Abstract
The clinical and necropsy records of 36 (25 male and 11 female) chimpanzees age 10 to 40 y old that died over a 6-y period (2001 to 2006) were reviewed. All animals had annual physical exams that included electrocardiograms and serial blood pressures. Nine of the 36 animals had a complete cardiac evaluation by a board certified veterinary cardiologist, and 7 of the 36 animals (19%) were diagnosed with some form of cardiomyopathy. Systemic hypertension was noted in 3 cases. Cardiac arrhythmias (ventricular ectopy) were seen in 15 (12 male and 3 female) of the 36 animals (42%). Sudden cardiac death (SCD) occurred in 13 (11 male and 2 female) chimps (36%) and was the leading cause of death (n = 13), followed by renal failure (n = 9) and septicemia (n = 3). Histologic examination of the hearts revealed interstitial myocardial fibrosis (IMF) in 29 chimpanzees (81%), and all of the animals that died suddenly due to cardiac causes had IMF to varying degrees. More data will be needed to identify the possible causes of IMF in captive chimpanzees, and IMF may be associated with arrhythmias and SCD in these animals.
Abbreviations: APF, Alamogordo Primate Facility; ECG, electrocardiography; IMF, interstitial myocardial fibrosis; SCD, sudden cardiac death
Myocardial fibrosis has been implicated as a factor in sudden cardiac death (SCD) in humans18 and can lead to heart failure, defects in conduction, and cardiac arrhythmias.40 SCD with concurrent myocardial fibrosis has not previously been described in chimpanzees but has been reported in other great apes.22,29 Myocardial fibrosis also has been reported in western lowland gorillas (Gorilla gorilla gorilla), orangutans (Pongo pygmaeus), white-handed gibbons (Hylobates lar), vervets (Cercopithecus aethiops), rhesus macaques (Macaca mulatta), and cynomolgus macaques (M. fascicularis).5,11,22,26,29,31
Because myocardial cells cannot regenerate, damaged myocardium is replaced by fibrous tissue, which is noncontractile collagenous tissue. Cardiac fibrosis occurs in humans secondary to systemic hypertension, myocarditis, and cardiomyopathy or as a result of any myocardial damage.18,38,39,40 An accumulation of extracellular matrix (particularly fibrillar type I and III collagen) in the cardiac interstitial space, a process defined as cardiac fibrosis), is now recognized as a major determinant of ventricular dysfunction.13 Fibrosing cardiomyopathy in great apes has been defined as myocardial replacement fibrosis with atrophy and hypertrophy of cardiac myocytes, absent or minimal myocardial inflammation, with no apparent etiology or associated disease.29 The 2 types of myocardial fibrosis are replacement (scarring) and reactive (interstitial) fibrosis. Scarring myocardial fibrosis is caused by the replacement of damaged myocardial cells with fibrous tissue, usually resulting from a vascular event such as myocardial infarction. Interstitial myocardial fibrosis (IMF) occurs when a network of collagen fibers spreads diffusely through the cardiac muscle fibers and can be caused by external stimuli such as pressure or volume overload. IMF is the form of myocardial fibrosis most often found histologically in chimpanzees.3
To the best of our knowledge, only 3 peer-reviewed publications report myocardial fibrosis in chimpanzees. One case report describes a male adult chimp that was diagnosed with hepatomegaly, congestive heart failure, and premature ventricular complexes.14 The second article describes 6 colony animals that died with myocardial disease; myocardial fibrosis was noted at necropsy, but the number of animals with this change is not mentioned.15 The third article describes myocardial fibrosis in a single chimpanzee that died suddenly at the National Zoo; this death was attributed to cardiac arrest.22 Here we review the clinical records and necropsy data for 36 captive chimpanzee that died during a 6-y period (2001 to 2006) at the Alamogordo Primate Facility (APF).
Materials and Methods
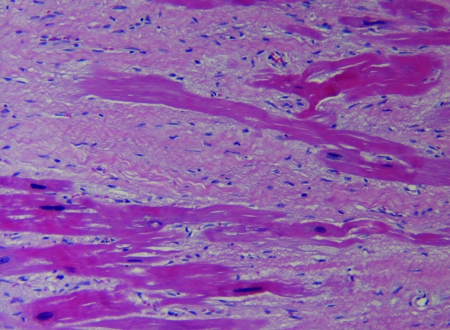
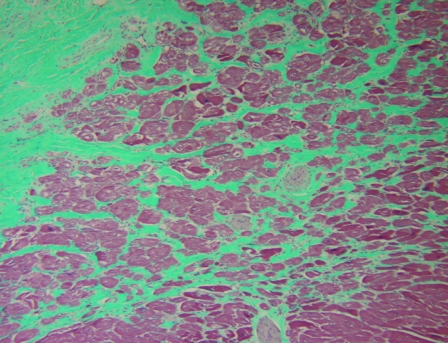
The clinical records including gross pathology and histopathology records were reviewed and evaluated for 36 captive chimpanzees that died between 2001 and 2006. Gross pathology and cardiac histopathology data were available for all 25 male chimpanzees and for 10 of the 11 female chimps in the study; histology information was not available for review in 1 of the female cases. Hematoxylin and eosin staining (Figure 1) was performed on myocardial samples from all chimpanzees, and 13 cases had Masson trichrome staining of myocardial samples to evaluate the presence of fibrosis (Figure 2).
Figure 1.
Moderate to severe myocardial fibrosis surrounding cardiac myocytes in a 33-y-old male chimpanzee. Hematoxylin and eosin stain; magnification, ×10.
Figure 2.
Mild myocardial fibrosis surrounding cardiac myocytes in a 28-y-old male chimpanzee. Masson trichrome stain; magnification, ×10.
While alive, all chimpanzees were maintained in same-sex group housing and received a commercial primate diet (Monkey Diet Jumbo 5LR2, Purina Mills International, Brentwood, MO). A maximum of 6 animals are maintained in each indoor den (180 ft2, 9.5 ft high) with radiant heated floor and air conditioning. In addition, 24-h access to an outdoor area (242 ft2, 12 ft high) is provided, along with weekly access to a Primadome (802 ft2; Brandes Brothers Constructors, Bee Cave, TX). Same-sex housing is maintained to comply with the 1997 enactment of the National Center for Research Resources breeding moratorium policy that no government-owned chimpanzees are to breed for research proposes.23 Chimpanzees at the APF are maintained in accordance with the Guide for the Care and Use of Animals.24 The facility and its program are fully accredited by AAALAC International. At the beginning of this study, the APF population consisted of 252 research veteran chimpanzees. No research occurs at APF. Each chimpanzee participates in enrichment programs and is observed every 2 h by experienced, AALAS-certified animal care technicians or clinical veterinarians. The enrichment program involves daily fruits and vegetables plus biweekly forage opportunities. Novelty items, such as blankets, magazines, and simulated termite mound feeders, are also provided. The animals are observed for general health, activity levels, elimination, exercise tolerance, and recovery rates. Each animal undergoes a complete physical examination annually while under anesthesia (3.5 mg/kg tiletamine hydrochloride–zolazepam [50 mg/ml tiletamine HCL and zolazepam HCL]), complete blood count, clinical chemistry, electrocardiogram (ECG), abdominal ultrasound, tuberculosis testing, dental prophylaxis, and blood pressure assessment. Electrocardiography (ECG) and blood pressure, partial pressure of O2, and core body temperature measurements are performed and recorded (Passport 2, Datascope, Mahwah, NJ). The ECG is monitored visually on the oscilloscope throughout anesthesia, and a 30-s representative strip is printed for each animal. Eight of the 36 animals had complete (including Doppler) serial echocardiograms performed by a board-certified veterinary cardiologist using a Prosound 5000 (Aloka, Tokyo, Japan) and a 2.5-mHz transducer.
All statistical analyses were performed with SYSTAT version 11.0 (SYSTAT Software, Chicago, IL ) by using ANOVA and the nonparametric Kruskal–Wallis test to model continuous variables and contingency table methods to model associations between categorical variables. The threshold for statistical significance was set at α = 0.05 and determined by inspection of F statistics, Pearson x2, the likelihood ratio (G2) statistic, and the Fisher exact test.1,10
Results
In all, 25 male and 11 female chimpanzees died over the 6-y period (2001 to 2006) and ranged in age from 10 to 40 y. The average age of death for male chimps was 25.5 y (median, 24.8 y) whereas that for female chimps was 21.0 y (median, 19.2 y). SCD and presumed fatal arrhythmia was the cause of death in 13 (11 male and 2 female) animals and was the most frequent cause of death (n = 13), followed by renal failure (n = 9), trauma (n = 3), septicemia (n = 3), thromboembolism (n = 2), anesthetic reaction (n = 2), neoplasia (n = 2), hyperadrenocortism (n = 1), and cerebral infarct (n = 1). Seventeen of the 36 chimps (47%) in this series had cardiac arrhythmias noted on ECG (ventricular ectopy). Twelve of the 13 chimpanzees (92%) that died of SCD had cardiac arrhythmias (ventricular ectopy). Nine of the 36 chimpanzees had complete cardiac examinations by a board certified veterinary cardiologist, and 7of these 9 animals (78%) were diagnosed with some form of cardiomyopathy, including dilated cardiomyopathy (n = 3). Other cardiac diagnoses included 1 male chimp with pulmonary hypertension and 3 (2 male, 1 female) chimpanzees with thromboembolic events (the left femoral artery was involved in all 3).
Heart weight (g):body weight (kg) ratios were estimated for all chimpanzees for which both measurements were known. These ratios were divided into 3 groups determined by cardiovascular antemortem and postmortem findings: animals with gross cardiac pathology with or without histologic myocardial fibrosis, animals with grossly normal hearts and myocardial fibrosis, and animals with grossly normal hearts without histologic evidence of myocardial fibrosis. The mean heart weight:body weight ratio in chimpanzees with gross anatomical heart disease was 0.0079 (range, 0.0067 to 0.0106; n = 9), whereas that for chimpanzees with completely normal hearts (gross and histologic analysis) was 0.0052 (range, 0.0045 to 0.0059; n = 4) and for animals with grossly normal hearts and myocardial fibrosis was 0.0056 (range, 0.0045 to 0.0087; n = 6; Table 1). One-way ANOVA of the heart weight:body weight ratio revealed statistically significant differences among heart disease categories (F2,11 = 4.57, P = 0.036), but the Kruskal–Wallis nonparametric test used to control for the presence of multiple outliers was not significant (g22 = 5.10, P = 0.078). Heart weight:body weight ratios for 17 animals were not included in the analysis because the heart was subjectively defined as hypertrophied on postmortem examination, but no other abnormality was found (postmortem contracture can make a normal heart appear hypertrophied).
Table 1.
Characteristics of the 36 chimpanzees that comprised the study population
| Animal no. | Sex | Age (y) | Heart weight (g) | Body weight (kg) | Heart weight: body weight ratio | Clinical finding | Myocardial fibrosisa | Hypertension | Myocarditis | VPCs |
| 1 | M | 33 | 463 | 60.5 | 0.0076 | SCD | +++ | yes | + | yes |
| 2 | M | 23 | 550 | 74.5 | 0.0075 | SCD | +++ | no | + | yes |
| 3 | M | 22 | 635 | 72.0 | 0.0088 | SCD | +++ | no | + | yes |
| 4 | M | 29 | 490 | 57.0 | 0.0085 | SCD | +++ | no | WNL | yes |
| 5 | M | 28 | 420 | 57.0 | 0.0074 | SCD | ++ | no | + | yes |
| 6 | M | 21 | 650 | 94.5 | 0.0069 | SCD | + | no | WNL | yes |
| 7 | M | 19 | 500 | 74.5 | 0.0067 | SCD | + | no | + | yes |
| 8 | M | 32 | 315 | 42.5 | 0.0074 | Septicemia | ++ | no | WNL | no |
| 9 | M | 39 | 350 | 40.5 | 0.0086 | Hyperadrenocortism | +++ | no | ++ | no |
| 10 | M | 27 | 530 | 74.0 | 0.0071 | Neoplasia | ++ | no | ++ | yes |
| 11 | M | 36 | 355 | 76.0 | 0.0046 | Thromboembolism | ++ | no | ++ | yes |
| 12 | M | 13 | 450 | 70.0 | 0.0064 | Septicemia | + | no | WNL | no |
| 13 | M | 25 | 660 | 62.0 | 0.0106 | SCD | +++ | yes | + | yes |
| 14 | M | 32 | 430 | 42.5 | 0.0101 | Anesthetic reaction | ++ | no | + | no |
| 15 | M | 16 | n/a | 52.0 | n/a | Septicemia | WNL | no | +++ | no |
| 16 | M | 35 | 495 | 61.0 | 0.0081 | SCD | +++ | no | WNL | no |
| 17 | M | 28 | 355 | 56.5 | 0.0063 | SCD | ++ | no | WNL | yes |
| 18 | M | 40 | 325 | 61.9 | 0.0053 | SCD | +++ | no | WNL | yes |
| 19 | M | 13 | n/a | 28.4 | n/a | Renal failure | WNL | no | WNL | no |
| 20 | M | 22 | 290 | 54.5 | 0.0053 | Trauma | ++ | no | WNL | no |
| 21 | M | 10 | 340 | 57.0 | 0.0059 | Trauma | WNL | no | WNL | no |
| 22 | M | 20 | 340 | 56.5 | 0.0060 | Trauma | ++ | no | + | no |
| 23 | M | 24 | 275 | 55.5 | 0.0049 | Thromboembolism | ++ | no | WNL | no |
| 24 | M | 30 | n/a | 47.4 | n/a | Renal failure | WNL | no | WNL | yes |
| 25 | M | 32 | n/a | 68.0 | n/a | Renal failure | ++ | no | + | yes |
| 26 | F | 25 | 360 | 49.3 | 0.0073 | Renal failure | ++ | no | ++ | no |
| 27 | F | 18 | 330 | 50.7 | 0.0065 | Renal failure | WNL | no | +++ | no |
| 28 | F | 16 | 415 | 44.0 | 0.0094 | Renal failure | ++ | no | WNL | no |
| 29 | F | 13 | 195 | 43.0 | 0.0045 | Neoplasia | + | no | WNL | no |
| 30 | F | 17 | 285 | 61.7 | 0.0046 | SCD | + | no | WNL | yes |
| 31 | F | 21 | 375 | 84.5 | 0.0044 | SCD | +++ | no | WNL | yes |
| 32 | F | 19 | 250 | 64.0 | 0.0039 | Renal failure | n/a | n/a | n/a | no |
| 33 | F | 29 | 340 | 39.0 | 0.0087 | Renal failure | + | no | WNL | yes |
| 34 | F | 31 | 263.5 | 59.0 | 0.0045 | Cerebral infarction | WNL | no | WNL | no |
| 35 | F | 18 | 250 | 37.0 | 0.0067 | Renal failure | ++ | no | WNL | no |
| 36 | F | 22 | 450 | 95.0 | 0.0047 | Anesthetic reaction | +++ | yes | WNL | no |
F, female; M, male; n/a, not available; SCD, sudden cardiac death; WNL, within normal limits
+, minimal; ++, mild; +++, moderate
b+, structural heart disease with or without myocardial fibrosis; ++, no structural heart disease and no myocardial fibrosis; +++, no structural heart disease with myocardial fibrosis
Histopathologically, all chimpanzees that were diagnosed with myocardial fibrosis had interstitial, focal, or multifocal fibrosis of the myocardium. The amount of IMF was categorized as either within normal limits, minimal, mild, or moderate (Table 1). Eight (7 male, 1 female) of the 13 animals (62%) that died suddenly had moderate amounts of IMF, which was identified in 29 (21 male, 8 female) of the 36 animals (81%). The youngest male with IMF was 13 y old whereas the oldest was 40 y (Table 1). IMF was not present in 3 male chimps (10, 13, and 16 y of age; 14% of the male animals), and none of these 3 chimps died of a cardiac event. Statistical analysis revealed that (as hypothesized) ventricular ectopy was strongly associated with SCD (G22 = 18.92, P < 0.000). The data were too sparse to reliably test for associations among the 4 levels of IMF and either ventricular premature contractions (VPC) and SCD in the full contingency table. Therefore, we hypothesized a fundamental distinction between the presence or absence of myocardial fibrosis, and collapsed myocardial fibrosis into a dichotomy (none versus some). The association between myocardial fibrosis and SCD was statistically significant (G21 = 6.29, P = 0.012), but reliability analysis indicated that this result was spurious (G21 = 2.19, P = 0.138). The association between myocardial fibrosis and VPC was not significant (G21 = 3.19, P = 0.086).
Five chimpanzees had minimal arteriosclerosis noted on histopathology, and 3 animals had atherosclerosis. Histopathologic examination revealed some degree of chronic myocardial inflammation in 13 male and 2 female chimps (Table 1). The myocarditis was described as minimal, mild, or moderate mononuclear cell infiltration and was focal or multifocal in distribution.
Ventricular ectopy was noted during physical or cardiac examinations ECG in 14 male and 3 female chimpanzees, of which 12 (10 male, 2 female) animals (86%) died from SCD. In addition, 12 animals with arrhythmias also had some amount of IMF, and 13 were noted to have structural heart disease at necropsy.
Four male chimpanzees with IMF in this study were virus-free, 7 were carriers of hepatitis C virus, 4 were positive for HIV1, and 1 was positive for hepatitis B virus. Another 5 male chimps were of mixed infectious status (positive for both hepatitis C virus and HIV), and 1 male chip was positive for both hepatitis B virus and HIV. Three female chimps with myocardial fibrosis were virus-free, 1 was a carrier for hepatitis C virus, another was infected with hepatitis B virus, and 2 others had mixed viral status (positive for both hepatitis C virus and HIV).
Other pathologic lesions included hepatocellullar carcinoma (3 animals), hepatic amyloidosis (2 animals), and hepatic abscess (1 animal). Seven of the 11 (63%) female chimps that died had renal disease, whereas 3 of the 25 (12%) male chimps had renal disease. Blood pressure data was available for all 36 chimpanzees. At APF, a systolic blood pressure of greater than or equal to 180 mm Hg or a diastolic blood pressure of greater than or equal to 90 mm Hg on 3 readings at 2 separate consecutive time points is defined as evidence of systemic hypertension necessitating treatment. Three (2 male and 1 female) animals were identified as hypertensive based on these criteria. Both hypertensive male chimps died suddenly, and a moderate amount of IMF with mild hypertrophy was noted at necropsy. The single female animal with systemic hypertension died from an anesthetic reaction and had a moderate amount of IMF.
Discussion
The most common cause of death in this group of chimpanzees was cardiac disease manifesting as SCD. SCD is an unexpected death in a subject with or without preexisting heart disease, within 1 h of onset of change in clinical status, usually resulting from a fatal arrhythmia.42 When due to a fatal arrhythmia, the cause of death is rarely evident on post mortem examination. The definition of SCD for chimpanzees at the APF differs only in the time frame, because animals are observed every 2 h during a 24-h period.
Myocardial fibrosis has occasionally been reported in captive chimpanzee populations,14,15,22 but the majority of cardiovascular information used in chimpanzee medicine has been extrapolated from literature for humans or other great apes. To our knowledge, this paper is the first description of IMF in chimpanzees that includes a considerable number of cases.
For the statistical analysis, the fundamental working model was that fibrosis leads to ventricular ectopy, which leads to SCD. This model was confirmed in part. Ventricular ectopy was strongly associated with SCD, as was noted previously in the colony,8 but we found no evidence for a causal prior association between myocardial fibrosis and VPC and only weak support for an association between myocardial fibrosis and SCD. Furthermore, post hoc analyses revealed a strong association between myocardial fibrosis and SCD, when contrasting the highest level of myocardial fibrosis to all lower levels (G21 = 11.15, P < 0.000). This result indicated a potentially strong causal relationship between interstitial myocardial fibrosis and SCD in the APF captive chimpanzee colony that demands further investigation.
Excessive myocardial fibrosis inhibits diastolic function in hypertensive human hearts.17,18 Comparative studies in rats have shown that an increased salt intake not only elevated blood pressure but also negatively affected the heart, vasculature, and kidneys with marked interstitial fibrosis of these organs.41 Normative blood pressure values for chimpanzees were reported 20 y ago, and the effect of sodium intake on systemic blood pressure has been studied more recently.6,9 However, an association between high blood pressure, heart disease, and IMF has not previously been evaluated in the chimpanzee. In our study, 3 of these 36 chimpanzees had systemic hypertension, and all 3 of them had histologic evidence of moderately severe IMF, suggesting an association between hypertension and IMF may exist in chimpanzees.
Renal failure was the second most common cause of death in this population of chimpanzees. Renal dysfunction can be associated with IMF. Renal disease has been linked to acute coronary syndromes, cardiac failure, and valvular heart disease in humans36 and the renin–angiotensin–aldosterone system plays an important role in the regulation of cardiac fibrosis.2 The hormones of the renin–angiotensin–aldosterone system are believed to modulate the synthetic activity of the cardiac fibroblast.39 Myocardial fibrosis commonly occurs in patients with end-stage renal failure, and it is an important predictor of cardiovascular events.32 In humans, activation of the renin–angiotensin–aldosterone system may promote the development of cardiac fibrosis independent of hemodynamic mechanisms.28 In human uremic patients, cardiac fibrosis is associated with an increased risk cardiac death due to arrhythmias.37 Nine (6 female and 3 male) of the 36 animals (25%) in the present study had renal disease, with 5 of these 9 animals having IMF at necropsy, suggesting a possible link between renal disease and IMF in chimpanzees.
Normal aging of the heart is associated with a number of characteristic morphologic, histologic, and biochemical changes.27 In humans, cardiac fibrosis is universal in the aging heart.2 Excessive deposition of extracellular matrix proteins during aging leads to progressive reduction of myocardial and arterial compliances.21 A collagen network, composed largely of type I and III fibrillar collagen, is present in the cardiac interstitial space, with type I collagen being the major component (approximately 75%) and determinant of tissue stiffness.35 Measurements of collagen content in myocardial tissue suggest that type I collagen fibers increase in number and thickness in the aged.7 Due to medical advances and excellent preventive medicine programs, captive chimpanzees are now living longer. Consequently, the increased life span creates a geriatric population with geriatric medical concerns. In this study, of the 4 chimpanzee males with no IMF, 3 were at least 10 y younger than the average age of male chimps at death. In addition, 1 of the 2 female chimps without IMF was younger than the average age of female chimps at death. Therefore, IMF may occur as an age-related change in chimpanzees.
The chimpanzees at APF have varied experimental backgrounds, including exposure to hepatitis viruses and HIV.8 HIV and hepatitis C virus both have been associated with cardiomyopathies in humans.4,16 SIV has been linked to myocarditis and cardiomyopathies in nonhuman primates.30 Further investigations are needed to correlate viral infection with cardiovascular disease in this population.
Interstitial myocardial fibrosis may be associated with an inflammatory response.25 The infiltration of inflammatory cells can play a critical part of the process resulting in cardiac fibrosis.19 Fifteen (13 male and 2 female) of the 26 animals (42%) had myocardial infiltration of inflammatory cells at necropsy; in addition, 13 of the 15 animals (87%) also had IMF. A multifocal, mononuclear cell infiltrate was the most commonly reported inflammatory histopathologic change.
The size of the adult chimpanzee heart corresponds with the relative heart weights calculated for adult human men.12 However, whereas the average adult human has a heart weight of 195 to 300 g,33 only 7 of our chimpanzees had heart weights that were within this normal human range, and 5 of these hearts had IMF. Because body weights in chimpanzees vary broadly, we attempted to normalize heart weight by looking at the heart weight:body weight ratio. Our results, although of marginal statistical significance, suggest that chimpanzees with gross structural heart disease may have greater heart weights, compared with those having normal hearts or hearts that are grossly normal but have concurrent histologic evidence of IMF (Table 1). Because heart weight can vary allometrically relative to body size,20 future studies should apply allometric approaches to better analyze the effects of heart disease in altering heart weight. Meanwhile, this information may be helpful for those performing necropsies on this species and attempting to determine whether heart enlargement is present.
Heart disease and renal disease are major causes of morbidity and mortality in the captive chimpanzee population, and the frequent finding of IMF in 92% of animals that died suddenly suggests that it is an important factor. In humans, cardiac fibrosis confers an increased risk for such adverse cardiovascular events as ventricular dysfunction and dysrhythmias.38 In vivo, the presence of fibrotic myocardium with heterogeneity of electrical properties greatly enhances the likelihood of reentrant tachyarrhythmias.2 Myocardial fibrosis has also been noted to cause sudden death in humans by predisposing the myocardium to electrical instability, reentry circuits, and fatal arrhythmias.18 Interstitial fibrosis reduces the electrical coupling between cardiac myocytes because fibroblasts produce smaller or larger collagenous septa, which electrically insulate cardiac cells or muscle bundles.2 Cardiac fibrosis has not been well documented in captive chimpanzees; however, numerous dysrhythmias have previously been noted in this colony, which includes animals previously used for research.8,34 This retrospective study suggests a link between ventricular dysrhythmias and IMF in chimpanzees.
This study was limited by the small number of subjects, its retrospective nature, the subjective assessment of fibrosis, and dependency on records, which may be incomplete. However, the data suggest an association between IMF, cardiac arrhythmias, and SCD in chimpanzees. Further evaluation of the pathophysiology and objective measurement of fibrosis will help elucidate the significance of IMF in the chimpanzee and also may result in useful information for humans with IMF.
Acknowledgments
This study was supported by NIH contract NO2-RR-1-209. The authors are grateful for the technical and scientific advice provided by Drs Dan Anderson, James Else, and James Herndon at the Yerkes Primate Center.
References
- 1.Agresti A. 2002. Categorical data analysis, 2nd ed New York: Wiley [Google Scholar]
- 2.Allessie M, Schotten U, Verheule S, Harks E. 2005. Gene therapy for repair of cardiac fibrosis: a long way to Tipperary. Circulation 111:391–393 [DOI] [PubMed] [Google Scholar]
- 3.Baskins G.2006. Personal communication.
- 4.Barbaro G. 2005. HIV-associated cardiomyopathy: etiopathegenesis and clinical aspects. Herz 30:486–492 [DOI] [PubMed] [Google Scholar]
- 5.Borkowski R, Taylor TG, Rush J. 2000. Cerebral infarction and myocardial fibrosis in a white-handed gibbon (Hylobates lar). J Zoo Wildl Med 31:65–70 [DOI] [PubMed] [Google Scholar]
- 6.Denton D, Weisinger R, Mundy NI, Wickings EJ, Dixson A, Moisson P, Pingard AM, Shade R, Carey D, Ardaillou R, Paillard F, Chapman J, Thillet J, Michel JB. 1995. The effects of salt intake on blood pressure of chimpanzees. Nat Med 1:1009–1016 [DOI] [PubMed] [Google Scholar]
- 7.de Souza RR. 2002. Aging of myocardial collagen. Biogerontology 3:325–335 [DOI] [PubMed] [Google Scholar]
- 8.Doane CJ, Lee DR, Sleeper MM. 2006. Electrocardiogram abnormalities in captive chimpanzees. Comp Med 56:512–518 [PubMed] [Google Scholar]
- 9.Eichberg JW, Shade RE. 1987. ‘Normal’ blood pressure in chimpanzees. J Med Primatol 16:317–321 [PubMed] [Google Scholar]
- 10.Fienberg SE. 1980. The analysis of cross-classified categorical data. Cambridge (MA): MIT Press [Google Scholar]
- 11.Fincham JE, Woodroof CW, van Wyk D, Capatos D, Weight MJ, Krichevsky D, Rossouw JE. 1987. Promotion and regression of atheroscerosis in vervet monkeys by diets realistic for westernized people. Atherosclerosis 66:205–213 [DOI] [PubMed] [Google Scholar]
- 12.Frick H. 1973. Heart of the chimpanzee. Bourne GH. The chimpanzee: immunology, infection, hormones, anatomy, and behavior, vol 3 Baltimore (MD): University Park Press; p 295–336 [Google Scholar]
- 13.Guntaka RV, Weber KT. 2005. Molecular strategies for the prevention of cardiac fibrosis. Villareal FJ. Interstitial fibrosis in heart failure. New York: Springer Media; p 329–341 [Google Scholar]
- 14.Hansen JF, Alford PL, Keeling ME. 1984. Diffuse myocardial fibrosis and congestive heart failure in an adult male chimpanzee. Vet Pathol 21:529–531 [DOI] [PubMed] [Google Scholar]
- 15.Hubbard GB, Lee DR, Eichberg JW. 1991. Diseases and pathology of chimpanzees at the Southwest Foundation for Biomedical Research. Am J Primatol 24:273–282 [Google Scholar]
- 16.Iskander SB, Loyd S, Downs CJ, Roy TM. 2004. Hepatitis C and dilated cardiomyopathy. TN Med 97:31–33 [PubMed] [Google Scholar]
- 17.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T. 2004. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension 43:739–745 [DOI] [PubMed] [Google Scholar]
- 18.Lecomte D, Fornes P, Fouret P, Nicolas G. 1993. Isolated myocardial fibrosis as a cause of sudden cardiac death and its possible relation to myocarditis. J Forensic Sci 38:617–621 [PubMed] [Google Scholar]
- 19.Levick SP, Loch DC, Taylor SM, Janicki JS. 2007. Arachidonic acid metabolism as a potential mediator of cardiac fibrosis associated with inflammation. J Immunol 178:641–646 [DOI] [PubMed] [Google Scholar]
- 20.Li JK-J, Zhu Y, Noordegraaf A. 2006. A comparative approach to analysis and modeling of cardiovascular function. : Bronzino JD. Biomedical engineering fundamentals. Boca Raton (FL): CRC Press; p 17.1–17.12 [Google Scholar]
- 21.Masson S, Latini R, Salio M, Fiordalsio F. 2005. Cardiac fibrosis and aging. : Razzaque M. Fibrogenesis: cellular and molecular basis. Austin (TX): Landes Bioscience; p 97–103 [Google Scholar]
- 22.Munson L, Montali RJ. 1990. Pathology and disease of great apes at the National Zoological Park. Zoo Biol 9:99–105 [Google Scholar]
- 23.National Research Council 1997. Chimpanzees in research: strategies for their ethical care, management, and use. Committee on Long-Term Care of Chimpanzees Institute for Laboratory Animal on Life Sciences. Commission on Life Sciences. Washington (DC): National Academy Press [Google Scholar]
- 24.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 25.Nicoletti A, Michel JB. 1999. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res 41:532–543 [DOI] [PubMed] [Google Scholar]
- 26.Pick R, Janicki JS, Weber KT. 1989. Myocardial fibosis in nonhuman primate with pressure overload hypertrophy. Am J Pathol 135:771–781 [PMC free article] [PubMed] [Google Scholar]
- 27.Roffe C. 1998. Ageing of the heart. Br J Biomed Sci 55:136–148 [PubMed] [Google Scholar]
- 28.Saeed A, Guron G, Oldfors A, Lindelow B, Herlitz H. 2006. Cardiac fibrosis triggered by the kidney: a case report. Nephrol Dial Transplant 21:1713–1715 [DOI] [PubMed] [Google Scholar]
- 29.Schulman FY, Farb A, Virmani R, Montali RJ. 1995. Fibrosing cardiomyopathy in captive western lowland gorillas (Gorilla gorilla gorilla) in the United States: a retrospective study. J Zoo Wildl Med 26:43–51 [Google Scholar]
- 30.Shannon RP. 2001. SIV cardiomyopathy in nonhuman primates. Trends Cardiovasc Med 11:242–246 [DOI] [PubMed] [Google Scholar]
- 31.Shannon RP, Simon MA, Mathier MA, Geng YJ, Mankad S, Lacker AA. 2000. Dilated cardiomyopathy associated with simian AIDS in nonhuman primates. Circulation 101:185–193 [DOI] [PubMed] [Google Scholar]
- 32.Shibasaki Y, Nishiue T, Masaki H, Yamura K, Matsumoto N, Mori Y, Nishikawa M, Matsubara H, Iwasaka T. 2005. Impact of angiotensin II receptor antagonist, losartan, on myocardial fibrosis in patients with end-stage renal failure: assessment by ultrasonic integrated backscatter and biochemical markers. Hypertens Res 28:787–795 [DOI] [PubMed] [Google Scholar]
- 33.Silver MA, Rowley NE. 1988. The functional anatomy of the human coronary sinus. Am Heart J 115:1080–1084 [DOI] [PubMed] [Google Scholar]
- 34.Sleeper MM, Doane CJ, Langner PH, Curtis SK, Avila K, Lee DR. 2005. Successful treatment of idiopathic dilated cardiomyopathy in an adult chimpanzee (Pan troglodytes). Comp Med 55:80–84 [PubMed] [Google Scholar]
- 35.Sun Y, Weber KT. 2005. Animal models of cardiac fibrosis. Methods Mol Med 117:273–290 [DOI] [PubMed] [Google Scholar]
- 36.Szczech L, Chew D, Coladonato J, Reddan D. 2002. The heart and the renal system. : Topol Textbook of cardiovascular disease, 2nd ed Philadelphia: Lippencott, Williams, and Wilkins; p 799–814 [Google Scholar]
- 37.Tyralla K, Amann K. 2002. Cardiovascular changes in renal failure. Blood Purif 20:462–465 [DOI] [PubMed] [Google Scholar]
- 38.Weber KT. 2004. Fibrosis in hypertensive heart disease: focus on cardiac fibroblasts. J Hypertens 22:47–50 [DOI] [PubMed] [Google Scholar]
- 39.Weber KT, Brilla CG. 1991. Pathological hypertrophy and cardiac interstitium. Fibrosis and the rennin–angiotensin–aldosterone system. Circulation 83:1849–1865 [DOI] [PubMed] [Google Scholar]
- 40.Wigley FM. 2004. Scleroderma (systemc sclerosis). : Goldman L, Ausiello D. Cecil textbook of medicine. Philadelphia: Saunders; p 1674–1675 [Google Scholar]
- 41.Yu HC, Burrell LM, Black MJ, Dilley RJ, Cooper ME, Johnston CI. 1998. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 98:2621–2628 [DOI] [PubMed] [Google Scholar]
- 42.Zipes DP, Rupart M. 2006. Neural modulation of cardiac arrhythmias and sudden death. Heart Rhythm 3:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]