Abstract
Despite enormous interest in membrane raft microdomains, no studies in any cell type have defined the relative compositions of the raft fractions on the basis of their major components—sterols, phospholipids, and proteins—or additional raft-associating lipids such as the ganglioside, GM1. Our previous localization data in live sperm showed that the plasma membrane overlying the acrosome represents a stabilized platform enriched in GM1 and sterols. These findings, along with the physiological requirement for sterol efflux for sperm to function, prompted us to characterize sperm membrane fractions biochemically. After confirming limitations of commonly-used detergent-based approaches, we utilized a non-detergent-based method, separating membrane fractions that were reproducibly distinct based on sterol, GM1, phospholipid and protein compositions (both mass amounts and molar ratios). Based on fraction buoyancy and biochemical composition, we identified at least three highly reproducible subtypes of membrane raft. Electron microscopy revealed that raft fractions were free of visible contaminants and were separated by buoyancy rather than morphology. Quantitative proteomic comparisons and fluorescence localization of lipids suggested that different organelles contributed differentially to individual raft sub-types, but that multiple membrane microdomain sub-types could exist within individual domains. This has important implications for scaffolding functions broadly associated with rafts. Most importantly, we show that the common practice of characterizing membrane domains as either “raft” or “non-raft” oversimplifies the actual biochemical complexity of cellular membranes.
Keywords: ganglioside GM1, membrane rafts, sperm, phospholipid, cholesterol
INTRODUCTION
Membrane rafts are defined as small regions of membrane that are enriched in sterols and sphingolipids relative to phospholipids, and which compartmentalize cellular processes (Pike, 2006). Despite recent technological advancements, imaging raft microdomains remains problematic because of their size and dynamic nature. Therefore, many studies of the organization and function of membrane rafts have utilized their resistance to solubilization when incubated at low temperatures with detergents such as Triton X-100 (TX-100) to isolate them biochemically (Brown and Rose, 1992). However, it has been cautioned that these detergent-resistant membranes (DRM) might not precisely represent pre-existing membrane rafts in living cells (Brown, 2006; Edidin, 2003; Heerklotz, 2002; Munro, 2003). That a given protein can demonstrate different solubility patterns with different detergents also underscores the difficulty in assigning physiological relevance to DRM (Drevot et al., 2002; Ilangumaran et al., 1999; Roper et al., 2000; Schuck et al., 2003).
On the other hand, membrane rafts can also be isolated without the use of detergents, on the basis of their buoyancy in density gradients. A discontinuous sucrose gradient of 5%/35%/45% has been widely used to partition the membrane domains, with rafts being collected at the interface between 5% and 35% (Garcia-Marcos et al., 2006a; Garcia-Marcos et al., 2006b). However, significant contamination with non-raft proteins can occur using this methodology (Foster et al., 2003). Although several studies using these isolation methods have been performed to characterize membrane raft proteins, there has been very little information on the comparative biochemical composition of membrane domains in their major lipids – sterols, gangliosides and phospholipids.
Of the cell types in which membrane domain organization has been studied, mammalian sperm are unique regarding the size and stability of the lipid segregations within their plasma membrane (Selvaraj et al., 2006). Although apparently at odds with the concept of nanometer-scale microdomains, larger-scale lipid segregations are consistent with the notion that rafts can be clustered in stabilized platforms (Pike, 2006). The plasma membrane of the sperm head is segregated into a domain overlying the acrosome (APM) and a post-acrosomal plasma membrane (PAPM). These are separated by a feature seen topographically on scanning electron microscopy, known as the “sub-acrosomal ring” (SAR) (Selvaraj et al., 2006). A number of studies using fixed cells showed that the APM was greatly enriched in sterols and that the PAPM had very low amounts of sterols (Friend, 1982; Lin and Kan, 1996; Pelletier and Friend, 1983; Visconti et al., 1999). Previously, we demonstrated in live murine sperm that GM1 localized to the APM domain (Selvaraj et al., 2006), as did caveolin-1 in fixed sperm (Travis et al., 2001b), and that these localizations are highly conserved among mammalian species (Buttke et al., 2006). However, these studies were susceptible to several of the common criticisms of studies of membrane rafts; namely, that they either involved fixed cells, or reagents such as cholera toxin B (CTB), which are capable of cross-linking even in live cell studies. Recent studies in our lab have verified the segregation of focal enrichments of sterols to the APM in live sperm of both human and mouse (Selvaraj et al., in revision). Together, these data support the notion of great enrichment of membrane raft components in the APM of live sperm.
The combined findings of our laboratory [(Buttke et al., 2006; Selvaraj et al., 2006; Selvaraj et al., 2007) and Selvaraj et al., in revision], and other laboratories (Bou Khalil et al., 2006; van Gestel et al., 2005) suggest that the APM itself might not be a single raft, but rather might be dynamic and include co-segregated smaller membrane heterogeneities. This possibility would make the sperm plasma membrane fit with prevailing concepts of the nature of membrane raft domains (Pike, 2006). Yet, even in this context, the APM would still represent an extreme example of a stabilized platform in terms of size and longevity, being maintained at least in part by a diffusion barrier at the level of the SAR (Selvaraj et al., in revision). Considering that sperm are transcriptionally and translationally inactive and are stored in the male tract in a quiescent state, this strategy of stable membrane segregation could be necessary to organize pre-assembled cellular pathways to specific compartments, in which they could perform specific functions later in the female reproductive tract.
In addition to the highly specialized organization of the plasma membrane in the sperm head, sperm also have membrane regions with distinctive lipid compositions in the flagellum. For example, the annulus and flagellar zipper were shown to have extremely low amounts of sterols relative to the midpiece and the rest of the principal piece (Lin and Kan, 1996; Pelletier and Friend, 1983). Moreover, the flagellar zipper was found to be enriched in lectin-binding glycoproteins (Enders et al., 1983). However, these studies also employed the use of fixatives. It is still unclear what functions might underlie such distinct membrane regions, or what mechanisms might exist to allow these large segregations on such stable temporal scales. We have shown previously that at least in part, this maintenance relies on protein disulfide bonds (Selvaraj et al., 2006). Involvement of proteins in lipid segregation is a feature of several models for membrane domain maintenance, including membrane compartmentation and lipid shells (Kusumi et al., 2004)..
Changes in sperm membrane lipids are critically important in conferring functional ability to these cells. Sterol efflux from the plasma membrane is a key stimulus in sperm capacitation, the process that renders them competent to fertilize an egg (Davis, 1976; Visconti et al., 1999). Bicarbonate and calcium influx are believed to be important regulators of phospholipid scramblase activity (Flesch et al., 2001). Upon receiving these signals and undergoing subsequent changes in membrane character, the sperm flagellum acquires a hyperactivated pattern of motility and the APM acquires the ability to fuse with the underlying outer acrosomal membrane in acrosomal exocytosis (Travis and Kopf, 2002).
Several studies have attempted to demonstrate possible roles for membrane rafts in mammalian sperm, pointing to potential roles in capacitation (Thaler et al., 2006), binding to the zona pellucida (Bou Khalil et al., 2006) and acrosomal exocytosis (Tsai et al., 2007). However, we have little information about the biochemical nature of sperm rafts because of the inherent limitations of detergent-based approaches (Kusumi and Suzuki, 2005). Previously, we utilized in murine sperm both detergent-based and non-detergent-based methods to demonstrate the presence of membrane rafts (Travis et al., 2001b). To improve our understanding of the composition of membrane domains in sperm, we now tested a detergent-based method and found it imprecise, and then utilized a non-detergent-based method for a detailed biochemical analysis of sperm membrane fractions. Our findings show that murine sperm had at least 3 distinct sub-types of membrane raft that differed reproducibly in their lipid and protein compositions. Quantitative proteomic comparisons of the fractions, along with fluorescence localization of sterols and GM1 during male germ cell development, suggest that specific organelles contribute differentially to the different raft sub-types. Of greater importance to the broader community interested in the composition and functions of cellular membranes, these data clearly show that binary division of membrane domains into either “raft” or “non-raft” oversimplifies actual membrane organization. Here we demonstrate that murine sperm possess multiple raft sub-types with distinct lipid and protein compositions.
MATERIALS AND METHODS
Reagents and Animals
All reagents were purchased from Sigma (St. Louis, MO), unless noted otherwise. Cholera toxin subunit B conjugated with horseradish peroxidase (CTB-HRP; Invitrogen, Eugene, OR) was reconstituted in PBS supplemented with 1% BSA and the aliquots were stored at −20°C until use. CTB conjugated with AlexaFluor 488 and 647 (CTB-488 and CTB-647) were reconstituted in PBS. The following polyclonal antibodies were used: caveolin-1 (Transduction Laboratories, Lexington, KY), N-ethylmaleimide-sensitive factor (NSF) and syntaxin-2 (Synaptic Systems, Göttingen, Germany), glucose transporter 3 (GLUT 3; Chemicon, Temecula, CA) and α-tubulin (Oncogene, San Diego, CA). A monoclonal antibody against murine erythroid cells was from eBioscience (TER-119, San Diego, CA). Antisera against phospholipase C-δ4 (PLC-δ4) and type I hexokinase (HK 1) were generous gifts from Dr. Kiyoko Fukami of Tokyo University of Pharmacy, and Dr. John Wilson of Michigan State University, respectively. Recombinant domain 4 of perfringolysin O conjugated with EGFP was synthesized and purified as described in our accompanying report (Selvaraj et al., in revision). Retired breeder CD1 mice were from Charles River Laboratories (Kingston, NY). All animal work was performed with the approval of the Institutional Animal Care and Use Committees of Cornell University or the University of Pennsylvania in accordance with the NIH guidelines for the Care and Use of Laboratory Animals.
Preparation of Sperm
Mature sperm were collected from the cauda epididymides of 20–30 mice by a swim-out procedure as described previously (Travis et al., 2001a). All washing steps were performed at 37°C. Sperm were initially centrifuged at 100 × g for 1 min to remove any tissue debris. The supernatants containing sperm were then diluted to 5 ml with PBS and spun at 500 × g for 6 minutes yielding “fluffy” pellets of loosely packed cells. The supernatants containing non-pelleted sperm were collected and kept aside at 37°C. The top of the sperm pellets were then carefully removed without disturbing any contaminating blood cells (if present) that sediment first at the bottom of the tube. Sperm were again diluted to 5 ml using PBS, and centrifuged at 500 × g for 3–6 minutes. The supernatants derived from these steps were combined and centrifuged at 1,000 × g for 10 minutes. The purity of the final sperm pellet was confirmed by both visual examination using a phase-contrast microscope and by specific testing for the lack of immunoreactivity for TER-119, an erythrocyte-specific membrane protein (data not shown; whole blood proteins were used as a positive control). Immunoblots to confirm purity were performed on every cell preparation, and new sperm membrane preparations were made for every fractionation replicate.
Isolation of Sperm Membranes
For both non-detergent-based and detergent-based membrane fractionations, all steps post-washing were carried out on ice or at 4°C, in the presence of 5x protease inhibitors (Complete protease inhibitor cocktail, Roche Applied Science, Indianapolis, IN). In both cases, cell membranes were first separated from other sub-cellular fractions without the use of detergents as described previously (Travis et al., 2001b), with some modification to the intensity of sonication as described here. The sperm (4.8–11.9 × 108 cells) were lysed by dounce homogenization in 4.5–5 ml PBS. The homogenate was then sonicated 10 times for 10 seconds at output 6, and then 10 times for 2 second bursts at outputs 7, 8 and 9 sequentially in a Branson Sonifier 450 (Branson Ultrasonics Corporation, Danbury, CT), taking care both to avoid frothing and to keep the sample cool. The cell lysate was centrifuged at 10,000 × g for 10 minutes and the top 4 ml of supernatant was collected, taking care to avoid any disturbance of the pellet. This supernatant contained both soluble proteins as well as sperm membranes.
Separation of Membrane Fractions Using a Detergent-Based Method
To examine the solubility of various membrane proteins in the presence of different detergents (Brown and Rose, 1992), the supernatant was centrifuged at 50,000 × g for 2 hours, and the pellet representing membrane vesicles and associated proteins was extracted with 0.5% TX-100 or 20 mM CHAPS at 4°C for 15 minutes, as described previously (Travis et al., 2001b). The detergent-soluble and -insoluble fractions were then collected by centrifugation again at 50,000 × g for 2 hours. A protein assay was performed on these fractions (Micro BCA Protein Assay, Pierce, Rockford, IL), and equivalent amounts of protein were then used for SDS-PAGE and immunoblotting as described below. Immunoblots with each antiserum were performed at least three separate times (representing at least three separate membrane preparations) with similar results.
Separation of Membrane Fractions Using a Non-detergent-based Method
To examine the biochemical characteristics of membrane domains partitioned without detergent, membranes were prepared as described above with the exception being that they were centrifuged at 100,000 × g for 1h. The pellet was sonicated at output 2 in TNE buffer (50 mM Tris, 150 mM NaCl, 1mM EDTA, pH 7.35) until it was completely homogenized and dispersed (approximately 30 one-second bursts). The membrane fraction was mixed with 80% sucrose to obtain a 45% (w/v) final sucrose concentration. This mixture (0.2 ml) was placed in the bottom of a tube and overlaid with a 10–30% continuous sucrose density gradient (4 ml) and then centrifuged at 100,000 × g for 28 hours. Fractions (0.4 ml) were collected from the top (designated 1 to 10 from top to bottom) by careful pipetting. The refractive index for each fraction was assessed using an ABBE-3L Refractometer (Thermo Electron Corporation, Waltham, MA) to ascertain the percent sucrose in each fraction and the linearity of the sucrose gradient. The fractions were then stored under nitrogen gas at −80°C to minimize any oxidation until analysis. Upon thawing, protein amount (Micro BCA Protein Assay Kit) and sterol content (Amplex® Red Cholesterol Assay kit, Invitrogen) were quantified based on manufacturer’s instructions. After preliminary trials, fractions 1 to 4, which had the highest buoyant densities and represented relatively low amounts of membrane, were pooled to improve the accuracy of analysis of these fractions. All fractions were then utilized for phospholipid and GM1 analysis as described below.
Phospholipid Analysis
Fractions were diluted with 4 ml TNE buffer and spun down at 275,000 × g for 90 minutes to remove sucrose. To validate that this dilution and ultracentrifugation allowed complete recovery of membranes, the contents of phosphatidylcholine, sphingomyelin and lysophosphatidylcholine in each undiluted versus washed sample were analyzed with an enzymatic assay (Phospholipids B test kit, Wako Chemicals, Richmond, VA; data not shown). An estimate of the amount of all phospholipid species was obtained based on the phosphorus content in the different fractions as described (Zhou and Arthur, 1992). Briefly, pellets were resuspended in 100 μl TNE by sonication and subjected to acid digestion by heating at 180°C for 2 hours with perchloric acid. The released phosphate was then allowed to react with ammonium molybdate and malachite green for 20 minutes, and absorbance was measured at 660 nm using an Ultraspec 2100 Pro (Amersham Biosciences, Piscatway, NJ). For constructing a standard curve, 439 μg/ml KH2PO4 (equivalent to 100 μg/ml of phosphate) was serially diluted to appropriate concentrations. The mass amount of phospholipid in each total fraction was calculated using a conversion factor of 25 as described (Walker, 1986).
GM1 Quantification
For the determination of amounts of GM1 in the different fractions, 20 μl of fractions 1–4, 5, 6, 7, 8, and 9, and 5 μl of fraction 10 were diluted with 130 and 145 μl of PBS, respectively; 10 μl aliquots of these initial dilutions were used to generate duplicate samples by further diluting them with 130 μl PBS in another set of tubes. A standard curve (1.64 × 10−7 to 1.87 μg/ml) was generated using serial dilutions of purified GM1 (Matreya LLC, Pleasant Gap, PA). The duplicate samples and GM1 standards were blotted using a Slot Blot Manifold (Hoefer, San Francisco, CA) by drawing the sample with gentle vacuum pressure onto a PVDF membrane (Immobilon-P, Milipore, Bedford, MA), that had been sequentially presoaked in methanol, Milli-Q water and then PBS. The membrane was removed, dried out, washed in PBS for 1 min, and then blocked with 5% BSA in PBS for 2–3 hours. After blocking, the membrane was incubated for 2 hours with 0.5 μg/ml CTB-HRP in PBS containing 1% BSA at room temperature. Washing to remove unbound CTB was performed 5 times with PBS for 15 minutes each. The GM1 expression was detected by chemiluminescence (ECL, GE Healthcare, Piscataway, NJ) and the resulting bands were scanned using an 8-bit gray scale and quantified by densitometry using Image J 1.36b software downloaded from the NIH website (http://rsb.info.nih.gov/ij/).
Electron Microscopy of Membrane Vesicles
After isolation of membrane fractions by non-detergent-based density gradient centrifugation, the membrane vesicles in 6 fractions (1–4, 5, 7, 8, 9 and 10) were spun down to remove the sucrose as described above. The pellets were resuspended by sonicating 10 times at output 2 for 1 second bursts in 30 μl PBS on ice. Droplets from the different fractions were placed on a dish and formvar/carbon coated 300-mesh copper grids were floated on the droplets for 3–5 minutes. The contents of the fractions adsorbed onto the grids were stained with 2% potassium phosphotungstic acid for 1 minute and then viewed with a transmission electron microscope (Tecnai 12 BioTwin, FEI Company, Hillsboro, OR). This procedure allowed visual inspection to compare size, shape and homogeneity of the membrane vesicles from the different fractions and also to verify that the membranes being analyzed were free from cytoskeletal debris and other cellular contaminants. This electron microscopic assessment was performed on two separate sample preparations with identical results. In addition, several more assessments were performed on the membrane pellet prior to sucrose density gradient centrifugation. These were also found to be free from organelles and had scant cytoskeletal contamination (data not shown).
Preparation of Sterol-Binding Protein
Perfringolysin O (PFO), a sterol-binding cytolysin, binds focal enrichments of membrane sterols through a specific domain (Shimada et al., 2002; Soltani et al., 2007). To label membrane regions having focal enrichment in sterols, we expressed the sterol-binding domain (domain 4) of PFO as a fusion protein with N-terminal EGFP (PFO-D4). All procedures were performed as described in our accompanying report (Selvaraj et al., submitted).
Localization of Lipids in Isolated Round Spermatids and Testis Sections
Male germ cells were prepared as described (Selvaraj et al., in revision). Briefly, decapsulated testes were treated with 0.75 mg/ml collagenase for 30 minutes at 33°C. The separated seminiferous tubules were minced and yielded a cell suspension that was filtered through 70 μm nylon mesh. The cells were incubated on coverslips for 10 minutes and fixed with 4% paraformaldehyde for 10 minutes. The cells were permeabilized with 1% TX-100 at room temperature or methanol at −20 °C for 1 minute. The cells were blocked by incubation with 10% goat serum for 1 hour at room temperature or overnight at 4°C. For dual labelings, the cells were first incubated with PFO-D4 (10 μg/ml) and 10 μM filipin overnight at 4°C and then incubated with CTB-647 and CTB-488 (5 μg/ml) in PBS, respectively. The PFO-D4 labeled spermatids were further incubated with Hoechst 33342 (Molecular Probes) for 2 hours at room temperature. The coverslips were mounted on slideglass using ProLong Gold Antifade (Molecular Probes). We observed no difference in localization or degree of the various probes’ signals when comparing the two methods of permeabilization (data not shown).
For immunohistochemistry, the testis paraffin sections, which were previously sliced at 4 μm thickness, were subjected to deparaffinization and rehydration, and then boiled for 10 minutes in 0.01M citrate buffer for antigen retrieval. The samples were blocked with 10% goat serum in PBS overnight at 4°C and then incubated overnight with CTB-647 at the same concentration used above. Nuclear staining was performed with SYTOX Green Dye (Molecular Probes) according to the manufacturer’s instructions. Coverslips were mounted as above.
Immunoblotting
Proteins from the fractions were extracted by boiling in sample buffer (Laemmli, 1970) and separated by SDS-PAGE. Transfer, blocking and immunodetection of specific proteins were performed largely as described previously (Travis et al., 1998). Dilutions used for the primary antisera were 1:1000 for anti-caveolin-1, PLC-δ4, syntaxin-2, and NSF, and 1:10,000 for anti-HK 1 and GLUT 3. Blocking was performed with 5% (w/v) non-fat milk, except for anti-PLC-δ4 (5% fish skin gelatin) and anti-NSF (1% polyvinyl pyrrolidone and 1% non-fat milk). A 1:5000 dilution was used for the secondary antiserum. Chemiluminescence was utilized to observe immunoreactivity. Image processing and figure preparation were performed using Adobe Photoshop (San Jose, CA) and Canvas software (Deneba, Miami, Fl). Adjustments made were always uniform across a blot or image such that all lanes were treated identically, with no selective enhancement or loss of data.
Preparation of Membrane Fraction Proteins for Quantitative Mass Spectrometry
To determine the relative enrichments of specific proteins between given fractions, we performed proteomic analysis with isobaric tagging relative quantification (iTRAQ). The membrane fractions were prepared as described above, and the buoyancy of each fraction was verified by measuring the refractive index for each fraction.
To increase yield, two membrane fractionations were performed (together representing membranes from 1.74 × 109 total sperm). The proteins in fractions 1–4, 5, 7, and 9 were precipitated with 10% trichloroacetic acid (TCA) alone on ice for 1 hour and then incubated with a 4-fold volume of acetone containing 10% TCA at −80°C overnight. The precipitates were pelleted at 40,000 × g for 2 hour and the pellets washed with acetone 3 times. The supernatants were removed and resultant pellets were dried with a centrifugal evaporator (Jouan RC1010, Jouan, Winchester, VA).
iTRAQ Labeling and Liquid Chromatography-MS/MS Analysis
The 4 samples (~75 μg total protein each) were reduced, alkylated, trypsin digested and derivatized with the 114, 115, 116 and 117 iTRAQ reagents (Ross et al., 2004) according to the standard protocol supplied with the iTRAQ™ kit (Applied Biosystems, Foster City, CA). The combined samples were separated by strong cation exchange (SCX) chromatography using a POROS™ 5-HS SCX cartridge (4 × 15 mm, Applied Biosystems). Samples were applied to the SCX cartridge in 2 ml of load buffer [10 mM potassium phosphate, 25% acetonitrile (ACN), pH 3.0], washed with 1 ml of load buffer, and the pass-through collected. The sample was then eluted using 0.5 ml elution buffer (10 mM potassium phosphate, 375 mM potassium chloride, 25% ACN, pH 3.0), and the eluate was dried by vacuum centrifugation.
Capillary LC-MS/MS analyses of the sample fractions were performed using a Waters CapLC™ chromatographic system and a Waters Q-Tof Premier™ mass spectrometry system (Waters Corporation, Milford, MA). The sample was dissolved in 200 μl of 2% ACN, 0.1% formic acid (solvent A) and utilized for two analyses using 40 μl and 80 μl injections onto a C-8 trapping column (Michrom Bioresources, Auburn, CA) at a flow rate of 3 μl/min of solvent A. The flow was then reversed through the trapping column and a 75 min gradient from 5 – 88% ACN in 0.1% formic acid at a flow rate of 350 nl/min was delivered through a 10 cm × 75μm C-18 Proteoprep 2™, PicoFrit™ column (New Objectives Woburn, MA) connected directly to the nanospray source of the mass spectrometer with 2.7 kV spray voltage applied through the column. The cone was maintained at 35 V. Data dependant acquisition (DDA) was used with 1 full survey scan from m/z 550 –1,500 followed by product ion scans (m/z 50 – 1,500) on the 3 most intense ions from the survey scan. Precursor ions were excluded from reselection for 1 min. Precursor ion charge state specific collision energy programs were used to provide high reporter ion intensity with high product ion sequence coverage. The total time to complete a DDA cycle was 13.4 sec.
Proteins were identified by database searching using the Protein Lynx Global Server™ (PLGS, Waters Corporation) search engine with a February 2007 download of a mouse subset of the IPI protein database. The two mass spectrometric runs were combined for data analysis. Determination of the iTRAQ reporter ion ratios was performed using the Expression Analysis routine in PLGS.
Statistical Analysis
After preliminary trials, data for biochemical analyses were gathered from 9 replications (i.e. 9 separate membrane preparations and corresponding non-detergent fractionations). All data from these analyses were mathematically normalized to the number of sperm (1 × 109 cells). This facilitated comparison between trials, which varied with regard to the number of sperm collected due to individual animal variation and our protocol to remove any contaminating blood cells. For ratiometric comparisons, the amounts of sterol, GM1 and phospholipid were transformed into mole units using their average molecular masses (385, 1545 and 750 Da, respectively). Statistical analysis across groups was carried out using a non-parametric Kruskal-Wallis test, and when significant differences were detected, pairwise comparisons between groups were performed by the method of Conover-Inman (Conover, 1999). In all cases, data are expressed as mean ± SEM. Probability values lower than 0.05 were considered to be significant.
RESULTS
Protein Partitioning Varied With the Nature of Detergent Used
Characterization of membrane rafts based on detergent insolubility has been a commonly used approach when examining the lipid and protein compositions of these membrane domains. Although studies of model membranes have supported the notion that liquid-ordered domains can resist solubilization by detergent (Schroeder et al., 1994), numerous questions have been raised about the physiological relevance of this approach. We carried out experiments isolating DRMs from whole membrane preparations using both TX-100 and CHAPS in order to examine whether the nature of the detergent affected sperm membrane protein partitioning. Equal amounts of protein from fractions representing total membranes, detergent-soluble membranes and DRM were separated by SDS-PAGE and then subjected to immunoblot analysis (Fig. 1). For both detergents used, caveolin-1 expression was exclusively observed in the DRM fraction. Proteins believed to be involved in the fusion of the plasma membrane and outer acrosomal membranes, PLC-δ4, syntaxin-2, and NSF, showed approximately equal distributions between detergent-soluble and -insoluble fractions. Metabolic proteins found in greater abundance in flagellar membranes, HK 1 and GLUT 3, partitioned predominantly into the soluble fractions when TX-100 was used, whereas in the CHAPS-based isolation they were almost equally distributed between the soluble and insoluble fractions. These results indicated that detergent solubility of membrane proteins differed depending on the detergents utilized, consistent with reports in other cell types (Roper et al., 2000; Schuck et al., 2003). Because it is unclear whether these differences represented actual membrane heterogeneities that could be discerned by the biochemical properties of the specific detergents (and if so, what those differences might be), the precise meaning of DRM in relation to actual cellular membranes remains unclear.
Fig. 1.

Comparison of membrane protein solubility when extracted with TX-100 versus CHAPS. Total sperm membranes (lanes A) were extracted using 0.5% TX-100 or 20 mM CHAPS for 15 min at 4°C and then separated into detergent-soluble and -insoluble fractions by centrifugation (lanes B and lanes C, respectively). Equal amounts of protein were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antisera. Note the difference in partitioning of GLUT 3 between the two detergents.
Biochemical Characteristics of Membrane Fractions Isolated Without Detergents
We previously demonstrated that sperm membrane vesicles isolated without detergents could be separated based on buoyancy by means of centrifugation on a continuous sucrose density gradient (Travis et al., 2001b). Using this method, we showed that sub-populations of caveolin-1 migrated with the most buoyant fractions (Travis et al., 2001b). It should be noted that the non-detergent methodology used was designed to yield very pure buoyant fractions. However, because detergent, pH, or salt extraction was not used at any stage, it is possible that associated protein complexes might have changed the apparent density with which a fraction migrated. In such cases, the low buoyancy, bottom (non-raft) fractions might contain rafts with associated protein complexes. This approach was chosen because purity of putative raft fractions was our target, and any errors associated with this approach would be conservative.
Using this methodology, we separated membrane fractions by their equilibration on a 10–30% linear sucrose density gradient. Each of the 10 fractions collected were assessed using refractometry to confirm the reproducibility of sucrose concentrations and the linearity of the gradients (Fig. 2A). Consistent with our previous results (Travis et al., 2001b), caveolin-1 migrated upward with the most buoyant fractions whereas α-tubulin was only present in the bottom fraction (Fig. 2F). This pattern of caveolin-1 partitioning throughout the gradient differed dramatically from our results using detergent extraction in Fig. 2. As will be seen below, this discrepancy likely reflects some combination of the presence of caveolin-1 in different sub-types of membrane rafts and the presence of rafts in the bottom, non-raft fractions; and/or highlights an artifactual effect of detergent on the membranes.
Fig. 2.
Quantitative biochemical characterization of membrane fractions isolated from murine sperm without detergent. Fractions of sperm membranes were separated based on their relative buoyancies. The fractions were collected from the sucrose density gradient and numbered 1–10, with fraction 10 representing the highest density, bottom fraction. Measurement of refractive indices of the fractions showed a highly reproducible and highly linear separation (A; mean values: fx1--11.2%, fx2--12.5%, fx3--14.6%, fx4--17.3%, fx5--20.2%, fx6--23.2%, fx7--26.1%, fx8--28.5%, fx9--30.9%). Quantification of mass amounts of sterol (B), GM1 (C), phospholipid (D) and protein (E) content were performed on 7 membrane fractions as described, and the results were normalized in each trial to 1 × 109 sperm. All data were expressed as mean ± SEM (n=9–10). The different letters denote significant differences between the fractions where found (P < 0.05). Immunoblotting showed a representative distribution of caveolin-1 and α-tubulin through the gradient. Caveolin-1 was present in high abundance in fraction 10, but is not shown in this panel to get a clear image of the neighboring lane.
Because of their low abundance, we pooled the most buoyant fractions, 1 to 4 (fx1-4) to bring them within linear analytical range. Although this risked missing additional raft sub-types that might exist in the most buoyant fractions, this step was required for analytical accuracy. We then quantified the amounts of sterol, GM1, phospholipid (PL) and protein in each of the resulting 7 membrane fractions. The mean values for mass amounts of both sterols and PL were lower in the top fractions as buoyancy increased (Fig. 2B and 3D, P < 0.05). However, the mean values for mass amounts of GM1 showed a more complex distribution, with higher peaks in fx7 and fx1-4 than nearby fxs 5 and 8, respectively (Fig. 2C). Mean values for mass amounts of protein were significantly lower in fxs 1 to 8 compared to 9 and 10, but there were no significant differences among fxs 1 to 8 (Fig. 2E).
Fig. 3.
Ratiometric comparisons of the components of the membrane fractions isolated from murine sperm without detergent. After quantitative analyses, mass amounts were converted into mole units using the average molecular masses. The ratios of sterol/phospholipid (A), GM1/phospholipid (B) and GM1/sterol (C) were estimated. Panel A contains the ratios for sterol/phospholipid for the various fractions. Note that the ratio of GM1/phospholipid in fx1-4 (0.44) and fx 7 (0.25) were higher than in the other fxs (which ranged from 0.03–0.07). A ratio of total protein/total lipid was estimated using the sum of phospholipid plus sterol as an estimation of total lipid in each fraction (D). Because of variation in molecular masses between different proteins and species of lipids, this final ratiometric comparison was made using mass amounts. Note that the total protein/total lipid ratio was higher in fx -4 (10.4) than in other fxs (which ranged from 2.2–4.2). The broken line in each panel demarcates the boundary between raft and non-raft fractions typically used when separating membrane fractions by buoyancy on a step gradient and also reflects the point of a 1:1 molar ratio of sterol:PL. This boundary was consistent with the data shown here, in that fractions that were more buoyant (fractions 1–7) had enrichments of sterols and/or GM1 relative to phospholipid. All results were expressed as mean ± SEM. The different letters denote significant differences between the fractions where found (P < 0.05).
The mass amounts of lipids and proteins in the different fractions potentially reflected some combination of both differing amounts of membrane vesicles having a given buoyancy, as well as differences in the compositions of the vesicles between given fractions. To better understand the biochemical nature of the vesicles in the different fractions, we performed a ratiometric comparison of sterols, GM1, and PL on a molar basis (Fig. 3A–C). The sterol to PL ratio was higher in the more buoyant fxs although no statistically significant differences were observed between fx1-4 to fx7 (Fig. 3A). However, the GM1 to PL ratio in fx1-4 and fx7 were higher than fx5, fx8, fx9 and fx10 (Fig. 3B, P < 0.05). A similar pattern of ratios was observed when comparing GM1 to sterols (Fig. 3C). Of note, the ratio of mass amount of protein to that of total lipid (estimated by PL plus sterol) was higher in fx1-4 than any others (Fig. 3D, P < 0.05).
These results demonstrated that the buoyant fractions of murine sperm possessed at least 4 types of membrane domains with different compositions: fx1-4 showed high sterol/PL, high GM1/PL, and high protein/total lipid ratios. Fractions 5 and 6 had high sterol/PL but lower GM1/PL and protein/total lipid ratios. Fraction 7 had a high GM1/PL ratio, but a lower enrichment in sterols and low protein/total lipid ratio. Fractions 8 and 9, consistent with their partitioning as low-buoyancy non-rafts, had low sterol/PL and GM1/PL ratios, and also had a low protein/total lipid ratio. Together, these results show that sperm had membrane domains reproducibly separable based on buoyancy, and that three distinct sub-types of domain existed that all had buoyancies and lipid compositions consistent with membrane rafts.
Morphological Characteristics of Membrane Vesicles Fractionated Without Detergents
We used negative staining and transmission electron microscopy to assess the morphology of the membrane vesicles that were separated by differences in buoyant density without detergents. Four fractions were negatively stained, including three fractions representing the different sub-types of the more buoyant vesicles (pooled fx1-4, fx5 and fx7) and a fraction having lower buoyancy (fx9). The membrane vesicles in the most buoyant membrane fractions (fx1-4 and fx5) showed a relatively homogenous size (100–250 nm diameter) compared to the vesicles in fx7, which displayed a slightly wider range of vesicle sizes (30–200 nm diameter; Fig. 4A, B and C). However, the least-buoyant fraction imaged, fx9, showed the highest incidence of smaller vesicles, although the vast majority still fell within the overall size range seen in the other fractions (30–200 nm diameter; Fig. 4D). Rare amounts of contaminating cytoskeletal elements were detected in fxs 8 and 9 whereas none of the more buoyant fractions had such contaminants (data not shown). These results demonstrated that the most buoyant membrane vesicles were slightly more homogenous as populations when compared with less buoyant fractions. Importantly, these results also demonstrated that upward migration through the density gradient was not correlated with their size/morphology, and that their flotation patterns truly reflected their composition, verifying the quality of this separation protocol for future proteomic and lipidomic analyses.
Fig. 4.
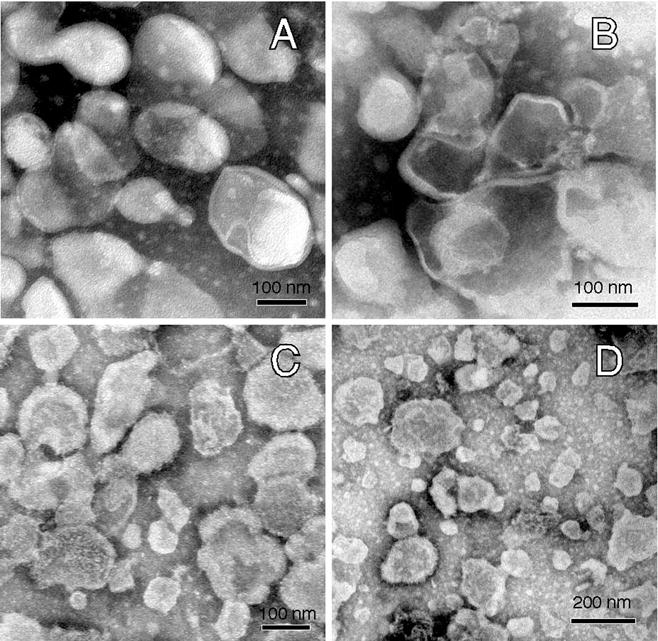
Morphological examination of membrane vesicles found in the various fractions obtained without detergent. Membrane vesicles from the 4 fractions analyzed were negative stained and subjected to electron microscopy as described. Images of vesicles from 4 fractions are depicted: pooled fractions 1–4 (A); fraction 5 (B); fraction 7 (C); and fraction 9 (D). Morphologically, the vesicles were similar in size in all fractions, with fraction 9 having increased abundance of smaller vesicles. Contamination of vesicles with other discernible sub-cellular structures was not visible in fractions 1–7; rare linear structures consistent with fragments of cytoskeletal elements of the sperm flagellum were seen in fraction 8, and these increased slightly in frequency in fraction 9.
Quantitative Proteomics of the Membrane Fractions
Based on our finding that membrane rafts in sperm are heterogeneous in composition, we performed mass spectrometry combined with iTRAQ on the three raft sub-types (fx1-4, 5, and 7) and a non-raft fraction (fx9). This unbiased approach allowed us to identify specific proteins, as well as their relative abundance between the fractions, affording insight into both potential functions and sub-cellular localizations of the different membrane domain sub-types. Database searching of the iTRAQ data matched 54 MS/MS spectra to a particular peptide sequence in a total of 25 proteins that were identified. The relative abundance of proteins was similar to that of the corresponding peptides obtained from reporter ion intensity analysis, verifying the accuracy of this quantification (data not shown).
We have used iTRAQ to identify and quantify the major proteins in these fractions. In separate studies, we are undertaking a shotgun proteomic analysis of the fractions to characterize more thoroughly the proteomes of each fraction. For our purposes in this manuscript we shall focus our attention on the eleven proteins for which a given peptide was found in all fractions and the presence of which has been demonstrated by previous reports in murine sperm (Cesario and Bartles, 1994; Eberspaecher et al., 1995; Ekstedt et al., 2004; Gibbons et al., 2005; Li et al., 1989; Radhakrishnan et al., 1992; Stein et al., 2006; Travis et al., 1998; Urner and Sakkas, 1999). Of these 11 proteins, SOSUI software analysis suggested that 7 had transmembrane helices (Hirokawa et al., 1998). Of the remaining 4, carbonic anhydrase 4 (CA-4), phosphatidylethanolamine binding protein 1 (PEBP-1) and HK 1 are known to associate with or localize to membranes (Ekstedt et al., 2004; Gibbons et al., 2005; Sleight et al., 2005; Travis et al., 1998; Travis et al., 1999).
Several of these proteins, including CA-4, cysteine rich secretory protein 1 (CRISP-1), PEBP-1, L lactate dehydrogenase C chain (LDH-C4), and CUB and zona pellucida-like domain containing protein 1 were relatively enriched in at least one of the raft sub-types. Interestingly, CA-4 was similarly enriched in fx1-4 and fx7, which both have relatively high levels of GM1, but was not enriched in fx5 that was enriched in sterols but had relatively lower GM1. Furthermore, CRISP-1, LDH-C4, and CUB and zona pellucida-like domain containing protein 1 were relatively enriched in fx1-4 and 5, which are both enriched in sterols. In contrast to this, PEBP-1, which is known to be involved in sperm decapacitation and has been reported to localize throughout the head and flagellum (Gibbons et al., 2005), was relatively uniformly enriched in all the buoyant fractions.
Not all proteins were enriched in the raft sub-types. Both the alpha and beta chains of the sodium-potassium transporting ATPase were found to be slightly enriched in non-raft fx9, and had remarkably consistent relative abundances in the different fractions serving as in internal control for the efficiency of the tagging. GLUT 3, HK 1, basigin, and epidermal growth factor precursor were all more notably enriched in the non-raft fraction as well. The fractionation data for both HK 1 and GLUT 3 correspond well with the detergent-based data from TX-100, providing additional support for this finding. These results suggest potential differences in protein targeting leading to functional differences between non-raft and raft fractions, as well as among raft sub-types, further underscoring the importance of not grouping buoyant membrane domain sub-types when investigating their potential functions.
Localization of GM1 and Sterol in Murine Germ Cells
The very close approximation of the plasma membrane and outer acrosomal membrane in mature sperm makes distinguishing localization to one or the other of these membranes difficult, even at the level of electron microscopy. Therefore, it is often beneficial to look at earlier stages of male germ cell development when there is more distance between these membranes. In an accompanying manuscript, we demonstrate that GM1 was highly enriched in the membranes of the developing acrosomal vesicle in early acrosome phase round spermatids (Selvaraj et al., in revision). Consistent with these data, we found using testis paraffin sections that the GM1 was highly abundant in the acrosomal membranes of golgi phase (Fig. 5A, a–c), cap phase (Fig. 5A, d–f) and acrosome phase (Fig. 5A, g–i) spermatids, whereas pachytene spermatocytes did not have membranes enriched in GM1 (Fig. 5A, f and i). Although GM1 was also abundant in specific regions of condensed spermatids (Fig. 5A, c), the membrane(s) to which GM1 localized were not identifiable in those cells because of the close apposition of the plasma and acrosomal membranes. However, in all these cells, the membranes enriched in GM1 were not recognized by PFO-D4, which recognizes focal enrichments of sterols (Fig. 5B). In contrast, this probe identified structures at the opposite pole consistent with membranous organelles, including the Golgi (Moreno et al., 2000a; Moreno et al., 2000b). Supporting this result, we further utilized filipin, a reagent that complexes with membrane sterols, to localize sterols s in round spermatids. We obtained a similar localization pattern to that seen with PFO-D4 (Fig. 5C). Our consistent results using PFO-D4 and filipin suggest that the acrosomal membranes were highly enriched in GM1 but not sterols, which is in accord with previous reports in the literature regarding the relatively low abundance of sterols in the acrosome (Toshimori et al., 1985; Toshimori et al., 1987). Together, our data suggest that acrosomal membranes contributed to fx7.
Fig. 5.

Localization of GM1- and sterol-enriched membranes in murine germ cells. Testis paraffin sections were subjected to antigen retrieval, and then labeled with CTB-647. SYTOX Green Dye was used for nuclear staining (A, a, d, and g). GM1 localized to the acrosomal membranes in round spermatids of golgi phase (A, b–c), cap phase (A, e–f), and acrosome phase (A, h–i), but was not observed in pachytene spermatocytes (A, f and i). GM1 enriched membranes were also observed in condensed spermatids (arrowheads, A, a–c). Focal enrichments of sterols (B) were localized using PFO-D4 to a structure at the opposite pole from the acrosomal membranes, consistent with the golgi in location and appearance (compare B, a–b). Hoechst 33342 was used for nuclear staining (B, c). The relative lack of co-localization of GM1 and sterols in the membranes of the developing acrosome were corroborated using CTB-488 and filipin (compare C, a–b). Merged images of CTB and sterol probes are shown in (B, d and C, c). DIC images of these cap phase spermatids are shown in (B, e and C, d). The bar is 10 μm in all panels.
DISCUSSION
Our findings demonstrate that binary division of biological membranes into “rafts” and “non-rafts” can markedly over-simplify their actual organization. The isolation of DRM using TX-100 at low temperature has been widely used for this purpose. In this study, we compared TX-100 and CHAPS because both detergents have been suggested to be relatively selective when isolating DRM proteins, and because they represent different classes of detergent (Pike, 2004; Schuck et al., 2003). Consistent with the current controversies surrounding DRM, we found great discrepancy in GLUT3 solubility between the detergents. It has been suggested that such discrepancies could reflect different raft populations (Pike, 2004). However, variation in partitioning of individual proteins between detergents is not a rigorous method of defining membrane domains, because such variations are unpredictable. The use of detergents can be used to identify proteins as “candidates” for localizing to rafts (Kusumi and Suzuki, 2005), but is not in and of itself sufficient to perform a biochemical characterization that might allow identification of potential raft sub-types.
Non-detergent-based methods have been suggested to yield native membrane raft fractions that more closely reflect the composition of pre-existing domains in live cells (Pike, 2003). Yet even using non-detergent methods, identification of rafts based on sterol abundance in a given membrane fraction can be flawed if there is not consideration of other lipids in that fraction. For example, increased “raft molecules” (e.g. sterols) in a fraction might indicate more true rafts, or might simply reflect more total membrane in a fraction (Subczynski and Kusumi, 2003). Similarly, comparisons of lipids to total protein in a fraction can be flawed because as seen here, protein:lipid ratios can vary between membrane domains, including between raft sub-types. Pooling of fractions is sometimes unavoidable because of analytical limitations, but can also mix (and mask) domain sub-types. Such considerations limit the interpretations one can draw from the few other biochemical analyses of membrane domains separated without detergent (Gaus et al., 2005; Pike et al., 2002; Pike et al., 2005). Therefore, we performed absolute and molar ratiometric comparative analyses of sterols, GM1, PL, and protein in each fraction. This is the first report of this approach, and coupled with our proteomic data, it revealed the presence of three distinct raft sub-types having both reproducible lipid and protein compositions. This approach and methodology should prove useful to other cell systems.
There is no refractive index value that by convention distinguishes rafts from non-rafts. Membrane rafts isolated from human macrophages without detergent had densities between 1.06–1.09 g/ml [16–23% (w/v) sucrose], based on distribution of raft and non-raft proteins (Gaus et al., 2005). Although our studies differ in several respects, our finding that sperm membrane rafts partitioned within sucrose densities of 11–26 % (w/v) is relatively similar. Our determination of the cut-off was based on both consistency with the practice of the step gradients most commonly used, and on the molar ratio of sterols:PL (1:1). In comparison with other studies, our immunoblot result that caveolin-1 was recovered through all fractions is quite interesting. This might be due to cell-type dependent differences in the molecular organization of membrane domains. Supporting this idea, in macrophages, 40% of lipids (sterol+phospholipid) were found in the raft fractions, whereas we found that only 9% of total lipids partitioned into the raft fractions in sperm.
Quantitative mass spectrometry is potentially a powerful tool to compare the complex profiles and relative abundances of proteins among membrane domain sub-types (Foster and Chan, 2007). In this study, we focused on 11 known sperm proteins that were found in all 4 fractions analyzed. Of the fertilization-associated proteins, PEBP-1, CRISP-1 and CA-4 are added to sperm during epididymal transit (Ekstedt et al., 2004; Jones and Hall, 1991; Rochwerger and Cuasnicu, 1992), suggesting an important targeting/docking function for rafts in sperm epididymal maturation. PEBP-1, which localizes to the plasma membrane overlying the apical acrosome (AA) and to the principal piece (Gibbons et al., 2005), was enriched in all raft sub-types (fx1-4, 5 and 7). This glycoprotein suppresses tyrosine phosphorylation at the membrane level, and is removed during sterol efflux in murine sperm (Nixon et al., 2006). Because sterol efflux is required for capacitation in both the head and flagellum, it is consistent that raft sub-types were found in both regions. CRISP-1 has been suggested to be involved in multiple stages of sperm function, including zona pellucida adhesion and gamete fusion (Busso et al., 2007; Cohen et al., 2007; Roberts et al., 2007). This glycoprotein, shown to localize to the AA (Cohen et al., 2000), was only enriched in fx1-4 and 5. In conjunction with our findings on the localization of focal enrichments of sterols (Selvaraj et al., in revision), this suggests that the AA contributed to fx1-4 and 5.
Of note, CA-4 was relatively enriched in fx1-4 and fx7, which were both highly enriched in GM1. This GPI-anchored carbonic anhydrase is known to catalyze reversible hydration of CO2/bicarbonate. Whether bicarbonate enters sperm by means of a sodium/bicarbonate co-transporter (Demarco et al., 2003), or it crosses the membrane as CO2 (Carlson et al., 2007), the partitioning of this protein to these fractions suggests their involvement with the stimulation of capacitation by bicarbonate. In addition to suggesting potential functions for raft sub-types, our results can be used as a point of reference for understanding how the membrane microenvironment might influence protein function. If used for this purpose, the differences in lipid content among domain sub-types might prove to be of significance.
An unexpected finding was the composition of fraction 7, which was greatly enriched in GM1 but much less so in sterols. Recently, our studies have shown that in developing male germ cells and mature sperm, the APM and the acrosomal membranes are enriched in GM1 [(Selvaraj et al., 2006) and Selvaraj et al., in revision]. In the accompanying study, we also confirmed using PFO-D4 that multiple sterol-enriched microdomains are segregated to the APM (Selvaraj et al., in revision). Therefore, we localized both GM1 and sterols in spermatids at several stages of spermiogenesis in order to examine the relative lipid compositions of acrosomal and other membranes. Unlike the APM, the acrosomal membranes did not have enrichments of sterols, suggesting that acrosomal membranes might contribute significantly to fraction 7. Supporting this idea, our shotgun proteomic analyses of the raft sub-types provide evidence that fraction 7 contains many vesicular-fusion associated proteins (Asano et al., manuscript in preparation).
The process of acrosomal biogenesis shares several characteristics with secretory granule formation in endocrine and exocrine cells. For example, the trans-golgi network (TGN) is the origin of membrane vesicles that contribute both to acrosomal development in spermatids (Burgos and Gutierrez, 1986; Griffiths et al., 1981; Ho et al., 1999; Huang and Ho, 2006), and to immature secretory granules in somatic cells (Schuck and Simons, 2004; Tooze, 1998). In epithelial cells, the TGN is suggested to be an important site for the packaging of specific subsets of lipids and proteins into membrane vesicles for targeted membrane trafficking (Schuck and Simons, 2004; Zegers and Hoekstra, 1998). Our localization data and biochemical analyses suggest that acrosomal membranes selectively retain GM1 in higher amounts than sterols. Such targeted trafficking and selective retention of membrane lipids can lead to polarized distributions of proteins and lipids (Matter, 2000; Yeaman et al., 1999), and different domain types and sub-types. Viewed from this perspective, the extraordinary compartmentalization of sperm membrane domains into not only specific sub-cellular organelles, but also into large domains and microdomains within specific membrane domains, suggests that investigations of membrane trafficking in these cells will be of great interest.
In summary, our results provide one of the first absolute and ratiometric comparative analyses of sterols, GM1, PL, and protein in membrane domains isolated by non-detergent-based methods in any cell type. They clearly demonstrate that sperm have at least three sub-types of membrane raft, showing that the common division of biological membranes into raft and non-raft fractions does not represent actual membrane complexity. These sub-types can reflect preferential contributions from specific sub-cellular organelles, and yet, single domains can contain multiple membrane microdomain sub-types. Biochemical, comparative proteomic, and cell biological data combine to show that the raft sub-types are positioned to be involved in several important aspects of sperm function, including acting as targets for the addition of epididymal proteins and acting in the regulation of capacitation. Using biochemical, proteomic and cytochemical approaches, we now demonstrate that murine sperm possess multiple raft sub-types with different lipid and protein compositions.
Table 1.
Quantitative mass spectrometric analysis of membrane fractions1
| Gene ID | Description | Relative abundance2 |
Pep3 | Tm4 | Ref5 | |||
|---|---|---|---|---|---|---|---|---|
| 1–4 | 5 | 7 | 9 | |||||
| ENZYME | ||||||||
| 12351 | Carbonic anhydrase 4 | 1.18 | 0.89 | 1.21 | 0.85 | 1 | No | Ekstedt et al., 2004 |
|
| ||||||||
| TRANSPORTER | ||||||||
| 232975 | Sodium potassium transporting ATPase alpha 4 chain | 0.69 | 0.69 | 1.02 | 1.17 | 4 | Yes | Stein et al., 2006 |
| 11933 | Sodium potassium transporting ATPase subunit beta 3 | 0.64 | 0.73 | 1.04 | 1.17 | 1 | Yes | Stein et al., 2006 |
| 20527 | Solute carrier family 2 facilitated glucose transporter member 3 | 0.56 | 0.77 | 0.86 | 1.30 | 5 | Yes | Urner and Sakkas, 1999 |
|
| ||||||||
| FERTILIZATION | ||||||||
| 11571 | Cysteine rich secretory protein 1 | 1.05 | 1.22 | 0.91 | 0.96 | 2 | Yes | Eberspaecher et al., 1995 |
| 12215 | Basigin isoform 2 | 0.27 | 0.39 | 0.95 | 1.45 | 1 | Yes | Cesario and Bartles, 1994 |
| 23980 | Phosphatidylethanolamine binding protein 1 | 1.15 | 1.08 | 1.10 | 0.87 | 1 | No | Gibbons et al., 2005 |
|
| ||||||||
| GLYCOLYSIS | ||||||||
| 15275 | Hk1 protein | 0.44 | 0.56 | 0.98 | 1.33 | 12 | No | Travis et al., 1998 |
| 16833 | L lactate dehydrogenase C chain | 1.34 | 1.47 | 0.84 | 0.85 | 1 | No | Li et al., 1989 |
|
| ||||||||
| PHOSPHORYLATION | ||||||||
| 13649 | Epidermal growth factor receptor precursor | 0.59 | 0.71 | 0.54 | 1.50 | 1 | Yes | Radhakrishnan et al., 1992 |
|
| ||||||||
| UNCLASSIFIED | ||||||||
| 16433 | CUB and zona pellucida like domains 1 | 1.10 | 1.22 | 0.91 | 0.96 | 1 | Yes | Stein et al., 2006 |
Membrane fractions were prepared by a non-detergent-based method as described. Proteomic analysis with iTRAQ was performed on fx1-4, 5, 7 and 9.
Relative abundance for each fraction was obtained from PLGS expression, with each fx normalized to the same net protein amount by use of the average expressions: 0.591(fx1-4), 0.518 (fx5), 1.095 (fx7) and 1.796 (fx9).
Number of peptides utilized to obtain PLGS protein expression data.
The presence of transmembrane helices in a protein predicted by SOSUI software.
Citations refer to demonstrations of the protein in murine sperm.
Acknowledgments
Contract grant sponsor: National Institute of Health; Contract grant number: K01-RR00188 and R01-HD-045664.
Contract grant sponsor: National Science Foundation; Contract grant number: DMR 0520404.
This work was supported by National Institute of Health grants K01-RR00188 and R01-HD-045664 (A.J.T). We thank Gregory Kopf, Marie Purdon, Tanya Merdiushev and John Northrop (University of Pennsylvania) for their assistance and the Keck Integrated Microscopy Facility (NSF-MRSEC program; DMR 0520404) at Cornell University for technical help with the FE-SEM imaging.
References
- Bou Khalil M, Chakrabandhu K, Xu H, Weerachatyanukul W, Buhr M, Berger T, Carmona E, Vuong N, Kumarathasan P, Wong PT, Carrier D, Tanphaichitr N. Sperm capacitation induces an increase in lipid rafts having zona pellucida binding ability and containing sulfogalactosylglycerolipid. Dev Biol. 2006;290(1):220–235. doi: 10.1016/j.ydbio.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology. 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Burgos MH, Gutierrez LS. The Golgi complex of the early spermatid in guinea pig. The Anatomical record. 1986;216(2):139–145. doi: 10.1002/ar.1092160205. [DOI] [PubMed] [Google Scholar]
- Busso D, Cohen DJ, Maldera JA, Dematteis A, Cuasnicu PS. A novel function for CRISP1 in rodent fertilization: involvement in sperm-zona pellucida interaction. Biol Reprod. 2007;77(5):848–854. doi: 10.1095/biolreprod.107.061788. [DOI] [PubMed] [Google Scholar]
- Buttke DE, Nelson JL, Schlegel PN, Hunnicutt GR, Travis AJ. Visualization of GM1 with cholera toxin B in live epididymal versus ejaculated bull, mouse, and human spermatozoa. Biol Reprod. 2006;74(5):889–895. doi: 10.1095/biolreprod.105.046219. [DOI] [PubMed] [Google Scholar]
- Carlson AE, Hille B, Babcock DF. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev Biol. 2007;312(1):183–192. doi: 10.1016/j.ydbio.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesario MM, Bartles JR. Compartmentalization, processing and redistribution of the plasma membrane protein CE9 on rodent spermatozoa. Relationship of the annulus to domain boundaries in the plasma membrane of the tail. J Cell Sci. 1994;107 (Pt 2):561–570. [PubMed] [Google Scholar]
- Cohen DJ, Da Ros VG, Busso D, Ellerman DA, Maldera JA, Goldweic N, Cuasnicu PS. Participation of epididymal cysteine-rich secretory proteins in sperm-egg fusion and their potential use for male fertility regulation. Asian J Androl. 2007;9(4):528–532. doi: 10.1111/j.1745-7262.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Ellerman DA, Cuasnicu PS. Mammalian Sperm-Egg Fusion: Evidence That Epididymal Protein DE Plays a Role in Mouse Gamete Fusion. Biol Reprod. 2000;63(2):462–468. doi: 10.1095/biolreprod63.2.462. [DOI] [PubMed] [Google Scholar]
- Conover W. In: Practical Nonparametric Statistics. 3. Conover WJ, editor. John Wiley and Sons; New York, NY: 1999. pp. 491–510. [Google Scholar]
- Davis BK. Influence of serum albumin on the fertilizing ability in vitro of rat spermatozoa. Proc Soc Exp Biol Med. 1976;151(2):240–243. doi: 10.3181/00379727-151-39182. [DOI] [PubMed] [Google Scholar]
- Demarco IA, Espinosa F, Edwards J, Sosnik J, De La Vega-Beltran JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE. Involvement of a Na+/HCO3− cotransporter in mouse sperm capacitation. J Biol Chem. 2003;278(9):7001–7009. doi: 10.1074/jbc.M206284200. [DOI] [PubMed] [Google Scholar]
- Drevot P, Langlet C, Guo XJ, Bernard AM, Colard O, Chauvin JP, Lasserre R, He HT. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. The EMBO J. 2002;21(8):1899–1908. doi: 10.1093/emboj/21.8.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberspaecher U, Roosterman D, Kratzschmar J, Haendler B, Habenicht UF, Becker A, Quensel C, Petri T, Schleuning WD, Donner P. Mouse androgen-dependent epididymal glycoprotein CRISP-1 (DE/AEG): isolation, biochemical characterization, and expression in recombinant form. Mol Reprod Dev. 1995;42(2):157–172. doi: 10.1002/mrd.1080420205. [DOI] [PubMed] [Google Scholar]
- Edidin M. The state of lipid rafts: From model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- Ekstedt E, Holm L, Ridderstråle Y. Carbonic Anhydrase in Mouse Testis and Epididymis; Transfer of Isozyme IV to Spermatozoa During Passage. J Mol Histol. 2004;35(2):167–173. doi: 10.1023/b:hijo.0000023387.02793.af. [DOI] [PubMed] [Google Scholar]
- Enders GC, Werb Z, Friend DS. Lectin binding to guinea-pig sperm zipper particles. J Cell Sci. 1983;60:303–329. doi: 10.1242/jcs.60.1.303. [DOI] [PubMed] [Google Scholar]
- Flesch FM, Brouwers JF, Nievelstein PF, Verkleij AJ, van Golde LM, Colenbrander B, Gadella BM. Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J Cell Sci. 2001;114(Pt 19):3543–3555. doi: 10.1242/jcs.114.19.3543. [DOI] [PubMed] [Google Scholar]
- Foster LJ, Chan QW. Lipid raft proteomics: more than just detergent-resistant membranes. Sub-cell biochem. 2007;43:35–47. doi: 10.1007/978-1-4020-5943-8_4. [DOI] [PubMed] [Google Scholar]
- Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A. 2003;100(10):5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DS. Plasma-membrane diversity in a highly polarized cell. J Cell Biol. 1982;93(2):243–249. doi: 10.1083/jcb.93.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marcos M, Perez-Andres E, Tandel S, Fontanils U, Kumps A, Kabre E, Gomez-Munoz A, Marino A, Dehaye JP, Pochet S. Coupling of two pools of P2X7 receptors to distinct intracellular signaling pathways in rat submandibular gland. J Lipid Res. 2006a;47(4):705–714. doi: 10.1194/jlr.M500408-JLR200. [DOI] [PubMed] [Google Scholar]
- Garcia-Marcos M, Pochet S, Tandel S, Fontanils U, Astigarraga E, Fernandez-Gonzalez JA, Kumps A, Marino A, Dehaye JP. Characterization and comparison of raft-like membranes isolated by two different methods from rat submandibular gland cells. Biochim Biophys Acta. 2006b;1758(6):796–806. doi: 10.1016/j.bbamem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Gaus K, Rodriguez M, Ruberu KR, Gelissen I, Sloane TM, Kritharides L, Jessup W. Domain-specific lipid distribution in macrophage plasma membranes. J Lipid Res. 2005;46(7):1526–1538. doi: 10.1194/jlr.M500103-JLR200. [DOI] [PubMed] [Google Scholar]
- Gibbons R, Adeoya-Osiguwa SA, Fraser LR. A mouse sperm decapacitation factor receptor is phosphatidylethanolamine-binding protein 1. Reproduction. 2005;130(4):497–508. doi: 10.1530/rep.1.00792. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Warren G, Stuhlfauth I, Jockusch BM. The role of clathrin-coated vesicles in acrosome formation. Eur J Cell Biol. 1981;26(1):52–60. [PubMed] [Google Scholar]
- Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J. 2002;83(5):2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa T, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14(4):378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- Ho HC, Tang CY, Suarez SS. Three-dimensional structure of the Golgi apparatus in mouse spermatids: a scanning electron microscopic study. Anato Rec. 1999;256(2):189–194. doi: 10.1002/(SICI)1097-0185(19991001)256:2<189::AID-AR9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Huang WP, Ho HC. Role of microtubule-dependent membrane trafficking in acrosomal biogenesis. Cell Tissue Res. 2006;323(3):495–503. doi: 10.1007/s00441-005-0097-9. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S, Arni S, van Echten-Deckert G, Borisch B, Hoessli DC. Microdomain-dependent regulation of Lck and Fyn protein-tyrosine kinases in T lymphocyte plasma membranes. Mol Biol Cell. 1999;10(4):891–905. doi: 10.1091/mbc.10.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R, Hall L. A 23 kDa protein from rat sperm plasma membranes shows sequence similarity and phospholipid binding properties to a bovine brain cytosolic protein. Biochim Biophys Acta. 1991;1080(1):78–82. doi: 10.1016/0167-4838(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Koyama-Honda I, Suzuki K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic. 2004;5(4):213–230. doi: 10.1111/j.1600-0854.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta. 2005;1746(3):234–251. doi: 10.1016/j.bbamcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li SS, O’Brien DA, Hou EW, Versola J, Rockett DL, Eddy EM. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol Reprod. 1989;40(1):173–180. doi: 10.1095/biolreprod40.1.173. [DOI] [PubMed] [Google Scholar]
- Lin Y, Kan FW. Regionalization and redistribution of membrane phospholipids and cholesterol in mouse spermatozoa during in vitro capacitation. Biol Reprod. 1996;55(5):1133–1146. doi: 10.1095/biolreprod55.5.1133. [DOI] [PubMed] [Google Scholar]
- Matter K. Epithelial polarity: sorting out the sorters. Curr Biol. 2000;10(1):R39–42. doi: 10.1016/s0960-9822(99)00256-0. [DOI] [PubMed] [Google Scholar]
- Moreno RD, Ramalho-Santos J, Chan EK, Wessel GM, Schatten G. The Golgi apparatus segregates from the lysosomal/acrosomal vesicle during rhesus spermiogenesis: structural alterations. Dev Biol. 2000a;219(2):334–349. doi: 10.1006/dbio.2000.9606. [DOI] [PubMed] [Google Scholar]
- Moreno RD, Ramalho-Santos J, Sutovsky P, Chan EK, Schatten G. Vesicular traffic and golgi apparatus dynamics during mammalian spermatogenesis: implications for acrosome architecture. Biol Reprod. 2000b;63(1):89–98. doi: 10.1095/biolreprod63.1.89. [DOI] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115(4):377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Nixon B, MacIntyre DA, Mitchell LA, Gibbs GM, O’Bryan M, Aitken RJ. The identification of mouse sperm-surface-associated proteins and characterization of their ability to act as decapacitation factors. Biol Reprod. 2006;74(2):275–287. doi: 10.1095/biolreprod.105.044644. [DOI] [PubMed] [Google Scholar]
- Pelletier RM, Friend DS. Development of membrane differentiations in the guinea pig spermatid during spermiogenesis. The American journal of anatomy. 1983;167(1):119–141. doi: 10.1002/aja.1001670110. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44(4):655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J. 2004;378(Pt 2):281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the Keystone symposium on lipid rafts and cell function. J Lipid Res. 2006;47(7):1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41(6):2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- Pike LJ, Han X, Gross RW. Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids: a shotgun lipidomics study. J Biol Chem. 2005;280(29):26796–26804. doi: 10.1074/jbc.M503805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan B, Oke BO, Papadopoulos V, DiAugustine RP, Suarez-Quian CA. Characterization of epidermal growth factor in mouse testis. Endocrinology. 1992;131(6):3091–3099. doi: 10.1210/endo.131.6.1446643. [DOI] [PubMed] [Google Scholar]
- Roberts KP, Johnston DS, Nolan MA, Wooters JL, Waxmonsky NC, Piehl LB, Ensrud-Bowlin KM, Hamilton DW. Structure and function of epididymal protein cysteine-rich secretory protein-1. Asian J Androl. 2007;9(4):508–514. doi: 10.1111/j.1745-7262.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- Rochwerger L, Cuasnicu PS. Redistribution of a rat sperm epididymal glycoprotein after in vitro and in vivo capacitation. Mol Reprod Dev. 1992;31(1):34–41. doi: 10.1002/mrd.1080310107. [DOI] [PubMed] [Google Scholar]
- Roper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2(9):582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3(12):1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci U S A. 1994;91(25):12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci U S A. 2003;100(10):5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, Simons K. Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J Cell Sci. 2004;117(Pt 25):5955–5964. doi: 10.1242/jcs.01596. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Asano A, Buttke DE, McElwee JL, Nelson JL, Wolff CA, Merdiushev T, Fornes MW, Cohen AW, Lisanti MP, Rothblat GH, Kopf GS, Travis AJ. Segregation of micron-scale membrane sub-domains in live murine sperm. J Cell Physiol. 2006;206(3):636–646. doi: 10.1002/jcp.20504. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Buttke DE, Asano A, McElwee JL, Wolff CA, Nelson JL, Klaus AV, Hunnicutt GR, Travis AJ. GM1 dynamics as a marker for membrane changes associated with the process of capacitation in murine and bovine spermatozoa. J Androl. 2007;28(4):588–599. doi: 10.2164/jandrol.106.002279. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Maruya M, Iwashita S, Ohno-Iwashita Y. The C-terminal domain of perfringolysin O is an essential cholesterol-binding unit targeting to cholesterol-rich microdomains. FEBS. 2002;269(24):6195–6203. doi: 10.1046/j.1432-1033.2002.03338.x. [DOI] [PubMed] [Google Scholar]
- Sleight SB, Miranda PV, Plaskett NW, Maier B, Lysiak J, Scrable H, Herr JC, Visconti PE. Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: evidence for dissociation of lipid rafts during capacitation. Biol Reprod. 2005;73(4):721–729. doi: 10.1095/biolreprod.105.041533. [DOI] [PubMed] [Google Scholar]
- Soltani CE, Hotze EM, Johnson AE, Tweten RK. Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc Natl Acad Sci U S A. 2007;104(51):20226–20231. doi: 10.1073/pnas.0708104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KK, Go JC, Lane WS, Primakoff P, Myles DG. Proteomic analysis of sperm regions that mediate sperm-egg interactions. Proteomics. 2006;6(12):3533–3543. doi: 10.1002/pmic.200500845. [DOI] [PubMed] [Google Scholar]
- Subczynski WK, Kusumi A. Dynamics of raft molecules in the cell and artificial membranes: approaches by pulse EPR spin labeling and single molecule optical microscopy. Biochim Biophys Acta. 2003;1610(2):231–243. doi: 10.1016/s0005-2736(03)00021-x. [DOI] [PubMed] [Google Scholar]
- Thaler CD, Thomas M, Ramalie JR. Reorganization of mouse sperm lipid rafts by capacitation. Mol Reprod Dev. 2006;73(12):1541–1549. doi: 10.1002/mrd.20540. [DOI] [PubMed] [Google Scholar]
- Tooze SA. Biogenesis of secretory granules in the trans-Golgi network of neuroendocrine and endocrine cells. Biochim Biophys Acta. 1998;1404(1–2):231–244. doi: 10.1016/S0167-4889(98)00059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshimori K, Higashi R, Oura C. Distribution of intramembranous particles and filipin-sterol complexes in mouse sperm membranes: polyene antibiotic filipin treatment. Ame J Anat. 1985;174(4):455–470. doi: 10.1002/aja.1001740408. [DOI] [PubMed] [Google Scholar]
- Toshimori K, Higashi R, Oura C. Filipin-sterol complexes in golden hamster sperm membranes with special reference to epididymal maturation. Cell Tissue Res. 1987;250(3):673–680. doi: 10.1007/BF00218962. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Foster JA, Rosenbaum NA, Visconti PE, Gerton GL, Kopf GS, Moss SB. Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol Biol Cell. 1998;9(2):263–276. doi: 10.1091/mbc.9.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 2001a;276(10):7630–7636. doi: 10.1074/jbc.M006217200. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Kopf GS. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest. 2002;110(6):731–736. doi: 10.1172/JCI16392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis AJ, Merdiushev T, Vargas LA, Jones BH, Purdon MA, Nipper RW, Galatioto J, Moss SB, Hunnicutt GR, Kopf GS. Expression and localization of caveolin-1, and the presence of membrane rafts, in mouse and Guinea pig spermatozoa. Dev Biol. 2001b;240(2):599–610. doi: 10.1006/dbio.2001.0475. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Sui D, Riedel KD, Hofmann NR, Moss SB, Wilson JE, Kopf GS. A novel NH(2)-terminal, nonhydrophobic motif targets a male germ cell-specific hexokinase to the endoplasmic reticulum and plasma membrane. J Biol Chem. 1999;274(48):34467–34475. doi: 10.1074/jbc.274.48.34467. [DOI] [PubMed] [Google Scholar]
- Tsai PS, De Vries KJ, De Boer-Brouwer M, Garcia-Gil N, Van Gestel RA, Colenbrander B, Gadella BM, Van Haeften T. Syntaxin and VAMP association with lipid rafts depends on cholesterol depletion in capacitating sperm cells. Mol Membr Biol. 2007;24(4):313–324. doi: 10.1080/09687860701228692. [DOI] [PubMed] [Google Scholar]
- Urner F, Sakkas D. A possible role for the pentose phosphate pathway of spermatozoa in gamete fusion in the mouse. Biol Reprod. 1999;60(3):733–739. doi: 10.1095/biolreprod60.3.733. [DOI] [PubMed] [Google Scholar]
- van Gestel RA, Brewis IA, Ashton PR, Helms JB, Brouwers JF, Gadella BM. Capacitation-dependent concentration of lipid rafts in the apical ridge head area of porcine sperm cells. Mol Hum Reprod. 2005;11(8):583–590. doi: 10.1093/molehr/gah200. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol. 1999;214(2):429–443. doi: 10.1006/dbio.1999.9428. [DOI] [PubMed] [Google Scholar]
- Walker FJ. Properties of chemically modified protein S: the effect of the conversion of .gamma.-carboxyglutamic acid to .gamma.-methyleneglutamic acid on functional properties. Biochemistry. 1986;25(20):6305–6311. doi: 10.1021/bi00368a071. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79(1):73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- Zegers MM, Hoekstra D. Mechanisms and functional features of polarized membrane traffic in epithelial and hepatic cells. Biochem J. 1998;336 (Pt 2):257–269. doi: 10.1042/bj3360257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Arthur G. Improved procedures for the determination of lipid phosphorus by malachite green. J Lipid Res. 1992;33(8):1233–1236. [PubMed] [Google Scholar]




