005;81:543–550. doi: 10.1016/j.pbb.2005.03.020. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Alomary AA, Vallée M, Koob GF, Fitzgerald RL, Purdy RH. Ethanol-induced increases in neuroactive steroids in the rat brain and plasma are absent in adrenalectomized and gonadectomized rats. Eur J Pharmacol. 2004a;484:241–247. doi: 10.1016/j.ejphar.2003.11.031. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004b;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Liang J, Cagetti E, Spigelman I. Plasticity of GABAA receptors in brains of rats treated with chronic intermittent ethanol. Neurochem Res. 2005;30:1579–1588. doi: 10.1007/s11064-005-8836-6. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Rassnick S, D'Amico E, Riley E, Koob GF. GABA antagonist and benzodiazepine partial inverse agonist reduce motivated responding for ethanol. Alcohol Clin Exp Res. 1993;17:124–130. doi: 10.1111/j.1530-0277.1993.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Pfeffer AO, Sadeghi KG, Mills FG. Oral ethanol reinforcement in the rat: effect of the partial inverse benzodiazepine agonist RO15-4513. Pharmacol Biochem Behav. 1987;27:517–519. doi: 10.1016/0091-3057(87)90357-1. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci AC, Mercado M, Bettica A, Cortes C, York D, Moody E. Residual behavioral and neuroanatomical effects of short-term chronic ethanol consumption in rats. Brain Res Cogn Brain Res. 2004;20:449–461. doi: 10.1016/j.cogbrainres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, O'Buckley T, Morrow AL, Matthews DB. Chronic intermittent ethanol exposure during adolescence reduces the effect of ethanol challenge on hippocampal allopregnanolone levels and Morris water maze task performance. Alcohol. 2006b;39:151–158. doi: 10.1016/j.alcohol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Olufsen K. Generalisation of ethanol with drug mixtures containing a positive modulator of the GABA(A) receptor and an NMDA antagonist. Neuropharmacology. 2001;40:123–130. doi: 10.1016/s0028-3908(00)00100-3. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986;234:1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, Silvers JM, Matthews DB. Chronic intermittent ethanol exposure during adolescence blocks ethanol-induced inhibition of spontaneously active hippocampal pyramidal neurons. Alcohol Clin Exp Res. 2006;30:1–6. doi: 10.1111/j.1530-0277.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Suruk M, Robledo S, Huber M, Wieland S, Lan NC, Gee KW, Wood PL, Carter RB. Positive allosteric modulators of the GABAA receptor: differential interaction of benzodiazepines and neuroactive steroids with ethanol. Psychopharmacology (Berl) 1999;141:77–82. doi: 10.1007/s002130050809. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Walker DW, Hunter BE, Abraham WC. Neuroanatomical and functional deficits subsequent to chronic ethanol administration in animals. Alcohol Clin Exp Res. 1981;5:267–282. doi: 10.1111/j.1530-0277.1981.tb04901.x. [DOI] [PubMed] [Google Scholar]
- White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcohol Clin Exp Res. 2002;26:960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]
- Wilder J. Stimulus and response: The law of initial value. John Wright; Bristol, England: 1967. [Google Scholar]