Abstract
Eosinophils are pleiotropic multi-functional leukocytes that are typically associated with the initiation and propagation of inflammatory responses, particularly helminth infection and allergic disease. However, expanding evidence supports a broader role for eosinophils in homeostatic function and organ development and modulation of local immune responses via interaction with other effector cells. In this review, the biology of eosinophils in the healthy gut is summarized. In particular, the molecular steps involved in eosinophil development and trafficking are described, with special attention to the important role of the transcription factor GATA-1, the eosinophil selective cytokine IL-5 and the eotaxin subfamily of chemokines. In addition, the regulation of eosinophil survival by inhibitory and death receptors and the expanding role for eosinophils in health and disease are reviewed.
Keywords: Eosinophils, gastrointestinal
Development and maturation of eosinophils
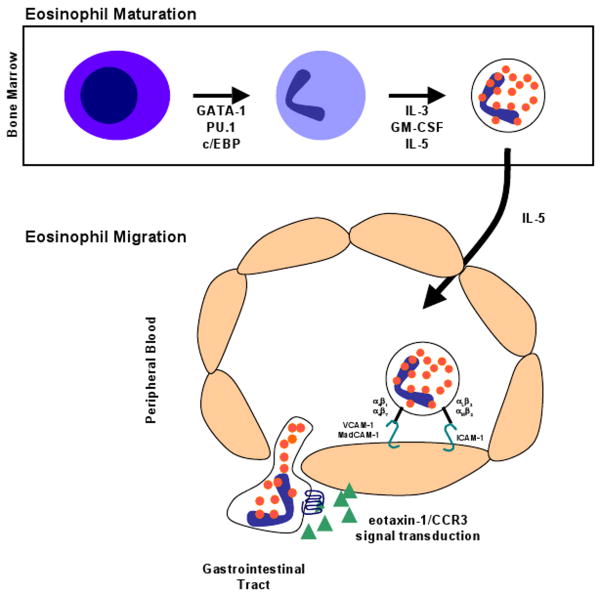
Eosinophils are generated from pluripotential hematopoietic stem cells in the bone marrow via a process that is highly regulated by transcription factors and cytokines (Figure 1) [1]. Eosinophil lineage development is dictated by an interplay of at least three classes of transcription factors including GATA-1 (a zinc family finger member), PU.1 (an ets family member), and C/EBP members (CCAAT/enhancer-binding protein family) [2]. While these three transcription factors are expressed in a variety of hematopoietic lineages, their mechanism of action in eosinophils is unique. In particular, the effects of PU.1 on specific lineage commitment is dosage-related with higher concentrations favoring myeloid differentiation [3,4]. Whereas GATA-1 and PU.1 antagonize each other in most cell types, they have synergistic activity in regulating eosinophil lineage specification (and eosinophil granule protein transcription) [4]. Of these transcription factors, GATA-1 is clearly the most important for eosinophil lineage specification [5]. While GATA-1 is expressed in erythroid cells, megakaryocytes, mast cells, and eosinophils, the specificity of GATA-1 activity for the eosinophil lineage is mediated by a high-affinity palindromic (or double) GATA binding site [6]. Targeted deletion of this double GATA binding site in the promoter for the transcription factor GATA-1 resulted in the loss of the eosinophil lineage in mice [6]. Further, this double GATA site is also present in the regulatory regions of eosinophil-specific genes, including the eotaxin receptor CC chemokine receptor 3 (CCR3), the granule protein major basic protein (MBP), and the IL-5 receptor alpha gene [7].
Figure 1.
Schematic representation of eosinophil maturation and trafficking. Eosinophils differentiate from hematopoietic progenitor cells into mature eosinophils in the bone marrow under the control of critical transcription factors, especially GATA-1. The eosinophil-promoting cytokines, IL-3, GM-CSF, and IL-5, regulate eosinophil expansion. Eosinophil migration from the bone marrow into the peripheral circulation is primarily regulated by IL-5. Circulating eosinophils interact with the endothelium in the gastrointestinal tract by a regulated process involving the coordinated interaction between adhesion molecules, chemokines and their receptors.
Three cytokines, interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are particularly important in regulating eosinophil development [8-10]. These eosinophil-promoting hematopoietic cytokines, or eosinophilpoietins, are encoded by closely linked genes on human chromosome 5q31 and signal through receptors that share a common beta chain but have unique alpha chains [11]. Eosinophilpoietins likely provide proliferative and differentiation signals specified by the transcription factors GATA-1, PU.1, and C/EBPs (Figure 1). Of these three cytokines, IL-5 is the most specific to the eosinophil lineage and is responsible for selective differentiation of eosinophils and stimulation of eosinophil release from the bone marrow into the peripheral circulation [12,13]. The overproduction of one or a combination of IL-5, IL-3 and GM-CSF occurs in humans with eosinophilia and diseases with selective eosinophilia are often accompanied by overproduction of IL-5 and IL-3 [14-16]. The critical role of IL-5 in regulating eosinophils in humans has been demonstrated by clinical trials with humanized anti-IL-5 antibody lowering eosinophil levels in the blood and in inflamed tissues, including lung, skin, nasal mucosa and esophagus [17-22].
Migration of eosinophils from the blood stream
Eosinophil trafficking from the circulating blood into peripheral tissues is a multi-step process involving rolling, tethering and firm adhesion to the vascular endothelium followed by transendothelial migration into the tissue. This complex process is orchestrated by the coordinated interaction between networks involving chemokine and cytokine signaling, adhesion molecules and their receptors expressed on vascular endothelial cells (Figure 1) [23]. Although the adhesion processes involved in eosinophil tissue accumulation have not been extensively elucidated in the gastrointestinal tract, they have been examined in vitro and in the lung [24]. The initial steps of eosinophil rolling and tethering are regulated by selectins, single-chain transmembrane glycoproteins, on the surface of eosinophils and their ligands expressed on the endothelium [25,26]. Eosinophils have been shown to constitutively express L-selectin which regulates eosinophil rolling on the endothelium [27,28]. Ligands for L-selectin include CD34 and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expressed by endothelial cells [29]. Eosinophils also express CD162 (P-selectin glycoprotein ligand-1 or PSGL-1) and sialyl-Lewis X (CD15s) which interact with E-selectin and P-selectins and contribute to eosinophil tethering to endothelium [30,31].
Integrins, another family of cell adhesion receptors involved in cell-extracellular matrix and cell-cell interactions, are heterodimeric surface molecules consisting of an α- and β-chain. Eosinophils express members of the β1 (α4β1 and α6β1), β2 (αLβ2, αMβ2, αXβ2 and αDβ2) and β7 (α4β7) integrin families [32,33]. These various integrin molecules also selectively interact with adhesion receptors expressed on the vascular endothelium. These adhesion interactions have been shown to be important for eosinophil recruitment into the lung and skin, but their role in eosinophil recruitment to the gastrointestinal tract has not been extensively evaluated [33,34]. The α4β7 molecule, which is co-expressed on lymphocytes and eosinophils, may be the most important integrin for gastrointestinal eosinophils. This integrin binds to MAdCAM-1, a major adhesion molecule expressed on high endothelial venules in the intestinal lamina propria, lymph nodes, and Peyer's patches [35,36]. In β7 gene targeted mice, there is a delay and reduced magnitude in the development of intestinal eosinophilia following Trichinella spiralis infection [37]. Recent experimental studies have demonstrated that eosinophil recruitment into different compartments of the gastrointestinal tract is regulated by differential adhesion pathways. For example, eosinophil recruitment to the small intestine of the gastrointestinal tract is MAdCAM-1/α4(β7-integrin-dependent, whereas eosinophil accumulation in the colon is regulated by a β2-integrin pathway (ICAM-1) and can occur independently of α4- and β7-integrin pathways [38,39]. Determining the role of the α4 and β7 integrins on gastrointestinal eosinophils has important therapeutic implication since there are several clinical agents that block these molecules; in particular, a recent study has shown a beneficial effect of anti-α4 integrin in patients with IBD [40,41]. Finally, eosinophils express several other adhesion receptors, including CD48, a CD2-subfamily receptor, CD44, a receptor for hyaluronan and members of the immunoglobulin receptor superfamily, including the sialic acid binding immunoglobulin-like lectin protein-8 (siglec-8), which have been shown to regulate multiple eosinophil responses [42-45].
Migration of eosinophils into the intestine
Eosinophils have been noted to be present at low levels in numerous tissues. Autopsy and biopsy specimens show tissue eosinophil infiltration at substantial levels in the gastrointestinal tract, spleen, lymph nodes, and thymus [46]. Whereas the healthy esophagus is essentially devoid of eosinophils, the rest of the gastrointestinal mucosa contains varying numbers of eosinophils. Examination of eosinophils throughout the gastrointestinal tract of conventional healthy mice (untreated mice maintained under specific pathogen-free conditions) has revealed that eosinophils are normally present in the lamina propria of the stomach, small intestine, cecum, and colon [47]. Prenatal mice have eosinophils located in similar regions and concentrations as observed in adult mice, providing evidence that eosinophil homing into the gastrointestinal tract occurs independent of endogenous flora [47]. In humans, the appendix, cecum and ascending colon contain the highest concentration of eosinophils in the gastrointestinal tract [48,49]. Interestingly, the number of eosinophils found in normal colon mucosa is influenced by geography, as there was a 35-fold increase in the number of eosinophils counted in colonic mucosal biopsy samples from asymptomatic patients in New Orleans compared to Boston [50]. Taken together, these studies suggest that a number of factors may account for resident gastrointestinal eosinophil populations, including local environmental exposures.
Numerous inflammatory mediators have been implicated in regulating eosinophil accumulation, including IL-1, IL-3, IL-4, IL-5, IL-13 and GM-CSF and the chemokines RANTES, monocyte chemoattractant protein (MCP)-3, MCP-4, macrophage inflammatory protein-1α and eotaxin-1, eotaxin-2 and eotaxin-3 [7,51]. In collaboration with IL-5, chemokines and lipid mediators (platelet activating factor and cysteinyl leukotriene [LT] C4) induce eosinophil trafficking by promoting chemoattraction into the gastrointestinal tract and other tissues. Of the mediators implicated in regulating eosinophil accumulation, IL-5 and the subfamily of eotaxin chemokines are relatively specific for eosinophils [52]. Studies suggest that eotaxin-1 has a particularly important role in the modulation of eosinophil accumulation in the gastrointestinal tract (Figure 1) [53]. For example, eotaxin-1-deficient mice have a defect in eosinophil trafficking to the gastrointestinal tract and are protected from experimental oral antigen-induced gastrointestinal pathology [54]. In contrast, IL-5-deficient mice fail to mount expansion of eosinophils in the bone marrow and blood, and have markedly impaired eosinophil accumulation in the allergen challenged lung [55]. These studies have identified IL-5 as a critical eosinophil growth factor and the eotaxin subfamily of chemokines as critical tissue recruitment factors.
Eotaxin-1 was initially discovered using a biological assay in guinea pigs designed to identify the molecules responsible for allergen-induced eosinophil accumulation in the lungs [52]. Subsequently, utilizing genomic analyses, two additional chemokines have been identified in the human genome that encode for CC chemokines with eosinophil-selective chemoattractant activity designated eotaxin-2 and eotaxin-3 [56-58]. The mouse genome was shown to only contain functional eotaxin-1 and eotaxin-2 [59,60]. Under baseline conditions, eotaxin-1 and eotaxin-2 are constitutively expressed in a variety of tissues, with especially high levels in the gastrointestinal tract and thymus [47,60,61]. The specific activity of all eotaxins is mediated by the selective expression of the seven-transmembrane spanning, G-protein coupled receptor CCR3, a receptor highly expressed on eosinophils [62,63]. Recently, CCR3-deficient mice have been developed, and have been shown to have a decreased level of jejunal eosinophils at baseline and following Trichinella spiralis infection [64]. The finding that gene targeting of the CCR3 locus (a locus not genetically linked to eotaxin-1) induces a phenotype similar to eotaxin-1-deficient mice definitively links the eotaxin/CCR3 pathway as a prime regulator of gastrointestinal eosinophils (Figure 1).
There is now substantial pre-clinical evidence supporting a role for eotaxins in human gastrointestinal disease, including markedly increased levels of eotaxin-1 mRNA in the lesions of patients with IBD [61]. In addition, a recent study correlated eotaxin-3 expression with mucosal eosinophilia in eosinophilic esophagitis [65]. Collectively, these studies and others have provided the impetus for the development of therapeutic agents aimed at blocking the action of eotaxins and/or CCR3. As such, small molecule inhibitors of CCR3 and a humanized anti-human eotaxin-1 antibody have been developed [66-69].
Effector functions of eosinophils
The beneficial function of eosinophils has been primarily attributed to their ability to defend the host against parasitic helminths. This is based on several lines of evidence including the ability of eosinophils to mediate antibody (or complement) dependent cellular toxicity against helminths in vitro, the observation that eosinophil levels increase during helminth infections, and that eosinophils aggregate and degranulate in the local vicinity of damaged parasites in vivo [70,71]. Although results in experimental models have largely supported this cytotoxic effector function, recent studies demonstrate an expanded role for eosinophils in organogenesis, tissue repair and remodeling and immune regulation (Figure 2) [72,73].
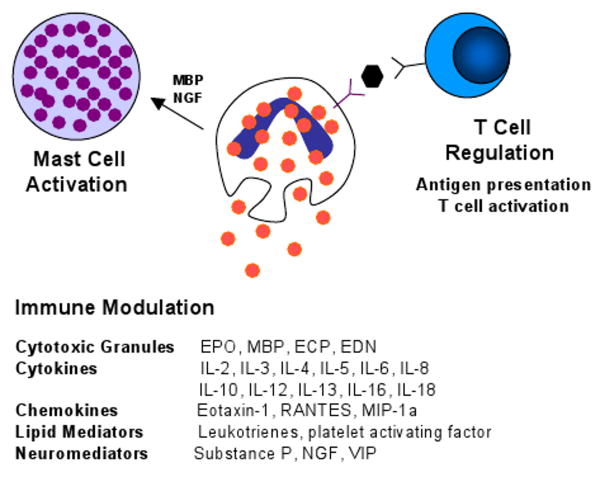
Figure 2.
Schematic diagram of an eosinophil and its multifunctional effects. Eosinophils release their preformed cytotoxic granules in response to diverse stimuli. In addition, eosinophils can modulate and perpetuate immune responses through the release of a variety of cytokines, chemokines, lipid mediators and neuromediators. Eosinophils regulate T cell activation via antigen presentation and cytokine production and activate mast cells through effects of MBP and NGF.
The finding that eosinophils home into the gastrointestinal tract during gestational development suggests that eosinophils may have a role in tissue or organ development [47]. A role for eosinophils in developmental processes in the gastrointestinal tract has not yet been identified. However, a physiological function for eosinophils in post-natal mammary gland development has been recently proposed [74]. In addition, the presence of constitutive eotaxin-1 and eosinophils in other endocrine organs (e.g. uterus), as well as in the gastrointestinal tract suggests that the involvement of tissue eosinophils in developmental processes is not likely to be restricted to the mammary gland [75].
Eosinophils are stimulated by a variety of triggers. In vitro studies have shown that eosinophil granule constituents are toxic to a variety of tissues including intestinal epithelium [76]. Eosinophil granules contain a crystalloid core composed of major basic protein (MBP)-1 (and MBP-2), and a matrix composed of eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), and eosinophil peroxidase (EPO). These cationic proteins share certain pro-inflammatory properties but differ in other ways. For example, MBP, EPO, and ECP have cytotoxic effects on epithelium, in concentrations similar to those found in biological fluids from patients with eosinophilia. Additionally, ECP and EDN belong to the Ribonuclease A superfamily and possess anti-viral and ribonuclease activity [77,78]. Further, eosinophils express a number of Toll-like receptors (TLR) important in innate immune responses [79]. Importantly, eosinophils have been shown to promote viral clearance from the lung via TLR-mediated activation [80].
Triggering of eosinophils by engagement of receptors for cytokines, immunoglobulins, and complement can lead to the generation of a wide range of inflammatory cytokines including IL-1, -3, -4, -5, -13, GM-CSF, transforming growth factors, TNF-α, RANTES, MIP-1α, vascular endothelial cell growth factor, and eotaxin-1, indicating that they have the potential to modulate multiple aspects of the immune response [81]. Further, eosinophil-derived transforming growth factor-β is linked with epithelial growth, fibrosis, and tissue remodeling [82,83].
Regulation of eosinophil life
Eosinophils are terminally differentiated granulocytes believed to possess a limited ability to survive in tissues in absence of survival promoting cytokines. There is increasing evidence that eosinophil numbers are regulated, not only by their production in the bone marrow, but also by the amount of programmed cell death or apoptosis. At sites of inflammation, eosinophil apoptosis can be delayed with production of survival factors (e.g. IL-5) by neighboring cells or by eosinophils themselves. Like many other cells, eosinophils do not only undergo apoptosis in the absence of survival factors, but can also be triggered to die via specific surface death receptors. One of these death receptors expressed by eosinophils is CD95 (Fas) [84,85]. The ligand of CD95 (CD95L/FasL) is highly expressed by activated T cells [86]. Thus, the same cells that produce eosinophil survival factors also express at least one death factor for eosinophils. Interestingly, activation of the CD95-mediated apoptotic pathway in eosinophils occurs even in the presence of eosinophil survival factors [84,85]. Similarly, signal transduction through other inhibitory receptors, including CD45, CD30, ICAM-3 and siglec-8, results in induction of apoptosis in eosinophils [87-90]. Eosinophils have been shown to express a number of other inhibitory receptors that can down regulate eosinophil function and inhibit survival [45]. These receptors likely represent an important regulatory mechanism controlling eosinophil accumulation at sites of tissue inflammation. Defining the signal transduction pathways and identifying the ligands for these inhibitory receptors could lead to new therapeutic targets in eosinophil-mediated diseases.
Interplay of eosinophils with other cells
In addition, to having a role in anti-parasite immunity, the localization of gastrointestinal eosinophils with lymphocytes suggests a functional interaction between these two leukocytes. While most studies have focused on the role of T lymphocytes in the regulation of eosinophils via elaboration of cytokines that promote eosinophil survival, activation and recruitment, it is likely that eosinophils also regulate immune responses through T cell recruitment and activation (Figure 2). Although antigen presentation to T cells is generally thought of as a process mediated by dendritic cells and macrophages, eosinophils have been demonstrated to be capable of inducing antigen-specific T-cell proliferation [91]. Further, eosinophils are known to express MCH II molecules and many of the costimulatory ligands (e.g. CD80, CD86) generally associated with antigen presentation and T-cell activation [92,93]. In addition, eosinophils secrete an array of cytokines capable of promoting T cell proliferation and activation of Th1 or Th2 polarization [73]. Importantly, infiltrating eosinophils have been shown to produce IL-4, IL-5 and IL-13, suggesting eosinophils are capable of modulating and sustaining a TH2 inflammatory response [94,95]. Further, murine eosinophils promote IL-4, IL-5 and IL-13 secretion by CD4+ T cells, suggesting a role for eosinophils in optimizing T cell inflammatory responses [96]. Additional support for an interaction between eosinophils and T cells has recently been derived from analysis of thymic eosinophils. As noted above, the thymus is a primary site for eosinophils under healthy conditions. In young mice, thymic eosinophils express IL-4 and IL-13, and are CD11b and CD11c positive (similar to dendritic cells) [97]. During experimental induction of tolerance, the level of thymic eosinophils increases and their location correlates with areas of active T cell apoptosis. Taken together, these studies indicate that regulation of T cell responses may be one of the physiological functions of eosinophils.
A substantial body of literature has emerged demonstrating that eosinophils have the capacity to regulate mast cell function (Figure 2). Notably, human umbilical cord blood derived mast cells can be activated by the eosinophil granule protein MBP to release histamine, PGD-2, GM-CSF, TNFα, and IL-8 [98]. The activation of mast cells by MBP elicits not only exocytosis, but also lipid mediator generation and cytokine production [98]. Eosinophils also produce nerve growth factor (NGF) [99], a cytokine not only involved in survival and functional maintenance of sympathetic neurons, but also in immune regulation, including promotion of mast cell survival and activation [100]. Interestingly, activation of eosinophils with the mast cell protease chymase promotes production of eosinophil derived stem cell factor, a critical mast cell growth factor. Thus, eosinophils and mast cells communicate in a bi-directional fashion.
Summary
At baseline healthy conditions, eosinophils normally account for a small subset of circulating blood cells and primarily reside in the gastrointestinal tract and hematopoietic organs. Their production is tightly regulated by the transcription factors GATA-1, GATA-2 and c/EBP that instruct the hematopoietic progenitor cell to differentiate into the eosinophil lineage and the expansion and tissue distribution of the eosinophil is primarily regulated by the cytokines IL-5 and eotaxin-1. As normal constituents of the mucosal immune system, particularly in the gastrointestinal tract, eosinophils are likely to have a physiological function. While the beneficial function of eosinophils is not very well understood, the cell appears to be involved in the optimal development of the female reproductive organs (e.g. mammary gland). In addition, evidence is emerging indicating that eosinophils contribute to the optimal innate immune response against certain parasitic and viral infections. Breakthroughs in identifying key eosinophil regulatory cytokines such as IL-5 and the eotaxin subfamily of chemokines have uncovered mechanisms that selectively regulate eosinophil production and localization at baseline and during inflammatory responses.
Practice Points
While eosinophil development in the bone marrow is orchestrated by a complicated network of cytokines and transcription factors, IL-5 is the most specific for the eosinophil lineage.
Therapy targeted against IL-5 lowers eosinophil levels in the blood and in inflamed tissues in eosinophil-associated diseases.
The gastrointestinal tract is the primary reservoir of eosinophils in humans.
Eotaxin-1 and its receptor CCR3 are critical for eosinophil trafficking into the intestine.
Small molecule inhibitors of CCR3 and anti-eotaxin directed therapies are in development for eosinophil-associated gastrointestinal diseases.
Regulation of T cell and mast cell activation is a physiological function of eosinophils.
Eosinophils have been shown to have a role in organogenesis, tissue repair and remodeling, viral clearance and local microenvironment immune regulation.
Research agenda
Detailed studies are necessary to define the potential of inhibitory receptors as therapeutic targets in eosinophil-mediated diseases.
The specific mechanism of eosinophil recruitment and activation in various disorders remains to be better elucidated.
The mechanisms that specifically control expression of the individual eotaxins are not yet defined.
Acknowledgments
This review was adopted from a number of prior reviews [7,51]. The authors are grateful to Nives Zimmermann and Simon Hogan for their contributions to this manuscript and for support from NIH grant R01 AI45898, the CURED (Campaign Urging Research for Eosinophilic Disease) Foundation, the Food Allergy Project, and the Buckeye Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Tenen DG, Hromas R, Licht JD, Zhang DE. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489–519. [PubMed] [Google Scholar]
- 2.McNagny K, Graf T. Making eosinophils through subtle shifts in transcription factor expression. J Exp Med. 2002;195:F43–F47. doi: 10.1084/jem.20020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeKoter RP, Singh H. Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science. 2000;288:1439–1441. doi: 10.1126/science.288.5470.1439. [DOI] [PubMed] [Google Scholar]
- 4.Du J, Stankiewicz MJ, Liu Y, Xi Q, et al. Novel combinatorial interactions of GATA-1, PU.1, and C/EBPepsilon isoforms regulate transcription of the gene encoding eosinophil granule major basic protein. J Biol Chem. 2002;277:43481–43494. doi: 10.1074/jbc.M204777200. [DOI] [PubMed] [Google Scholar]
- 5.Hirasawa R, Shimizu R, Takahashi S, Osawa M, et al. Essential and instructive roles of GATA factors in eosinophil development. J Exp Med. 2002;195:1379–1386. doi: 10.1084/jem.20020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu C, Cantor AB, Yang H, Browne C, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothenberg ME, Hogan SP. The Eosinophil. Annu Rev Immunol. 2005 doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 8.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, et al. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonoda Y, Arai N, Ogawa M. Humoral regulation of eosinophilopoiesis in vitro: analysis of the targets of interleukin-3, granulocyte/macrophage colony-stimulating factor (GM-CSF), and interleukin-5. Leukemia. 1989;3:14–18. [PubMed] [Google Scholar]
- 10.Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989;73:1504–1512. [PubMed] [Google Scholar]
- 11.Vadas M, Lopez A, Gamble J, Khew-Goodall Y, et al. Cytokines and allergy. J Allergy Clin Immunol. 1994;94:1289–1293. doi: 10.1016/0091-6749(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 12.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 13.Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, et al. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon D, Simon HU. Eosinophilic disorders. J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Owen WF, Rothenberg ME, Petersen J, Weller PF, et al. Interleukin 5 and phenotypically altered eosinophils in the blood of patients with the idiopathic hypereosinophilic syndrome. J Exp Med. 1989;170:343–348. doi: 10.1084/jem.170.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassina EM, Yousefi S, Simon D, Zwicky C, et al. cIAP-2 and survivin contribute to cytokine-mediated delayed eosinophil apoptosis. Eur J Immunol. 2006;36:1975–1984. doi: 10.1002/eji.200635943. [DOI] [PubMed] [Google Scholar]
- 17.Leckie MJ, ten Brinke A, Khan J, Diamant Z, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 18.Kips JC, O'Connor BJ, Langley SJ, Woodcock A, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 2003;167:1655–1659. doi: 10.1164/rccm.200206-525OC. [DOI] [PubMed] [Google Scholar]
- 19.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrett JK, Jameson SC, Thomson B, Collins MH, et al. Anti-interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol. 2004;113:115–119. doi: 10.1016/j.jaci.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 21.Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–1141. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Stein ML, Collins MH, Villanueva JM, Kushner JP, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312–1319. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Ebnet K, Kaldjian EP, Anderson AO, Shaw S. Orchestrated information transfer underlying leukocyte endothelial interactions. Annu Rev Immunol. 1996;14:155–177. doi: 10.1146/annurev.immunol.14.1.155. [DOI] [PubMed] [Google Scholar]
- 24.Resnick MB, Weller PF. Mechanisms of eosinophil recruitment. Am J Respir Cell Mol Biol. 1993;8:349–355. doi: 10.1165/ajrcmb/8.4.349. [DOI] [PubMed] [Google Scholar]
- 25.Wardlaw A. Eosinophil trafficking: new answers to old questions. Clin Exp Allergy. 2004;34:676–679. doi: 10.1111/j.1365-2222.2004.1944.x. [DOI] [PubMed] [Google Scholar]
- 26.Wardlaw AJ. The role of adhesion in eosinophil function. Chem Immunol. 2000;78:93–111. doi: 10.1159/000058820. [DOI] [PubMed] [Google Scholar]
- 27.Sriramarao P, von Andrian UH, Butcher EC, Bourdon MA, et al. L-selectin and very late antigen-4 integrin promote eosinophil rolling at physiological shear rates in vivo. J Immunol. 1994;153:4238–4246. [PubMed] [Google Scholar]
- 28.Georas SN, Liu MC, Newman W, Beall LD, et al. Altered adhesion molecule expression and endothelial cell activation accompany the recruitment of human granulocytes to the lung after segmental antigen challenge. Am J Respir Cell Mol Biol. 1992;7:261–269. doi: 10.1165/ajrcmb/7.3.261. [DOI] [PubMed] [Google Scholar]
- 29.Berg EL, McEvoy LM, Berlin C, Bargatze RF, et al. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 30.Symon FA, Lawrence MB, Williamson ML, Walsh GM, et al. Functional and structural characterization of the eosinophil P-selectin ligand. J Immunol. 1996;157:1711–1719. [PubMed] [Google Scholar]
- 31.Wardlaw AJ, Walsh GM, Symon FA. Adhesion interactions involved in eosinophil migration through vascular endothelium. Ann N Y Acad Sci. 1996;796:124–137. doi: 10.1111/j.1749-6632.1996.tb32574.x. [DOI] [PubMed] [Google Scholar]
- 32.Tachimoto H, Bochner BS. The surface phenotype of human eosinophils. Chem Immunol. 2000;76:45–62. doi: 10.1159/000058780. [DOI] [PubMed] [Google Scholar]
- 33.Tachimoto H, Ebisawa M, Bochner BS. Cross-talk between integrins and chemokines that influences eosinophil adhesion and migration. Int Arch Allergy Immunol. 2002;128 1:18–20. doi: 10.1159/000059414. [DOI] [PubMed] [Google Scholar]
- 34.Broide D, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunol Rev. 2001;179:163–172. doi: 10.1034/j.1600-065x.2001.790116.x. [DOI] [PubMed] [Google Scholar]
- 35.Shaw SK, Brenner MB. The beta 7 integrins in mucosal homing and retention. Semin Immunol. 1995;7:335–342. doi: 10.1016/1044-5323(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 36.Butcher EC, Williams M, Youngman K, Rott L, et al. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 37.Artis D, Humphreys NE, Potten CS, Wagner N, et al. Beta7 integrin-deficient mice: delayed leukocyte recruitment and attenuated protective immunity in the small intestine during enteric helminth infection. Eur J Immunol. 2000;30:1656–1664. doi: 10.1002/1521-4141(200006)30:6<1656::AID-IMMU1656>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 38.Mishra A, Hogan SP, Brandt EB, Wagner N, et al. Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol Chem. 2002;277:4406–4412. doi: 10.1074/jbc.M110424200. [DOI] [PubMed] [Google Scholar]
- 39.Forbes E, Hulett M, Ahrens R, Wagner N, et al. ICAM-1-dependent pathways regulate colonic eosinophilic inflammation. J Leukoc Biol. 2006;80:330–341. doi: 10.1189/jlb.1105643. [DOI] [PubMed] [Google Scholar]
- 40.Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122:1592–1608. doi: 10.1053/gast.2002.33426. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh S, Goldin E, Gordon FH, Malchow HA, et al. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 42.Floyd H, Ni J, Cornish AL, Zeng Z, et al. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 43.Kikly KK, Bochner BS, Freeman SD, Tan KB, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 2000;105:1093–1100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 44.Rothenberg ME. CD44--a sticky target for asthma. J Clin Invest. 2003;111:1460–1462. doi: 10.1172/JCI18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munitz A, Levi-Schaffer F. Inhibitory receptors on eosinophils: A direct hit to a possible Achilles heel. J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 46.Kato M, Kephart GM, Talley NJ, Wagner JM, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998;252:418–425. doi: 10.1002/(SICI)1097-0185(199811)252:3<418::AID-AR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 47.Mishra A, Hogan SP, Lee JJ, Foster PS, et al. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103:1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowichik A, Weinberg AG. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol. 1996;9:110–114. [PubMed] [Google Scholar]
- 49.DeBrosse CW, Case JW, Putnam PE, Collins MH, et al. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9:210–218. doi: 10.2350/11-05-0130.1. [DOI] [PubMed] [Google Scholar]
- 50.Pascal RR, Gramlich TL, Parker KM, Gansler TS. Geographic variations in eosinophil concentration in normal colonic mucosa. Mod Pathol. 1997;10:363–365. [PubMed] [Google Scholar]
- 51.Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID) J Allergy Clin Immunol. 2004;113:11–28. doi: 10.1016/j.jaci.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 52.Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matthews AN, Friend DS, Zimmermann N, Sarafi MN, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A. 1998;95:6273–6278. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hogan SP, Mishra A, Brandt EB, Royalty MP, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353–360. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 55.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, et al. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forssmann U, Uguccioni M, Loetscher P, Dahinden CA, et al. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J Exp Med. 1997;185:2171–2176. doi: 10.1084/jem.185.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shinkai A, Yoshisue H, Koike M, Shoji E, et al. A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J Immunol. 1999;163:1602–1610. [PubMed] [Google Scholar]
- 58.Kitaura M, Suzuki N, Imai T, Takagi S, et al. Molecular cloning of a novel human CC chemokine (Eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J Biol Chem. 1999;274:27975–27980. doi: 10.1074/jbc.274.39.27975. [DOI] [PubMed] [Google Scholar]
- 59.Pope SM, Fulkerson PC, Blanchard C, Akei HS, et al. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005;280:13952–13961. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- 60.Zimmermann N, Hogan SP, Mishra A, Brandt EB, et al. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J Immunol. 2000;165:5839–5846. doi: 10.4049/jimmunol.165.10.5839. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, et al. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 62.Ponath PD, Qin S, Post TW, Wang J, et al. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, et al. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–2354. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gurish MF, Humbles A, Tao H, Finkelstein S, et al. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. J Immunol. 2002;168:5730–5736. doi: 10.4049/jimmunol.168.11.5730. [DOI] [PubMed] [Google Scholar]
- 65.Blanchard C, Wang N, Stringer KF, Mishra A, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabroe I, Peck MJ, Van Keulen BJ, Jorritsma A, et al. A small molecule antagonist of chemokine receptors CCR1 and CCR3. Potent inhibition of eosinophil function and CCR3-mediated HIV-1 entry. J Biol Chem. 2000;275:25985–25992. doi: 10.1074/jbc.M908864199. [DOI] [PubMed] [Google Scholar]
- 67.Main S, Handy R, Wilton J, Smith S, et al. A potent human anti-eotaxin1 antibody, CAT-213: isolation by phage display and in vitro and in vivo efficacy. J Pharmacol Exp Ther. 2006;319:1395–1404. doi: 10.1124/jpet.106.110734. [DOI] [PubMed] [Google Scholar]
- 68.De Lucca GV. Recent developments in CCR3 antagonists. Curr Opin Drug Discov Devel. 2006;9:516–524. [PubMed] [Google Scholar]
- 69.Morokata T, Suzuki K, Masunaga Y, Taguchi K, et al. A novel, selective, and orally available antagonist for CC chemokine receptor 3. J Pharmacol Exp Ther. 2006;317:244–250. doi: 10.1124/jpet.105.097048. [DOI] [PubMed] [Google Scholar]
- 70.Butterworth AE. The eosinophil and its role in immunity to helminth infection. Curr Top Microbiol Immunol. 1977;77:127–168. doi: 10.1007/978-3-642-66740-4_5. [DOI] [PubMed] [Google Scholar]
- 71.Butterworth AE. Cell-mediated damage to helminths. Adv Parasitol. 1984;23:143–235. doi: 10.1016/s0065-308x(08)60287-0. [DOI] [PubMed] [Google Scholar]
- 72.Behm CA, Ovington KS. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol Today. 2000;16:202–209. doi: 10.1016/s0169-4758(99)01620-8. [DOI] [PubMed] [Google Scholar]
- 73.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: Singularly destructive effector cells or purveyors of immunoregulation. J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 74.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J, Lathbury LJ, Salamonsen LA. Expression of the chemokine eotaxin and its receptor, CCR3, in human endometrium. Biol Reprod. 2000;62:404–411. doi: 10.1095/biolreprod62.2.404. [DOI] [PubMed] [Google Scholar]
- 76.Gleich GJ, Frigas E, Loegering DA, Wassom DL, et al. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979;123:2925–2927. [PubMed] [Google Scholar]
- 77.Slifman NR, Loegering DA, McKean DJ, Gleich GJ. Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein. J Immunol. 1986;137:2913–2917. [PubMed] [Google Scholar]
- 78.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- 79.Nagase H, Okugawa S, Ota Y, Yamaguchi M, et al. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 80.Phipps S, Lam CE, Mahalingam S, Newhouse M, et al. Eosinophils contribute to innate anti-viral immunity and promote clearance of respiratory syncytial virus. Blood. 2007 doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 81.Kita H. The eosinophil: a cytokine-producing cell? J Allergy Clin Immunol. 1996;97:889–892. doi: 10.1016/s0091-6749(96)80061-3. [DOI] [PubMed] [Google Scholar]
- 82.Gharaee-Kermani M, Phan SH. The role of eosinophils in pulmonary fibrosis (Review) Int J Mol Med. 1998;1:43–53. [PubMed] [Google Scholar]
- 83.Phipps S, Ying S, Wangoo A, Ong YE, et al. The relationship between allergen-induced tissue eosinophilia and markers of repair and remodeling in human atopic skin. J Immunol. 2002;169:4604–4612. doi: 10.4049/jimmunol.169.8.4604. [DOI] [PubMed] [Google Scholar]
- 84.Matsumoto K, Schleimer RP, Saito H, Iikura Y, et al. Induction of apoptosis in human eosinophils by anti-Fas antibody treatment in vitro. Blood. 1995;86:1437–1443. [PubMed] [Google Scholar]
- 85.Tsuyuki S, Bertrand C, Erard F, Trifilieff A, et al. Activation of the Fas receptor on lung eosinophils leads to apoptosis and the resolution of eosinophilic inflammation of the airways. J Clin Invest. 1995;96:2924–2931. doi: 10.1172/JCI118364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Green DR, Ware CF. Fas-ligand: privilege and peril. Proc Natl Acad Sci U S A. 1997;94:5986–5990. doi: 10.1073/pnas.94.12.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 88.Kessel JM, Sedgwick JB, Busse WW. Ligation of intercellular adhesion molecule 3 induces apoptosis of human blood eosinophils and neutrophils. J Allergy Clin Immunol. 2006;118:831–836. doi: 10.1016/j.jaci.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 89.Blaylock MG, Sexton DW, Walsh GM. Ligation of CD45 and the isoforms CD45RA and CD45RB accelerates the rate of constitutive apoptosis in human eosinophils. J Allergy Clin Immunol. 1999;104:1244–1250. doi: 10.1016/s0091-6749(99)70020-5. [DOI] [PubMed] [Google Scholar]
- 90.Matsumoto K, Terakawa M, Miura K, Fukuda S, et al. Extremely rapid and intense induction of apoptosis in human eosinophils by anti-CD30 antibody treatment in vitro. J Immunol. 2004;172:2186–2193. doi: 10.4049/jimmunol.172.4.2186. [DOI] [PubMed] [Google Scholar]
- 91.Del PV, De AB, Martin E, Cardaba B, et al. Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing. Eur J Immunol. 1992;22:1919–1925. doi: 10.1002/eji.1830220736. [DOI] [PubMed] [Google Scholar]
- 92.Lucey DR, Nicholson-Weller A, Weller PF. Mature human eosinophils have the capacity to express HLA-DR. Proc Natl Acad Sci U S A. 1989;86:1348–1351. doi: 10.1073/pnas.86.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tamura N, Ishii N, Nakazawa M, Nagoya M, et al. Requirement of CD80 and CD86 molecules for antigen presentation by eosinophils. Scand J Immunol. 1996;44:229–238. doi: 10.1046/j.1365-3083.1996.d01-303.x. [DOI] [PubMed] [Google Scholar]
- 94.Justice JP, Borchers MT, Lee JJ, Rowan WH, et al. Ragweed-induced expression of GATA-3, IL-4, and IL-5 by eosinophils in the lungs of allergic C57BL/6J mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L302–L309. doi: 10.1152/ajplung.00158.2001. [DOI] [PubMed] [Google Scholar]
- 95.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 96.MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol. 2001;167:3146–3155. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- 97.Throsby M, Herbelin A, Pleau JM, Dardenne M. CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J Immunol. 2000;165:1965–1975. doi: 10.4049/jimmunol.165.4.1965. [DOI] [PubMed] [Google Scholar]
- 98.Piliponsky AM, Gleich GJ, Bar I, Levi-Schaffer F. Effects of eosinophils on mast cells: a new pathway for the perpetuation of allergic inflammation. Mol Immunol. 2002;38:1369. doi: 10.1016/s0161-5890(02)00090-1. [DOI] [PubMed] [Google Scholar]
- 99.Solomon A, Aloe L, Pe'er J, Frucht-Pery J, et al. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J Allergy Clin Immunol. 1998;102:454–460. doi: 10.1016/s0091-6749(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 100.Bullock ED, Johnson EM., Jr Nerve growth factor induces the expression of certain cytokine genes and bcl-2 in mast cells. Potential role in survival promotion. J Biol Chem. 1996;271:27500–27508. doi: 10.1074/jbc.271.44.27500. [DOI] [PubMed] [Google Scholar]