Abstract
β1-integrin engagement on normal (NL) CD34+ cells increases levels of the cyclin-dependent kinase inhibitor (cdki), p27Kip, decreases cdk2 activity, and inhibits G1/S-phase progression. In contrast, β1-integrin engagement on chronic myelogenous leukemia (CML) CD34+ cells does not inhibit G1/S progression. We now show that, in CML, baseline p27Kip levels are significantly higher than in NL CD34+ cells, but adhesion to fibronectin (FN) does not increase p27Kip levels. p27Kip mRNA levels are similar in CML and NL CD34+ cells and remain unchanged after adhesion, suggesting posttranscriptional regulation. Despite the elevated p27Kip levels, cdk2 kinase activity is similar in CML and NL CD34+ cells. In NL CD34+ cells, >90% of p27Kip is located in the nucleus, where it binds to cdk2 after integrin engagement. In CML CD34+ cells, however, >80% of p27Kip is located in the cytoplasm even in FN-adherent cells, and significantly less p27Kip is bound to cdk2. Thus, presence of BCR/ABL induces elevated levels of p27Kip and relocation of p27Kip to the cytoplasm, which contributes to the loss of integrin-mediated proliferation inhibition, characteristic of CML.
The β1-integrins are responsible for adhesion of normal (NL) human CD34+ cells to fibronectin (FN) and to VCAM (vascular cell adhesion molecule) (1–4). We have recently shown that engagement of β1-integrins regulates proliferation of NL CD34+ cells (5, 6) by up-regulating p27Kip protein levels and inhibiting cdk2 kinase activity (7). Phosphorylation of the retinoblastoma protein (Rb) by cyclin D-cdk 4/6 and cyclin E-cdk2 controls progression through the G1/S-phase of the cell cycle (8–10). Cdk-inhibitors (11–13), including members of the Cip/Kip family and the Ink4 protein family, negatively regulate the activity of the cdk-cyclin complexes. Binding of p27Kip to cyclin E-cdk2 prevents cells from entering the S-phase of the cell cycle, and binding of p27Kip to cyclin-A-cdk2 prevents passage through the S-phase. p27Kip plays an important role in contact-mediated growth arrest (14). The role of p27Kip in contact inhibition has also been elegantly illustrated in p27Kip–null mice that display generalized increased body size (15). Thus, contact between NL CD34+ cells and their microenvironment inhibits transition from G1 to S, similar to what has been shown in other biological systems (7). Of note, β1-integrin-mediated inhibition of S-phase entry can be overridden by addition of supraphysiological concentrations of a number of cytokines, including IL-3, stem cell factor (SCF), fetal liver-tyrosine kinase-ligand-3 (Flt3-L), and granulocyte–macrophage colony-stimulating factor (GM-CSF) (7). We showed that IL-3 and SCF prevent up-regulation of p27Kip protein levels and suppression of cdk2 kinase activity after integrin engagement.
Chronic myelogenous leukemia (CML) is a malignant disease of the human hematopoietic stem cell characterized by the Philadelphia chromosome (Ph) and the BCR/ABL gene rearrangement (16–18). p210BCR/ABL is necessary and sufficient for the malignant transformation of hematopoietic cells (19–21). Clinically, CML is characterized by abnormal, premature circulation of an expanded immature malignant progenitor population (22). Although integrins are expressed on CML progenitors, adhesion of CML progenitors to FN is significantly decreased (23, 24), and engagement of integrins does not inhibit CML progenitor proliferation (25). The mechanism(s) underlying the loss of integrin engagement-mediated proliferation seen in CML is unknown. We have recently shown restoration of integrin-mediated proliferation inhibition when levels of p210BCR/ABL protein (26) or the p210BCR/ABL kinase (27) are decreased. These findings demonstrate that BCR/ABL is directly responsible for this defect characteristic for CML.
A number of investigators have shown that IL-3 (28) and SCF (29) activate signal pathways that are similar to those activated by the oncoprotein, p210BCR/ABL. Like p210BCR/ABL, IL-3 and SCF override β1-integrin-mediated proliferation inhibition. We therefore examined whether p210BCR/ABL prevents β1-integrin-mediated p27Kip up-regulation and proliferation inhibition in CML, as we have shown for IL-3 and SCF (7). Surprisingly, we found that p27Kip protein levels are elevated in CML CD34+ cells independent of cell adhesion. CML cells continue to proliferate despite the high levels of p27Kip, because the majority of p27Kip molecules are located in the cell cytoplasm where they cannot bind to and inactivate the function of cdks such as cdk2.
Materials and Methods
Reagents.
Adhesive ligands.
Plasma fibronectin (FN), poly-l-lysin (PLL), and BSA (98% pure) were purchased from Sigma.
Cytokines.
IL-3, IL-6, leukemia inhibitory factor (LIF), macrophage-inflammatory protein (MIP-1α) were purchased from R&D Systems; SCF was a kind gift from Amgen Biologicals; fetal liver-tyrosine kinase-ligand-3 (Flt3-L) was a kind gift from Immunex; thrombopoietin was a kind gift from Kirin Brewery Co., Gunma, Japan; GM-CSF was purchased from Immunex, and G-CSF from Amgen.
Antibodies.
Antibodies used in FACS analysis against p27Kip and cyclin-E, FITC-coupled antibodies against cyclin-A and cyclin-D1+2+3, as well as secondary goat anti-mouse-FITC antibodies and isotype control antibodies, were obtained from PharMingen. Anti-CD34-allophycocyanin (APC) or phycoerythrin (PE) were obtained from Becton Dickinson. Antibodies used in Western blot and immunoprecipitation against p27Kip, cdk2, cdk4, and β-actin were obtained from PharMingen. Anti-cyclin-H and anti-human ABL antibodies were purchased from Santa Cruz Biotechnology Inc., Santa Cruz Biotechnology. Secondary goat anti-mouse horseradish peroxidase (HRP)-conjugated antibodies were obtained from PharMingen. The activating anti-β1-integrin antibody, 8A2, was a kind gift from Dr. N. Kovach, University of Washington, Seattle, WA (30).
Low-dose-cytokine-free, serum-free medium.
We used Iscove's modified Dulbecco's medium (IMDM, GIBCO-BRL) containing 20 mg/ml BSA, 10 μg/ml insulin (Sigma), 200 μg/ml transferrin (Sigma), 10−4 M 2-mercaptoethanol (Bio-Rad), 100 units/ml penicillin and streptomycin (GIBCO-BRL). We used the following cytokines: 200 pg/ml GM-CSF, 1,000 pg/ml G-CSF, 200 pg/ml SCF, 50 pg/ml LIF, 200 pg/ml MIP-1α, and 1,000 pg/ml IL-6 (31).
NL and CML CD34+ cells.
All samples were obtained with informed consent obtained according to Guidelines from the Committee for the Protection of Human Subjects at the University of Minnesota. Twelve NL donors, selected by using standard criteria of the American Association of Blood Banks for blood donors, received a daily dose of 10 μg/kg/day G-CSF s.c. for 5 days. On day +6, donors underwent an apheresis procedure as described (32). Mobilized peripheral blood was used, because larger numbers of CD34+ cells can be obtained for studies done. We have previously shown that, after short-term culture in “low dose cytokine” medium as we do here, adhesive and proliferative behavior of normal blood-derived CD34+ cells is equivalent to that from steady state marrow (7). Cord blood samples were obtained from full-term pregnancies by standard procedures used for umbilical cord banking. Marrow or blood was obtained from 15 CML patients with BCR/ABL positive chronic phase disease, which had most recently been treated with hydroxyurea only. Some samples were obtained from marrow harvests. CD34+ cells were obtained by sequential Ficoll Hypaque centrifugation (specific gravity, 1077) (Sigma) and immunoselection by either two passages over the MACS CD34 Isolation Kit (Miltenyi Biotec, Sunnyvale, CA) or sequential selection with the Ceprate SC device for clinical scale stem cell concentration (CellPro, Bothell, WA) followed by the MACS CD34 Isolation Kit. CD34+ populations were >95% pure.
Adhesion and Proliferation Assays.
Adhesion assays to FN- or BSA-coated wells were done by using 51Cr-labeled CD34+ cells suspended in serum-free medium and low doses of cytokines as described (7). In some studies, we added the β1-integrin-activating antibody, 8A2, to increase the fraction of adherent cells (30). We have previously shown that treatment with 8A2 increases CD34+ cell adhesion from CML and NL blood and marrow to FN but not adhesion-mediated proliferation regulation (7, 29). To assess the effect of adhesion on cell cycle status and cell cycle protein levels, CD34+ cells were allowed to adhere to FN or PLL for 12 h. Adherent and nonadherent cells were collected separately, and cell cycle analysis, as well as levels of cell cycle proteins expressed, was evaluated by FACS or Western blot (see below) as described (7).
When we analyzed the cell cycle status of MSCV-eGFP or MSCV-p210-eGFP-transduced cord blood cells, we labeled transduced cells immediately after transduction or after they were recovered from adhesion assays with anti-CD34-APC (Becton Dickinson). Cells were fixed and permeabilized with 1% paraformaldehyde and 80 μg/ml lysolesithin in PBS for 5 min at 4°C. Cells were washed and then incubated with anti-p27Kip antibodies, washed, and incubated with PE-conjugated goat anti-mouse Ig for 30 min. CD34+ eGFP+ cells and CD34+ eGFP− cells were gated, and p27Kip levels were compared. Alternatively, after permeabilization, cells were labeled with 50 ng/ml propidium iodide for 10 min and were cell cycle analyzed by FACS. Data were interpreted by using modfit program.
Western Blotting and Immunoprecipitation.
Protein extracts.
Cells recovered in the adherent and nonadherent fraction of adhesion assays were lysed in Nonidet P-40 lysis buffer, as described (7), and lysates were recovered by centrifugation. Protein was quantitated by using the Bradford assay. To obtain nuclear and cytoplasmic proteins separately, 5 × 106 to 107 CD34+ cells were resuspended in cold buffer [10 mM Hepes (pH 7.9)/1 mM EDTA/60 mM KCl/1 mM DTT/1 mM PMSF/1 μg/ml antipain/1 μg/ml leupeptin/1 μM pepstain A/100 μg/ml chymostatin/1 μg/ml aprotinin, all from Sigma], and incubated on ice for 5 min. Nonidet P-40 was added to a final concentration of 0.1%, the mixture again incubated on ice for 3 min, and spun at 1200 × g for 5 min at 4°C. Supernatants were used to recover cytoplasmic proteins, and the pellets were used to obtain nuclear proteins.
Nuclear protein preparation.
The pellet was washed with lysis buffer without Nonidet P-40 and spun at 1200 × g for 5 min at 4°C. The pellet was resuspended in ice cold nuclear resuspension buffer [250 mM Tris⋅HCl (pH7.8)/60 mM KCl/10% glycerol/1 mM DTT/1 mM PMSF/1 μg/ml antipain/1 μg/ml leupeptin/1 μM pepstain A/100 μg/ml chymostatin/1 μg/ml aprotinin, all from Sigma] and immersed in dry ice/ethanol (Sigma). Nuclei were freeze/thawed three times, the thawed suspension was spun at 7000 × g for 15 min at 4°C, and the supernatant containing nuclear proteins was collected.
Cytoplasmic protein preparation.
Nuclear resuspension buffer with 20% glycerol was added to the supernatant containing cytoplasmic proteins. The suspension was spun at full speed in a microcentrifuge for 15 min at 4°C, and the supernatant containing cytoplasmic proteins was collected.
Western blotting.
Protein lysates were separated by SDS/PAGE and transferred onto nitrocellulose. Blots were probed with anti-p27Kip, cyclin-E, cdk2, cdk4, β-actin, and secondary goat anti-mouse HRP-conjugated antibodies as described (7). Bands were visualized by using an ECL detection system (DuPont). Some of the blots were stripped and reprobed with antibody against other cell cycle proteins or β-actin.
Immunoprecipitation.
Immunoprecipitations were done with protein G-agarose beads (Boehringer Mannheim) as described (7). Antibodies used included anti-cdk2, anti-cdk4, or, as control, polyclonal mouse Ig. Immune-complexes were resolved by SDS/PAGE, and blots were probed with anti-cdk2 or cdk4 antibodies, or anti-p27Kip antibodies, as described above.
cdk2 kinase assay.
cdk2-associated kinase activity was assayed by measuring the ability of cdk2 to phosphorylate histone H1, as described (7).
cdk4 kinase assay.
CD34+ cells were washed with cold PBS and lysed with cdk4 lysis buffer [50 mM Hepes (pH 7.5)/10% glycerol/150 mM NaCl/1 mM EDTA/2.5 mM EGTA/1 mM DTT/0.1% Tween 20/5 mM NaF/0.1 mM sodium orthovanadate/5 μg/ml leupeptin/10 μg/ml aprotinin/50 μg/ml PMSF/5 μg/ml pepstatin A. cdk4-containing complexes were immunoprecipitated by using rabbit polyclonal anti-cdk4 antibody. The cdk4 kinase activity was assayed in 50 μl of glutathione S-transferase (GST)-Rb kinase buffer with 10 μg of GST-Rb, 2 mM EGTA, and 10 μCi of [γ-32P]ATP. Reactions were incubated for 30 min at 30°C, and cold ATP (final concentration, 30 μM) was added. The reaction was stopped by adding Laemmli sample buffer and boiling for 3 min. Reaction products were resolved by SDS/PAGE. The gel was dried and exposed to x-ray film.
Quantitation of protein levels.
Differences between different products on SDS/PAGE gels were evaluated by scanning images by using a GS-700 Imaging Densitometer (Bio-Rad), which were then quantified by using molecular analyst software (Bio-Rad).
RNase Protection Assay.
High-specific-activity biotin-labeled, anti-sense RNA probes were synthesized from the hCC-1 Human Cell Cycle Multiprobe Template Set (PharMingen) by using the MAXIscript In Vitro Transcription Kit (Ambion, Austin, TX). The probes hybridize with target human mRNAs encoding cdk1, cdk2, cdk3, cdk4, p27Kip, p21Cop, PISSLRE, p16INK, and the housekeeping gene products L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Total RNA was extracted from CD34+ cells by using RNeasy Mini Kit (Qiagen, Chatsworth, CA). The labeled probes were hybridized to CD34+ cell-derived RNA in solution (56°C, overnight). Free probe and remaining single-stranded mRNA were digested with RNase at 30°C for 45 min. The remaining “RNase-protected” probes were resolved on denaturing polyacrylamide gels, transferred to a positively charged nylon-membrane by electroblotting, and cross-linked to the membrane. The nonisotopic probe was visualized with Ambion's BrightStar BioDetect Kit, and differences were evaluated by scanning images by using a GS-700 Imaging Densitometer, which were then quantified by using molecular analyst software.
PCR and Sequencing of the p27Kip Gene.
Genomic DNA was prepared from one normal donor and four CML patients by using DNeasy Tissue kit from Qiagen. Primers were synthesized at the Microchemical Facility, University of Minnesota based on the published sequence for p27Kip (33). For exon-1: exon-1a, 5′-AGT-CCA-TTT-GAT-CAG-CGG-AG-3′, 5′-GTC-CGA-CGG-ATC-AGT-CTT-TG-3′ produces a 678-bp fragment; exon-1b, 5′-ATT-CTA-TGG-TTG-GGG-AAG-GGT-3′, 5′-AGT-ACG-AGT-GGC-AAG-AGG-TG-3′ produces a 286-bp fragment; Exon-2, 5′-GTT-TAC-GTT-TGA-CGT-CTT-CTG-AG-3′, 5′-GTT-TTT-TCT-AAT-AAA-GAT-TGT-GTG-TTC-3′ produces a 179-bp fragment. Each 50 μl of PCR reaction contained 0.5 μl Taq polymerase, 0.5 μM of each primer, 0.2 mM dNTP, and 1.5 mM MgCl2 (GIBCO-BRL) using the following cycles: 2 min denaturation at 94°C, 35 cycles for 30 sec denatured at 94°C, 45 sec annealing at 55°C (for exon-1a and exon-2) or at 60°C (for exon-1b), 45 sec extension at 72°C, and 10 min postextension at 72°C. PCR products were purified by using the QIAquick Gel Extraction (Qiagen) kit and sent to the Microchemical Facility for sequencing.
Statistical Analysis.
Results of experimental points obtained from multiple experiments were reported as the mean ± SEM. Significance levels were determined by two-sided Student's t test.
Results and Discussion
Adhesion to FN Does Not Affect CML CD34+ Cell Proliferation.
We have previously shown that CML clonogenic progenitors adhere poorly to FN (23, 24) and that adhesion to FN or direct antibody mediated β1-integrin engagement does not affect the proliferation of clonogenic progenitors in CML marrow (25). Consistent with this finding we now demonstrate that significantly fewer CML CD34+ cells (2 ± 2%, n = 4) than NL CD34+ cells (10 ± 1%, n = 4; as previously reported (7) adhere to FN (P < 0.01). When NL CD34+ cells (43 ± 4.1%, n = 4; as previously reported (7) or CML CD34+ cells (42 ± 3.6%, n = 4) were treated with the β1-integrin activating antibody 8A2, a similar fraction of cells adhered to FN, consistent with previous studies from by our group (30). Because 8A2 does not affect cell proliferation (7), all subsequent studies were done by using 8A2 to increase the fraction of adherent cells.
We examined the effect of adhesion to FN on the cell cycle status of CML CD34+ cells. After culture in low dose cytokine medium 22 ± 3% of CML CD34+ cells (n = 4) were in S phase [similar to that seen for NL CD34+ cells after 48-h culture: 23 ± 3%, n = 4 (7)]. In contrast to NL CD34+ cells, where adhesion to FN causes a 50% decrease in the fraction of cells that is in S-phase (7), the percentage of CML CD34+ cells in the FN-adherent (FN-A) fraction that was in S phase (18 ± 2%, n = 3) was equivalent to that in FN-nonadherent (NA) cells (2 ± 1%), PLL-A (20 ± 3%), or PLL-NA (21 ± 2%) cells.
Adhesion to FN Does Not Affect p27Kip Levels in CML CD34+ Cells.
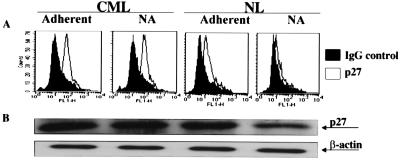
We have published that levels of p27Kip are markedly elevated and that levels of cyclin-E and cyclin-A are lower in NL FN-A CD34+ cells compared with FN-NA or PLL-A (7). We therefore examined by FACS levels of p21Cip, p27Kip, cyclin-A, cyclin-D1+2+3 and cyclin-E in CML CD34+ cells before and after adhesion to FN. We found that no significant differences in the level of p27Kip (Fig. 1A) exist between FN-A and FN-NA CML CD34+ cells. Surprisingly, the baseline levels of p27Kip were higher in CML CD34+ cells compared with NL CD34+ cells (Fig. 1A). Levels of cyclin-A, cyclin-D1+2+3, cyclin-E, or p21 did not change when CML CD34+ cells adhered to FN (n = 3; not shown). Baseline levels of these four cell cycle regulatory proteins were similar in CML or NL CD34+ cells. Results from FACS analysis were confirmed by Western blot. As shown in Fig. 1B, p27Kip levels were significantly higher in CML CD34+ cells than NL CD34+ cells but did not change after adhesion of CML CD34+ cells to FN. Cyclin-E levels remain unchanged when CML CD34+ cells adhere to FN (not shown). Several studies have shown that signal pathways that are activated both p210BCR/ABL are similar to those activated by cytokines, such as IL-3 and SCF (28). However, we show here that the block in adhesion-mediated proliferation inhibition caused by p210BCR/ABL and cytokines is different. Like IL-3 and SCF (7), p27Kip levels in CML CD34+ cells do not change after engagement of integrins, but, in contrast to cytokine-treated cells, levels of p27Kip in BCR/ABL-containing cells are markedly elevated. In contrast, we have previously shown that p27Kip levels are low in IL-3- or SCF-treated NL CD34+ cells whether or not they are adherent to FN (7).
Figure 1.
p27Kip levels are elevated in CML CD34+ cells irrespective of β1-integrin engagement. Freshly selected CML (n = 3) and NL (n = 3) CD34+ cells or adherent and nonadherent cells that had been collected separately 12 h after coculture with FN or PLL (not shown) were analyzed by FACS or by Western blot to determine levels of p27Kip. Results for normal donors have previously been reported (7). (A) Cells were fixed, permeabilized, and stained with anti-p27Kip antibodies (open graph) or control Ig (filled graph). Cells were then incubated with goat-anti-mouse-FITC antibodies. A representative example of three separate experiments is shown. (B) Proteins were extracted from CML or NL CD34+ cells. Proteins were separated by SDS/PAGE. Blots were probed with antibodies against p27Kip, or β-actin as control for loading, and goat anti-mouse HRP-conjugated antibody. A representative example of three separate experiments is shown.
Levels of p27Kip Protein Are Elevated Without Significant Change in p27Kip mRNA Levels.
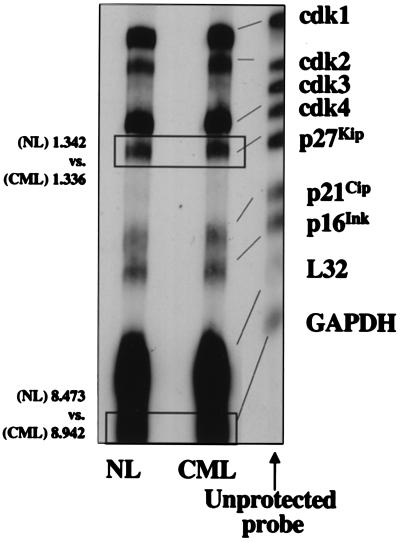
To assess whether increased levels of p27Kip are caused by increased transcription, we examined p27Kip mRNA levels in NL and CML CD34+ cells by RNase protection assay. p27Kip mRNA levels were similar in NL and CML CD34+ cells (n = 3; Fig. 2), and this independent of adhesion to FN (not shown). Thus, regulation of p27Kip levels in CML and NL CD34+ cells may not be due to changes in p27Kip transcription but are to changes in posttranscriptional processing. However, further studies will be needed to prove this notion conclusively. In addition, mRNA levels of cdk2, cdk4, p21Cip, and p16Ink were similar between CML and NL CD34+ cells (Fig. 2) and did not change with adhesion (not shown).
Figure 2.
p27Kip mRNA levels are not significantly different in CML and NL CD34+ cells. High-specific-activity biotin-labeled, anti-sense RNA probes that hybridize with target human mRNAs encoding cdk1, cdk2, cdk3, cdk4, p27Kip, p21Cip, PISSLRE, p16INK and the housekeeping gene products L32 and GAPDH, were synthesized from the hCC-1 Human Cell Cycle Multiprobe Template Set. Total RNA was extracted from CML or NL CD34+ cells and hybridized to the labeled probes. Free probe and remaining single-stranded mRNA were digested with RNase, and the “RNase-protected” probes were resolved on denaturing polyacrylamide gels and transferred by electroblotting to a positively charged nylon membrane. The nonisotopic probe was visualized with Ambion's BrightStar BioDetect Kit. A representative example of three separate experiments is shown. Quantitative differences in protein levels were evaluated by scanning images by using a GS-700 Imaging Densitometer and quantitated by using molecular analyst software. Compared with normal CD34+ cells, levels of p27Kip and GADPH in CML cells were 1.02-fold and 1.05-fold higher (densitometry values added to the figure: values are OD × mm).
Elevated Levels of p27Kip Are Not Associated with Decreased cdk2 Activity in CML.
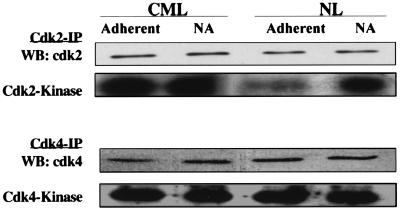
Even though high levels of p27Kip are present in CML CD34+ cells, they proliferate more than NL CD34+ cells, and CML CD34+ cell proliferation is not inhibited by adhesion to FN. To inhibit cell cycle progression, p27Kip must bind to cyclin/cdk complexes and inactivate cdks, including cdk2 and to a lesser extent cdk4. Cell proliferation in the setting of high levels of p27Kip therefore suggests that p27Kip does not inhibit cdk2 and/or cdk4. There is evidence from other transformed cells that increased expression of cyclin-A, cyclin-D, or cyclin-E, or cdks functionally inactivates p27Kip (34). We have no evidence in CML CD34+ cells that cyclin-A, cyclin-D, cyclin-E, or cdk2 levels are elevated (FACS analysis and Western blot; data not shown). A second possibility is that p27Kip binds to the cdks but cannot inactivate the kinase, or does not bind to the cdks and therefore cannot inactivate them. To examine this hypothesis further, we first measured the kinase activity of cdk2 and cdk4 in CML and normal cells. The cdks were immunoprecipitated from FN-A and FN-NA, and PLL-A and PLL-NA CML or NL CD34+ cells and kinase activity was measured. Total levels of cdk2 or cdk4 were not significantly different between CML and NL CD34+ cells (n = 3, Fig. 3). cdk2 and cdk4 activity was similar in PLL-NA and PLL-A CML or NL CD34+ cells (not shown). In contrast to NL CD34+ cells where adhesion to FN inhibits cdk2 but not cdk4 activity, adhesion of CML CD34+ cells to FN did not change cdk2 nor cdk4 activity.
Figure 3.
Elevated levels of p27Kip do not inhibit cdk2 or cdk4 activity. Proteins were extracted from 5–10 × 106 FN-A, FN-NA, PLL-A (not shown), and PLL-NA (not shown) CML or NL CD34+ cells. cdk2 and cdk4 were immunoprecipitated from 500 μg protein by using anti-cdk2 or anti-cdk4 antibodies, or control IgG and protein-G-agarose beads, separated by SDS/PAGE and blots probed with anti-cdk2 or anti-cdk4 and goat anti-mouse HRP antibodies. cdk2 and cdk4 activity was assayed by adding 5 μg histone or GST-Rb and 10 μCi [r-32P] to immune complexes. Reaction products were resolved by SDS/PAGE, and the gel was exposed to x-ray film. A representative example of three separate experiments is shown.
p27Kip Is Relocated in the Cytoplasm of CML CD34+ Cells.
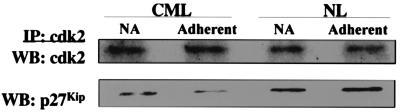
That p27Kip does not inhibit cdk activity could be caused by the inability of p27Kip to bind to and inactivate cdk-cyclin complexes. We therefore evaluated whether p27Kip immunoprecipitates with cdk2. Shown in Fig. 4, despite the higher levels of p27Kip present in CML than NL CD34+ cells, less p27Kip coimmunoprecipitated with cdk2 in CML than NL CD34+ cells. Further, 2- to 3-fold more p27Kip was bound to cdk2 in NL CD34+ cells adherent to FN than in nonadherent cells whereas the amount of p27Kip bound to cdk2 in CML CD34+ cells did not increase after adhesion to FN. Thus, inability of p27Kip to bind to cdk2 underlies the inability of p27Kip to inhibit cdk activity and G1/S progression in CML CD34+ cells.
Figure 4.
Despite the elevated levels of p27Kip, less p27Kip is bound to cdk2 in CML CD34+ cells. Proteins were extracted from 5–10 × 106 FN-A, FN-NA, PLL-A (not shown), and PLL-NA (not shown) CML or NL CD34+ cells. cdk2 was immunoprecipitated from 100 μg of protein by using anti-cdk2 antibodies, or control IgG. To determine the amount of cdk2 present, the immune complexes were separated by SDS/PAGE and blots were probed with anti-cdk2 antibodies and goat anti-mouse HRP-conjugated antibody. To determine the amount of p27Kip bound to cdk2, blots were stripped and reprobed with anti-p27Kip and goat anti-mouse HRP antibodies. A representative example of three experiments is shown.
One possibility would be that, in CML, mutations have occurred in the binding domain, contained within amino acid residues 53–85 that is associated not only with the catalytic activity but also inactivation of cdk activity (35). Western blot analysis demonstrated a similar size of p27Kip in CML and NL CD34+ cells. Likewise, the RNase protection assay demonstrated that the p27Kip probe bound to the target mRNA from CML CD34+ cells, suggesting that no gross abnormalities exist in p27Kip. However, the possibility remained that point mutations in the binding domain may be responsible for the decreased binding of p27Kip to cdk2 in CML. To address this possibility, we PCR amplified p27Kip DNA from CD34+ cells from two normal donors and three patients with CML, followed by sequencing of the PCR products. In all five samples, we detected the polymorphism previously described at codon 109, causing a Val→Gly change, with no known functional consequences (36). Consistent with published observation that the p27Kip and p21Cip of cdki are rarely mutated in human malignancies, no mutations were seen in the cyclin–cdk binding domain (33).
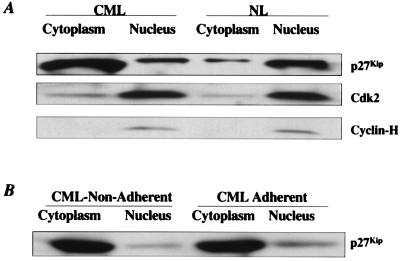
An alternative explanation for the inability of p27Kip to bind to cdk2 would be that p27Kip and cdk2 are not present in the same cell compartment (37–39). We extracted proteins from the cell cytoplasm and nucleus separately, and measured p27Kip, cdk2,and cyclin-H levels by Western blot. Whereas >80% of all p27Kip protein is in the nucleus of NL CD34+ cells, >90% of p27Kip is located in the cytoplasm of CML CD34+ cells (Fig. 5A). In contrast, cdk2, as well as cyclin-H (37), was present in the nucleus in either CML and NL CD34+ cells. After adhesion to FN, p27Kip is not relocated to the nucleus of CML CD34+ cells (Fig. 5B). This is consistent with other models of transformation where a similar dislocation of p27Kip to the cytoplasm has been described (37–39). As we saw in NL CD34+ cells, p27Kip is usually localized in the nucleus. p27Kip contains a nuclear localization site (NLS), which facilitates nuclear entry of p27Kip (34). Sequence analysis of p27Kip in three of three CML patients did not show mutations in the NLS domain. Why the reentry of p27Kip in CML is defective is not known.
Figure 5.
In CML CD34+ cells, >80% of p27Kip is located in the cytoplasm and does not relocate to the nucleus after adhesion of cells to FN. Nuclear and cytoplasmic proteins were isolated separately from 107 CD34+ cells either freshly selected (A), or recovered in the adherent and nonadherent fraction of adhesion assays (B). Proteins present in cytoplasm or nucleus were resolved by SDS/PAGE, and blots were probed with anti-p27Kip antibodies and goat anti-mouse HRP-conjugated antibody. Blots were then stripped and reprobed with anti-cdk2 antibodies and goat anti-mouse HRP-conjugated antibody, and stripped again and probed with anti-cyclin-H antibodies and goat anti-mouse HRP-conjugated antibody. A representative example of three experiments is shown.
Conclusions
We show that, because p27Kip is relocated in the cell cytoplasm where it cannot interact with cdk2 that is located almost exclusively in the nucleus, functional inactivation of p27Kip in CML CD34+ cells is in part responsible for the lack of integrin-mediated growth regulation of BCR/ABL positive CD34+ cells, characteristic for CML. Why the total level of p27Kip in CML cells is elevated remains to be determined. Regulation of p27Kip is complex. Increased transcription, mRNA stabilization, or decreased protein degradation may all lead to elevated p27Kip level (11). We showed by RNase protection assay that transcription of p27Kip is similar in NL and CML CD34+ cells, suggesting that posttranscriptional regulation of p27Kip in CML may be altered. p27Kip elimination involves the ubiquitin-proteasome pathway. Dai et al. showed that, although presence of BCR/ABL triggers the destruction of the Abi proteins, a family of ABL-interacting proteins, through the ubiquitin-proteasome pathway (40), the increased proteasome activity in BCR/ABL-containing cells did not affect other molecules such as IκB-α and p27Kip. We show here that introduction of BCR/ABL causes increased, not decreased, levels of p27Kip. p27Kip degradation needs formation of a p27Kip/cyclin/cdk complex that results in phosphorylation of p27Kip on threonine 187, which is required for the recognition of p27Kip by the ubiquitin ligase (41, 42). In CML CD34+ cells, p27Kip is relocated to the cell cytoplasm, and only a small fraction is bound in cdk2-based complexes. It is thus possible that the majority of p27Kip molecules in CML CD34+ cells cannot be phosphorylated by the cyclin-E-cdk2 complexes, effectively preventing ubiquitin/proteasome degradation (investigation of which warrants further research). Finally, how BCR/ABL causes the relocation of p27Kip will also need further investigation. Likewise, whether BCR/ABL is responsible for relocation and functional inactivation or constitutive activation of other molecules that regulate cell growth or cell survival also needs to be examined.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 HL-49930 and RO1-DK-53673, and the University of Minnesota Bone Marrow Transplant Research Fund. C.M.V. is a Scholar of the Leukemia Society of America.
Abbreviations
- NL
normal
- FN
fibronectin
- Rb
retinoblastoma protein
- SCF
stem cell factor
- CML
chronic myelogenous leukemia
- PLL
poly-l-lysin
- HRP
horseradish peroxidase
- FN-NA
FN nonadherent
- PLL-NA
PLL nonadherent
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190104497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190104497
References
- 1.Verfaillie C, McCarthy J, McGlave P. J Exp Med. 1991;174:693–703. doi: 10.1084/jem.174.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons P, Masinovsky B, Longenecker B, Berenson R, Torok-Storb B, Gallatin W. Blood. 1992;80:388. [PubMed] [Google Scholar]
- 3.Williams D A, Rios M, Stephens C, Patel V P. Nature (London) 1991;352:438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- 4.Papayannopoulou T, Craddock C. Acta Haematol. 1997;97:97–104. doi: 10.1159/000203665. [DOI] [PubMed] [Google Scholar]
- 5.Hurley R W, McCarthy J B, Verfaillie C. J Clin Invest. 1995;96:511–512. doi: 10.1172/JCI118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurley R, McCarthy J, Verfaillie C. Exp Hematol. 1997;25:321–328. [PubMed] [Google Scholar]
- 7.Jiang Y, Prosper F, Verfaillie C. Blood. 2000;95:846–854. [PubMed] [Google Scholar]
- 8.Zhu X, Ohtsubo M, Bàhmer R, Roberts J, Assosian R. J Cell Biol. 1996;133:391. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- 10.Hatakeyama M, Weinberg R. Prog Cell Cycle Res. 1995;1:9–19. doi: 10.1007/978-1-4615-1809-9_2. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd R, Erickson L, Jin L, Kulig E, Qian X, Cheville J, Cheithauer B. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liggett W J, Sidransky D. J Clin Oncol. 1996;16:1197–1206. doi: 10.1200/JCO.1998.16.3.1197. [DOI] [PubMed] [Google Scholar]
- 13.Shiohara M, Koike K, Komiyama A, Koeffler H. Leuk Lymphoma. 1997;26:35–41. doi: 10.3109/10428199709109155. [DOI] [PubMed] [Google Scholar]
- 14.Polyak K, Kato J, Solomon M, Sherr C, Massague J, Roberts J, Koff A. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh D, Nakayama K. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 16.Nowell P, Hungerford D. Science. 1960;132:1497–1501. [Google Scholar]
- 17.Heisterkamp N, Stam K, Groffen J, de Klein A, Grosveld G. Nature (London) 1985;315:758–762. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- 18.Rowley J. Cancer. 1990;65:2178–2184. doi: 10.1002/1097-0142(19900515)65:10<2178::aid-cncr2820651004>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Daley G Q, Van Etten R A, Baltimore D. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 20.Elefanty A G, Hariharan I K, Cory S. EMBO J. 1990;9:1069–1078. doi: 10.1002/j.1460-2075.1990.tb08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heisterkamp N, Jenster G, ten Hoeve J, Zovich D, Pattengale P K, Groffen J. Nature (London) 1990;344:251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- 22.Kantarjian H M, Smith T L, McCredie K B, Keating M J, Walters R S, Talpaz M, Hester J P, Bligham G, Gehan E, Freireich E J. Blood. 1985;66:1326–1335. [PubMed] [Google Scholar]
- 23.Verfaillie C M, McCarthy J B, McGlave P B. J Clin Invest. 1992;90:1232–1241. doi: 10.1172/JCI115985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia R, Wayner E A, McGlave P B, Verfaillie C M. J Clin Invest. 1994;94:384–391. doi: 10.1172/JCI117333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatia R, McCarthy J B, Verfaillie C M. Blood. 1996;87:3883–3891. [PubMed] [Google Scholar]
- 26.Bhatia R, Verfaillie C. Blood. 1998;91:3414–3422. [PubMed] [Google Scholar]
- 27.Bhatia R, Munthe H, Verfaillie C. Leukemia. 1998;12:1708–1717. doi: 10.1038/sj.leu.2401193. [DOI] [PubMed] [Google Scholar]
- 28.Matulonis U, Salgia R, Okuda K, Druker B, Griffin J D. Exp Hematol. 1993;21:1460–1466. [PubMed] [Google Scholar]
- 29.Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. Cell. 1997;88:197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 30.Lundell B I, McCarthy J B, Kovach N L, Verfaillie C M. Blood. 1996;87:2450–2458. [PubMed] [Google Scholar]
- 31.Gupta P, McCarthy J, Verfaillie C. Blood. 1996;87:3229–3236. [PubMed] [Google Scholar]
- 32.Prosper F, Stroncek D, Verfaillie C M. Blood. 1996;88:2033–2042. [PubMed] [Google Scholar]
- 33.Takeuchi S, Koeffler H P, Hinton D R, Miyoshi I, Melmed S, Shimon I. J Endocrinol. 1998;157:337–341. doi: 10.1677/joe.0.1570337. [DOI] [PubMed] [Google Scholar]
- 34.Baldassarre G, Belletti B, Bruni P, Boccia A, Trapasso F, Pentimalli F, Barone M, Chiappetta G, Vento M, Spiezia S, Fusco A, Viglietto G. J Clin Invest. 1999;104:865–874. doi: 10.1172/JCI6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon T, Buchholz M, Nordin A. Biochem Biophys Res Commun. 1996;220:703–709. doi: 10.1006/bbrc.1996.0468. [DOI] [PubMed] [Google Scholar]
- 36.Kawamata N, Morosetti R, Miller C W, Park D, Spirin K S, Nakamaki T, Takeuchi S, Hatta Y, Simpson J, Wilcyznski S, Koeffler H P. Cancer Res. 1995;55:2266–2269. [PubMed] [Google Scholar]
- 37.Sgambato A, Ratto C, Faraglia B, Merico M, Ardito R, Schinzari G, Romano G, Cittadini A. Mol Carcinog. 1999;26:172–179. doi: 10.1002/(sici)1098-2744(199911)26:3<172::aid-mc6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Orend G, Hunter T, Ruoslahti E. Oncogene. 1998;6:2575–2583. doi: 10.1038/sj.onc.1201791. [DOI] [PubMed] [Google Scholar]
- 39.Singh S, Lipman J, Goldman H, Ellis F J, Aizenman L, Cangi M, Signoretti S, Chiaur D, Pagano M, Loda M. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- 40.Dai Z, Quackenbush R, Courtney K, Grove M, Cortez D, Reuther G, Pendergast A. Genes Dev. 1998;12:1415–1424. doi: 10.1101/gad.12.10.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X, Nakano T, Wick S, Dubay M, Brizuela L. Biochemistry. 1999;38:8713–8722. doi: 10.1021/bi9903446. [DOI] [PubMed] [Google Scholar]
- 42.Montagnoli A, Fiore F, Eytan E, Carrano A, Draetta G, Hershko A, Pagano M. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]