Abstract
Background
Although useful for probing bacterial pathogenesis and physiology, current random mutagenesis systems suffer limitations for studying the toxin-producing bacterium Clostridium perfringens.
Methodology/Principal Findings
An EZ-Tn5-based random mutagenesis approach was developed for use in C. perfringens. This mutagenesis system identified a new regulatory locus controlling toxin production by strain 13, a C. perfringens type A strain. The novel locus, encoding proteins with homology to the AgrB and AgrD components of the Agr quorum sensing system of Staphylococcus aureus and two hypothetical proteins, was found to regulate early production of both alpha toxin and perfringolysin O (PFO) by strain 13. PFO production by the strain 13 ΔagrB mutant could be restored by genetic complementation or by physical complementation, i.e. by co-culture of the strain 13 ΔagrB mutant with a pfoA mutant of either strain 13 or C. perfringens type C CN3685. A similar AgrB- and AgrD-encoding locus is identifiable in all sequenced C. perfringens strains, including type B, C, D, and E isolates, suggesting this regulatory locus contributes to toxin regulation by most C. perfringens strains. In strain 13, the agrB and agrD genes were found to be co-transcribed in an operon with two upstream genes encoding hypothetical proteins.
Conclusions/Significance
The new Tn5-based random mutagenesis system developed in this study is more efficient and random than previously reported C. perfringens random mutagenesis approaches. It allowed identification of a novel C. perfringens toxin regulatory locus with homology to the Agr system of S. aureus and which functions as expected of an Agr-like quorum sensing system. Since previous studies have shown that alpha toxin and perfringolysin O are responsible for strain 13-induced clostridial myonecrosis in the mouse model, the new agr regulatory locus may have importance for strain 13 virulence.
Introduction
Clostridium perfringens is a major pathogen of humans and other animals, causing a spectrum of serious enteric and histotoxic infections ranging from clostridial myonecrosis to Clostridium perfringens type A food poisoning [1]. The virulence of this Gram-positive, spore-forming anaerobe is largely attributable to its prodigious toxin production, with the literature reporting at least 17 different C. perfringens toxins [1]. However, toxin production varies from strain-to-strain, allowing individual C. perfringens isolates to be classified into types A–E, based upon their production of four typing toxins (alpha, beta, iota and epsilon toxins).
Besides being an important pathogen, C. perfringens is also ubiquitously distributed in the environment [1]. This bacterium is commonly found amongst the normal intestinal flora of most animal species, including humans [1], [2]. C. perfringens is also a common inhabitant of soils, both in its spore and vegetative forms [3]. Due to its presence in feces and ability to form resistant spores, C. perfringens has been used as an indicator organism for fecal water pollution [4].
C. perfringens is the most genetically tractable of all pathogenic clostridial species. Using allelic exchange-based techniques, it has been possible for >15 years to construct directed null mutants in transformable strains of this bacterium [5]. More recently, adaptation of group II introns (Targetrons) has greatly improved the efficacy of directed C. perfringens mutant construction [5]–[7]. For example, Targetron technology facilitated rapid construction of several C. perfringens single and double toxin null mutants [6], [8], [9], or mutants unable to express an acid soluble protein important for spore resistance properties [10], providing new understanding of C. perfringens virulence, pathogenesis and physiology.
Despite these recent improvements in directed mutant construction, there are still technical limits for performing genetics in C. perfringens. For example, random mutagenesis is a powerful tool for providing insights into bacterial gene function, but this technique remains suboptimal for all pathogenic clostridial species, including C. perfringens. While, Tn916 has been successfully used for many years as a mutagenic tool for C. perfringens [11]–[13], this transposon suffers from several significant limitations. One problem is Tn916's tendency towards multiple insertions, with several studies detecting multiple Tn916 insertions in 65–75% of all isolated C. perfringens mutants [12], [13]. In addition, Tn916 remains active after integration, resulting in unstable mutants [14]. A final problem is that Tn916 insertion can be followed by a deletion event that removes DNA regions, rather than inactivating a specific gene [12].
Because of those Tn916 limitations, there is interest in identifying alternative transposon tools for C. perfringens random mutagenesis. To that purpose, another group recently reported development of a phage Mu-based, random transposition mutagenesis system for C. perfringens [15], which proved relatively efficient and produced mutants with only a single transposon insertion. Although not directly addressed in that study, C. perfringens phage Mu-system mutants should also be stable based upon results with other bacteria [16]. However, the new phage Mu system still exhibited limitations when used in C. perfringens, including, i) nearly half of all the obtained mutants carried their transposon insertion in a rRNA gene [15] and ii) the number of C. perfringens mutants obtained per µg of Erm-Mu transposase DNA was relatively low, e.g., 239 transformant colonies/µg DNA for strain JIR325 (a strain 13 derivative) [15].
EZ-Tn5 random mutagenesis (Epicentre®) is an alternative approach to phage Mu-based systems for random mutagenesis. The EZ-Tn5 system has allowed random mutagenesis in many bacterial species (www.epibio.com) and retains the simplicity of phage Mu-based mutagenesis. However the EZ-Tn5 system possesses two important advantages, i) a high transposition frequency (e.g., reportedly ∼100-fold higher than phage Mu-based systems) and ii) the highest degree of “randomness” for insertions among commercially-available transposition systems, including phage Mu (www.epibio.com).
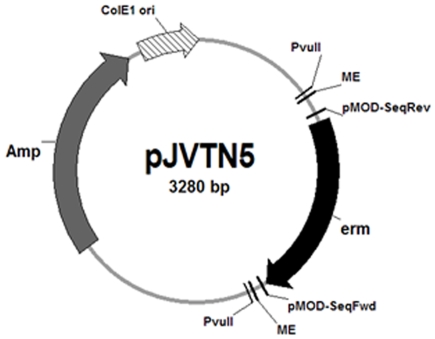
Transformants carrying a EZ-Tn5 insertion are easily identifiable by antibiotic selection. However, commercial EZ-Tn5 transposons are sold with antibiotic resistance determinants that either do not work in C. perfringens or would impart novel resistance characteristics to this organism. Therefore, to explore the potential usefulness of the EZ-Tn5 system for improving random mutagenesis in C. perfringens, we modified a commercial EZ-Tn5 pMOD vector by inserting a C. perfringens erythromycin resistance determinant (Fig. 1). This modified EZ-Tn5 transposon proved highly useful for random mutagenesis of C. perfringens, identifying a novel locus regulating the production of both α toxin and perfringolysin O (PFO) in strain 13.
Figure 1. Modification of the EZ-Tn5-encoding vector for random mutagenesis in C. perfringens.
To allow selection of C. perfringens transformants after electroporation with the EZ-Tn5 transposon, a C. perfringens erythromycin resistance determinant (erm) was cloned into the multiple cloning site in the Epicentre EZ-TN5-encoding pMOD-2 vector creating pJVTN5. This plasmid also contains PvuII-recognized sequences flanking the mosaic end (ME) sites, which are specifically recognized by the EZ-Tn5 transposase.
Results
Development of an EZ-Tn5-based random mutagenesis system for C. perfringens
A transposome mixture (containing the erm-carrying transposon DNA+EZ−Tn5 transposase) was electroporated into strain 13. A total of ∼280 erythromycin (Erm)-resistant transformants were obtained in each of three independent transformation experiments with strain 13. PCR reactions using primers erm-Fwd-EcoRI and erm-Rev-HindIII confirmed that the erm gene, which is present in the modified Tn5 transposon but is not naturally encoded by C. perfringens strain 13, was carried by all strain 13 transformants growing on BHI plates containing Erm (data not shown). Thus, for strain 13, the efficiency of the erm-carrying EZ-Tn5 transposon insertion was ∼11,200 transformants/µg transposon DNA.
Random insertion of the erm-carrying transposon in C. perfringens
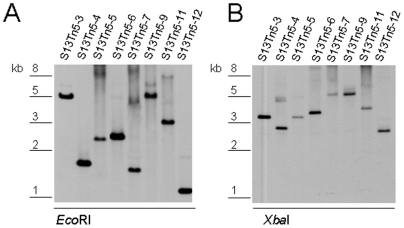
Southern hybridization with an erm probe was used to assess the “randomness” of transposon insertion in the strain 13 mutants. Only a single copy of the erm gene was detected in 8 different C. perfringens strain 13 transposon mutants (Fig. 2). These Southern blot analyses also suggested that the erm gene had apparently inserted into different DNA regions in these mutants (Fig. 2). To confirm that EZ-Tn5 insertions are random, upstream and downstream regions flanking the transposon were sequenced for 11 arbitrarily selected C. perfringens strain 13 mutants. Table 1 results confirmed that the transposon insertions were random and had occurred within ORF sequences, including ORFs encoding a putative virulence factor (9%), hypothetical proteins (27%), genes encoding proteins of metabolic pathways or protein biosynthesis (45%) and rRNA genes (18%).
Figure 2. Southern blot analyses of C. perfringens random mutants obtained after electroporation with EZ-Tn5 transposomes.
After selection on BHI plates containing Erm (40 µg/ml), DNA was extracted from strain 13 transformants. Following digestion with EcoRI (A) or XbaI (B), the digested DNA was electrophoresed and blotted to a nylon membrane. DNA on the membranes was then hybridized with a Dig-labeled erm probe, as found in the C. perfringens-modified EZ-Tn5, and blots were developed as described in the Materials and Methods. Size of DNA fragments, in kilobases (kb), is shown at left.
Table 1. Target gene of the EZ-Tn5 based transposon in C. perfringens strain 13.
| Mutant | Gene name or locus tag, description |
| S13Tn5-01 (CPJV501) | CPE1561 (agrB), quorum sensing regulatory protein |
| S13Tn5-03 | rrnB-16S (CPEr004), 16S ribosomal RNA, |
| S13Tn5-04 | CPE2407, elongation factor Tu |
| S13Tn5-05 | CPE1142, hypothetical protein |
| S13Tn5-06 | 16S RNAr |
| S13Tn5-07 | CPE1892, 50S ribosomal protein L20 |
| S13Tn5-09 | CPE1736, ribulose phosphate 3-epimerase |
| S13Tn5-10 | CPE0027, hypothetical protein |
| S13Tn5-11 | CPE2524, methionyl-tRNA synthetase |
| S13Tn5-12 | CPE0643, hypothetical protein |
| S13Tn5-13 | CPE2348, phosphate butyryltransferase |
Disruption of the C. perfringens agrB gene using EZ-Tn5 transposon mutagenesis
To demonstrate the usefulness of the new EZ-Tn5 system, our strain 13 mutant library was screened by growth on blood agar plates or egg yolk agar plates for reduced or lost PFO-induced β-hemolysis or CPA phospolipase activity, respectively. One EZ-Tn5-carrying mutant, named CPJV501, exhibited a complete loss of PFO-induced β-hemolysis halo when growing on blood agar plates and a reduced phospholipase C (alpha toxin)-induced halo when growing on egg yolk agar plates (data not shown). PCR analyses, using primers shown in Table 2, indicated (data not shown) that the transposon present in this mutant had not disrupted either its pfoA gene (including its promoter and virR boxes), the plc gene, or the virS/virR operon that had previously been shown to regulate pfoA and plc transcription [11], [17], [18].
Table 2. Primers used in this study.
| Primer | Sequence | Reference |
| erm-Fwd-EcoRI | AAGGGAATTCCTAAAAATTTGTAATTAAGAAGGAGT | This study |
| erm-Rev-HindIII | AAGGAAGCTTCCAAATTTACAAAAGCGACTCATA | |
| pMOD-SeqFwd | GCCAACGACTACGCACTAGCCAAC | Epicentre |
| pMOD-SeqRev | GAGCCAATATGCGAGAACACCCGAGAA | |
| agrBFwd | GATTGAGAATATATCGAAGTTAAT | This study |
| agrBRev | TATGTAGGTTAGAGTCATACATTGC | |
| agrBF | TTACGAATTCGATGTTAGCCATGTATGCTTTCG | This study |
| agrBR | TAGAGGATCCTCATTTAACTCATCCCCTCAAG | |
| agrF1 | TTACGAATTCTTAGCTCTTTATATTGGATATACAG | This study |
| agrR1 | TAGAGGATCCCCGGTTTAAAACCGACCTTTAG | |
| pfoAF1 | ATCCAACCTATGGAAAAGTTTCTGG | [22] |
| pfoAR1 | CCTCCTAAAACTACTGCTGTGAAGG | |
| cpaF | GCTAATGTTACTGCCGTTGA | [45] |
| cpaR | CCTCTGATACATCGTGTAAG | |
| polCJVL | AATATATGATACTGAAGAGAGAGTAA | This study |
| polCJVR | TCTAAATTATCTAAATCTATGTCTACT | |
| agr101L | TAAATTTGCTCCAGTAGATACTAA | This study |
| agrDR | TATTCATCTCTTAAAGATTTTGGT | |
| agr102L | TTCAAGTTTGATATTGGTATTAGT | This study |
| agr101R | CAAAGCTTCTAAAGCTATATTAAA | |
| agr103L | ATGATAGGAACAAGTACAGTAAAA | This study |
| agr102R | AACTTGAAATTAAATATTCCTTCT | |
| agr104L | AAATTTAAAACTTGTTATTGGAGT | This study |
| agr103R | GGCTTTAAACTATATCCTTTTATT | |
| pfoA81L | CCCAGTTATTCACGATTAAAG | This study |
| pfoA82R | AGTAATACTAGATCCAGGGTATAAA |
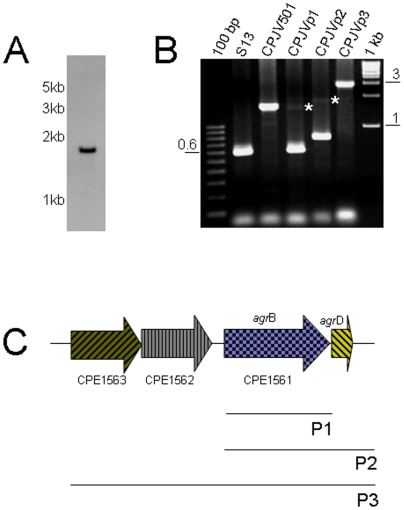
Therefore, the DNA flanking the erm-modified EZ-Tn5 transposon in CPJV501 was sequenced, which revealed that the transposon had inserted into strain 13 ORF CPE1561 (Table 1). Southern blot analyses showed that this mutant carries only one copy of the transposon integrated in its genome (Fig. 3A). PCR analysis using primers that amplify the wt CPE1561 gene (642 bp) in strain 13 then further demonstrated the presence of the transposon-disrupted CPE1561 gene (1600 bp) in CPJV501 (Fig. 3B).
Figure 3. Generation of a C. perfringens agrB mutant and complementing strains.
A) Southern blot analyses, as described in Fig. 2, using EcoRI-digested DNA from CPJV501 and a Dig-labeled probe that detected a single copy of the erm gene. Size of DNA fragments, in kilobases (kb) is shown at left. B) PCR was performed with DNA extracted from the indicated strain and the following pair of primers, agrBFwd and agrBRev in reactions containing DNA from strain 13 (S13), CPJV501 and CPJVp1; agrBFwd and argDR for CPJVp2 and agrF1 and agrD100R for CPJVp3. DNA ladders (100 bp or 1 kb) were included in the first and last lane of the gel. Asterisks show the expected PCR product when the primers amplified the Tn5-disprupted agrB gene. C) Genes cloned in the E. coli-C. perfringens shuttle plasmid pJIR750 to complement the agrB transposon mutant. As shown, P1 encodes the agrB gene alone, P2 the agrB and agrD genes and P3 encodes two-genes (CPE1562 and CPE1563) upstream the agrB gene (CPE1561) and agrB and agrD.
A homolog of the putative protein encoded by the CPE1561 gene has been annotated as AgrB for C. perfringens strain ATCC 13124 [19], which is found in other Gram-positive bacteria, including some other clostridial species. In S. aureus, AgrB and the secreted peptide AgrD are part of a well-characterized quorum sensing (QS) system that is involved in regulating the expression of virulence genes, including several toxins [20]. Bioinformatics analyses using the Pathema website further revealed that a sequence with 100% homology to the agrB ORF in C. perfringens strain 13 and ATCC 13124 is also present in all other sequenced C. perfringens isolates, including those classifying as types A–E (data not shown). Moreover, the region between ORFs CPE1561 and CPE1560 in strain 13 contains a small (135 bp) ORF (Fig. 3C) sharing ∼99% homology with a putative agrD gene from C. perfringens type A strain SM101 and ATCC13124 [21].
The C. perfringens agr locus regulates PFO production
Results presented above suggested that PFO production is regulated by the C. perfringens agr locus. A hemoglobin release (Hb) assay [22] was used to examine the kinetics of this regulation. In this assay, the ability of C. perfringens supernatants to lyse horse erythrocytes and release Hb is specifically attributable to PFO activity [11], as further supported by our observation that the culture supernatant of a C. perfringens strain 13 pfoA-null mutant is incapable of inducing Hb release from horse erythrocytes (Fig. 4A).
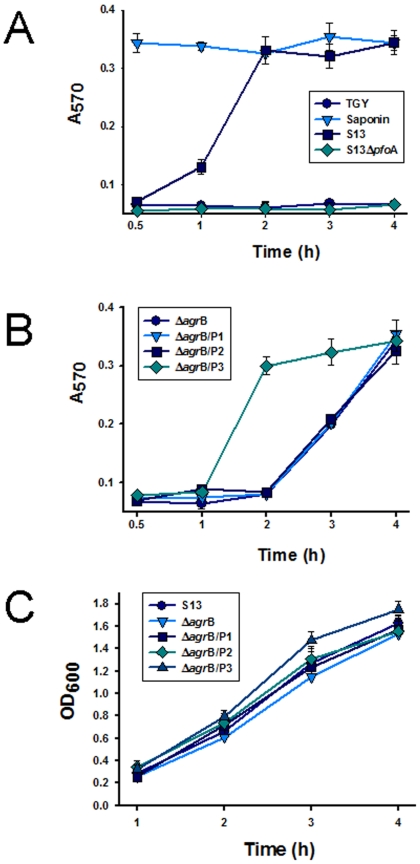
Figure 4. The C. perfringens agrB locus regulates PFO production.
A and B) Hemoglobin (Hb) release assay. Culture supernatants obtained, at the indicated time point, from strain 13 (S13), S13 pfoA-null mutant (S13ΔpfoA), CPJV501 (ΔagrB), CPJVp1 (ΔagrB/P1), CPJVp2 (ΔagrB/P2) or CPJVp3 (ΔagrB/P3), were incubated (1∶1) with a 1% suspension of horse red blood cells for 30 min at 37°C. Non-inoculated TGY or 0.1% saponin (Saponin) was included as negative or positive control, respectively. PFO-induced Hb release was detected by obtaining the absorbance at 570 (A570). C) For each time point, the OD600 of the cultures is shown. For all panels, error bars represent the standard error of the mean calculated using data from three independent experiments.
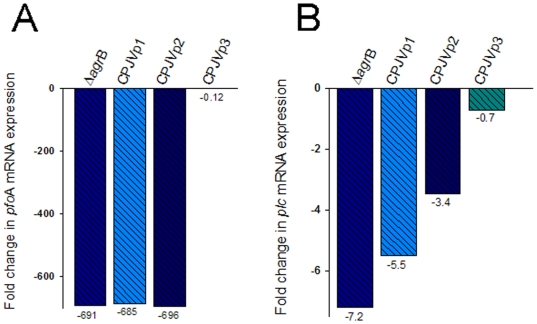
Using this horse erythrocyte assay, the appearance of PFO activity in the supernatant of CPJV501 was delayed by 2 h compared to wt strain 13 (Fig. 4A and 4B). This difference was not due to growth differences between CPJV501 and wt strain 13 (Fig. 4C). The absence of PFO activity in the early growth phase supernatants of CPJV501 involved an inhibition of early pfoA transcription in CPJV501. Specifically, quantitative RT-PCR analyses revealed that a 2 hour CPJV501 culture contains ∼700-fold less pfoA mRNA than does the equivalent culture of wt strain 13 (Fig. 5A).
Figure 5. Early transcription of pfoA and plc genes is regulated by the C. perfringens agr locus.
Total RNA was extracted from a 2 h TGY culture of the wt strain 13 (S13), CPJV501 (ΔagrB), CPJVp1, CPJVp2 or CPJVp3. Quantitative RT-PCR was then performed with 20 ng of each RNA and primers that amplified the (A) pfoA gen (pfoAF1 and pfoAR1) or the (B) plc gene (cpaF and cpaR). Average CT values were normalized to the polC gene and the fold differences were calculated using the comparative CT method (2−ΔΔC T) [44]. Values below each bar indicate the calculated fold change relative to the wt strain 13. Panels shown are representative of three independent experiments.
Co-culture with ΔpfoA strains can restore PFO production by strain 13 agrB mutant
In S. aureus, AgrD is a secreted factor that activates the agr-mediated regulatory network [23]. In the current study, we found that PFO production by CPJV501 could be restored by coincubating CPJV501 with either a strain 13 pfoA-null mutant or a C. perfringens type C CN3685 pfoA-null mutant, both of which retain an intact agr locus (Fig. 6A and 6B). These physical complementation results suggested that toxin regulation by the agr locus involves either cell to cell contact or a secreted factor(s). To distinguish between those two possibilities, CPJV501 and strain 13 pfoA-null mutant were inoculated into the same 100 mm tissue culture dish but physically separated by a Transwell filter. As shown in Fig. 6C, inoculation of the strain 13 pfoA-null mutant into the top well of the dish, and CPJV501 into the bottom well of the dish, restored PFO activity to similar levels as shown by the wt strain 13, i.e. this physical complementation involves a secreted factor produced by both strain 13 and CN3685.
Figure 6. A C. perfringens secreted factor(s) regulates PFO production.
A and B) Physical complementation of the ΔagrB mutant by co-culture with a ΔpfoA mutant of strain 13 or CN3685. C. perfringens strain 13 (S13), CPJV501 (ΔagrB), S13 pfoA-null mutant (S13ΔpfoA), CPJV501 and S13 pfoA-null mutant (ΔagrB/S13ΔpfoA) or CPJV501 and CN3685 pfoA-null mutant (ΔagrB/CN3685ΔpfoA) were inoculated in TGY and incubated at 37°C for the indicated time. Culture supernatants obtained, at the indicated time point, were incubated (1∶1) with a 1% suspension of horse red blood cells for 30 min at 37°C. Non-inoculated TGY or 0.1% saponin was included as negative or positive control, respectively (not shown). PFO-induced Hb release was detected by obtaining the absorbance at 570 nm (A570). C) The physical complementation shown in panels A and B requires a secreted factor to regulate PFO production. Strain 13 (S13), CPJV501 (ΔagrB) or S13 pfoA-null mutant (ΔpfoA) was inoculated in 100 mm tissue culture dishes containing 25 ml of TGY. Another 100 mm tissue culture dish containing a transwell filter device (0.4 µm pore size) received 25 ml of TGY. Then, the S13 pfoA-null mutant was inoculated into the top chamber and CPJV501 was inoculated into the bottom chamber of the dish (bottom ΔagrB/Top S13ΔpfoA) and incubated for the indicated time. Culture supernatants obtained at the indicated time points were incubated (1∶1) with a 1% suspension of horse red blood cells for 30 min at 37°C. PFO-induced Hb release was detected by obtaining the absorbance at 570 nm (A570). For all panels, error bars represent the standard error of the mean calculated using data from three independent experiments.
The C. perfringens agr locus also regulates CPA production
Relative to wt strain 13, production of CPA in culture supernatants was also reduced in 2 h cultures of CPJV501, as detected by an ELISA assay (Fig. 7A). Western blot analyses of bacterial lysates demonstrated that this reduction of CPA supernatant levels was due to decreased intracellular production of CPA by CPJV501, rather than impaired CPA secretion (Fig. 7B). qRT-PCR analyses demonstrated that this reduced CPA production involved transcriptional regulation; compared to wt strain 13, levels of cpa mRNA were reduced about 7-fold in 2 hour cultures of this agrB mutant (Fig. 5B). Together, these results indicated that the agr locus is involved in regulating pfoA and cpa transcription, and thus PFO and CPA production, during the early logarithmic growth phase.
Figure 7. The C. perfringens agrB locus regulates CPA production.
A) ELISA analyses. Culture supernatants obtained, at the indicated time point, from strain 13 (S13), CPJV501 (ΔagrB), CPJVp1 (ΔagrB/P1), CPJVp2 (ΔagrB/P2), CPJVp3 (ΔagrB/P3) or purified CPA was used to coat a 96-well microplate overnight at 4°C. The wells were incubated with a mouse monoclonal anti-CPA antibody followed by a HRP-conjugated anti-mouse antibody. The bound antibody was detected with a TMB substrate solution and the color reaction stopped with sulphuric acid (0.18 M). A450 was determined using an ELISA reader. Error bars represent the standard error of the mean calculated using data from three independent experiments. B) Western blot showing the agr locus regulates production of CPA. Strain 13 (S13), CPJV501 (ΔagrB) or CPJVp3 (ΔagrB/P3) was inoculated in TGY and incubated at 37°C for 4 h. Bacteria were then pelleted by centrifugation, resuspended in lysis buffer and sonicated. Equal amount (25 µl) of bacterial lysates was run in a 12% SDS-PAGE, transferred to nitrocellulose membrane and western blotted with a monoclonal anti-CPA antibody. As a control, 25 µl of CPA-containing concentrated supernatant proteins was added to the gel. The expected molecular weight in kDa of CPA is shown at the left. Shown is a representative figure of three independent experiments.
Evidence that C. perfringens agrB and agrD gens are co-transcribed in an operon
In S. aureus, the agr locus is encoded by an operon consisting of four genes, agrB, agrD, agrC, and agrA [23]–[25]. The agrA and agrC genes encode a response regulator and histidine kinase, respectively, of a two-component regulatory system (TCRS). This TCRS responds to a small peptide named an autoinducer (AI), which is encoded by the agrD gene. The agrB gene encodes the enzyme cleaving and modifying the AI [24].
To confirm that the C. perfringens agr locus contributes to early regulation of PFO and CPA expression in strain 13, and to assess whether agrB and agrD are expressed by C. perfringens as part of an operon, the agrB gene alone, the agrB and agrD genes alone, or the agrB, agrD and two ORFs (CPE1563 and CPE1562, which are annotated as encoding hypothetical proteins) that lie upstream of agrB were cloned into the E. coli-C. perfringens shuttle vector pJIR750, creating the plasmids P1, P2 or P3 respectively (Fig. 3C). Those plasmids were then individually electroporated into CPJV501 to create the strains CPJVp1, CPJVp2 or CPJVp3. PCR analyses confirmed the genotype of these CPJV501 complementing strains (Fig. 3B).
Neither CPJVp1 (encoding agrB alone) nor CPJVp2 (encoding agrB and agrD) were able to restore PFO or CPA production (Figs. 4B and 7A). However, complementation with CPJVp3 (encoding CPE1563, CPE1562, agrB and agrD) did restore 2 h supernatant PFO activity and CPA levels to approximately those found in culture supernatants of the wt strain 13 (Figs. 4B and 7A). qRT-PCR analyses confirmed that 2 h cultures of CPJVp3 exhibited similar levels of pfoA and cpa mRNA as found in 2 h cultures of the wt strain 13 (Fig. 5). In contrast, CPJVp1 and CPJVp2 exhibited substantially less, if any, complementation in these qRT-PCR analyses.
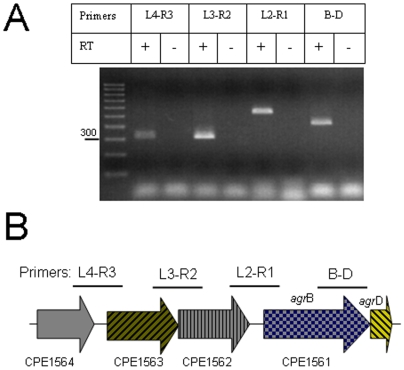
Results presented above suggested that agrB and agrD may be transcribed as an operon that also includes the two upstream ORFs CPE1563 and CPE1562, which are annotated as encoding hypothetical proteins (Fig. 8). To assess further whether agrB and agrD might be transcribed as part of an operon, RT-PCR analyses were performed using RNA extracted form the wt strain 13. These RT-PCR analyses also used primers that would produce a product only if every two ORF's from CPE1564 through agrD are co-transcribed. Fig. 8 results showed evidence for significant levels of co-transcription of CPE1563 and CPE1562, CPE1562 and agrB (CPE1561), and agrB and agrD, strongly suggesting that all four of these genes are co-transcribed in an operon. Interestingly, mRNA transcript was also detected for CPE1564 and CPE1563, but the signal was less intense than for the other transcripts, possibly suggesting a weak promoter can also independently co-transcribe these two ORFs.
Figure 8. Organization and RT-PCR analysis of the agr operon.
A) RT-PCR reactions were performed with 50 ng of RNA extracted from an overnight TGY culture of the wt strain 13. RT-PCR reactions included (+) or not (−) retrotranscriptase (RT). The following pair of primers were used to detect mRNA transcripts from every two-adjacent ORF's, agr104L and agr103R (L4-R3, which should generate a 321 bp PCR product), agr103L and agr102R (L3-R2, which should generate a 315 bp PCR product), agr102L and agr101R (L2-R1, which should generate a 420 bp PCR product) or agr101L and agrDR (B–D, which should generate a 520 bp PCR product). A 100-bp DNA ladder is shown at left. B) Schematic representation of the agr locus showing primers used for RT-PCR reactions.
Discussion
This study reports the development of a simple EZ-Tn5-based approach for random mutagenesis in Clostridium perfringens. All screened EZ-Tn5 mutants obtained by this method contained only a single transposon insertion and were stable over at least 10 sequential overnight culturings. This new approach produced mutants at high efficiency, i.e., 11,200 CFU/µg DNA for strain 13. This mutant yield was 46-fold higher than recently reported for phage Mu-based random mutagenesis of a C. perfringens strain 13 derivative [15], which is consistent with previous reports comparing mutant yields for other bacteria when using EZ-Tn5 vs. phage Mu-based random mutagenesis approaches (www.epibio.com).
Besides better efficiency, EZ-Tn5 random mutagenesis possesses a second advantage over the phage Mu-based system. When applied to C. perfringens, phage Mu-based transpositions favored insertion into rRNA genes, with nearly 45% of the phage Mu-based C. perfringens mutants carrying an insertion into a rRNA gene [15]. Since another 12% of those phage Mu-based mutants carried an insertion into an intergenic region, only ∼45% of the C. perfringens mutants in that phage Mu-based library had the desired outcome, i.e., a transposon insertion into a protein-encoding ORF. While the EZ-Tn5 transposon exhibited somewhat higher insertion rates into C. perfringens rRNA genes than would be expected by mere chance, this preference was much less than observed for phage Mu-based random mutagenesis. Specifically, only 18% of the screened EZ-Tn5 C. perfringens mutants carried a transposon insertion into a rRNA gene (rRNA genes represent about 1.5% of total genes in C. perfringens). Since none of the EZ-Tn5 C. perfringens strain 13 mutants happened to carry a transposon insertion in an intergenic region (although limited intergenic EZ-Tn5 insertion was observed with another C. perfringens strain, data not shown), ∼73% of the transposons in the screened C. perfringens strain 13 EZ-Tn5 carrying mutants had single insertions in a protein encoding gene.
The current study then directly demonstrated the utility of the new EZ-Tn5 random mutagenesis system by identifying a new locus involved in controlling early log-phase production of α toxin and PFO by C. perfringens strain 13. Prior to the current work, regulation of PFO and α toxin expression in strain 13 was known to involve a classical bacterial two component regulatory system named VirS/VirR, where VirS is the membrane sensor and VirR is the transcriptional regulator [11], [17], [18], [26]–[30]. When phosphorylated, the VirR protein binds directly to VirR boxes located upstream of the pfoA gene encoding PFO. However, VirR boxes are not present upstream of the plc gene encoding α toxin [31]–[34]; instead, a regulatory RNA (named VR-RNA), whose transcription is itself regulated by VirS/VirR, is involved in control of α toxin expression [28]. In addition, previous studies have implicated the LuxS quorum sensing system in the regulation of α toxin and PFO expression by strain 13 [35].
The current study reveals a new level of complexity in the regulation of α toxin and PFO expression by strain 13. Specifically, the current results demonstrated that the early log-phase regulation of α toxin and PFO expression by this C. perfringens strain involves a locus containing ORFs with homology to S. aureus agrB and agrD. A recent bioinformatics search had identified the presence of agr ORFs in many firmicutes, including C. perfringens [21], but it had not yet been evaluated whether this system is functional or important for regulating C. perfringens virulence factor expression. As mentioned in the Results, a similar agr locus is well-established in quorum sensing regulation of S. aureus virulence, where the agr locus controls expression of several toxins, as well as some surface virulence factors [20]. A similar agr locus was also recently implicated in Listeria monocytogenes virulence, where agrD-dependent quorum sensing regulates biofilm formation, Caco-2 cell invasion and mouse virulence [36].
In S. aureus, the agr locus is a four gene operon transcribed primarily from a promoter named P2 [20]. This S. aureus agr operon encodes for a TCRS (that includes the AgrA transcriptional regulator and the AgrC membrane sensor), the agrD signaling peptide, and an AgrB transmembrane protein involved in AgrD processing. Once activated and secreted, extracellular AgrD binds to (and activates) AgrC, which then phosphorylates AgrA. The phosphorylated AgrA then binds to P2 and to another promoter named P3 [25], [37]. This P3 binding leads to production of a regulatory RNA (named RNAIII) encoded by a gene adjacent to the agr locus. RNAIII then modulates expression of several exotoxins and surface proteins.
The agr systems of C. perfringens and S. aureus apparently share some similarities. Our results clearly demonstrated that, as in S. aureus, the agr locus (via a secreted factor) transcriptionally regulates toxin production by C. perfringens strain 13. Since the agr locus is involved in controlling early log-phase expression of α-toxin and PFO and these two toxins are important for C. perfringens-induced gas gangrene [12], [38], [39], the agr locus may play an important role in C. perfringens virulence, although this needs to be experimentally confirmed. Another similarity, revealed by our RT-PCR and complementation results, is that both C. perfringens and S. aureus co-transcribe agrB and agrD as part of an operon.
There also appear to be some differences between the agr systems of S. aureus versus C. perfringens. One variation concerns the arrangement of the agrB and agrD genes within the agr operon. In S. aureus, agrB and agrD are the first two transcribed genes in the operon [20], [25], but in C. perfringens these two agr genes appear to be downstream of other genes in the operon. It is unclear whether the two ORFs adjacent to agrB and agrD in C. perfringens encode a TCRS, as in S. aureus, although those two ORFs co-transcribed with agrB and agrD in the C. perfringens agr operon are currently annotated as encoding “hypothetical proteins” and thus do not possess obvious characteristics of a TCRS. However, future studies should evaluate this possibility. A final difference between the agr systems of C. perfringens versus S. aureus is that the RNAIII-encoding gene lies in close proximity to the agr operon in S. aureus [25], but no readily identifiable homolog of the RNAIII-encoding gene is located near the C. perfringens agr operon or is readily identifiable elsewhere in the strain 13 genome.
If the two upstream ORFs co-transcribed with the agrB and agrD genes in C. perfringens do not encode a TCRS, sorting out the signaling cascade may require some effort. The strain 13 genome contains 28 known or putative histidine kinase sensors [40], which coupled with one or more of the 20 known or putative response regulators, could mediate AgrD signaling. Whether the C. perfringens agr locus acts via the VirS/VirR TCRS, which is known to regulate α toxin and PFO production, should be assessed. If C. perfringens resembles S. aureus by using its Agr system to upregulate the production of a regulatory RNA, that regulatory RNA should be identified. A previous study [28] identified a C. perfringens regulatory RNA named VR-RNA that is involved in strain 13 production of α toxin. However, VR-RNA alone would not appear to readily explain agr-mediated signaling since, this regulatory RNA is not known to control PFO production [28], yet our agrB mutant cannot produce PFO during early log-phase growth. Therefore, further studies might evaluate whether several other putative regulatory RNAs of C. perfringens [18] mediate the agr locus signal. Also, sorting out the hierarchy of toxin expression control between the agr locus, LuxS and the VirS/VirR TCRS will require further studies to fully understand how C. perfringens regulates production of its toxins. Additionally, future studies should also examine whether expression of other toxins produced by some C. perfringens strains are regulated by the Agr system. Finally, it would be interesting to identify the environmental cues that signal the onset of agr operon transcription in C. perfringens. While many additional studies are clearly needed to fully understand it roles in C. perfringens, linkage of the agr locus to PFO and α toxin production opens a new chapter towards understanding toxin gene regulation by the important pathogen C. perfringens.
Materials and Methods
Strains and bacterial culture media
Strain 13, a genome-sequenced, highly transformable C. perfringens type A strain [40] was used for transposon mutagenesis experiments. A strain 13 pfoA-null mutant and CN3685 pfoA-null mutant were constructed using our previously described Targetron® technology [6], [8]. The bacterial culture media used throughout this study included FTG (fluid thioglycolate medium; Difco Laboratories), TGY (3% tryptic soy broth [Becton-Dickinson]; 2% glucose [Sigma Aldrich], 1% yeast extract [Becton-Dickinson], 0.1% sodium thioglycolate [Sigma Aldrich]), TSC agar medium (SFP agar [Difco Laboratories], supplemented with 0.04% of D-cycloserine [Sigma Aldrich]), Luria-Bertani (LB) broth (1% tryptone [Becton-Dickinson], 0.5% yeast extract, 1% NaCl), LB agar (1.5% agar [Becton-Dickinson]) and brain heart infusion (BHI) agar (Becton-Dickinson). E. coli Top10 cells (Invitrogen) were used as the cloning host. When indicated, ampicillin (Amp, 100 µg/ml), erythromycin (Erm [100 µg/ml] or [40 µg/ml]) or chloramphenicol (Cm [15 µg/ml]) was added to the culture medium.
Construction of the modified EZ-Tn5 transposon vector and transposome preparation
To modify the EZ-Tn5-carrying plasmid pMOD-2 (Epicentre) for use in C. perfringens, a single colony of an E. coli strain encoding the EZ-Tn5 pMOD-2 vector was inoculated into 10 ml of LB broth supplemented with ampicillin (LBA) and then incubated overnight at 37°C with shaking (250 RPM). The plasmid was extracted with a QIAprep Spin plasmid extraction kit (Qiagen) and simultaneously digested with EcoRI and HindIII (New England Biolabs). The erythromycin resistance gene (erm) from the E. coli-C. perfringens shuttle vector pJIR751 [41] was amplified by PCR using JumpStart REDTaq ready mix (Sigma-Aldrich) and primers erm-Fwd-EcoRI and erm-Rev-HindIII (Table 2). The PCR product was run on a 1.5% agarose gel, purified using a QIAquick gel extraction kit (Qiagen), and then simultaneously digested with EcoRI and HindIII. The digested EZ-Tn5-carrying pMOD-2 plasmid and the erm gene PCR product were ligated overnight at 4°C with T4 DNA ligase (New England Biolab). The resulting plasmid, named pJVTN5, was then transformed into chemically competent E. coli Top10 cells (Invitrogen) and inoculated onto LB agar plates supplemented with erythromycin and ampicillin. To prepare the transposome, pJVTN5 was digested with PvuII at 37°C for 1 h (see Fig. 1). The erm-carrying EZ-Tn5 transposon fragment (∼900 bp) was purified from an agarose gel as described earlier and DNA concentration was quantified. Two µl of the erm-modified EZ-Tn5 Transposon DNA (100 µg/ml in TE Buffer [10 mM Tris-HCl (pH 7.5), 1 mM EDTA]) were mixed with 4 µl of the EZ-Tn5 transposase (Epicentre) and 2 µl of glycerol. The mixture was incubated for 30 min at room temperature, to allow the transposase to stably bind to the erm-modified EZ-Tn5 Transposon DNA; that mixture was then stored at −20°C.
Transposome electroporation into C. perfringens strains
Following a standard procedure [6], [42], 1 µl of the transposome was electroporated into a 4 hour TGY culture of highly transformable C. perfringens strain 13 [40]. For this purpose, electrocompetent cells (400 µl) were mixed, in a 0.2 cm electroporation cuvette (Biorad), with 1 µl of the transposome and incubated 5 min at 4°C. Electroporation was performed using a BioRad Gene Pulser™ with pulse controller set at 200Ω, 25 µF and 1.5 kV. Electroporated transposome-containing bacteria were grown in 3 ml of pre-warmed TGY for 3 h at 37°C to allow them complete recovery, plated onto BHI agar plates with erythromycin (40 µg/ml), and incubated at 37°C for 18 h under anaerobic conditions. All colonies were propagated in BHI agar plates with erythromycin (40 µg/ml). To confirm the presence of the erm gene, a PCR was performed with 2 µl of cell lysate as DNA template. This PCR used primers erm-Fwd-EcoRI and erm-Rev-HindIII and the following PCR conditions: 1 cycle of 95°C for 5 min, 35 cycles of 95°C for 30 s, 55°C for 45 s, and 68°C for 1 min; and a single extension of 68°C for 10 min.
Sequencing of the EZ-Tn5 target gene in selected mutants
After electroporation of the transposon, C. perfringens strain 13 Erm-resistant transformants, which were also erm-positive by PCR, were randomly chosen for sequencing. Total DNA was extracted and 1 µg of each DNA was mixed with 10 pmol of primers pMOD-SeqFwd or pMOD-SeqRev (Epicentre) and sent for sequencing at the University of Pittsburgh Genomics and Proteomics Core Laboratory. C. perfringens sequences flanking the erm-carrying transposon were determined using the nucleotide BLAST program on the National Center for Biotechnology Information (NCBI) web site and the J. Craig Venter Institute's Pathema website programs.
Complementation of the agrB mutant
DNA was isolated from strain 13 using a Master Pure™ Gram Positive DNA purification Kit (Epicentre). The primers agrBF and agrBR (Table 2) were added (at a 5 µM final concentration) to a PCR mixture containing 1 µl of purified DNA template and 25 µl 2×Taq mixture (NEB). Those reaction mixtures, with a total volume of 50 µl, were placed in a thermal cycler (Techne) and subjected to the following amplification conditions: 1 cycle of 95°C for 2 min, 35 cycles of 95°C for 30 s, 55°C for 40 s, and 68°C for 3 min, and a single extension of 68°C for 5 min. The PCR products were cloned into a TOPO vector (Invitrogen) and sequenced at the University of Pittsburgh Core Sequencing Facility. Using EcoRI and BamHI, the insert was removed from the TOPO vector and ligated into pJIR750, forming a plasmid named P1 (which is 1072 bp and contains agrB and a 403 bp upstream sequence). Using the same method, two other complementing plasmids were created, including P2 (created using agrBF-agrR1 primers and which has a 1230 bp insert containing agrB and agrD, along with a 403 bp upstream sequence) and P3 (created using agrF1-agrR1 and which has a 2893 bp insert containing agrB, agrD and two upstream ORFs encoding hypothetical proteins). Plasmids P1, P2 and P3 were separately introduced, by our standard electroporation techniques, into the agrB mutant of strain 13. Chloramphenicol (15 µg/ml) resistant transformants were then selected. The resultant transformants were designated CPJVp1, CPJVp2 and CPJVp3.
Southern blot analyses
C. perfringens DNA was isolated using the MasterPure gram-positive DNA purification kit (Epicentre, Wisconsin). Each isolated DNA sample (2.5 µg) was then digested overnight with EcoRI or XbaI, according to the manufacturer's (New England Biolabs) instructions. The digested DNA samples were electrophoresed on a conventional 1% agarose gel, and the separated DNA digestion products were then transferred onto nylon membranes (Roche) for hybridization with an erm-specific probe. After hybridization of the erm probe, the Southern blots were developed using reagents from the DIG DNA labeling and detection kit (Roche), according to the manufacturer's instruction.
Hemoglobin (Hb) release assay for PFO activity
C. perfringens strain 13 (S13), S13 pfoA-null mutant, CPJV501, CPJVp1, CPJVp2, CPJVp3, or a combination of CPJV501 and S13 pfoA-null mutant (at a 1∶1 ratio), or CPJV501 and CN3685 pfoA-null mutant (at a 1∶1 ratio), were inoculated into 10 ml of FTG and grown overnight at 37°C. An aliquot (100 µl) of this overnight culture was then inoculated into a test tube containing 10 ml of sterile TGY (OD600∼0.05) and grown for the indicated times. At each time point, the OD600 of the culture was recorded. In other experiments, 100 mm tissue culture dishes (Costar) containing 25 ml of TGY were inoculated (OD600∼0.05) with strain 13 or CPJV501 and incubated at 37°C for the indicated times. Another experiment used a 100 mm tissue culture dish containing a Transwell® filter (Costar) and 25 ml of TGY; strain 13 pfoA-null mutant was inoculated into the top well of the dish and CPJV501 was inoculated into the bottom chamber of the culture dish (at a 2∶1 ratio). This culture was also incubated at 37°C for different times. The culture supernatant was obtained and filter sterilized using a 0.45 µm filter (Millipore). The Hb release assay was then performed essentially as previously described [43].
ELISA
C. perfringens alpha toxin (1 µg/ml) purchased from Sigma Aldrich, or culture supernatants, were analyzed by ELISA using mouse monoclonal anti-CPA antibody (kindly provided by Dr. Paul Hauer), as previously described [43].
Western blot
Strain 13 (S13), CPJV501 (ΔagrB) or CPJVp3 (ΔagrB/P3) was inoculated in 10 ml of TGY and incubated at 37°C for 4 h. Bacteria were then pelleted by centrifugation at 8000×g at 4°C for 30 min. The bacterial pellet was resuspended in 500 µl of ice-cold lysis buffer [40 mM Tris-HCl pH 7.5, 100 mM NaCl, 1× protease inhibitors (Roche), 1 mM DTT and 1% Triton X-100] and then sonicated. Equal amounts (25 µl) of bacterial lysates were run in a 12% SDS-PAGE and transferred to nitrocellulose membranes. As control, 25 µl of 100× ammonium sulfate supernatant-concentrated proteins of an overnight culture of strain 13 was also run in the gel. Those membranes were blocked with PBS-Tween 20 (0.05% v/v) and non fat dry milk (5% wt/v) for 1 h and then probed with a mouse monoclonal anti-CPA antibody. Bound antibody was then detected after incubation with a horseradish peroxidase-conjugated secondary anti-species specific antibody and addition of Chemiluminescent Substrate (Pierce).
RT-PCR and qRT-PCR
Total C. perfringens RNA was extracted from 2 ml of an overnight TGY culture by the following procedure. After centrifugation of that culture (10,000×g at 4°C), the pellet was resuspended in 200 µl of acetate solution (20 mM sodium acetate [pH 5], 1 mM EDTA, 0.5% sodium dodecyl sulfate [SDS, Bio-Rad]). The suspension received 200 µl of saturated phenol (Fisher scientific) and was thoroughly resuspended before incubation at 60°C in a water bath with vigorous shaking for 5 min. After centrifugation (10,000×g at 4°C for 5 min), the nucleic acid-containing supernatant received cold ethanol and that sample was mixed well. The mixed sample was centrifuged (10,000×g at 4°C) for 5 min to obtain the RNA pellet. This pellet was washed two times with cold 70% ethanol and finally resuspended in 100 µl of DNase-free, RNase-free water. All RNA samples were additionally treated with 2 U of DNase I (Promega) at 37°C for 30 min. To stop DNase I activity, DNase I inhibitor (Promega) was added to the reaction tube. RNA was quantified by absorbance at 260 nm and stored in 50 ml aliquots at −80°C.
RT-PCR reactions were then performed on those DNase-treated RNA samples using the AccesQuick RT-PCR system (Promega). Briefly, 50 ng of each RNA sample were reversed-transcribed to cDNA at 45°C for 45 min and then used as template for PCR reactions (denaturing at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1 min) with the gene-specific primers. Control RT-PCR reactions were similarly performed, except for the omission of reverse transcriptase.
Quantitative RT-PCR (qRT-PCR) was performed using the iScript One-Step RT-PCR kit with SYBR Green (Bio-Rad) and the iCycler thermal cycler with a 96-well reaction module (Bio-Rad). qRT-PCR reactions were performed in triplicate with 20 ng of total RNA, 500 nM concentration of each primer (Table 2) and the following conditions; 1 cycle at 50°C for 30 min, 1 cycle at 95°C for 10 min and 40 cycles of 95°C for 15, 55°C for 1 min. Melting curves were generated by a cycle of 95°C for 1 min, 55°C for 1 min and 80 cycles of 55°C with 0.5°C increments. The relative quantitation of mRNA expression was normalized to the constitutive expression of the housekeeping polC gene and calculated by the comparative CT (2−ΔΔCT) method [44].
Note added during revision
During preparation of the revised version of this paper, Ohtani et al. (2009) published work also implicating the C. perfringens agr locus in control of PFO and CPA production by strain 13 (Ohtani, K., et al. J. Bacteriology. 2009. 191(12):3919–27).
Acknowledgments
We thank Dr. J. Carroll and C. L. Nolder for their assistance and helpful advice with qRT-PCR experiments. The authors also thank Dr. P. Hauer for supplying the monoclonal anti-CPA antibody.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grant R01 AI056177-06 from National Institute of Allergy and Infectious Diseases. JEV thanks a generous support from the Mexican National Council of Science and Technology (CONACyT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McClane BA, Uzal FA, Fernandez-Miyakawa M, Lyerly D, Wilkins TD. The Enterotoxigenic Clostridia. In: Dworkin SF M, Rosenburg E, Schleifer KF, Stackebrandt E, editors. The Prokaryotes. New York, NY: Springer-Verlag; 2004. pp. 698–752. [Google Scholar]
- 2.Carman RJ, Sayeed S, Li J, Genheimer CW, Hiltonsmith MF, et al. Clostridium perfringens toxin genotypes in the feces of healthy North Americans. Anaerobe. 2008;14:102–108. doi: 10.1016/j.anaerobe.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Sayeed S, McClane BA. Prevalence of enterotoxigenic Clostridium perfringens Isolates in Pittsburgh (Pennsylvania) area soils and home kitchens. Appl Environ Microbiol. 2007;73:7218–7224. doi: 10.1128/AEM.01075-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briancesco R. Microbial indicators and fresh water quality assessment. Ann Ist Super Sanita. 2005;41:353–358. [PubMed] [Google Scholar]
- 5.Heap JT, Cartman ST, Pennington OJ, Cooksley CM, Scott JC, Blount B, Burns DA, Minton NP. Development of genetic knock-out systems for clostridia. In: Bruggemann HaGG., editor. Clostridia: molecular biology in the post-genomic era. Norfolk, UK: Caister Acadeic Press; 2009. pp. 179–198. [Google Scholar]
- 6.Chen Y, McClane BA, Fisher DJ, Rood JI, Gupta P. Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl Environ Microbiol. 2005;71:7542–7547. doi: 10.1128/AEM.71.11.7542-7547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta P, Chen Y. Chromosomal engineering of Clostridium perfringens using group II introns. Methods Mol Biol. 2008;435:217–228. doi: 10.1007/978-1-59745-232-8_16. [DOI] [PubMed] [Google Scholar]
- 8.Sayeed S, Uzal FA, Fisher DJ, Saputo J, Vidal JE, et al. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol Microbiol. 2008;67:15–30. doi: 10.1111/j.1365-2958.2007.06007.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Caruso L, McClane B, Fisher D, Gupta P. Disruption of a toxin gene by introduction of a foreign gene into the chromosome of Clostridium perfringens using targetron-induced mutagenesis. Plasmid. 2007;58:182–189. doi: 10.1016/j.plasmid.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, McClane BA. A novel small acid soluble protein variant is important for spore resistance of most Clostridium perfringens food poisoning isolates. PLoS Pathog. 2008;4:e1000056. doi: 10.1371/journal.ppat.1000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyristis M, Bryant AE, Sloan J, Awad MM, Nisbet IT, et al. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol Microbiol. 1994;12:761–777. doi: 10.1111/j.1365-2958.1994.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 12.Awad MM, Rood JI. Isolation of alpha-toxin, theta-toxin and kappa-toxin mutants of Clostridium perfringens by Tn916 mutagenesis. Microb Pathog. 1997;22:275–284. doi: 10.1006/mpat.1996.0115. [DOI] [PubMed] [Google Scholar]
- 13.Briolat V, Reysset G. Identification of the Clostridium perfringens genes involved in the adaptive response to oxidative stress. J Bacteriol. 2002;184:2333–2343. doi: 10.1128/JB.184.9.2333-2343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smidt H, Song D, van Der Oost J, de Vos WM. Random transposition by Tn916 in Desulfitobacterium dehalogenans allows for isolation and characterization of halorespiration-deficient mutants. J Bacteriol. 1999;181:6882–6888. doi: 10.1128/jb.181.22.6882-6888.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanckriet A, Timbermont L, Happonen LJ, Pajunen MI, Pasmans F, et al. Generation of single-copy transposon insertions in Clostridium perfringens by electroporation of phage mu DNA transposition complexes. Appl Environ Microbiol. 2009;75:2638–2642. doi: 10.1128/AEM.02214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pajunen MI, Pulliainen AT, Finne J, Savilahti H. Generation of transposon insertion mutant libraries for Gram-positive bacteria by electroporation of phage Mu DNA transposition complexes. Microbiology. 2005;151:1209–1218. doi: 10.1099/mic.0.27807-0. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu T, Ba-Thein W, Tamaki M, Hayashi H. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J Bacteriol. 1994;176:1616–1623. doi: 10.1128/jb.176.6.1616-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okumura K, Ohtani K, Hayashi H, Shimizu T. Characterization of genes regulated directly by the VirR/VirS system in Clostridium perfringens. J Bacteriol. 2008;190:7719–7727. doi: 10.1128/JB.01573-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers GS, Rasko DA, Cheung JK, Ravel J, Seshadri R, et al. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Res. 2006;16:1031–1040. doi: 10.1101/gr.5238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 21.Wuster A, Babu MM. Conservation and evolutionary dynamics of the agr cell-to-cell communication system across firmicutes. J Bacteriol. 2008;190:743–746. doi: 10.1128/JB.01135-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher DJ, Fernandez-Miyakawa ME, Sayeed S, Poon R, Adams V, et al. Dissecting the contributions of Clostridium perfringens type C toxins to lethality in the mouse intravenous injection model. Infect Immun. 2006;74:5200–5210. doi: 10.1128/IAI.00534-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji G, Beavis RC, Novick RP. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci U S A. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 25.Novick RP, Projan SJ, Kornblum J, Ross HF, Ji G, et al. The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet. 1995;248:446–458. doi: 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 26.Ohtani K, Kawsar HI, Okumura K, Hayashi H, Shimizu T. The VirR/VirS regulatory cascade affects transcription of plasmid-encoded putative virulence genes in Clostridium perfringens strain 13. FEMS Microbiol Lett. 2003;222:137–141. doi: 10.1016/S0378-1097(03)00255-6. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu T, Shima K, Yoshino K, Yonezawa K, Shimizu T, et al. Proteome and transcriptome analysis of the virulence genes regulated by the VirR/VirS system in Clostridium perfringens. J Bacteriol. 2002;184:2587–2594. doi: 10.1128/JB.184.10.2587-2594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu T, Yaguchi H, Ohtani K, Banu S, Hayashi H. Clostridial VirR/VirS regulon involves a regulatory RNA molecule for expression of toxins. Mol Microbiol. 2002;43:257–265. doi: 10.1046/j.1365-2958.2002.02743.x. [DOI] [PubMed] [Google Scholar]
- 29.Ba-Thein W, Lyristis M, Ohtani K, Nisbet IT, Hayashi H, et al. The virR/virS locus regulates the transcription of genes encoding extracellular toxin production in Clostridium perfringens. J Bacteriol. 1996;178:2514–2520. doi: 10.1128/jb.178.9.2514-2520.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung JK, Awad MM, McGowan S, Rood JI. Functional analysis of the VirSR phosphorelay from Clostridium perfringens. PLoS ONE. 2009;4:e5849. doi: 10.1371/journal.pone.0005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung JK, Rood JI. The VirR response regulator from Clostridium perfringens binds independently to two imperfect direct repeats located upstream of the pfoA promoter. J Bacteriol. 2000;182:57–66. doi: 10.1128/jb.182.1.57-66.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung JK, Dupuy B, Deveson DS, Rood JI. The spatial organization of the VirR boxes is critical for VirR-mediated expression of the perfringolysin O gene, pfoA, from Clostridium perfringens. J Bacteriol. 2004;186:3321–3330. doi: 10.1128/JB.186.11.3321-3330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGowan S, Lucet IS, Cheung JK, Awad MM, Whisstock JC, et al. The FxRxHrS motif: a conserved region essential for DNA binding of the VirR response regulator from Clostridium perfringens. J Mol Biol. 2002;322:997–1011. doi: 10.1016/s0022-2836(02)00850-1. [DOI] [PubMed] [Google Scholar]
- 34.McGowan S, O'Connor JR, Cheung JK, Rood JI. The SKHR motif is required for biological function of the VirR response regulator from Clostridium perfringens. J Bacteriol. 2003;185:6205–6208. doi: 10.1128/JB.185.20.6205-6208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtani K, Hayashi H, Shimizu T. The luxS gene is involved in cell-cell signalling for toxin production in Clostridium perfringens. Mol Microbiol. 2002;44:171–179. doi: 10.1046/j.1365-2958.2002.02863.x. [DOI] [PubMed] [Google Scholar]
- 36.Riedel CU, Monk IR, Casey PG, Waidmann MS, Gahan CG, et al. AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes. Mol Microbiol. 2009;71:1177–1189. doi: 10.1111/j.1365-2958.2008.06589.x. [DOI] [PubMed] [Google Scholar]
- 37.Koenig RL, Ray JL, Maleki SJ, Smeltzer MS, Hurlburt BK. Staphylococcus aureus AgrA binding to the RNAIII-agr regulatory region. J Bacteriol. 2004;186:7549–7555. doi: 10.1128/JB.186.22.7549-7555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickey MJ, Kwan RY, Awad MM, Kennedy CL, Young LF, et al. Molecular and cellular basis of microvascular perfusion deficits induced by Clostridium perfringens and Clostridium septicum. PLoS Pathog. 2008;4:e1000045. doi: 10.1371/journal.ppat.1000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Awad MM, Bryant AE, Stevens DL, Rood JI. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol Microbiol. 1995;15:191–202. doi: 10.1111/j.1365-2958.1995.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, et al. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci U S A. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bannam TL, Rood JI. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid. 1993;29:233–235. doi: 10.1006/plas.1993.1025. [DOI] [PubMed] [Google Scholar]
- 42.Chen CK, Boucle CM, Blaschek HP. Factors involved in the transformation of previously non-transformable Clostridium perfringens type B. FEMS Microbiol Lett. 1996;140:185–191. doi: 10.1111/j.1574-6968.1996.tb08334.x. [DOI] [PubMed] [Google Scholar]
- 43.Vidal JE, Ohtani K, Shimizu T, McClane BA. Contact with enterocyte-like Caco-2 cells induces rapid upregulation of toxin production by Clostridium perfringens type C isolates. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01332.x. DOI: 10.1111/j.1462-5822.2009.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the (2−ΔΔC T) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Garmory HS, Chanter N, French NP, Bueschel D, Songer JG, et al. Occurrence of Clostridium perfringens beta2-toxin amongst animals, determined using genotyping and subtyping PCR assays. Epidemiol Infect. 2000;124:61–67. doi: 10.1017/s0950268899003295. [DOI] [PMC free article] [PubMed] [Google Scholar]