Abstract
The activities of the Raf kinase family proteins control extracellular signal-regulated kinase (ERK) activation in many aspects of cellular responses. However, the relative contributions of individual isozymes to cellular functions including T cell responses are still unclear. In addition to Raf-1, another Raf family kinase, B-Raf, is expressed in murine thymocytes and peripheral T cells, and its activation was induced by TCR stimulation. Here, we investigated the function of B-Raf in development of T cells by generating chimeric mice in which a T cell-compromised host was reconstituted with fetal liver-derived cells from embryonic lethal B-Raf-deficient mice. Although B-Raf was dispensable for normal T cell lineage differentiation at the CD4−CD8− double-negative stage, thymocytes in the chimeric mice derived from B-Raf−/− cells exhibited a drastic arrest of differentiation at the CD4+CD8+ double-positive stage, suggesting that B-Raf is crucial for Tcell development, especially for the transition to CD4+ and CD8+ single-positive thymocytes. Regarding intracellular signaling, we found that activation of ERK following TCR stimulation was impaired in the thymocytes from the chimeric mice. In conclusion, we present first evidence for the important role of B-Raf-mediated signaling in T cell development.
Keywords: B-Raf, Cell differentiation, T cells, T cell receptors
Introduction
In thymus, thymocyte development is responsible for generation of mature T cells. Developing thymocytes can be classified into three stages of increasing maturity, CD4−CD8− double-negative (DN), CD4+CD8+ double-positive (DP) and CD4+ or CD8+ single-positive (SP) subsets [1, 2]. TCR signaling mediates these processes, especially positive and negative selection at the DP stage. A favored hypothesis, the avidity model, postulates that positively selecting peptide/MHC complexes interact with TCR with low avidity, delivering a weak signal that rescues cells from their default fate (death by neglect) without inducing apoptotic cell death of thymocytes [2, 3]. On the other hand, negatively selecting peptide/MHC complexes interact with TCR with high avidity, delivering an intense signal, which induces apoptosis [2, 3].
A number of studies have revealed that the MAPK pathway consisting of p21Ras, Raf, MEK and ERK represents one of the important signaling cascades coupled to TCR-mediated responses, including the determination of cell fate at various stages of thymic selection [4, 5]. Transgenic expression of dominant-negative or constitutively active forms of Ras, Raf or MEK1 all revealed that the MAPK pathway is implicated in positive selection at the CD4+CD8+ DP stage [6–9]. Consistent with these reports, ERK activation is involved in the most critical events during TCR signaling and plays a crucial role in positive selection [4, 5]. In addition to positive selection, ERK activation is also known to be indispensable for the intracellular signals promoting the first β-selection checkpoint [10] and lineage commitment of mature CD4+ or CD8+ SP cells [11]. Therefore, understanding how TCR signaling in thymocytes results in ERK activation is important for elucidation of the mechanisms of thymocyte development.
A-Raf, B-Raf and C-Raf (also called Raf-1) constitute a Raf kinase family that contributes to activation of the MEK/ERK pathway in various aspects of proliferation, differentiation and apoptosis in mammalian cells [12–15]. Although the individual Raf genes have some apparent functional redundancies with each other in ERK regulation, the different phenotypes of each Raf kinase-deficient mouse suggest that they have specific and non-redundant functions [12, 16–19], which seem to be due to their distinct expression patterns [20] and differences in the thresholds for activation [15, 21, 22].
In T cells, Raf-1 is thought to regulate TCR-mediated activation [23], whereas the expression and function of B-Raf remain controversial. Previously we reported that B-Raf activation is regulated by Ras and mediates sustained ERK activation and subsequent IL-2 production upon TCR stimulation in human T cells [22, 24]. In addition, enforced expression of B-Raf in mouse CD4+ T cells resulted in augmentation of their proliferation and prevention of anergy induction [25]. Reciprocally, it was demonstrated that superantigen-induced anergic T cells down-regulate the expression of B-Raf in vivo [26]. Taken together, current data in T cells indicate that B-Raf may be an important signaling molecule in TCR-mediated responses.
Although B-Raf-deficient T cells would be a powerful tool for analyses of B-Raf function, T cells cannot be obtained from B-Raf-null mice, since targeted disruption of the murine B-Raf gene results in embryonic lethality caused by defects of vasculargenesis during mid-gestation [19]. Therefore, the in vivo role of B-Raf on development and activation of T cells remains to be elucidated. To overcome the early embryonic lethality of B-Raf−/− mice, we generated chimeric RAG2−/− mice in which T cells were reconstituted using hematopoietic stem cells (HSC) or T cell precursor cells derived from fetal liver (FL) cells in B-Raf−/− mice and examined the role of B-Raf in T cell development. These approaches demonstrated that B-Raf is a positive regulator of T cell development. Furthermore, we found that B-Raf contributes to the promotion of TCR-mediated ERK activation at the DP stage. Our data provide evidence that B-Raf plays a specific and crucial role in T cell development in vivo.
Results
Expression and activation of B-Raf in thymocytes
To examine B-Raf expression, DN-arrested thymocytes from RAG2−/− mice [27], purified CD4+CD8+ DP thymocytes and peripheral T cells from wild-type C57BL/6 mice were immunoblotted with anti-B-Raf Ab (Fig. 1A). Substantial expression of B-Raf protein could be detected in all subsets of immature thymocytes and peripheral T cells. In addition, it was shown that T cells isolated from B-Raf+/− mice express less B-Raf protein than T cells from wild-type mice. B-Raf−/− T cells isolated from RAG2−/− chimeric mice that had been reconstituted with B-Raf-deficient HSC (see section Requirement of B-Raf for the development of single-positive thymocytes) did not exhibit B-Raf expression but did express Raf-1 and ERK. No compensatory increase in Raf-1 expression was observed in B-Raf-deficient Tcells. B-Raf protein was highly expressed in PC12 cells and B-Raf-transfected Jurkat cells, which were utilized as positive controls [24, 28].
Figure 1. Expression and TCR-mediated activation of B-Raf in T cells.
(A) Western blotting analysis of B-Raf expression in thymocytes fromRAG2−/− mice, DP and SP thymocytes sorted from C57BL/6 mice, peripheral (splenic) T cells fromRAG2−/− chimeras reconstituted with B-Raf+/+, B-Raf +/− or B-Raf−/− FL cells, as well as mock- and B-Raf-transfected Jurkat cells. Raf-1 and ERK blottings are shown as loading controls. PC12 and NIH3T3 were utilized as positive and negative controls of B-Raf expression, respectively. (B, C) In vitro kinase assays for Raf-1 and B-Raf isolated from thymocytes stimulated with 10 µg/mL anti-CD3 Ab or 25 ng/mL PMA for 10 min (B) or anti-CD3Ab for the indicated times (C). Recombinant GST-MEK was used as a substrate, and incorporated 32P radioactivity was visualized by autoradiography (upper left panel). ERK phosphorylation was determined by blotting with an antibody specific for phosphorylated ERK. Equal loading of each Raf isoform and ERK was confirmed (lower left panels). (D) Splenic T cells were stimulated with 5 µg/mL anti-CD3 Ab for the indicated times in an in vitro kinase assay for B-Raf and Raf-1 performed as described in (B).
Next we examined whether B-Raf activity is regulated by TCR stimulation. In thymocytes, TCR stimulation induced up-regulation of the kinase activity of B-Raf. MEK phosphorylation was confirmed using Ab specific for phosphorylated MEK (data not shown). This activation was prolonged for up to 60 min and was coincident with ERK activation (Fig. 1B, C). As expected, PMA stimulation also induced B-Raf activation (Fig. 1B). However, surprisingly, Raf-1 activation was not detected up to 60 min after TCR stimulation, although substantial ERK activation was observed under the same stimulatory conditions (Fig. 1C), while Raf-1 activation could be observed in PMA-stimulated thymocytes (Fig. 1B). In TCR-stimulated peripheral T cells, activation of both Raf-1 and B-Raf was induced (Fig. 1D). These results could be attributed to the lower activation threshold of B-Raf than Raf-1 in response to TCR stimulation [22]. The findings of TCR stimulation-inducible B-Raf activation prompted us to hypothesize that B-Raf could play a role in TCR-mediated T cell responses in thymocytes.
β-selection proceeds normally in vitro and in vivo in B-Raf-deficient T cell progenitors
T cell progenitors enter the thymus as CD44+CD25− cells and soon acquire expression of CD25. The next transition, from CD44−CD25+ (DN3 stage) to CD44−CD25− (DN4 stage), is termed β-selection; this step requires pre-TCR-mediated signals and is coincident with extensive proliferation [29]. These developmental events of T cell progenitors at the DN stage are mimicked in an in vitro co-culture system using OP9 cells expressing delta-like1 (OP9-DL1) [30, 31]. Using this system, we assessed the requirement for B-Raf during developmental progression of the DN subpopulation and in β-selection.
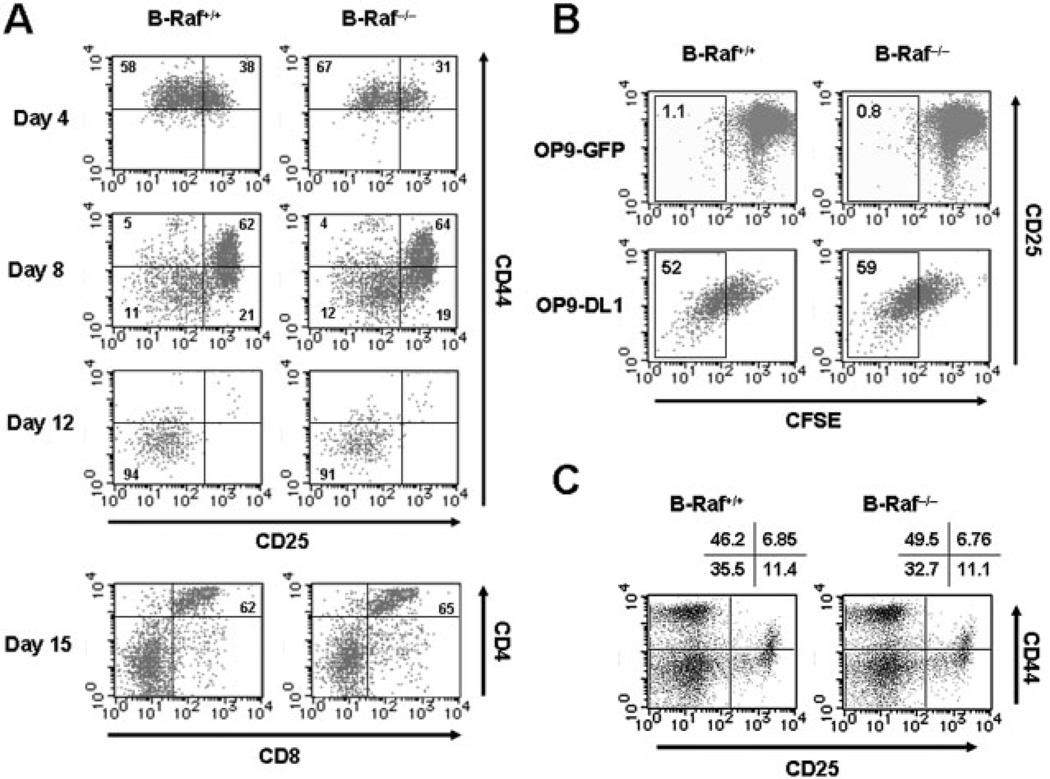
Given that by embryonic day 11–12, definitive hematopoietic progenitor cells capable of generating T, B and myeloid cells colonize the FL [32], we utilized FL cells from B-Raf−/− fetuses prior to embryonic death (embryonic day 11.5–12.5) as a source of B-Raf-deficient HSC. As shown in Fig. 2A, kinetic analysis of differentiation from the FL cells co-cultured with OP9-DL1 demonstrated that both B-Raf+/+ and B-Raf−/− DN cells acquire DN3–4 phenotypes with loss of CD44 expression at day 8 of the co-culture. At this time point, there were comparably low frequencies of NK1.1+ cells (<3%) and B220+ cells (<0.5%) in B-Raf+/+ and B-Raf−/− FL cell cultures (data not shown). Moreover, at day 15 of co-culture, regardless of the B-Raf status of the T cell progenitors, CD4+CD8+ DP cells were efficiently induced (Fig. 2A, lower panels). The number of harvested B-Raf−/− Thy-1+ cells decreased as compared with B-Raf+/+ cells, which might be the consequence of a lower precursor frequency in B-Raf−/− FL cells rather than differentiation defects of B-Raf−/− DN cells ([16] and Supporting Information Fig. 1).
Figure 2. Pre-TCR-mediated β-selection is not affected by B-Raf deficiency.
(A) Phenotypic changes of DN cells differentiated from wild-type and B-Raf−/− FL-derived progenitors in co-culture with OP9-DL1 for various times. Developmental progression of the T cell lineage was assessed on days 4, 8 and 12 by CD25 and CD44 expression profiles on CD4−CD8− DN-gated cells. In the lowest panels, percentages of CD4+CD8+ DP cells co-cultured with OP9-DL1 for 15 days are indicated. Lymphoid cells were gated based on their SSC and FSC profiles. (B) DN T cell progenitors derived from B-Raf+/+ and B-Raf−/− FL cells co-cultured with OP9-DL1 for 8 days were loaded with CFSE and re-seeded on OP9 GFP (upper panels) or OP9 DL1 (lower panels). After 48 h, proliferation indicated by CFSE dilution and CD25 expression on DN cells during β-selection were determined. Dot plots show cells gated to eliminate CD4+CD8+ DP and OP9-DL1 cells. (C) Thymocytes from B-Raf+/+ and B-Raf−/− chimeric mice 7 wk after transfer of FL cells into RAG2−/− mice were isolated by positively sorting with anti-Thy-1 magnetic beads. The plots for CD44 and CD25 expression were gated on the CD4−CD8− DN population. The data are representative of three animals per group in two independent experiments with similar results, and the percentages of gated cells that fall into each quadrant are shown.
Because cellular expansion is one of the hallmarks of β-selection [29, 33], we examined the requirement of B-Raf for this event. For this purpose, cells harvested at day 8 of OP9-DL1 co-culture (CD25+ DN2–3 stage) were labeled with a mitotic tracker, CFSE, and reseeded onto OP9-DL1. OP9 cells expressing GFP (OP9-GFP) were utilized as a mock-transfected control of OP9-DL1 [31]. As shown in Fig. 2B, the degree of dilution of CFSE revealed that B-Raf deficiency was permissive for pre-TCR-mediated proliferation during the transition from CD25+ DN3 to CD25− DN4 stage in a delta-like1-dependent manner.
To determine the dependency of in vivo development of the T cell lineage on B-Raf, we generated chimeric mice by transferring HSC from FL of B-Raf−/− fetuses into T cell-compromised RAG2−/− mice. In support of the results obtained from the in vitro culture system of DN cell differentiation, B-Raf-deficient thymocytes underwent normal phenotypic progression to DN4 stage, as did B-Raf+/+ thymocytes reconstituted in the FL chimeric mice (Fig. 2C). Taken together, these results suggest that B-Raf is not necessary for T cell development at the DN stages and pre-TCR-mediated β-selection both in vitro and in vivo.
Requirement of B-Raf for the development of single-positive thymocytes
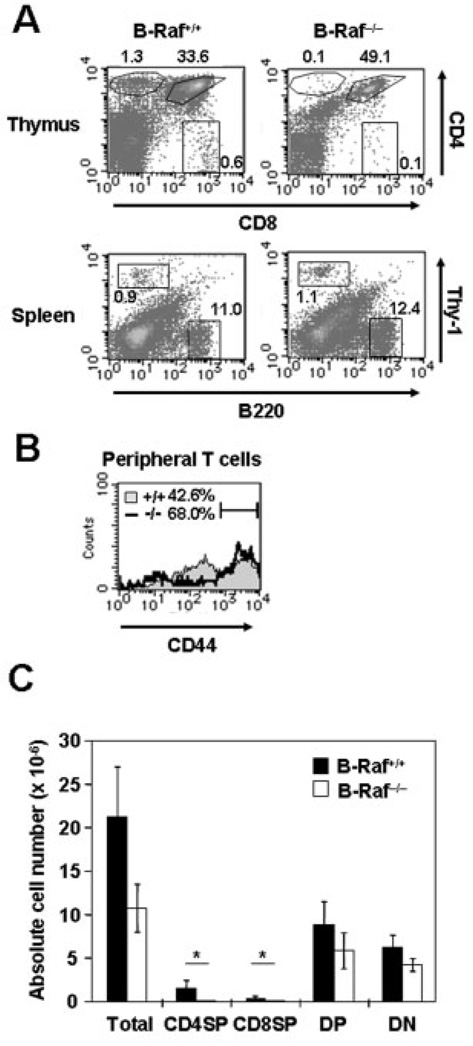
To address whether B-Raf is required for further T cell development, we first examined the CD4 and CD8 expression profiles of B-Raf +/+ and B-Raf−/− thymocytes in FL chimeras. Surprisingly, we found that B-Raf−/− FL chimeras showed accumulation of CD4+CD8+ DP cells, and both CD4+ SP and CD8+ SP thymocytes derived from B-Raf−/− cells were markedly decreased as compared with B-Raf +/+ controls (Fig. 3A, upper panels). On the other hand, detectable Thy-1+ T cells and B220+ B cells were reconstituted to similar levels in spleen in both chimeric mice (Fig. 3A, lower panels). Despite the lower thymic output of T cells in B-Raf−/− FL chimeras, accumulation of T cells in the periphery was observed at a similar proportion as in control mice; this seemed to be due to lymphopenia-induced proliferation of a small number of B-Raf−/− T cells to complement the limited T cell niche, leading to accumulation in the periphery [34]. In fact, compared to control mice, B-Raf−/− chimeric mice exhibited an increased CD44hi population, which is reminiscent of T cells undergoing lymphopenia-induced proliferation (Fig. 3B).
Figure 3. Inefficient production of CD4+ and CD8+ SP cells in B-Raf-deficient thymocytes.
(A) Single-cell suspensions of thymocytes (upper panels) and spleen cells (lower panels) were isolated from age-matched B-Raf+/+ and B-Raf−/− FL chimeric mice 8 wk after transfer, stained with Ab against CD4, CD8, B220 and TCR-β chain, and analyzed by flow cytometry. Lymphoid cells were gated based on SSC and FSC. The percentages of total thymocytes and spleen cells that fall into each region are indicated. (B) Cell surface expression of CD44 on peripheral T cells isolated from spleen and lymph nodes of B-Raf +/+ (filled gray) and B-Raf−/− (bold line) FL chimeric mice. (C) Thymi were harvested 8–12 wk after transfer and the cell suspension enumerated. After calculating the percentage of lymphoid cells (~85% of total) on the basis of FSC/SSC, the numbers of the indicated population were calculated (mean ± SD). Data are based on four to five samples of thymus (*p<0.01). All data are representative of at least three B-Raf +/+ and B-Raf−/− chimeric mice in two independent experiments.
Thymic cellularity of B-Raf−/− chimeras was typically about half of that of B-Raf+/+ mice, and the absolute numbers of CD4−CD8− DN and CD4+CD8+ DP B-Raf−/− cells were slightly decreased as compared with control mice (Fig. 3C). Furthermore, significantly reduced numbers of CD4+ SP and CD8+ SP cells were observed in B-Raf−/− thymocytes. These results raised the possibility that B-Raf is required for further development of thymocytes after β-selection.
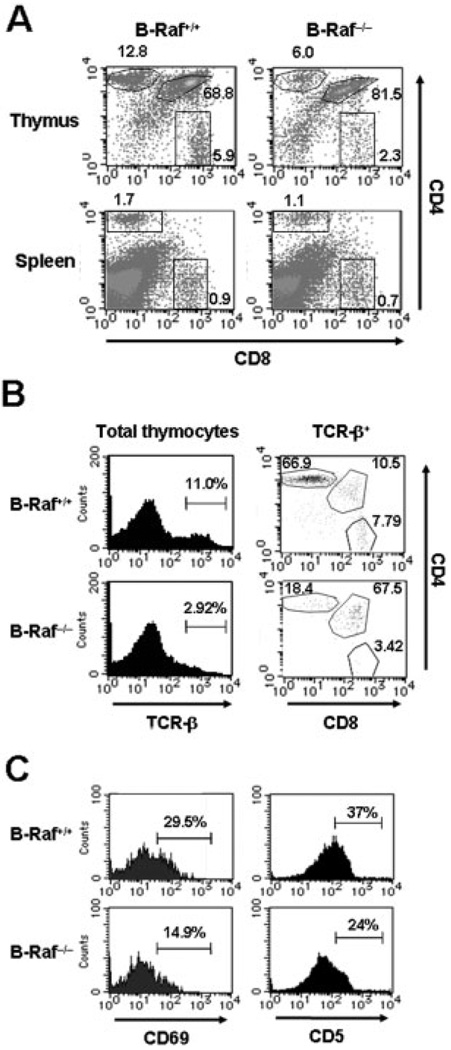
The results from such FL chimeras generated by reconstitution with HSC have to be interpreted with caution, especially with regard to the possibility of extrinsic effects of non-T cell lineages and of the difference in the frequency of HSC giving lymphoid subsets between mutant and wild-type FL cells [32]. To confirm the T cell autonomous effects of B-Raf deficiency on thymocyte development at the DP stage, we reconstituted RAG2−/− mice with the B-Raf−/− T cell lineage from committed T progenitor cells atDN2–3stage generated by 8 days co-culture with OP9-DL1 (Fig. 2). Recapitulating the results from FL chimeras, when RAG2−/− mice were reconstituted with DN cells, the majority of B-Raf−/− thymocytes were retained in the CD4+CD8+ DP stage (B-Raf+/+, 69% versus B-Raf−/−, 82%), and CD4+ or CD8+SP populations were decreased (Fig. 4A, upper panels). Moreover, significant decreases in SP cell numbers were observed in the B-Raf−/− chimeras (Supporting Information Fig. 2A). In the periphery, the two chimeric mice possessed comparable CD4+ SP and CD8+ SP frequencies and numbers (Fig. 4A, lower panels and Supporting Information Fig. 2B). As expected and in contrast to FL chimeras, we observed transient but not long-term repopulation of DP thymocytes derived from transferred DN cells in B-Raf+/+ chimeras (measured 4 wk after transfer), whereas B-Raf−/− cells remained in the DP stage up to 6 wk after transfer (data not shown).
Figure 4. Attenuated thymocyte development in B-Raf−/− chimeric mice.
(A) CD4 and CD8 staining on total thymocytes (upper panels) and spleen cells (lower panels) from chimeric mice 4 wk after transfer of B-Raf+/+ or B-Raf−/− DN cells generated by co-culture with OP9-DL1. The cells were analyzed by flow cytometry. The percentages of cells in each population are indicated within each dot plot. (B) Representative histograms of TCR-β chain expression on total thymocytes (left panels) and dot plots of CD4 and CD8 staining on cells that express high levels of TCR (right panels). (C) Representative histograms of CD69 (left panels) and CD5 (right panels) expression on thymocytes gated on CD4+CD8+ DP cells from the B-Raf+/+ and B-Raf−/− chimeric mice described in (A). All data are representative of at least three independent experiments.
After DP cells undergo positive selection, the expression of TCR increases, and thus TCR+ DP and CD4+ or CD8+ SP cells are expected to be positively selected [3, 7, 8]. We therefore examined the TCR expression on B-Raf +/+ and B-Raf−/− thymocytes. As shown in Fig. 4B, B-Raf−/− TCRhi thymocytes were reduced by approximately 40%, with a concomitant decrease in mature CD4+ or CD8+ SP TCRhi thymocytes in B-Raf−/− chimeras.
We next hypothesized that the failure of DP cells to efficiently undergo further differentiation in the absence of B-Raf could be due to a defect in the responsiveness of the DP cells to TCR signaling. We investigated this hypothesis by staining for CD69 and CD5 on B-Raf+/+ and B-Raf−/− thymocytes, because up-regulation of these two markers on thymocytes is dependent on productive TCR signaling, and DP thymocytes that have undergone positive selection can be enumerated by measuring the relative expression levels of CD69 and CD5 [3, 35, 36]. As shown in Fig. 4C and Table 1, wild-type DP cells exhibited higher frequencies of CD69+ and CD5+ cells than their B-Raf−/− counterparts. These results suggested that the abnormal phenotype of B-Raf−/− thymocytes is T cell autonomous, and B-Raf−/− DP thymocytes do not transmit sufficient TCR signals to lead to thymocyte maturation, resulting in the DP arrest.
Table 1.
Decreased frequency of mature thymocytes in B-Raf−/− chimeric mice.
| Cell Subset | B-Raf+/+ chimeras | B-Raf−/− chimerasa) | p valuesb) |
|---|---|---|---|
| TCR-βhi (% in total)c) | 9.58±1.6 | 3.40±1.3 | <0.01 |
| CD69+ (% in DP)c) | 26.9±3.0 | 15.9±2.6 | <0.01 |
| CD5+ (% in DP)c) | 36.2±3.5 | 25.9±4.7 | <0.05 |
Thymi from control B-Raf+/+ chimeras (n=4) or B-Raf−/− chimeras (n=4) were harvested 8–12 wk after transfer, and cells were stained with the indicated subset marker.
p values were calculated for frequencies using a two-tailed Student's t-test, which was considered significant at p<0.05.
Each value represents the frequency of the indicated population within total thymocytes or the CD4+CD8+ DP population. Mean percentages of the indicated populations are shown with SD.
B-Raf is a positive regulator of ERK activation in thymocytes
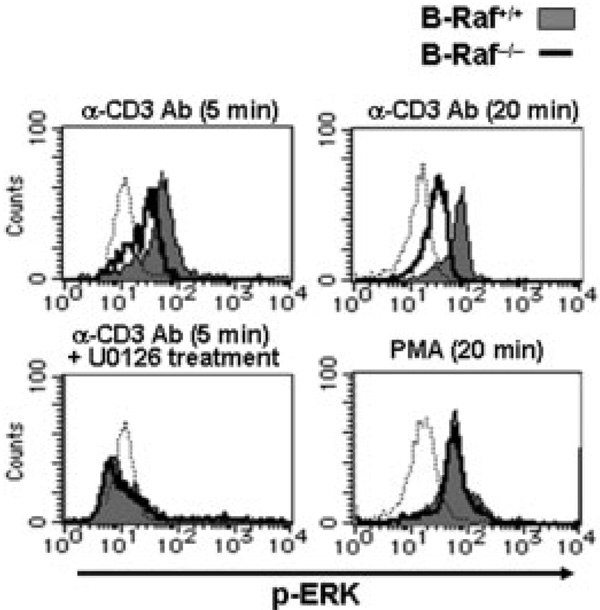
Progress of thymocyte differentiation at the DP stage is critically dependent on TCR-mediated intracellular signaling [3]. To evaluate whether the inability of B-Raf-deficient thymocytes to differentiate into the SP stage is due to impaired signaling through the TCR, we measured the activation status of ERK following TCR stimulation, since it has been shown that ERK activity is regulated by TCR-mediated signaling and acts as an important regulator of thymic selection [4]. To determine the levels of ERK activation in thymocytes, we performed a flow cytometry-based analyses using a specific anti-phospho-ERK Ab. As shown in Fig. 5, TCR stimulation induced ERK activation in wild-type DP thymocytes, and the ERK activation was drastically attenuated in B-Raf−/− DP cells at 5 and 20 min after stimulation. As expected, TCR-mediated ERK activation in both wild-type and B-Raf−/− thymocytes was completely blocked by the treatment of cells with the MEK inhibitor U0216. In contrast to TCR stimulation, stimulation with PMA evoked comparable ERK activation in B-Raf−/− and B-Raf+/+ thymocytes (Fig. 5). It was demonstrated that B-Raf deficiency specifically reduced TCR-mediated ERK activation in the DP thymocytes, while PMA stimulation bypassed B-Raf function and induced full ERK activation comparable to that of the TCR-stimulated B-Raf+/+ DP thymocytes. Collectively, these results suggest that the developmental arrest of SP transition in B-Raf-deficient thymocytes reflects, at least in part, a defective cellular response to the TCR-mediated ERK activation pathway.
Figure 5.
Total thymocytes were stimulated by crosslinking of CD3 with 10 µg/mL anti-CD3 Ab and anti-hamster Ig Ab for 5 or 20 min (upper panels) or by incubation with 25 ng/mL PMA for 20 min (lower right panel). Cells were treated for 30 min with 10 µM U0126 prior to TCR stimulation (lower left panel). Histograms show profiles of phospho-ERK in B-Raf+/+ (filled gray) and B-Raf−/− (bold line) cells gated on CD4+CD8+ DP cells from DN cell-chimeric mice. Dotted lines indicate the status of phospho-ERK in unstimulated B-Raf+/+ DP cells. The data are from one representative of three independent experiments.
Discussion
In this study, we provide convincing evidence for B-Raf expression in thymocytes and peripheral T cells. Furthermore, the analyses of T cells lacking B-Raf demonstrates an appreciable impact of B-Raf in thymocyte development.
It has previously been reported that B-Raf is not expressed in T cells and, therefore, might not play a role for T cells [25, 37]. This lack of observed B-Raf expression in T cells can be explained by a much lower expression level of B-Raf in human and murine T cells when compared with the robust expression of B-Raf in PC12 cells (Fig. 1 and [25]); there might be a relative difficulty to detect B-Raf expression in T cells under conditions in which comparable loading of total protein is done for T cells and PC12 cells. However, supporting our observations, several groups independently reported that B-Raf is expressed in human and murine T cells [24, 26, 38–40]. Based on our findings and these reports showing B-Raf expression, we have concluded that both murine thymocytes and peripheral T cells express B-Raf, which prompted us to investigate the function of B-Raf in T cells.
The reconstitution of immunocompromised RAG2−/− hosts with B-Raf−/− HSC or T cell progenitors allowed us to dissect the contribution of B-Raf to thymocyte development and T cell activation. Thymocytes lacking B-Raf showed a profound increase in the DP fraction and a proportional decrease in both the SP fractions and TCR-β+ mature thymocytes, suggesting an important role for B-Raf in T cells during late thymic development, particularly in the transition from DP thymocytes to the SP stage. Furthermore, ERK activation was impaired in B-Raf-deficient DP thymocytes (Fig. 4). These results support the notion that ERK is a critical molecule for efficient transition from the DP to SP stage [4, 5]. However, unlike ERK−/− thymocytes, the developmental progression from DN3 to DN4 stage (β-selection) was not attenuated in B-Raf−/− thymocytes (Fig. 2 and [4]). This raises the possibility that ERK activation is complemented by other up-stream molecules at this checkpoint. The most likely candidate is Raf-1, whose activation may overcome B-Raf deficiency during β-selection. Supporting this interpretation, it has been reported that active Raf-1 is sufficient to mediate β-selection even in the absence of pre-TCR signaling [41].
In contrast, the prominent phenotype in B-Raf−/− thymocytes at the DP stage seemed to be due to the inability of Raf-1 to compensate for B-Raf function. Indeed, Raf-1 activation did not coincide with ERK activation in TCR-stimulated thymocytes (Fig. 1 and [39]), and B-Raf was necessary for TCR-stimulated ERK activation in DP thymocytes (Fig. 4). The defect of ERK activation was not observed in B-Raf−/− thymocytes treated with PMA, which provoked substantial activation of the Raf-1-ERK pathway. It is interesting to note that thymocytes expressing dominant-interfering mutants of Raf and Ras also exhibited DP arrest [6–8] grossly similar to that of B-Raf−/− thymocytes. This allows us to speculate that the Ras-Raf-1-ERK pathway exerts a promoting effect during DP-SP transition. However, results using dominant-interfering mutants must be interpreted with caution, as the specificity of Raf isoforms varies, and the specificity of the dominant-interfering mutant of Raf has not yet been elucidated [8, 42]; thus, this mutant may interfere with not only Raf-1 but also B-Raf function. Therefore, the DP arrest of thymocytes expressing the mutant Raf protein may be due to interference with B-Raf function, which can be supportive evidence of our results. Furthermore, it was reported that maximal activation of B-Raf requires Ras activation [15], and we have demonstrated that B-Raf activity is regulated by Ras in TCR-stimulated T cells [24]. Based on these results, the defect in thymocyte differentiation from the DP to SP stage (positive selection) in mice expressing a dominant-negative mutant of Ras could be partly explained by impaired B-Raf activation. Compared with the approaches using constitutively active or dominant-negative mutants of Raf, our specific, non-pharmacological approach provides a more convincing assessment of the function of B-Raf in T cell differentiation and raises the possibility that the Ras-B-Raf-ERK pathway is critical for differentiation of thymocytes. It is implied that the Raf isoforms would either function synergistically under certain circumstances or function specifically at different time points of thymocyte development.
We found that B-Raf acts as a positive regulator for TCR-dependent ERK activation in DP thymocytes, which has an obligatory role in positive selection [4, 5]. A currently favored model postulates that the strength, kinetics and compartmentalization of ERK activation dictates the thresholds for both positive and negative selection of thymocytes [9, 43, 44]. According to this viewpoint, the developmental defect observed in B-Raf−/− thymocytes can be partly explained by the reduction in TCR-mediated ERK activation, which is necessary for thymocytes to pass through the checkpoint of positive selection. Furthermore, the leaky formation of SP thymocytes in B-Raf−/− chimeric mice may be a result of thymocytes employing alternative signaling molecules such as Raf-1 and/or a reflection of biased thresholds of positive and negative selection. Further studies regarding the effects of B-Raf on thymic selection are needed to prove this point.
Materials and methods
Reagents and flow cytometry
FITC-, Cy5- or PE-conjugated anti-mouse CD44, CD25 and Thy1.2 mAb and anti-CD3ε mAb (145–2C11) were purchased from BD PharMingen (San Diego, CA). Anti-B220 mAb was purchased from Miltenyi Biotec (Auburn, CA), and FITC-, Cy5- or PE-conjugated anti-mouse CD4, CD8, TCR-β chain, CD5 and CD69 mAb were from eBioscience (San Diego, CA). Polyclonal anti-ERK, Raf-1 and B-Raf Ab were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal anti-phospho-ERK Ab and the MEK inhibitor U0126 were purchased from Cell Signaling Technology (Beverly, MA). HRP-conjugated rabbit anti-mouse and donkey anti-rabbit IgG were from Amersham Bioscience (Arlington Heights, IL). PMA and anti-hamster Ig Ab were purchased from Sigma (St. Louis, MO). Intracellular staining for phospho-ERK was done using IntraPrep (Immunotech, Marseille, France) according to the manufacturer's instructions. Acquisition of data was performed on a FACScan, and the data were analyzed with CELLQuest software (Becton Dickinson, San Jose, CA).
Cell stimulation, Western blotting and in vitro kinase assay
Jurkat cells transfected with B-Raf (or mock-transfected) were prepared as described [24]. Thymic DP and SP cells were purified by cell sorting using a BD FACSVantage (>98% CD4+CD8+ DP, >95% CD4+ SP or CD8+ SP). For TCR stimulation, cells were preincubated on ice for 20 min, and then soluble anti-CD3 mAb was added. After 15 min incubation, anti-hamster Ig Ab was added for cross-linking of CD3 and incubated for 5 min. Stimulation was performed by incubation at 37°C for the indicated time. Western blottings, immunoprecipitations, in vitro kinase assays for Raf-1 and B-Raf, and intracellular stainings were carried out as previously described [22, 24].
Preparation of fetal liver cells and co-culture with OP9-DL1
The day of vaginal plug discovery was designated as embryonic day 0.5. Genotyping of B-Raf−/− fetuses was retrospectively performed on DNA isolated from fetal bodies by PCR. The primers for PCR have been described [19]. Single-cell suspensions of FL cells (day 11.5–12.5 of gestation) were cultured in 12-well tissue culture plates seeded with a confluent monolayer of OP9-DL1 to be differentiated into the T cell lineage as described [31]. Briefly, the co-culture was performed in the presence of 5 ng/mL IL-7 and 5 ng/mL fms-like tyrosine kinase-3 ligand (Peprotech, London, UK). Co-cultured cells were harvested by forceful pipetting at the indicated time points and then reseeded onto new OP9-DL1 or OP9-GFP or stained for flow cytometric analyses.
Generation of chimeric mice
C57BL/6 mice, RAG2−/− mice [27] and B-Raf+/− mice [19] were bred on the C57BL/6 background and maintained in specific pathogen-free conditions at a barrier facility in accordance with the Animal Experiment Committee of Kumamoto University. RAG2−/− mice (6–8 wk old) irradiated with 5 Gy 1 day before the injection were used as recipients for lymphoid reconstitution with B-Raf-deficient and wild-type control cells. FL cells (1 × 106)were isolated from B-Raf +/+ or B-Raf−/− fetuses on day 11.5–12.5 of gestation and then injected intravenously into the recipient mice. OP9-DL1-induced DN cells were negatively selected by magnetic separation using anti-CD4 and anti-CD8 beads and positively selected using anti-Thy1.2 beads (Miltenyi Biotec) (>95% CD4−CD8−, Thy1.2+). Then 5 × 106 cells were i.v. injected into the 5 Gy-irradiated recipients. Cells from thymus, spleen and lymph nodes of the reconstituted recipient RAG2−/− mice were harvested and analyzed 7–9 wk or 4–6 wk after the transfer of FL or DN cells, respectively. Donor thymocytes and T cells were recognized by the expression of the Thy-1 or TCR-β chain.
Quantitative analyses of T cell proliferation
For the analysis of cell division, T cell progenitor cells from the OP9-DL1 co-culture were labeled with 1 µM CFSE (Molecular Probes, Eugene, OR) in PBS for 10 min at 37°C. Cells were washed in RPMI containing 10% FCS and reseeded onto OP9-DL1 or OP9-GFP. After 2 days of additional co-culture with OP9-DL1, the cells were harvested and analyzed for the intensity of CFSE dye together with other cell surface markers.
Acknowledgements
We thank Dr. Carlos Zuniga-Pflucker for the generous gift of OP9-DL1 and OP9-GFP cell lines. We also acknowledge Midori Fukuda for her secretarial assistance. This work was supported by Grants-in-Aid Nos. 14370115, 15510165 and 17510167 to Y. Nishimura from the Ministry of Education, Science, Technology, Sports and Culture, Japan.
Abbreviations
- DN
double negative
- DP
double positive
- FL
fetal liver
- OP9-DL1
OP9 cells expressing delta-like1
- SP
single positive
Footnotes
Supporting Information for this article is available at http://www.wiley-vch.de/contents/jc_2040/2008/37430_s.pdf
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Mason CS, Springer CJ, Cooper RG, Superti-Furga G, Marshall CJ, Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 1999;18:2137–2148. doi: 10.1093/emboj/18.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 3.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 4.Fischer AM, Katayama CD, Page G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Pages G, Guerin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouyssegur J. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 6.Alberola-Ila J, Hogquist KA, Swan KA, Bevan MJ, Perlmutter RM. Positive and negative selection invoke distinct signaling pathways. J. Exp. Med. 1996;184:9–18. doi: 10.1084/jem.184.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swan KA, Alberola-Ila J, Gross JA, Appleby MW, Forbush KA, Thomas JF, Perlmutter RM. Involvement of p21ras distinguishes positive and negative selection in thymocytes. EMBO J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Shea CC, Crompton T, Rosewell IR, Hayday AC, Owen MJ. Raf regulates positive selection. Eur. J. Immunol. 1996;26:2350–2355. doi: 10.1002/eji.1830261012. [DOI] [PubMed] [Google Scholar]
- 9.Alberola-Ila J, Hernandez-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 2003;191:79–96. doi: 10.1034/j.1600-065x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 10.Crompton T, Gilmour KC, Owen MJ. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell. 1996;86:243–251. doi: 10.1016/s0092-8674(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 11.Bommhardt U, Basson MA, Krummrei U, Zamoyska R. Activation of the extracellular signal-related kinase/mitogen-activated protein kinase pathway discriminates CD4 versus CD8 lineage commitment in the thymus. J. Immunol. 1999;163:715–722. [PubMed] [Google Scholar]
- 12.Pritchard CA, Bolin L, Slattery R, Murray R, McMahon M. Post-natal lethality and neurological and gastrointestinal defects in mice with targeted disruption of the A-Raf protein kinase gene. Curr. Biol. 1996;6:614–617. doi: 10.1016/s0960-9822(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 13.Brummer T, Shaw PE, Reth M, Misawa Y. Inducible gene deletion reveals different roles for B-Raf and Raf-1 in B-cell antigen receptor signalling. EMBO J. 2002;21:5611–5622. doi: 10.1093/emboj/cdf588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagemann C, Rapp UR. Isotype-specific functions of Raf kinases. Exp. Cell Res. 1999;253:34–46. doi: 10.1006/excr.1999.4689. [DOI] [PubMed] [Google Scholar]
- 15.Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J. Biol. Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 16.Kamata T, Kang J, Lee TH, Wojnowski L, Pritchard CA, Leavitt AD. A critical function for B-Raf at multiple stages of myelopoiesis. Blood. 2005;106:833–840. doi: 10.1182/blood-2004-11-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikula M, Schreiber M, Husak Z, Kucerova L, Ruth J, Wieser R, Zatloukal K, et al. Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. EMBO J. 2001;20:1952–1962. doi: 10.1093/emboj/20.8.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wojnowski L, Stancato LF, Larner AC, Rapp UR, Zimmer A. Overlapping and specific functions of Braf and Craf-1 proto-oncogenes during mouse embryogenesis. Mech. Dev. 2000;91:97–104. doi: 10.1016/s0925-4773(99)00276-2. [DOI] [PubMed] [Google Scholar]
- 19.Wojnowski L, Zimmer AM, Beck TW, Hahn H, Bernal R, Rapp UR, Zimmer A. Endothelial apoptosis in Braf-deficient mice. Nat. Genet. 1997;16:293–297. doi: 10.1038/ng0797-293. [DOI] [PubMed] [Google Scholar]
- 20.Barnier JV, Papin C, Eychene A, Lecoq O, Calothy G. The mouse B-raf gene encodes multiple protein isoforms with tissue-specific expression. J. Biol. Chem. 1995;270:23381–23389. doi: 10.1074/jbc.270.40.23381. [DOI] [PubMed] [Google Scholar]
- 21.Emuss V, Garnett M, Mason C, Marais R. Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF. Cancer Res. 2005;65:9719–9726. doi: 10.1158/0008-5472.CAN-05-1683. [DOI] [PubMed] [Google Scholar]
- 22.Tsukamoto H, Irie A, Chen YZ, Takeshita K, Kim JR, Nishimura Y. TCR ligand avidity determines the modeof B-Raf/Raf-1/ERK activation leading to the activation of human CD4+ T cell clone. Eur. J. Immunol. 2006;36:1926–1937. doi: 10.1002/eji.200535803. [DOI] [PubMed] [Google Scholar]
- 23.Owaki H, Varma R, Gillis B, Bruder JT, Rapp UR, Davis LS, Geppert TD. Raf-1 is required for T cell IL2 production. EMBO J. 1993;12:4367–4373. doi: 10.1002/j.1460-2075.1993.tb06121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukamoto H, Irie A, Nishimura Y. B-Raf contributes to sustained extracellular signal-regulated kinase activation associated with interleukin-2 production stimulated through the T cell receptor. J. Biol. Chem. 2004;279:48457–48465. doi: 10.1074/jbc.M403087200. [DOI] [PubMed] [Google Scholar]
- 25.Dillon TJ, Karpitski V, Wetzel SA, Parker DC, Shaw AS, Stork PJ. Ectopic B-Raf expression enhances extracellular signal-regulated kinase (ERK) signaling in T cells and prevents antigen-presenting cell-induced anergy. J. Biol. Chem. 2003;278:35940–35949. doi: 10.1074/jbc.M301506200. [DOI] [PubMed] [Google Scholar]
- 26.Kurella S, Yaciuk JC, Dozmorov I, Frank MB, Centola M, Farris AD. Transcriptional modulation of TCR, Notch and Wnt signaling pathways in SEB-anergized CD4+ T cells. Genes Immun. 2005;6:596–608. doi: 10.1038/sj.gene.6364245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 28.Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 29.von Boehmer H, Aifantis I, Feinberg J, Lechner O, Saint-Ruf C, Walter U, Buer J, Azogui O. Pleiotropic changes controlled by the pre-T-cell receptor. Curr. Opin. Immunol. 1999;11:135–142. doi: 10.1016/s0952-7915(99)80024-7. [DOI] [PubMed] [Google Scholar]
- 30.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat. Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 32.Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 33.Ciofani M, Schmitt TM, Ciofani A, Michie AM, Cuburu N, Aublin A, Maryanski JL, Zuniga-Pflucker JC. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J. Immunol. 2004;172:5230–5239. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 34.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 35.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swat W, Dessing M, von Boehmer H, Kisielow P. CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur. J. Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- 37.Carey KD, Dillon TJ, Schmitt JM, Baird AM, Holdorf AD, Straus DB, Shaw AS, Stork PJ. CD28 and the tyrosine kinase lck stimulate mitogen-activated protein kinase activity in T cells via inhibition of the small G protein Rap1. Mol. Cell. Biol. 2000;20:8409–8419. doi: 10.1128/mcb.20.22.8409-8419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagy ZS, Wang Y, Erwin-Cohen RA, Aradi J, Monia B, Wang LH, Stepkowski SM, et al. Interleukin-2 family cytokines stimulate phosphorylation of the Pro-Ser-Pro motif of Stat5 transcription factors in human T cells: resistance to suppression of multiple serine kinase pathways. J. Leukoc. Biol. 2002;72:819–828. [PubMed] [Google Scholar]
- 39.Reynolds LF, de Bettignies C, Norton T, Beeser A, Chernoff J, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J. Biol. Chem. 2004;279:18239–18246. doi: 10.1074/jbc.M400257200. [DOI] [PubMed] [Google Scholar]
- 40.Eychene A, Dusanter-Fourt I, Barnier JV, Papin C, Charon M, Gisselbrecht S, Calothy G. Expression and activation of B-Raf kinase isoforms in human and murine leukemia cell lines. Oncogene. 1995;10:1159–1165. [PubMed] [Google Scholar]
- 41.Iritani BM, Alberola-Ila J, Forbush KA, Perimutter RM. Distinct signals mediate maturation and allelic exclusion in lymphocyte progenitors. Immunity. 1999;10:713–722. doi: 10.1016/s1074-7613(00)80070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wotton D, Ways DK, Parker PJ, Owen MJ. Activity of both Raf and Ras is necessary for activation of transcription of the human T cell receptor beta gene by protein kinase C, Ras plays multiple roles. J. Biol. Chem. 1993;268:17975–17982. [PubMed] [Google Scholar]
- 43.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]