Abstract
Minute virus of mice (MVM) is a major concern for laboratory animal facilities because it remains with considerably high prevalence despite strict barrier systems. The aim of this study was to elucidate potential risks associated with MVM infection by investigating the role of the genetic background on antibody production and persistence as well as viral shedding. Mice of various strains and stocks were inoculated oronasally with the immunosuppressive strain MVMi; in addition, natural infection was modeled through contact exposure. As determined by serology, seroconversion and serum levels of IgG differed considerably among strains and stocks, especially in the contact-exposed group. For example, C57BL/6J mice responded well to exposure in contrast to FVB/N, NMRI, ICR, and C3H/HeN mice. Titration studies indicated that the viral dose necessary to induce seroconversion was strain-dependent. Experiments to dissect the role of the major histocompatibility complex haplotype in the response to MVMi gave inconclusive results. To detect viral persistence, spleens and feces were analyzed by PCR at 16 wk after exposure, and the infectivity of PCR-positive spleens was investigated by IP and oronasal inoculation of naive mice. Although DNA was detected in the spleens of some mice, feces remained negative, and naive mice were not infected by inoculation. In addition, viral shedding declined rapidly after day 20 postinoculation. In summary, the data show that seroconversion and antibody response to MVMi infection depend on the genetic background of mice, with the infective dose being a critical factor. The role of viral DNA in chronically infected mice will require further elucidation.
Abbreviations: B6, C57BL/6J; BQ, B10/Q-H2q/SgAi; ICR, CRL:CD1 ICR; IFA, immunofluroescent assay; MHC, major histocompatibility complex; MPV, mouse parvovirus; MVM, minute virus of mice; NS, nonstructural protein; VP, virion protein
Minute virus of mice (MVM) is one of the most frequent viral pathogens in laboratory mice. Interestingly, this high prevalence occurs not only in conventionally housed mice but also in mice maintained behind strict barrier systems and monitored by extensive health surveillance programs. Therefore, parvoviruses remain a major threat to laboratory mouse colonies.
The prototype strain MVMp was originally isolated in 1966.6 The immunosuppressive variant MVMi was isolated from a transplantable mouse lymphoma and replicates in T-lymphocytes.4 Natural hosts of MVM are laboratory and wild mice, although hamsters and rats are susceptible to experimental infection.14,23 MVM is highly resistant in the environment and a common contaminant of cell lines and transplantable tumors.20
Although clinical signs and morbidity can occur after experimental infection with MVM, natural infection in mice is usually asymptomatic.5,11 However, MVM might interfere considerably with research, because this virus has strong inhibitory effects on allogeneic mixed lymphocyte cultures and inhibits various T-lymphocyte functions in vitro, namely antigen-induced proliferation, generation of cytolytic T cell activity, and T helper cell function.6,7 In addition, MVM mediates acute myelosuppression in vitro and in vivo and is a potential oncosuppressive agent.8,15,18,22
Because MVM infection lacks characteristic clinical signs and pathologic lesions, monitoring of mouse populations usually relies on serologic testing. Indirect immunofluorescent assay (IFA) exhibits some degree of cross reactivity with mouse parvovirus (MPV) and other closely related parvoviruses because of high conservation of nonstructural proteins (NS). In contrast, assays for virion proteins (VP), such as the hemagglutination inhibition assay and recombinant VP2 ELISA, are specific for MVM.17 When serologic methods are used for health surveillance, mouse strain-specific antibody responses to parvoviral infection (as shown for MPV1)2 should be considered. Recently, several PCR assays valuable for confirming serologic results or detecting viral DNA in feces from infected mice have become available.1,21,27
The aim of the present study was to evaluate potential concerns regarding MVM infection of experimental mouse populations. We investigated whether antibody production to MVMi depends on the genetics of the mouse strain, especially the haplotype of the major histocompatibility complex (MHC), and therefore might interfere with health monitoring. Furthermore, we assessed whether MVMi establishes a persistent infection in mice by evaluating the persistence of viral DNA, infectious virus, and viral shedding and whether persistence depends on the genetic background of mice.
Materials and Methods
Mice.
Female C57BL/6J (B6), FVB/N, BALB/c, NMRI, DBA/2J, AKR/N, and C3H/HeN mice (age, 6 to 9 wk) were obtained from the Central Animal Facility (Hannover Medical School, Hannover, Germany), and 6-wk-old female CRL:CD1 ICR (ICR) mice were purchased from Charles River Laboratories (Sulzfeld, Germany). Six-wk-old B10/Q-H2q/SgAi (BQ) and C57BL/10 (B10) mice were purchased from Taconic (Hudson, New York, USA).Routine microbiologic monitoring according to recommendations of the Federation of European Laboratory Animal Science Associations did not reveal any evidence of infection with common murine pathogens.19 For infection experiments, mice were maintained under negative pressure in plastic film isolators (Metall + Plastik GmbH, Radolfzell-Stahringen, Germany) or in static microisolators (located in a room with controlled environment: 21 ± 2 °C; relative humidity, 55% ± 5%; 12:12-h light:dark cycle, 12 to 14 air changes hourly). Pelleted irradiated diet (ssniff M-Z, Soest, Germany) containing 22.0% protein, 4.5% fat and 3.9% fiber and autoclaved distilled water were provided ad libitum.
This study was conducted in accordance with German law for animal protection and with the European Communities Council Directive 86/609/EEC for the protection of animals used for experimental purposes. All experiments were approved by the Local Institutional Animal Care and Research Advisory committee and permitted by the local government.
Virus.
MVMi was propagated in NBK newborn kidney cells (both virus and cells were kindly provided by W Nicklas, German Cancer Research Centre, Heidelberg, Germany). NBK cells were maintained in 75 cm2 culture flasks in M199 medium supplemented with 2% heat-inactivated FCS. Subconfluent cell cultures were infected with MVMi for 2 to 3 h, after which virus suspension was removed and replaced with M199. At complete cytopathic effect, cells underwent 3 freeze–thaw cycles. Contents of cell culture flasks were centrifuged at 5000 x g for 5 min to pellet cell debris. Resulting viral stocks were stored at –80 °C until use. Titration of virus was performed as described previously.9 Briefly, NBK cells were seeded in flat-bottomed 96-well plates (7.5 × 103 cells/well) and cultured overnight in M199 medium supplemented with 10% FCS. After removal of culture medium, cells were infected with 300 µl of 10-fold dilutions of virus using 10 wells for each dilution. Cytopathic effect was determined on the sixth day of culture and was defined as complete loss of cell-to-cell contact (cell detachment). The mean tissue culture infective dose (TCID50) was calculated according to the Spearman–Kaerber method.13,25
Infection experiments.
Mice were separated into 3 groups: inoculated animals, contact-exposed animals, and controls. To obtain contact-exposed mice, 5 noninoculated animals were placed in the cage of 5 inoculated mice of the same strain directly after inoculation. Mice were inoculated oronasally (each inoculated mouse received 7 × 102 TCID50) by placing 10 µl on the external nares and applying 50 µl orally. The control group for each strain comprised 2 mice that were housed separately from exposed animals. Blood samples were taken from the retroorbital sinus of ether-anesthetized mice at 5, 9, 13, and 16 wk after exposure. The resulting sera of all experiments were heat-inactivated at 56 °C for 30 min and stored at –20 °C until use. At the end of the experiments (16 wk postexposure), all mice were euthanized by CO2 inhalation and cardiocentesis. All samples (including spleens and feces) harvested during necropsies were stored at –20 °C until use. All manipulations on animals were performed under sterile conditions in a class II biological safety cabinet.
Because we noted during the study that the antibody responses of contact-exposed but not inoculated FVB/N mice were markedly lower than those of B6 mice, we performed an additional experiment. Two FVB/N mice were inoculated as described earlier and housed with 4 noninoculated B6 mice, and 2 B6 mice were inoculated and kept together with 4 noninoculated FVB/N mice. Blood samples were taken at 5, 9, and 14 wk after exposure.
Titration studies in B6 and FVB/N mice.
To determine the dose of viral inoculum required for seroconversion of B6 and FVB/N mice, 6-wk-old mice (3 mice/group) were infected oronasally with MVMi at 7 × 102 TCID50, 3.5 × 102 TCID50, 35 TCID50, 3.5 TCID50, and 0 TCID50 (negative control; M199 medium only). After 4 wk all mice were euthanized and bled by cardiocentesis during necropsy.
Inoculation of MHC-congenic mice.
After noting that contact-exposed mouse strains with low antibody responses shared the MHC-q haplotype, we performed an additional experiment to determine the role of the MHC haplotype in the antibody response to MVMi. Because contact-exposed B6 mice (MHC-b haplotype) showed high antibody responses, 5 B6 mice were inoculated as described earlier and housed with 5 noninoculated B10/Q-H2q/SgAi (BQ) mice, which express the MHC-q haplotype on a B10 background. To verify that B6 and B10 mice produce comparable antibody responses, a control group of 5 inoculated B6 mice was housed with 5 noninoculated B10 mice. After 4 wk, all mice were euthanized and bled by cardiocentesis during necropsy.
Studies of fecal viral shedding in FVB/N mice.
Mature female and male FVB/N mice, 10 to 12 wk old, were inoculated oronasally with 3.5 × 102 TCID50 and maintained in static microisolators as described earlier. Spleens, feces, and blood from 3 mice of each gender were harvested every other day from day 1 until day 15 and every fifth day until day 30 after inoculation. Viral DNA was extracted from fecal pellets by using the PSP Spin Stool DNA Kit (Invitek, Berlin, Germany) according to the manufacturer's instructions. DNA was prepared from spleens as described later for PCR analysis. Serum was harvested from blood samples and treated as described earlier.
Mouse antibody production test.
Each of the 4 female B6 mice (6 wk old) in 2 groups received a homogenized spleen by both IP and intranasal routes. Spleens were derived from PCR-positive inoculated FVB/N mice euthanized at 16 wk postexposure. Mice in the control group each received a suspension containing a PCR-positive spleen of a FVB/N mouse euthanized at day 11 postinoculation. Mice were maintained in static microisolators under the husbandry conditions as described earlier, and after 4 wk spleens, mesenteric lymph nodes, feces, and blood were harvested and stored as described.
ELISA.
The rVP2 ELISA17 was purchased from Charles River Laboratories (Maastricht, Netherlands) and performed according to the manufacturer's instructions. Briefly, 50 µl of serum, diluted 1:50 in calcium- and magnesium-free PBS supplemented with 5% FCS, was added to the antigen and tissue control wells, and plates were covered and incubated at 37 °C for 40 min. Subsequently, plates were washed with 100 μl PBS containing Tween 20 and incubated for 3 min at room temperature; this process was repeated twice for a total of 3 washes. To each well, 50 µl of affinity-purified, peroxidase-conjugated goat antimouse IgG (Charles River Laboratories), diluted 1:5000 in PBS, was added. After incubation for 40 min at 37 °C, plates were washed 3 times as described above, and 100 µl of 0.4 mM ABTS–2 mM H2O2 chromogenic substrate was added to each well. Plates were covered and incubated for 40 min at room temperature, and absorbance was measured at a wavelength of 405 nm. The net absorbance values (antigen well minus tissue control well) were converted to scores by dividing by 0.13. A net score of 3 or greater was considered positive, according to the manufacturer's recommendations and own experience. Positive and negative control samples were included in each assay.
IFA.
MVM IFA slides were prepared by acetone fixation of MVM-infected L cells (the prototype strain of MVM [ATCC VR663] was used to infect murine fibroblast L929 cells), propagated on Teflon-coated 8-well slides. For detection of MVM-specific antibodies, 32 µl of serum diluted 1:20 was added to each well. Slides were incubated for 20 min at room temperature, washed twice for 10 min in PBS, and air dried. Affinity-purified fluoroscein isothiocyanate-labeled goat antimouse IgG (diluted 1:40, 25 µl) was added to each well. After 20 min of incubation at room temperature, slides were washed twice for 10 min in PBS and counterstained for 5 min with 0.0005% aqueous Evans Blue solution. Slides were examined by microscopy (model DM 2000, Leica, Microsystems AG, Wetzlar, Germany). By comparing the intensity and distribution of the resulting fluorescence with that of positive and negative control wells, the fluorescence of samples was scored as 0 (negative), 1 (weak), 2 (pronounced), or 3 (very strong).
PCR.
DNA was extracted from mouse spleens by use of the NucleoSpin Tissue kit (Macherey–Nagel, Düren, Germany) in accordance with manufacturer's instructions. DNA was assessed after extraction by electrophoresis on a 1.0% SeaKem agarose gel (Biozym, Hamburg, Germany) stained with ethidium bromide (Roth, Karlsruhe, Germany). Viral DNA was amplified by using a parvovirus PCR assay specific for a 310-bp product from the conserved NS gene region.27 Primer sequences were MVM-1458f (5′ACC AGC CAG CAC AGG CAA ATC TAT3′) and MVM-1791r (5′CAT TCT GTC TCT GAT TGG TTG AGT3′); the annealing temperature was set to 61.5 °C. Positive results were confirmed by using a PCR assay specific for MVM.1 The primers used to amplify a 735-bp region from the VP2 gene of MVM were: MVM-2929f (5′AAA TTA CTG CAC TAG CAA CTA GAC3′) and MVM-3713r (5′CTT CAG GAA AGG TTG ACA GCA 3′), with an annealing temperature of 60 °C. All amplification products were loaded onto a 1.5% SeaKem agarose gel (Biozym) containing SYBR Green (Gel Star, 4 µl/100 ml; Biozym) and visualized by using UV light. Molecular weight markers were used to confirm the sizes of amplicons.
Statistics.
ANOVA and subsequent Bonferroni multiple comparison tests were used to determine whether results obtained by ELISA differed significantly between the different groups of mice (significance levels were set to 5%). To detect differences in semiquantitative fluorescence intensity scores among the groups of animals, Kruskal–Wallis and subsequent Mann–Whitney U tests were performed, with the significance level adapted by using Bonferroni correction (P = 0.05 divided by the number of comparisons). A Pearson test was used to determine whether IFA and ELISA results correlated significantly. All statistical analyses were performed by use of GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
Results
The proportion of mice that seroconverted varied among strains.
To determine whether antibody production to MVMi depends on the genetic background, mice of 8 strains and stocks were either inoculated or contact-exposed as described above. After 5, 9, 13, and 16 wk, all mice were tested by serology for MVM-specific antibodies. The percentage of seroconversion (qualitative results) at 5 wk postexposure was determined and the levels of MVM-specific antibodies were compared semiquantitatively by using IFA and ELISA.
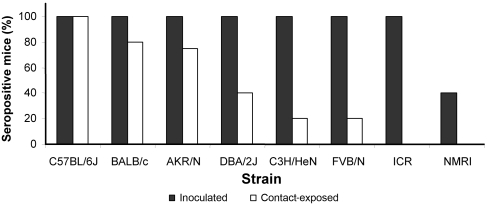
Seroconversion at 5 wk postexposure varied among mouse strains and between inoculated and contact-exposed mice. As determined by IFA, all inoculated mice had seroconverted 5 wk postexposure, except for NMRI mice (only 40% seropositive). Among contact-exposed mice, the percentage of seroconversion varied from 100% in B6 mice to only 20% in FVB/N mice and 0% in NMRI and ICR animals (Figure 1).
Figure 1.
Effect of mouse strain on seroconversion at 5 wk after exposure to MVMi in inoculated and contact-exposed mice by IFA. Seroconversion varied among mouse strains and between inoculated and contact-exposed mice.
Similar results were obtained by ELISA. Seroconversion at 5 wk postexposure was detected in all inoculated mice, except for NMRI mice (60% seropositive) and DBA/2J (80% seropositive). In contact-exposed mice, seroconversion was detected in 80% of C57BL/6J, 60% of BALB/c, 40% of DBA/2J, 25% of AKR/N (1 animal had to be euthanized due to causes not related to infection), 20% of NMRI, and 20% of C3H/HeN mice; no seroconversion occurred in FVB/N or ICR mice. Results of IFA and ELISA correlated (P < 0.0001; r = 0.76), but slightly more seropositive animals were detected using IFA; therefore, IFA results are shown in Figure 1.
Serum levels of MVMi-specific antibodies depended on the genetic background of mice.
IFA and ELISA were used to quantify MVM-specific antibodies in experimentally and contact-exposed mice of all strains at 5, 9, 13, and 16 wk post exposure. The results of IFA and ELISA correlated significantly (P = 0.0001, r = 0.7598) as determined by the Pearson test. Again, IFA identified a few more positive mice than did ELISA.
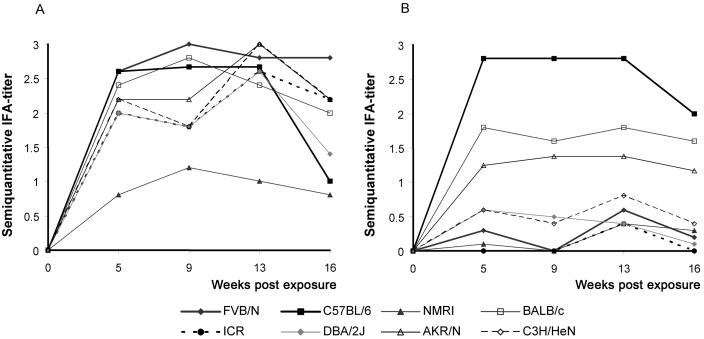
As detected by IFA, a high antibody response at 5 wk postexposure was detected among inoculated animals(with the exception of NMRI mice) but only in contact-exposed B6 mice (Figure 2). Contact-exposed FVB/N, NMRI, ICR, C3H/HeN, and DBA/2J mice showed low or no antibody response and BALB/c and AKR/N mice showed intermediate antibody responses at all times investigated. These findings were consistent over the 16-wk observation period, with the exception of intermediate responses in inoculated ICR, DBA/2J, and C3H mice at 9 wk postexposure and B6 and DBA/2J mice at 16 wk. Therefore, the B6 and DBA/2J strains showed a drop in antibody levels over the evaluation period.
Figure 2.
Mean semiquantitative IFA score of (A) inoculated and (B) contact-exposed mouse strains at 5, 9, 13, and 16 wk after exposure.
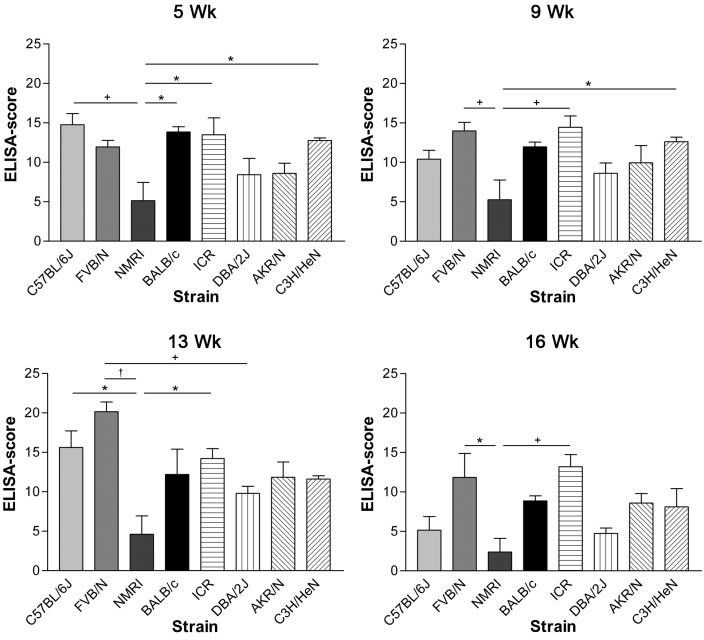
Results obtained by ELISA are shown in Figures 3 (inoculated mice) and 4 (contact-exposed mice). In accordance with IFA results, inoculated NMRI mice showed lower antibody responses than did most other strains; the differences in responses were significant (P < 0.05) when compared with those of BALB/c mice at 5 wk postinoculation; C3H/HeN mice at 5 and 9 wk; B6 mice at 5 and 13 wk; FVB/N mice at 9, 13, and 16 wk; and ICR mice at all time points. In addition, DBA/2J mice showed significantly (P < 0.05) lower antibody responses than did FVB/N mice at 13 wk post inoculation.
Figure 3.
Serum antibody levels (mean + SD) detected by ELISA in inoculated mouse strains at 5, 9, 13, and 16 wk postexposure. ANOVA revealed differences in the antibody responses at all times investigated (5 wk, P = 0.001; 9 wk, P = 0.0032; 13 wk, P = 0.0002; 16 wk, P= 0.0052). Significance levels of subsequent Bonferroni multiple comparison tests were: *, P < 0.05; +, P < 0.01; and †, P < 0.001. Except for NMRI mice, all inoculated animals showed high serum antibody levels at all time points.
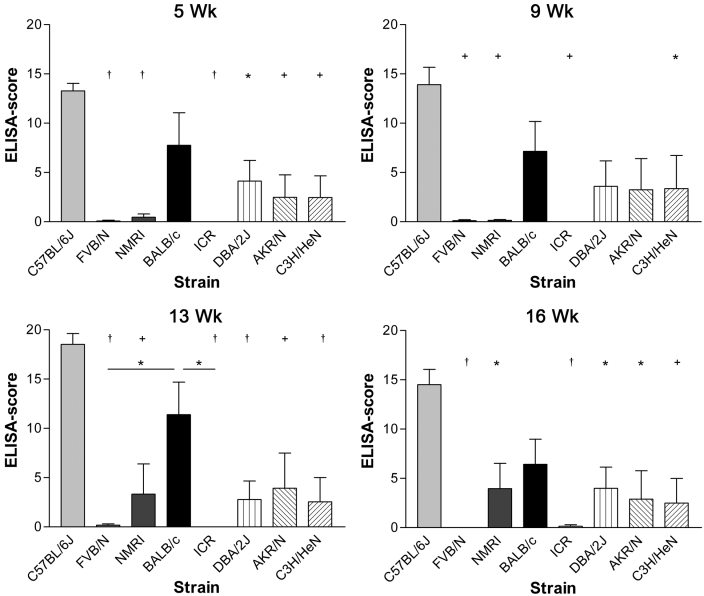
Figure 4.
Serum antibody levels (mean + SD) detected by ELISA in contact-exposed mouse strains at 5, 9, 13, and 16 wk postexposure. ANOVA revealed differences in the antibody responses at all times investigated (5 wk, P < 0001; 9 wk, P = 0.002; 13 wk, P < 0.0001; and 16 wk, P = 0.0004). Significance levels of subsequent Bonferroni multiple comparison tests were: *, P < 0.05; +, P < 0.01; and †, P < 0.001. Significant differences between each strain and B6 are shown; in addition, differences between BALB/c and FVB/N and BALB/c and ICR at 13 wk are displayed.
Among contact-exposed mice (Figure 4), the antibody responses of B6 mice were significantly (P < 0.05) higher at all times evaluated than those of all other strains investigated except BALB/c. Most mice that showed seroconversion after 5 wk remained seropositive at the 16-wk time point.
Strain-dependent seroconversion depended on the viral load used for inoculation.
Mice of most strains showed high antibody responses when inoculated, but only B6 mice had high serum antibodies when contact-exposed. To elucidate whether the low antibody response in contact-exposed mice was due to decreased viral shedding of inoculated mice compared with B6 mice or whether low-responding strains needed higher viral loads for seroconversion, we performed further experiments with B6 mice (which showed high antibody responses in both inoculated and contact-exposed animals) and FVB/N mice (which had a high response in inoculated mice but a negligible response in contact-exposed animals). When FVB/N mice were inoculated and housed together with noninoculated B6 mice, all animals seroconverted after 5 wk and showed high antibody responses over the 14-wk observation period. Therefore, FVB/N mice were able to infect B6 mice. However, when B6 mice were inoculated and kept together with noninoculated FVB/N mice, none of the FVB/N mice seroconverted during the 14-wk period, suggesting that the dose necessary to induce seroconversion is strain-dependent.
To confirm this hypothesis, titration studies were performed in B6 and FVB/N mice. All animals of both strains seroconverted (and had high antibody levels) after inoculation with 7 × 102 TCID50 and 3.5 × 102 TCID50. However, when inoculated with 35 TCID50, only B6 mice seroconverted and displayed high levels of antibody. In contrast, none of the FVB/N mice showed any antibody response. None of the B6 and FVB/N mice seroconverted after inoculation with 3.5 TCID50, or the negative control.
Influence of MHC haplotype on antibody response.
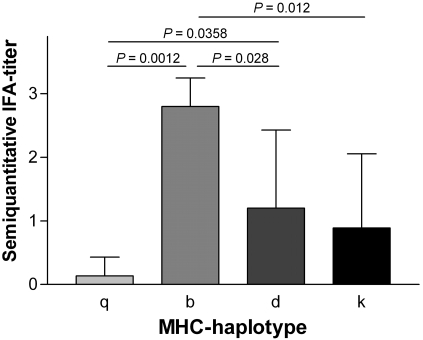
Because the genetic background of mice influenced the antibody response to MVMi, we questioned whether the MHC haplotype might contribute to this effect. We therefore sorted the antibody response (determined by IFA) of contact-exposed mice at 5 wk postexposure by the MHC haplotype of the strain (Figure 5). Mouse strains with low antibody responses (FVB/N, NMRI, ICR) shared the MHC-q haplotype, whereas B6 mice (MHC-b haplotype) showed a high antibody response.
Figure 5.
Antibody response (mean + SD) determined by IFA at 5 wk postexposure sorted by MHC haplotype. Mice of the q haplotype (FVB/N, NMRI, ICR) showed lower antibody levels than did mice of the b (C57BL/6J) and d (BALB/c, DBA2J) haplotype. Furthermore, mice of the b haplotype responded with increased levels of specific antibodies compared with those of the intermediate-responding mice of the d and k (AKR/N, C3H/HeN) haplotypes. Differences were determined by the Kruskal–Wallis test (P = 0.0009) and subsequent Mann–Whitney U tests.
To further elucidate the role of the MHC haplotype in antibody response to MVMi, naive B10/Q-H2q/SgAi (BQ) mice were housed together with inoculated B6 mice; naive B10 and B6 mice housed with inoculated B6 mice served as an internal control. In contrast to FVB/N, NMRI, and ICR mice, BQ mice responded to MVMi exposure with considerably high antibody levels, comparable to the antibody response seen in contact-exposed B6 mice. The antibody levels of B10 mice were slightly lower than those of B6 and BQ mice (data not shown).
Detection of viral DNA at 16 wk postexposure.
To investigate whether MVMi produces a persistent infection in mice, we harvested spleens and feces from inoculated and contact-exposed mice at 16 wk postexposure. PCR analyses were used to detect viral DNA in the organ and fecal samples, and homogenates of spleens that tested positive by PCR were used for inoculation of mice to investigate whether virus remained infectious. We further investigated the course of viral shedding in the feces of inoculated FVB/N mice.
Both VP2 and NS1 PCR assays detected viral DNA in the spleens of both inoculated and contact-exposed mice at 16 wk postexposure. The NS1 parvovirus PCR assay proved to be slightly more sensitive in our hands (data not shown). PCR revealed viral DNA in the spleens of inoculated FVB/N, C57BL/6J, ICR, BALB/c, DBA/2J, AKR/N, and C3H/HeN mice and of contact-exposed C57BL/6J, NMRI, BALB/c, ICR, DBA/2, and AKR/N mice (Table 1). The antibody levels at 16 wk postinoculation seem to parallel the number of animals positive by PCR.
Table 1.
Viral positivity according to MVM (VP2)- and parvovirus (NS1)-specific PCR assays of spleens of inoculated and contact-exposed mice at 16 wk after exposure
| FVB/N | C57BL/6J | NMRI | BALB/c | ICR | DBA/2J | AKR/N | C3H/HeN | ||
| Inoculated | |||||||||
| VP2 | 5 / 5 | 0 / 4 | 0 / 5 | 4 / 5 | 1 / 5 | 1 / 5 | 1 / 5 | 2 / 5 | |
| NS1 | 5 / 5 | 1 / 4 | 0 / 5 | 3 / 5 | 4 / 5 | 1 / 5 | 3 / 5 | 2 / 5 | |
| Contact-exposed | |||||||||
| VP2 | 0 / 5 | 2 / 5 | 0 / 5 | 2 / 5 | 0 / 5 | 1 / 5 | 1 / 3 | 0 / 5 | |
| NS1 | 0 / 5 | 3 / 5 | 1 / 5 | 1 / 5 | 1 / 5 | 0 / 5 | 2 / 3 | 0 /5 |
Data are given as no. of virus-positive mice/no. of mice exposed.
Fecal samples from PCR-positive FVB/N, B6, and BALB/c mice were tested by VP2 and NS1 PCR assays, but viral DNA was not detected (data not shown).
Fecal shedding of MVMi in FVB/N mice.
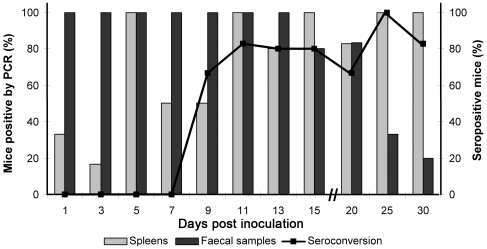
To determine the duration of viral shedding, feces from FVB/N mice were harvested every other day from day 1 until day 15 after inoculation and every fifth day until day 30 (Figure 6). Consistent with previously reported data,24 seroconversion occurred between days 7 and 9 postinoculation. Spleens from some mice were positive from day 1 postinoculation and from day 5 until day 30 in the majority of animals. MVM DNA was detected in feces as soon as day 1 postinoculation. Fecal samples from all mice were positive for viral DNA from day 1 until day 13, and 80% of samples were DNA-positive on day 15. Viral shedding declined rapidly after day 20 postinoculation, with only 1 of 5 samples positive for viral DNA on day 30.
Figure 6.
Detection of viral shedding in feces of FVB/N mice on days 1 through 30 after inoculation. The proportion of mice that shed viral DNA is given as a percentage of the mice inoculated. Two additional parameters of viral infection, seroconversion and detection of viral DNA in spleen, are shown also.
Use of mouse antibody production tests to determine viral infectivity.
Our studies showed that viral shedding in the feces of FVB/N mice declined rapidly after day 20 postinoculation and could not be detected in fecal samples at 16 wk postexposure. However, comparison of the persistence of viral DNA and the levels of antibody responses at 16 wk postinoculation suggested an association between these parameters. Therefore, the question arose whether the viral DNA detected in spleens at 16 wk postexposure represented infectious virus or inactive remnants. We approached this question by performing mouse antibody production tests.
B6 mice that received spleen homogenates derived from FVB/N mice euthanized on day 11 postinoculation (control group) were seropositive by IFA. Semiquantitative PCR detected low levels of viral DNA in the spleens or mesenteric lymph nodes but not the feces of these B6 mice (data not shown). However, B6 mice that received spleen homogenates from FVB/N mice euthanized at 16 wk postexposure did not seroconvert, and viral DNA was not detected in organs or feces (data not shown), indicating that the viral DNA detected at 16 wk postexposure does not represent sufficient infectious virus to establish a productive infection in mice.
Discussion
Parvoviruses are among the most common pathogens in laboratory mice. Monitoring of populations is based on serologic diagnosis, because serology remains the most effective method to screen mouse populations for viruses. However, several previously published PCR assays offer opportunities to confirm serologic results.1,21,27 In the present study, antibody response, viral shedding, and viral persistence were investigated in several mouse strains by experimental inoculation and by contact exposure.
Antibody response varied among mouse strains, especially in contact-exposed mice. Seroconversion could be detected in all inoculated animals except some NMRI mice. In contrast, among contact-exposed animals, all C57BL/6J mice but only some BALB/c, AKR/N, DBA/2J, FVB/N, and C3H/HeN mice seroconverted. No seroconversion could be detected in NMRI and ICR mice by IFA, although ELISA identified seroconversion in a single NMRI mouse. These findings indicate that MVM infection might remain undetected in animal facilities, especially when sentinel mice are used for surveillance, because some mouse strains may not be suitable as sentinels. This inconsistency should be considered when interpreting results of serologic tests and sentinel programs.
Contact-exposed but not inoculated FVB/N mice showed a markedly lower antibody response than B6 mice (Figure 2). Therefore, we investigated whether viral shedding of inoculated FVB/N mice was insufficient to infect other mice or whether FVB/N mice required higher infectious doses of MVMi. Because inoculated FVB/N mice infected naive B6 mice, but inoculated B6 mice did not infect FVB/N mice, the dose required for seroconversion likely is higher for FVB/N than for B6 mice. Our titration study, in which the dose to induce seroconversion was 10-fold higher in FVB/N mice than in B6 mice, confirmed this hypothesis.
Another factor to consider regarding the influence of genetic background on antibody responses to MVM is the potential for fecal viral loads to be decreased in certain mouse strains. Decreased fecal viral loads might also influence viral transmission; for example, a highly robust antibody response could lead to decreased viral replication and shedding.
An influence of genetic background on the antibody response to parvoviral infection has been shown for MPV1, a related murine parvovirus.2 Seroconversion to MPV1 was detected in all C3H/HeN mice investigated, but only in some BALB/c, ICR, and DBA/2N mice and not in C57BL/6N mice at same dose; these mice seroconverted only after the dose of viral inoculum was increased. Therefore, investigation of strain-specific susceptibilities have yielded conflicting results concerning the B6 strain: whereas no seroconversion could be detected after experimental infection of C57BL/6N mice with MPV1, both inoculated and contact-exposed C57BL/6J mice showed a high antibody response to MVMi. This apparent inconsistency might be a consequence of the different B6 substrains used in the 2 studies or may be due to differences between MVMi and MPV1.
A recent study demonstrated that gender influences the antibody response to MVMi in C57BL/6NCr and C57BL/6Tac mice, with males showing more frequent seroconversion.26 However, we noted that all female C57BL/6J mice seroconverted. Together with the earlier cited findings,2 these results might indicate that the B6/N and B6/J substrains differ in their susceptibility to parvovirus infection.
Strain-specific antibody responses might be related to the predominant T-helper phenotype (Th1 or Th2 response) generated to MPV1, because B10.D2 mice generated a Th1 response when stimulated by antigens that do not initiate strong T-helper phenotype-directing signals, whereas BALB/c mice were primed to generate a Th2 response.2,10 B6 mice also show a predominant Th1 response to different pathogens, prompting the notion that strains of mice primed to generate a Th1 response might require more antigenic stimulation (for example, a higher dose of virus) to generate a detectable humoral immune response to MPV 1.3 However, we could not confirm this scenario for MVMi infection, given that contact-exposed BALB/c mice showed an intermediate and B6 mice a high antibody response (and even seroconverted to 35 TCID50).
We hypothesized that the different antibody responses observed in the present study might be influenced by the MHC haplotype of the mouse strains. Inoculated and contact-exposed B6 mice, which carry the MHC-b haplotype, showed a high antibody response, whereas strains carrying the MHC-q haplotype (NMRI, ICR, FVB/N) showed no or only weak antibody response after contact exposure. However, an experiment using MHC congenic mice revealed that when housed with inoculated B6 mice, BQ mice (which carry the MHC-q haplotype on a B10 background) had an antibody response similar to that of B6 mice and a slightly higher response than that of B10 mice (MHC-b haplotype). Therefore, the MHC-q haplotype did not appear responsible for a low antibody response toward MVMi. However, titration studies were not performed using MHC congenic mice.
As shown for human B19 virus and rat virus, parvoviruses can persist after acute infection, but the precise mechanisms of persistence are not fully understood.12,16 Only contradictory data are available for MVM regarding the establishment of a persistent infection in immunocompetent mice. We approached this question by investigating whether viral DNA is detectable in organs at16 wk postexposure, whether PCR-positive samples are infectious, and how long virus is shed in feces. MVM DNA was detected in spleen at 16 wk postexposure in some mouse strains (these animals were otherwise free of common murine pathogens), indicating that MVM might indeed cause persistent infections. PCR analysis revealed different viral DNA loads in the spleens of inoculated and contact-exposed mice. Although we noted an association between viral DNA and antibody levels at 16 wk postinoculation, that between the genetic background of mice and viral DNA persistence was not as pronounced. Notably, MPV1 DNA was present at 4 wk postinoculation in all strains investigated except DBA/2.2
Viral DNA was detectable for as long as 35 wk in the mesenteric lymph nodes of members of an endemically infected colony.1 In that study, fecal shedding of MVM was investigated in an endemically infected colony positive for multiple murine pathogens including murine hepatitis virus, Theiler murine encephalomyelitis virus, Sendai virus, and MPV.1 The mice shed virus for as long as 6 wk until an age of 11 wk. Although no viral shedding occurred in older animals, MVM was still present in the mesenteric lymph nodes in the majority of animals. However, coinfection with multiple pathogens may interfere with viral shedding and viral persistence of MVM, and others have identified viral shedding for only 9 to 12 d in the fecal samples of B6 mice.26
In our study MVM shedding in the feces of FVB/N mice was detected as soon as day 1 postinoculation but declined rapidly after day 20. However, that we inoculated mice that were 10 to 12 wk old might have an influence on the duration of fecal shedding, because older mice might be more resistant to infection. Furthermore viral DNA was not detected in fecal samples collected at 16 wk postexposure, indicating that viral shedding did not occur at this time.
Because we observed persistence of viral DNA in spleens of mice for 16 wk but no viral shedding in PCR-positive animals, we questioned whether viral DNA represented infectious virus. MAP tests revealed that organ homogenates positive for MVM by PCR did not contain infectious virus as determined by serology and PCR. This result might indicate either very low viral loads insufficient to establish an infection or the presence of viral remnants. The relevance of viral DNA detected in spleens of mice at 16 wk postexposure has to be determined in further studies.
Our data and recent studies indicate that several factors, including age, dose, coinfection, mouse strain, and for some strains gender, influence the rate of seroconversion to MVMi, duration of viral shedding, and persistence of viral DNA. These observations demonstrate the complex nature of parvovirus infection in mice. This complexity has to be considered in the design of health monitoring programs for laboratory mouse populations and may account for the relatively high prevalence of parvovirus infections in specific pathogen-free colonies. This complexity might also be relevant to parvoviral infection in other species.
Acknowledgment
This work was supported by a grant from the Society of Laboratory Animal Science (to AB). The authors thank W. Nicklas for providing MVMi and NBK-cells.
References
- 1.Bauer BA, Riley LK. 2006. Antemortem detection of mouse parvovirus and mice minute virus by polymerase chain reaction (PCR) of faecal samples. Lab Anim 40:144–152 [DOI] [PubMed] [Google Scholar]
- 2.Besselsen DG, Wagner AM, Loganbill JK. 2000. Effect of mouse strain and age on detection of mouse parvovirus 1 by use of serologic testing and polymerase chain reaction analysis. Comp Med 50:498–502 [PubMed] [Google Scholar]
- 3.Bleich A, Mähler M. 2005. Environment as a critical factor for the pathogenesis and outcome of gastrointestinal disease: experimental and human inflammatory bowel disease and Helicobacter-induced gastritis. Pathobiology 72:293–307 [DOI] [PubMed] [Google Scholar]
- 4.Bonnard GD, Manders EK, Campell DA, Jr, Herberman RB, Collins MJ., Jr 1976. Immunosuppressive activity of a subline of the mouse EL4 lymphoma. Evidence for minute virus of mice causing the inhibition. J Exp Med 143:187–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownstein DG, Smith AL, Jacoby RO, Johnson EA, Hansen G, Tattersall P. 1991. Pathogenesis of infection with a virulent allotropic variant of minute virus of mice and regulation by host genotype. Lab Invest 65:357–364 [PubMed] [Google Scholar]
- 6.Crawford LV. 1966. A minute virus of mice. Virology 29:605–612 [DOI] [PubMed] [Google Scholar]
- 7.Engers HD, Louis JA, Zubler RH, Hirt B. 1981. Inhibition of T cell-mediated functions by MVM(i), a parvovirus closely related to minute virus of mice. J Immunol 127:2280–2285 [PubMed] [Google Scholar]
- 8.Guetta E, Graziani Y, Tal J. 1986. Suppression of Ehrlich ascites tumors in mice by minute virus of mice. J Natl Cancer Inst 76:1177–1180 [PubMed] [Google Scholar]
- 9.Heldt CL, Hernandez R, Mudiganti U, Gurgel PV, Brown DT, Carbonell RG. 2006. A colorimetric assay for viral agents that produce cytopathic effects. J Virol Methods 135:56–65 [DOI] [PubMed] [Google Scholar]
- 10.Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. 1995. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med 181:713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacoby RO, Ball-Goodrich LJ, Besselsen DG, McKisic MD, Riley LK, Smith AL. 1996. Rodent parvovirus infections. Lab Anim Sci 46:370–380 [PubMed] [Google Scholar]
- 12.Jacoby RO, Johnson EA, Paturzo FX, Ball-Goodrich L. 2000. Persistent rat virus infection in smooth muscle of euthymic and athymic rats. J Virol 74:11841–11848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaerber G.1931. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch Exp Pathol Pharmakol 162:480–483 [Google Scholar]
- 14.Kilham L, Margolis G. 1970. Pathogenicity of minute virus of mice (MVM) for rats, mice and hamsters. Proc Soc Exp Biol Med 133:1447–1452 [DOI] [PubMed] [Google Scholar]
- 15.Lamana ML, Albella B, Bueren JA, Segovia JC. 2001. In vitro and in vivo susceptibility of mouse megakaryocytic progenitors to strain i of parvovirus minute virus of mice. Exp Hematol 29:1303–1309 [DOI] [PubMed] [Google Scholar]
- 16.Lefrere JJ, Servant-Delmas A, Candotti D, Mariotti M, Thomas I, Brossard Y, Lefrere F, Girot R, Allain JP, Laperche S. 2005. Persistent B19 infection in immunocompetent individuals: implications for transfusion safety. Blood 106:2890–2895 [DOI] [PubMed] [Google Scholar]
- 17.Livingston RS, Besselsen DG, Steffen EK, Besch-Williford CL, Franklin CL, Riley LK. 2002. Serodiagnosis of mice minute virus and mouse parvovirus infections in mice by enzyme-linked immunosorbent assay with baculovirus-expressed recombinant VP2 proteins. Clin Diagn Lab Immunol 9:1025–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mousset S, Rommelaere J. 1982. Minute virus of mice inhibits cell transformation by simian virus 40. Nature 300:537–539 [DOI] [PubMed] [Google Scholar]
- 19.Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke BFELASA. 2002. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim 36:20–42 [DOI] [PubMed] [Google Scholar]
- 20.Nicklas W, Kraft V, Meyer B. 1993. Contamination of transplantable tumors, cell lines, and monoclonal antibodies with rodent viruses. Lab Anim Sci 43:296–300 [PubMed] [Google Scholar]
- 21.Redig AJ, Besselsen DG. 2001. Detection of rodent parvoviruses by use of fluorogenic nuclease polymerase chain reaction assays. Comp Med 51:326–331 [PubMed] [Google Scholar]
- 22.Segovia JC, Real A, Bueren JA, Almendral JM. 1991. In vitro myelosuppressive effects of the parvovirus minute virus of mice (MVMi) on hematopoietic stem and committed progenitor cells. Blood 77:980–988 [PubMed] [Google Scholar]
- 23.Singleton GR, Smith AL, Shellam GR, Fitzgerald N, Muller WJ. 1993. Prevalence of viral antibodies and helminths in field populations of house mice (Mus domesticus) in southeastern Australia. Epidemiol Infect 110:399–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith AL. 1983. Response of weanling random-bred mice to inoculation with minute virus of mice. Lab Anim Sci 33:37–40 [PubMed] [Google Scholar]
- 25.Spearman C. 1908. The method of ‘right and wrong cases’(constant stimuli) without Gauss's formulae. Br J Psychol 2:227–242 [Google Scholar]
- 26.Thomas ML, 3rd, Morse BC, O'Malley J, Davis JA, St Claire MB, Cole MN. 2007. Gender influences infectivity in C57BL/6 mice exposed to mouse minute virus. Comp Med 57:74–81 [PubMed] [Google Scholar]
- 27.Wan CH, Söderlund-Venermo M, Pintel DJ, Riley LK. 2002. Molecular characterization of three newly recognized rat parvoviruses. J Gen Virol 83:2075–2083 [DOI] [PubMed] [Google Scholar]