Abstract
Emerging evidence suggests that despite testicular androgen ablation, residual androgens, likely of adrenal---though potentially of prostatic---origin, play a critical role in the progression of prostate cancer to recurrent ‘castration-resistant’ disease. Thus, a reassessment of the concept of total androgen deprivation is warranted. Current treatment strategies may not only lack optimal efficacy, but may actually contribute to the selection of neoplastic clones adapted to exist and proliferate in a low (but not zero) androgen environment. Moreover, the adequacy of AR pathway inhibition cannot be surmised from serum or plasma androgen levels, but must be ascertained at the tissue and molecular level prior to drawing conclusions regarding clinical efficacy or failure. Recent studies by our group and others indicate that prostate cancers undergo an adaptive response to castration that is associated with the upregulation of transcripts encoding enzymes involved in the biosynthesis of androgens. Targeting these metabolic enzymes either individually or using combinations of agents to inhibit testicular, adrenal and intracrine sources may provide enhanced clinical responses in the setting of both localized and metastatic disease.
Keywords: Prostate cancer, hormone therapy, castration resistant, metastatic, androgen metabolism, intracrinology, steroidogenesis
INTRODUCTION
Since the landmark studies of Huggins and Hodges demonstrated that prostate cancers were influenced by androgens (1), therapies directed toward suppressing systemic testosterone levels have remained the choice for the initial treatment of metastatic disease. Although initially highly effective, hormonal suppression for metastatic prostate cancer fails to effect durable complete remissions, with the predictable emergence of progressive disease over a period of 18–20 months that is variously termed hormone independent, hormone refractory, or castration resistant prostate cancer (CRPC). The median survival in the setting of castration-resistant metastasis is between 1–2 years. An important aspect of CRPC centers on the fact that androgen-receptor signaling is usually reactivated in men with this disease stage, a finding that is easily substantiated through measurements of high concentrations of prostate specific antigen (PSA), a protein known to be regulated by androgens. Further, several studies have documented that many genes known to be regulated via androgen receptor (AR) signaling are expressed in these tumors.
The observation that androgen-regulated genes are almost universally measurable in ‘hormone-refractory/castration-adapted’ prostate cancer has prompted a search for processes that contribute to androgen receptor (AR) activation in the castrate environment. Proposed mechanisms include: [1] amplification/over-expression of the AR, [2] AR gene mutations leading to promiscuous ligand or cofactor interaction, [3] enhanced AR signal transduction through alterations in coactivators/corepressors, and [4] activation of the AR or downstream regulatory molecules by “cross talk” with other signaling pathways (e.g. Her2, IL6) (2). Studies of these mechanisms represent important avenues of investigation as they each may account for a component of what is viewed as androgen-independent disease. While there certainly may be pathways leading to prostate cancer growth---and AR signaling---that are entirely ligand-independent (3, 4), many of the processes identified to date either still require or are enhanced by the presence of ligand. As shown by Chen et al, prostate cancer growth in “castrate” animal models induced by AR overexpression is still ligand dependent (5). Thus, the most straightforward explanation for persistent/recurrent AR signaling is the persistence of androgenic ligands at levels adequate to engage a wild-type AR. The following sections provide data generated by our group and others that support this possibility. Further, data will be presented detailing mechanisms involving the adaptation of androgen metabolic pathways through which tumoral androgen levels may be maintained despite castration. These include the utilization of adrenal androgens and the possible contribution of de novo intratumoral steroidogenesis.
Intraprostatic Androgens Following Systemic Testosterone Suppression
A substantial body of data generated over the past 30 years indicates that in the setting of ‘castrate’ serum testosterone concentrations, levels of intra-prostatic androgens are reproducibly measurable at concentrations sufficient to activate the AR. In the decades spanning the 1970s and 1980s, Geller and colleagues published several studies in which intra-prostatic DHT levels were quantitated by radioimmunoassay (RIA) in patients treated with a variety of hormonal regimens. Several key observations were made: [1] systemic testosterone suppression resulting from orchiectomy or megace plus DES reduced prostatic DHT levels by 75–80% to about 1 ng/g in many but not all patients; [2] prostatic epithelial and stromal cell protein synthesis was strongly correlated with tissue DHT levels; and [3] prostatic dihydrotestosterone (DHT) levels were further reduced when castration was combined with adrenal androgen blockade by the addition of ketoconazole (6–8). From these studies, and others reported by Labrie and co-workers (9), the investigators concluded that the low, but measurable intraprostatic concentrations of DHT following androgen suppressing therapies was sufficient to stimulate tumor growth, and that treatments should be designed to suppress intraprostatic DHT as low as possible (10–12).
More recently, Mohler et al reported that patients with recurrent prostate cancer treated with therapies resulting in castrate serum testosterone levels, had intra-prostatic testosterone concentrations equivalent to those of eugonadal patients with benign prostatic hyperplasia (BPH), and that intraprostatic DHT levels were reduced to only 80% (~ 0.4 ng/g) of untreated individuals (13). Contemporary studies reported by Nishiyama et al have confirmed these findings (14). Further, tumor differentiation, as determined by Gleason grading, was correlated with the extent of change in tissue DHT, with an 85% decrease measured in Gleason 6 cancers, but only a 60% decrement found in Gleason 7–10 tumors (15). This finding indicates that tumor type-specific changes in androgen metabolism (synthesis or utilization) may impact responses to systemic testosterone suppression.
In support of the findings detailed in the studies described above, we have reported that androgen signaling is maintained in neoplastic prostate epithelial cells in men with localized prostate cancer following short-term (3 months) and longer term (9 months) castration (16). Remarkably, we also found that short term castration therapy in normal healthy men, resulting in serum levels of testosterone below the limits of detection, did not effectively eliminate intraprostatic androgens and androgen-regulated gene expression, as determined by quantitating transcript levels of known AR-regulated genes such as PSA (17). Thus, the intraprostatic androgen levels achieved by standard medical and surgical methods of castration are inadequate to fully suppress critical physiologic processes regulated by androgens. These processes include the synthesis of proteins such as PSA that contribute to the normal secretory output of prostate epithelium as well as fundamental cellular events maintaining survival and proliferation.
Together, these data suggest that prostate cancer cells may resist androgen suppression therapies through a process of metabolic adaptation rather than through genomic events such as mutation, translocation, or the amplification of chromosomal loci. Genomic alterations occur rarely in a population of tumor cells and become dominant through a selective growth advantage. This process would manifest as a rare population of resistant cells early in the selection process followed by increasing numbers of these clonally-derived cells expressing AR-responsive genes over time. In contrast, metabolic adaptation would be reflected by large numbers of tumor cells, representing different clones, each capable of expressing androgen-regulated genes at all time-points evaluated post-castration. The latter explanation best approximates the findings we and others have reported.
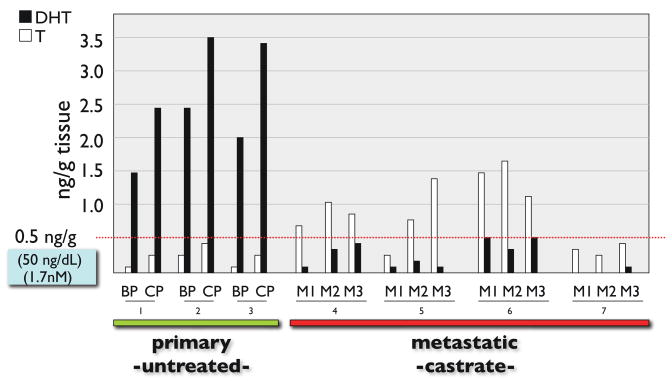
While the majority of data analyzing tissue androgen levels are derived from in-traprostatic measurements, recent reports have described concordant findings in prostate cancer metastasis. The vast majority of men with castration-resistant metastatic prostate cancer have elevated levels of the androgen-regulated gene, PSA, in the blood. Further, immunohistochemical studies of metastatic prostate tumor samples readily detect proteins encoded by androgen-regulated genes. A comprehensive transcript profiling study of castration-resistant bone metastasis reported by Stanbrough et al found abundant transcripts for androgen regulated genes in most of the tumor samples (18). These and other studies demonstrate that AR-signaling is active, but the mechanism(s) underlying AR activation have not been conclusively elucidated. To determine if intratumoral androgens could contribute to AR activation in metastatic tumors, we conducted an analysis of multiple distinct metastatic prostate tumors obtained through a rapid autopsy protocol from anorchid men with CRPC. We found that testosterone levels within metastases from the castrate men exceeded serum levels and were approximately 3-fold higher than levels within primary prostate tumors from untreated (eugonadal) patients (Figure 1). The androgen concentrations measured in these metastasis were at levels known to be sufficient for activating AR target genes (19).
Figure 1. Quantitation of Androgens in Prostate Tissue.
Measurements of tissue testosterone (T) and dihydrotestosterone (DHT) in prostate tissues. Concentrations are reported as ng of steroid per gram of tissue and extrapolated to a serum concentration commonly reported as ng/dL (blue box). All metastatic tissue samples (3 metastasis from each of 4 different individuals) were acquired from subjects with documented anorchid serum testosterone levels.
The data acquired from tissue androgen measurements in both local and metastatic prostate tumors clearly demonstrate that achieving castrate levels of circulating testosterone does not entirely eradicate androgens from the prostate tumor microenvironment. The persistent intratumoral androgen levels observed in CRPC, coupled with evidence of AR-regulated gene expression measured after both short-term and long-term androgen suppression suggests that alterations in androgen metabolism within the tumoral tissue may contribute to these residual tissue androgen levels observed in the castrate setting.
Activity and Significance of Intratumoral Androgens in Crpc
Data acquired from in vitro and in vivo studies of AR activation have found that DHT levels in the 0.5 to 1.0 nM range, a concentration measured in the prostatic tissue of castrate patients, are adequate to activate the AR, stimulate expression of androgen regulated genes, and promote neoplastic epithelial cell growth and survival (20, 21). Of the proposed mechanisms explaining persistent AR activation in the setting of castration (e.g. AR mutation), most still depend upon the presence of AR ligands for maximal effect (5, 22). Although not usually sustained, prostate cancer that recurs following castration will typically respond to a variety of secondary hormonal manipulations (23), suggesting that recurrent disease maintains at least some degree of androgen sensitivity.
The importance of residual androgens in mediating castration-resistant tumor growth is supported by numerous clinical studies designed to evaluate the effects of suppressing AR signaling by combining castration with steroidal or non-steroidal inhibitors of the AR, a strategy termed combined androgen blockade (CAB) (24). Despite numerous trials, the therapeutic advantage of CAB remains highly debated. While the initial studies utilizing either surgical or medical castration in combination with an anti-androgen suggested significant improvements in survival compared to historical controls (9), subsequent randomized trials have not been conclusive. The systematic reviews and meta-analyses performed to date have consistently identified small improvements in 5-year survival rates, on the order of 5%, in favor of CAB over castration alone (Figure 2). For example, a meta-analysis limited to trials using a non-steroidal anti-androgen in the CAB arm, involving 2922 patients, reported statistically significant relative risks for survival and time to progression in favor of CAB (RR survival =0.78 [95% CI 0.67–0.90] and RR TTP = 0.74 [95%CI 0.63–0.86]) (25). A Cochrane Collaborative Review of 20 trials using a non-steroidal anti-androgen, involving 6320 patients, reported an odds ratio for survival at 5 years favoring CAB (OR = 1.29 [95% CI 1.11–1.50], with a 5% improvement in 5-year survival for CAB (from 25% to 30%) (26).
Figure 2. Survival Outcomes with Combined Androgen Blockade.
Results of clinical trials of testosterone suppression alone (e.g. orchiectomy) versus testosterone suppression combined with an androgen receptor (AR) antagonist. Though variable, the overall results indicate a survival advantage with the addition of an AR antagonist. The figure is modified from studies reported in Samson et al (56).
Although the overall benefit attributed to anti-androgens such as flutamide or bicalutamide is limited, the near-uniformity of the observations made in the meta-analyses, both in the direction and magnitude of benefit, suggests that although small, CAB does impart an improvement in survival compared to monotherapy. A major problem in the interpretation of these results centers on the lack of data actually demonstrating on-target activity. Studies of non-steroidal AR-antagonists have not systematically assessed intra-prostatic effects on AR-signaling inhibition. The currently available anti-androgens, such as bicalutamide, exhibit only moderate affinity for the AR (27) with affinities 50–100 times less than DHT (28). Further, these anti-androgens can function as AR agonists in the setting of low androgen levels or amplified AR. Thus more effective ablation of the androgen axis could potentially improve the clinical benefit observed.
The potential for adrenal androgens to influence prostate cancer growth was recognized decades ago. Bilateral surgical adrenalectomy or hypophysectomy procedures were performed in progressive disease and resulted in pain relief in the majority of patients and objective responses in some, but the approach was associated with significant morbidity (29). Pharmacological ‘adrenalectomy’ using aminoglutethimide or ketoconazole produces response rates of about 40–50% in men with disease progression following castration (7, 30, 31). However, these inhibitors only partially suppress adrenal androgen production (by about 50%), and responses have been correlated with the magnitude of adrenal suppression (30, 32). Small et al have reported improved PSA and objective responses in patients treated with a combination of anti-androgen withdrawal and ketoconazole compared to anti-androgen withdrawal alone (33).
A valid criticism concerns the lack of more substantial efficacy with the radical procedures of adrenalectomy and hypophesectomy which, while demonstrating clinical responses, fall far short of curative therapy. One hypothesis explaining these clinical observations is the possibility that prostate adenocarcinomas are capable of de novo androgen biosynthesis from cholesterol precursors, and thus in some cases do not require adrenal androgens as substrates. In support of this possibility are data reported by Holzerbeierlein et al showing the enhanced expression of enzymes in the cholesterol bio-synthetic pathway in castration adapted prostate cancer relative to prostate cancers analyzed from untreated patients (34).
Endocrine and Intracrine Sources of Tissue Androgens after Castration
The sources of residual intraprostatic androgens detected following castration have yet to be definitively determined. However, the origin most commonly cited is the adrenal gland which provides precursors for uptake into the prostate gland and conversion to more potent steroids (35). Intraprostatic adrenal androgens have been detected at significant levels (50–60% of normal) in patients undergoing androgen deprivation (13, 36, 37). In castrate prostate tumor tissues, levels of DHEA, DHEA-S and AED decrease to about 50% of those measured in untreated prostate tissue, and exceed the concentrations of testosterone and DHT (13). Of interest, a study of prostate tissues after castration reported by Mizokami and colleagues found no decreases in tissue levels of 5-androstenediol, a primary metabolite of DHEA and a direct precursor of testosterone (37). This finding is of importance as this androgen is capable of activating wild type AR and is not inhibited by flutamide or bicalutamide (38). Finally, Koh et al and others have demonstrated the conversion of the adrenal androgens DHEA and androstenedione (A-dione) to DHT in prostate cancer cell lines and benign prostate tissue (39, 40).
An alternative mechanism accounting for the presence of intratumoral androgens is the de novo or intracrine synthesis from cholesterol or other ubiquitous molecular precursors (Figure 3). In this situation prostate cancers would not require adrenal androgens as substrates. In this respect, Holzerbeierlein et al have shown the enhanced expression of transcripts encoding enzymes in the cholesterol and steroid precursor bio-synthetic pathway in castration resistant cancers versus prostate cancers analyzed from untreated patients (34). We and others have reported that compared to primary prostate tumors, castration-resistant primary cancers and metastases display alterations in the expression of genes encoding many steroidogenic enzymes, including the upregulated expression of FASN, CYP17A1, HSD3B1, HSD17B3, CYP19A1 and UBT2B17 (18, 41). Alterations in the expression of 17β-hydroxysteroid dehydrogenase family members (HSD17B) are of particular interest in view of their potential to be targeted by inhibitory drugs. In general, prostate cancers have been shown to increase expression of HSD17B enzymes that catalyze the reductive conversion of precursors to highly active androgens (e.g. HSD17B3 and HSD17B5 -also known as aldo-keto reductase AKR1C3), and decrease expression of enzymes catalyzing the reverse oxidative reaction (e.g. HSD17B2) (42–44). These changes would produce a shift in tumoral androgen metabolism favoring the synthesis of testosterone and DHT.
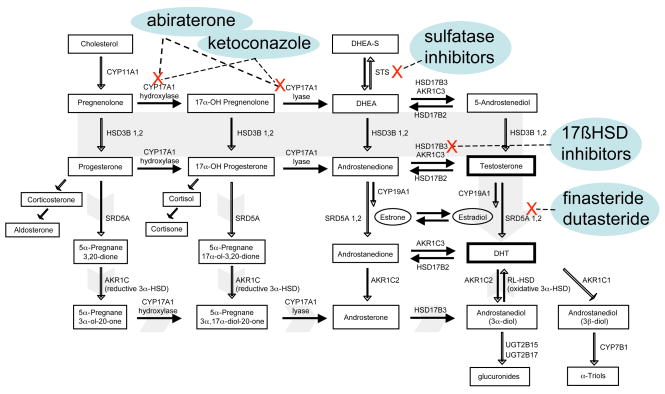
Figure 3. The classical and backdoor pathways of androgen biosynthesis and key enzymes for therapeutic inhibition.
The classical pathway (solid gray arrow) involves the conversion of C21 precursors (pregnenolone and progesterone) to the C19 adrenal androgens DHEA and androstenedione (AED) by the hydroxylase and lyase activity of CYP17A1. Adrenal androgens present in the circulation (including the sulfated form of DHEA, DHEA-S), enter the prostate gland and may be converted to testosterone through a cascade of enzymatic steps modulated by the HSD3B, HSD17B and AKR1C enzymes. Testosterone is subsequently reduced to the potent androgen DHT by members of the SRD5A family. The ‘backdoor’ pathway to DHT formation (short gray arrows) involves the enzymatic conversion of C21 precursors by SRD5A and the reductive 3a-HSD activity of AKR1C family members, with the subsequent metabolism to C19 androgens via the CYP17A lyase activity, followed by conversion to DHT through interactions with HSD17B3 and an oxidative 3a-HSD enzyme.
Intraprostatic androgens may also be produced via a ‘backdoor pathway’, wherein steroid metabolism to DHT bypasses the ‘forward’ or classical molecular intermediates of AED and testosterone (Figure 3). In the classical pathway, C21 steroids such as pregnenolone and progesterone are initially converted to the C19 steroids DHEA and AED via the hydroxylase and lyase activity of CYP17A. These reactions are followed by metabolic reactions involving HSD17B and SRD5A. In cells or tissues expressing both CYP17A and SRD5A, DHT synthesis can occur through the initial reactions of C21 steroids with SRD5A, and subsequently with CYP17A and HSD17B (45).
Though yet to be definitively proven, testosterone and DHT biosynthesis within prostate tumors is possible, either through the utilization of adrenal androgen precursors or through the metabolism of precursors incorporated earlier in the biosynthetic pathway. Tumor-associated molecular alterations in enzymes involved in androgen metabolism, resulting in biologically-relevant concentrations of ligand for AR activation, could explain the failure of castration therapy and afford opportunities for the development of targeted therapeutics.
Implications of Intracrine Androgen Metabolism for Prostate Cancer Therapy
Several approaches have been taken to reduce the contribution of adrenal androgens in the setting of castration-resistant prostate cancer progression. Surgical approaches involving adrenalectomy and hypophysectomy met with limited success and suffered from substantial morbidity. The use of ketoconazole and glutethamide as inhibitors of adrenal androgen biosynthesis also have substantial side effects, but ketoconazole (a weak inhibitor of CYP11A and CYP17A) in particular remains in active use due to the significant benefits exhibited in a subset of patients (46). These activities have prompted the development of additional agents targeting enzymes involved in androgen biosynthesis.
CYP17A catalyzes sequential steps in the conversion of C21 precursors to the C19 adrenal androgens, DHEA and AED. Several CYP17A inhibitors have been developed (see (47), including a subset of compounds exhibiting both CYP17A antagonism and anti-androgen activity (48). Abiraterone, an orally-bioavailable CYP17A inhibitor has been evaluated in phase I studies, and demonstrated substantial activity in castration resistant prostate cancer, even in many individuals progressing despite additional secondary and tertiary hormone manipulations. A report by Ryan and colleagues treated 16 patients with CRPC with abiraterone 500 mg orally per day for 28 days. Of 9 patients refractory to ketoconazole, 5 experienced a >50% decline in PSA (49). A report from Attard and colleagues described a phase I study of abiraterone in which men with metastatic CRPC were treated for up to 14 months. PSA declines of greater than 50% were measured in 21 of the 34 patients. Importantly, of 21 patients evaluable by RECIST criteria, 12 exhibited partial responses and 8 had disease stabilization (50).
The HSD17B3 and AKR1C3 enzymes reduce 17-ketosteroids such as AED and androstanedione to the 17-OH androgens testosterone and DHT. Elevated levels of these enzymes in prostate cancers could contribute to the generation of potent AR ligands serving to maintain cancer cell survival in the setting of low systemic androgen concentrations, and in this context represent potential therapeutic targets. Small molecule inhibitors of HSD17B3 have been developed and tested using in vitro and in vivo systems and have been shown to reduce systemic androgen levels (51, 52). However, to date no studies in prostate cancer have been reported.
The enzyme encoded by the steroid sulfatase (STS) gene hydrolyzes inactive sulfates of estrogen and DHEA to steroids with greater biological activity at the estrogen and androgen receptors. Inhibitors of STS have been evaluated in patients with breast cancer (53) but have yet to be explored as a therapy for prostate cancer. STS enzyme activity has been measured in prostate cancer cell lines and in prostate tissue homogenates (54), and the expression of STS has been found in the majority of localized prostate cancers (55). In clinical applications for castration resistant prostate cancer, STS inhibition could reduce prostatic utilization of the adrenal androgen DHEA, which primarily exists as the inactive sulfate form, DHEA-S.
Conclusions
There is substantial evidence that activation of the AR continues despite current approaches designed to inhibit the AR signaling axis. Continued activation of the AR may play the central role in prostate cancer progression despite the achievement of a clinically defined “castrate” level of androgens, i.e. castration resistant prostate cancer. The marginal (but consistent and positive) additional benefit of adrenal hormone suppression and AR-antagonists could be due to: 1) sub-optimal inhibition of adrenal androgens; 2) active transport or sequestration of androgens by tumor cells; 3) de-novo androgen biosynthesis (e.g. intracrine model); 4) sub-optimal competitive activities of AR-antagonists; and 5) agonist activities of AR-antagonists in the setting of low androgen. In light of these possibilities, a reassessment of the concept of tissue androgen deprivation is warranted. Establishing if intraprostatic androgen levels can be reduced below a threshold resulting in prostate cancer cell apoptosis has never been addressed utilizing assays for tissue androgens and markers for target effect. The lack of correlation between serum and tissue androgen levels mandates that the effectiveness of androgen suppression should be evaluated quantitatively, using tissue rather than serum to establish whether more effective inhibition of hormone concentrations (and AR signaling with anti-androgens) can be achieved. Further studies are required to determine which steroidogenic enzymes represent the most critical targets for inhibition.
Acknowledgments
We thank Stephanie Page, Alvin Matsumoto, William Bremner, David Hess, and Thomas Kalhorn for helpful discussions, and Robert Vessella and the members of the tissue acquisition program at the University of Washington and the patients and their families who agreed to participate in these studies. This work was supported by a Career Development Award from the Prostate Cancer Foundation, a Young Investigator Award from the American Society of Clinical Oncology, and NIH grant 5K23 CA122820-02 (all to E.A.M.); and the NIH/NCI Pacific Northwest Prostate Cancer SPORE grant P50CA97186 (P.S.N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huggins C, Hodges CV. Studies on prostate cancer 1: the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research. 1941;1:293–7. [Google Scholar]
- 2.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 3.Debes JD, Comuzzi B, Schmidt LJ, Dehm SM, Culig Z, Tindall DJ. p300 regulates androgen receptor-independent expression of prostate-specific antigen in prostate cancer cells treated chronically with interleukin-6. Cancer Res. 2005;65:5965–73. doi: 10.1158/0008-5472.CAN-04-2837. [DOI] [PubMed] [Google Scholar]
- 4.Gao H, Ouyang X, Banach-Petrosky WA, Shen MM, Abate-Shen C. Emergence of androgen independence at early stages of prostate cancer progression in nkx3.1; pten mice. Cancer Res. 2006;66:7929–33. doi: 10.1158/0008-5472.CAN-06-1637. [DOI] [PubMed] [Google Scholar]
- 5.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. Epub 2003 Dec 21. [DOI] [PubMed] [Google Scholar]
- 6.Geller J, Liu J, Albert J, Fay W, Berry CC, Weis P. Relationship between human prostatic epithelial cell protein synthesis and tissue dihydrotestosterone level. Clin Endocrinol (Oxf) 1987;26:155–61. doi: 10.1111/j.1365-2265.1987.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Albert J, Geller J. Effects of androgen blockade with ketoconazole and megestrol acetate on human prostatic protein patterns. Prostate. 1986;9:199–205. doi: 10.1002/pros.2990090210. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Geller J, Albert J, Kirshner M. Acute effects of testicular and adrenal cortical blockade on protein synthesis and dihydrotestosterone content of human prostate tissue. J Clin Endocrinol Metab. 1985;61:129–33. doi: 10.1210/jcem-61-1-129. [DOI] [PubMed] [Google Scholar]
- 9.Labrie F, Dupont A, Belanger A, et al. Combination therapy with flutamide and castration (LHRH agonist or orchiectomy) in advanced prostate cancer: a marked improvement in response and survival. J Steroid Biochem. 1985;23:833–41. doi: 10.1016/s0022-4731(85)80024-8. [DOI] [PubMed] [Google Scholar]
- 10.Geller J, Albert J, Nachtsheim D, Loza D, Lippman S. Steroid levels in cancer of the prostate--markers of tumor differentiation and adequacy of anti-androgen therapy. Prog Clin Biol Res. 1979;33:103–11. [PubMed] [Google Scholar]
- 11.Geller J, Albert J, Vik A. Advantages of total androgen blockade in the treatment of advanced prostate cancer. Semin Oncol. 1988;15:53–61. [PubMed] [Google Scholar]
- 12.Geller J. Review of assessment of total androgen blockade as treatment of metastatic prostate cancer. J Endocrinol Invest. 1991;14:881–91. doi: 10.1007/BF03347954. [DOI] [PubMed] [Google Scholar]
- 13.Mohler JL, Gregory CW, Ford OH, 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10:7121–6. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama T, Ikarashi T, Hashimoto Y, Wako K, Takahashi K. The change in the dihydrotestosterone level in the prostate before and after androgen deprivation therapy in connection with prostate cancer aggressiveness using the Gleason score. J Urol. 2007;178:1282–9. doi: 10.1016/j.juro.2007.05.138. [DOI] [PubMed] [Google Scholar]
- 16.Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–41. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 17.Page ST, Lin DW, Mostaghel EA, et al. Persistent Intraprostatic Androgen Concentrations after Medical Castration in Healthy Men. J Clin Endocrinol Metab. 2006;1:1. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 18.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–8. [PubMed] [Google Scholar]
- 21.Culig Z, Hoffmann J, Erdel M, et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer. 1999;81:242–51. doi: 10.1038/sj.bjc.6690684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer. 2004;11:459–76. doi: 10.1677/erc.1.00525. [DOI] [PubMed] [Google Scholar]
- 23.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 24.Labrie F, Dupont A, Belanger A, et al. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin Invest Med. 1982;5:267–75. [PubMed] [Google Scholar]
- 25.Caubet JF, Tosteson TD, Dong EW, et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology. 1997;49:71–8. doi: 10.1016/S0090-4295(96)00325-1. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt B, Bennett C, Seidenfeld J, Samson D, Wilt T. Maximal androgen blockade for advanced prostate cancer. Cochrane Database Syst Rev. 2000:CD001526. doi: 10.1002/14651858.CD001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens): structure-activity relationships. Curr Med Chem. 2000;7:211–47. doi: 10.2174/0929867003375371. [DOI] [PubMed] [Google Scholar]
- 28.Kolvenbag GJ, Furr BJ, Blackledge GR. Receptor affinity and potency of non-steroidal antiandrogens: translation of preclinical findings into clinical activity. Prostate Cancer Prostatic Dis. 1998;1:307–14. doi: 10.1038/sj.pcan.4500262. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg E. Endocrine therapy in the management of prostatic cancer. Clin Endocrinol Metab. 1980;9:369–81. doi: 10.1016/s0300-595x(80)80039-9. [DOI] [PubMed] [Google Scholar]
- 30.Robinson MR, Shearer RJ, Fergusson JD. Adrenal suppression in the treatment of carcinoma of the prostate. Br J Urol. 1974;46:555–9. doi: 10.1111/j.1464-410x.1974.tb03856.x. [DOI] [PubMed] [Google Scholar]
- 31.Harris KA, Weinberg V, Bok RA, Kakefuda M, Small EJ. Low dose ketoconazole with replacement doses of hydrocortisone in patients with progressive androgen independent prostate cancer. J Urol. 2002;168:542–5. [PubMed] [Google Scholar]
- 32.Shearer RJ, Hendry WF, Sommerville IF, Fergusson JD. Plasma testosterone: an accurate monitor of hormone treatment in prostatic cancer. Br J Urol. 1973;45:668–77. doi: 10.1111/j.1464-410x.1973.tb12238.x. [DOI] [PubMed] [Google Scholar]
- 33.Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–33. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 34.Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–27. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med. 2004;22:299–309. doi: 10.1055/s-2004-861547. [DOI] [PubMed] [Google Scholar]
- 36.Belanger B, Belanger A, Labrie F, Dupont A, Cusan L, Monfette G. Comparison of residual C-19 steroids in plasma and prostatic tissue of human, rat and guinea pig after castration: unique importance of extratesticular androgens in men. J Steroid Biochem. 1989;32:695–8. doi: 10.1016/0022-4731(89)90514-1. [DOI] [PubMed] [Google Scholar]
- 37.Mizokami A, Koh E, Fujita H, et al. The adrenal androgen androstenediol is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen receptor. Cancer Res. 2004;64:765–71. doi: 10.1158/0008-5472.can-03-0130. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto H, Yeh S, Lardy H, Messing E, Chang C. Delta5-androstenediol is a natural hormone with androgenic activity in human prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95:11083–8. doi: 10.1073/pnas.95.19.11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh E, Kanaya J, Namiki M. Adrenal steroids in human prostatic cancer cell lines. Arch Androl. 2001;46:117–25. doi: 10.1080/01485010151094010. [DOI] [PubMed] [Google Scholar]
- 40.Harper ME, Pike A, Peeling WB, Griffiths K. Steroids of adrenal origin metabolized by human prostatic tissue both in vivo and in vitro. J Endocrinol. 1974;60:117–25. doi: 10.1677/joe.0.0600117. [DOI] [PubMed] [Google Scholar]
- 41.Montgomery B, Mostaghel E, Vessella R, et al. Androgen synthesis in castration-adapted metastatic prostate cancer. Journal of Clinical Oncology; ASCO Annual Meeeting Proceedings 2007; 2007. p. 98. [Google Scholar]
- 42.Koh E, Noda T, Kanaya J, Namiki M. Differential expression of 17beta-hydroxysteroid dehydrogenase isozyme genes in prostate cancer and noncancer tissues. Prostate. 2002;53:154–9. doi: 10.1002/pros.10139. [DOI] [PubMed] [Google Scholar]
- 43.Ashida S, Nakagawa H, Katagiri T, et al. Molecular features of the transition from prostatic intraepithelial neoplasia (PIN) to prostate cancer: genome-wide gene-expression profiles of prostate cancers and PINs. Cancer Res. 2004;64:5963–72. doi: 10.1158/0008-5472.CAN-04-0020. [DOI] [PubMed] [Google Scholar]
- 44.Fung KM, Samara EN, Wong C, et al. Increased expression of type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer. 2006;13:169–80. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- 45.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15:432–8. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Small EJ, Baron AD, Fippin L, Apodaca D. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J Urol. 1997;157:1204–7. [PubMed] [Google Scholar]
- 47.Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96:1241–6. doi: 10.1111/j.1464-410X.2005.05821.x. [DOI] [PubMed] [Google Scholar]
- 48.Handratta VD, Vasaitis TS, Njar VC, et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J Med Chem. 2005;48:2972–84. doi: 10.1021/jm040202w. [DOI] [PubMed] [Google Scholar]
- 49.Ryan CJ. Phase I evaluation of abiraterone acetate (CB7630), a 17 alpha hydroxylase C17,20-Lyase inhibitor in androgen-independent prostate cancer (AiPC). Journal of Clinical Oncology; ASCO Annual Meeting Proceedings 2007; 2007. p. 5064. Part 1. [Google Scholar]
- 50.Attard G. Phase I study of continuous oral dosing of an irreversible CYP17 inhibitor, abiraterone (A), in castration refractory prostate cancer (CRPC) patients (p) incorporating the evaluation of androgens and steroid metabolites in plasma and tumor. Journal of Clinical Oncology; ASCO Annual Meeting Proceedings 2007; 2007. p. 5063. Part 1. [Google Scholar]
- 51.Berube M, Poirier D. Chemical synthesis and in vitro biological evaluation of a phosphorylated bisubstrate inhibitor of type 3 17beta-hydroxysteroid dehydrogenase. J Enzyme Inhib Med Chem. 2007;22:201–11. doi: 10.1080/14756360601051423. [DOI] [PubMed] [Google Scholar]
- 52.Spires TE, Fink BE, Kick EK, et al. Identification of novel functional inhibitors of 17beta-hydroxysteroid dehydrogenase type III (17beta-HSD3) Prostate. 2005;65:159–70. doi: 10.1002/pros.20279. [DOI] [PubMed] [Google Scholar]
- 53.Stanway SJ, Purohit A, Woo LW, et al. Phase I study of STX 64 (667 Coumate) in breast cancer patients: the first study of a steroid sulfatase inhibitor. Clin Cancer Res. 2006;12:1585–92. doi: 10.1158/1078-0432.CCR-05-1996. [DOI] [PubMed] [Google Scholar]
- 54.Klein H, Molwitz T, Bartsch W. Steroid sulfate sulfatase in human benign prostatic hyperplasia: characterization and quantification of the enzyme in epithelium and stroma. J Steroid Biochem. 1989;33:195–200. doi: 10.1016/0022-4731(89)90294-x. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura Y, Suzuki T, Fukuda T, et al. Steroid sulfatase and estrogen sulfotransferase in human prostate cancer. Prostate. 2006;66:1005–12. doi: 10.1002/pros.20426. [DOI] [PubMed] [Google Scholar]
- 56.Samson DJ, Seidenfeld J, Schmitt B, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer. 2002;95:361–76. doi: 10.1002/cncr.10647. [DOI] [PubMed] [Google Scholar]