Abstract
Prenatal protein malnutrition continues to be a significant problem in the world today. Exposure to prenatal protein malnutrition increases the risk of a number of neuropsychiatric disorders in adulthood including depression, schizophrenia and attentional deficit disorder. In the present experiment we have examined the effects of stress on extracellular serotonin (5-HT) and dopamine in the medial prefrontal cortex and dorsal hippocampus of rats exposed in utero to protein malnutrition. The medial prefrontal cortex and dorsal hippocampus were chosen as two limbic forebrain regions involved in learning and memory, attention and the stress response. Extracellular 5-HT and dopamine were determined in the medial prefrontal cortex and dorsal hippocampus of adult male Sprague-Dawley rats using dual probe in vivo microdialysis. Basal extracellular 5-HT did not differ between malnourished and well-nourished controls in either the medial prefrontal cortex or the dorsal hippocampus. Basal extracellular dopamine was significantly decreased in the medial prefrontal cortex of malnourished animals. Restraint stress (20 m) produced a significant rise in extracellular dopamine in the medial prefrontal cortex of well-nourished rats but did not alter release in malnourished rats. In malnourished rats, stress produced an increase in 5-HT in the hippocampus, whereas stress produced a decrease in 5-HT in the hippocampus of well-nourished rats. These data demonstrate that prenatal protein malnutrition alters dopaminergic neurotransmission in the medial prefrontal cortex as well as altering the dopaminergic and serotonergic response to stress. These changes may provide part of the bases for alterations in malnourished animals’ response to stress.
Keywords: In vivo microdialysis, dopamine, serotonin, prenatal protein malnutrition, medial prefrontal cortex, dorsal hippocampus, stress, dual-probe microdialysis
1. INTRODUCTION
Prenatal protein malnutrition affects a significant portion of the world’s population. Our group has attempted to understand the consequences of malnutrition on the development of the brain in a rat model of prenatal protein malnutrition which exposes rats in utero to a low (6%) casein diet (Morgane et al., 1993; Morgane et al., 2002; Tonkiss et al., 1993). We have found that prenatal protein malnutrition, though affecting broad areas of the brain, particularly impacts the limbic formation (Morgane et al., 2002).
Protein malnutrition has been shown to alter brain development in a number of ways. A clear and continuous finding in prenatally malnourished animals has been alterations in the serotonergic systems of the brain (Blatt et al., 1994; Díaz-Cintra et al., 1981; Galler et al., 1996; Miller et al., 1977; Mokler et al., 1999; Mokler et al., 2003; Morgane et al., 1999; Morgane et al., 2002; Resnick and Morgane, 1984). Neuroanatomical findings by our group have shown diminished growth and arborization of serotonin neurons of the dorsal raphé nucleus (Blatt et al., 1994; Cintra et al., 1997; Díaz-Cintra et al., 1981). Further studies have shown decreases in serotonergic nerve terminals in the hippocampus as reflected by decreased 5-hydroxytryptamine (5-HT) transporters (5-HTT) and 5-HT1A receptors (Blatt et al., 1994). Our neurochemical studies of malnourished brains, on the other hand, have reported increased 5-HT levels throughout the brain during development (Resnick and Morgane, 1984; Stern et al., 1975) and more recently increased extracellular 5-HT in the dorsal hippocampus as determined by in vivo microdialysis (Mokler et al., 1999; Mokler et al., 2003). However, other studies using the same model of prenatal protein malnutrition have not reported changes in tissue levels of 5-HT in the hippocampus (Blatt et al., 1994) or the hippocampus, striatum, brainstem and cerebral cortex (Chen et al., 1997). Never the less, exposure to prenatal protein malnutrition substantially alters serotonergic systems in the brain, in particular the hippocampal formation. In the present study we have extended this research in two directions, into another key area of the limbic forebrain, the medial prefrontal cortex (mPFC) and another key limbic system neurotransmitter, dopamine.
In clinical studies, children exposed to prenatal or early postnatal malnutrition show behavioral changes throughout development and into adulthood (Galler et al., 2005; Galler et al., 2006; Galler and Ramsey, 1989). These changes include attentional problems, increased aggression, hyperactivity, and conduct disorders (Liu et al., 2004). In addition, exposure to prenatal malnutrition increases the risk of development of psychiatric disorders such as depression (Neugebauer et al., 1999; Susser et al., 1998) and schizophrenia (St Clair et al., 2005). Behavioral studies in rats have shown alterations in learning and memory as well as decreased sensitivity to benzodiazepines in prenatally malnourished animals (Almeida et al., 1996; Tonkiss et al., 2000a). Rosene et al. (2004) have shown that restraint stress for 20 min increases c-Fos expression in the anterior cingulate cortex and medial prefrontal cortices of malnourished animals greater than animals exposed to a control diet. Since the complex functions of learning and memory are widely distributed (Morgane et al., 2005; Morgane and Mokler, 2006), this suggests that other brain areas in addition to the hippocampal formation are impacted by protein malnutrition during the prenatal period. In order to investigate this hypothesis further we have used dual-probe in vivo microdialysis to examine the release of 5-HT in the mPFC and dorsal hippocampus of adult rats exposed to prenatal protein malnutrition. We have also examined the changes in extracellular dopamine in the mPFC given the role of dopamine in attentional processes (Dalley et al., 2004; Seamans and Yang, 2004; Sullivan, 2004).
Studies have shown an important role of the mPFC and hippocampal formation in the stress response (Deutch and Roth, 1990; Mizoguchi et al., 2000; Mizoguchi et al., 2003; Morgane et al., 2005; Sorg and Kalivas, 1993; Sullivan, 2004). Furthermore, exposure to prenatal protein malnutrition alters the behavioral and neural response to stress (Duran et al., 2006; King et al., 2004; Rosene et al., 2004; Trzcinska et al., 1999). Thus, we have included in the current experiments a restraint stress to examine how the malnourished brain responds to stress. Finally, given the important roles of the mPFC and hippocampus in the stress response, we have examined the changes in dialysate dopamine and 5-HT following restraint stress.
2. RESULTS
Histological verification of all probe tracks showed them located in the dorsal hippocampus or the medial prefrontal cortex. Representative photomicrographs showing the location of the probes are shown in Figure 1(A and B). In each photomicrograph the top of the photo shows the track of the guide cannula. The track of the microdialysis probe placement is seen below the track of the guide cannula.
Figure 1.
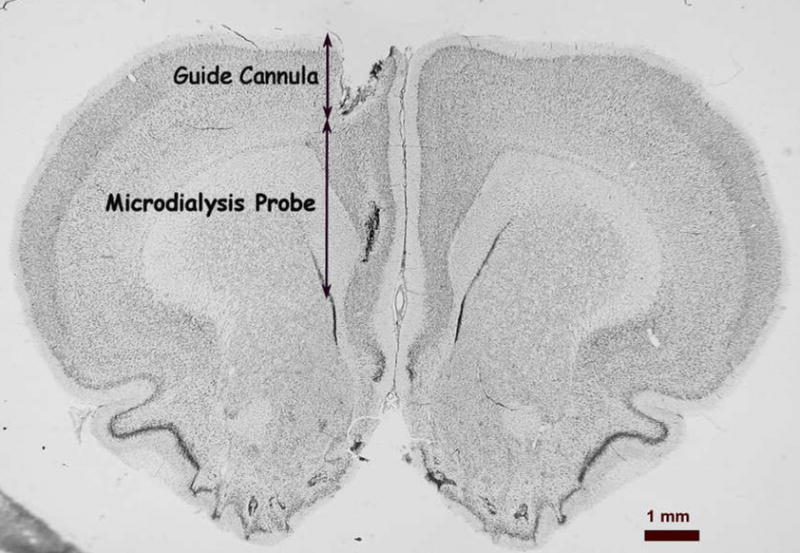
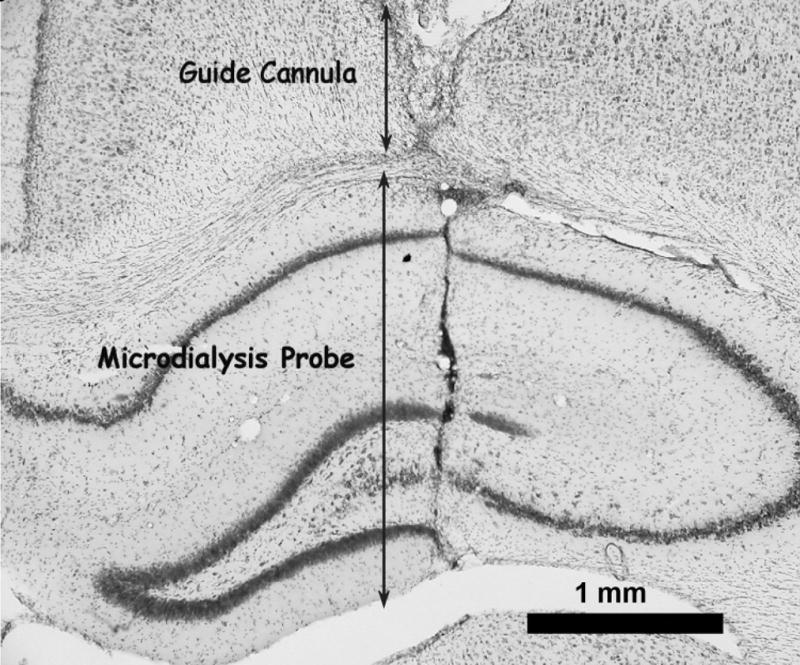
Representative photomicrographs of probe locations in the medial prefrontal cortex (A) and dorsal hippocampus (B). Arrows show the extent of the track for the guide cannula and the 3 mm microdialysis probe.
In order to determine how extracellular 5-HT and dopamine change over the course of 6 hours after probe implantation, in a control experiment we placed a microdialysis probe into the mPFC and perfused the mPFC for 6 hours (2 hours for stabilization of extracellular dopamine and 5-HT followed by 4 hours of sample collection). Extracellular 5-HT and dopamine in the mPFC did not change significantly over a 4-hour period. There was a slow decline in the concentrations of dopamine and 5-HT in dialysate over the 4-hour period which reach a nadir of 60% of control after 3 hours (Figure 2; a 2-hour period preceded the collection of samples for stabilization of extracellular dopamine and 5-HT after probe insertion).
Fig. 2.
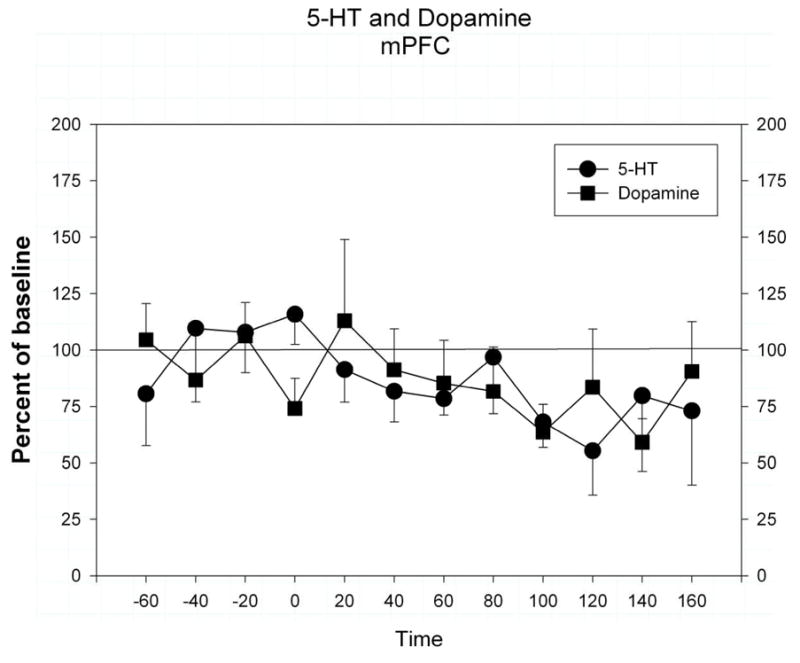
Extracellular dopamine and 5-HT in the medial prefrontal cortex over a 4 hour period. Data is expressed as mean ± s.e.m. of percent of baseline control values. Baseline control values were determined by the average of the period from −60 to −20 min.
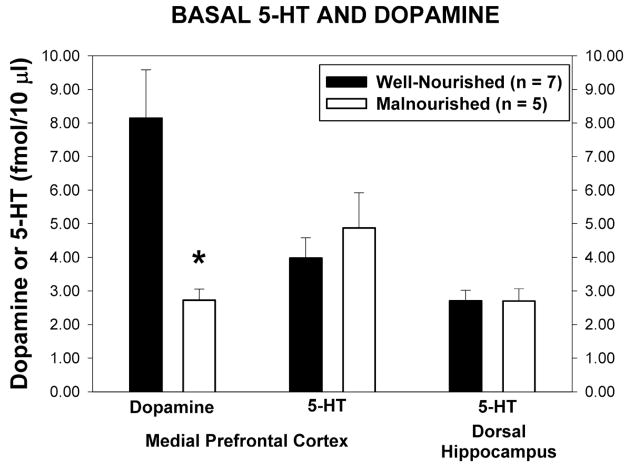
Basal dialysate levels of dopamine and 5-HT in the mPFC and 5-HT in the dorsal hippocampus are shown in Figure 3. Basal extracellular dopamine in the mPFC was significantly lower in malnourished animals than in control animals (2.73 ± 0.32 fmol/10 μl vs 8.14 ± 1.44 fmol/10 μl, t = 3.425, p<0.05). Basal extracellular levels of 5-HT in the mPFC were unchanged in animals exposed to prenatal protein malnutrition compared with control animals (4.88 ± 1.05 fmol/10 μl vs. 3.98 ± 0.60 fmol/10 μl., t = 0.80, p = 0.43) There was also no difference between well-nourished and malnourished animals in the basal extracellular levels of 5-HT in the dorsal hippocampus (2.71 ± 0.31 fmol/10 μl vs 2.70 ± 0.37 fmol/10 μl, t = 0.02, p = 0.99). Dopamine, being low in the hippocampus, was not measured.
Fig 3.
Basal extracellular concentrations of dopamine and 5-HT from the mPFC and hippocampus of well-nourished (closed bars, n = 7) and malnourished (open bars, n = 5) rats. * Significantly different from well-nourished group, t-test, p<0.05.
Exposure to 20 min of restraint stress significantly increased the release of dopamine in the mPFC of well-nourished but not malnourished animals (Figure 4). Normalization of extracellular dopamine as a percent of control when compared to well-nourished controls (Fig 4A) demonstrates no significant differences between the two nutritional groups (F(1,8) = 3.78, p = 0.088) but shows a significant time effect (F(12,96) = 8.01, p<0.05). This was seen as a significant increase in dialysate dopamine in well-nourished animals during exposure to the stressor. There was also a significant decline in extracellular dopamine in the malnourished animals beginning 2 hr following the stress with a maximal decrease to 48% of control at 3 hours post stress.
Figure 4.
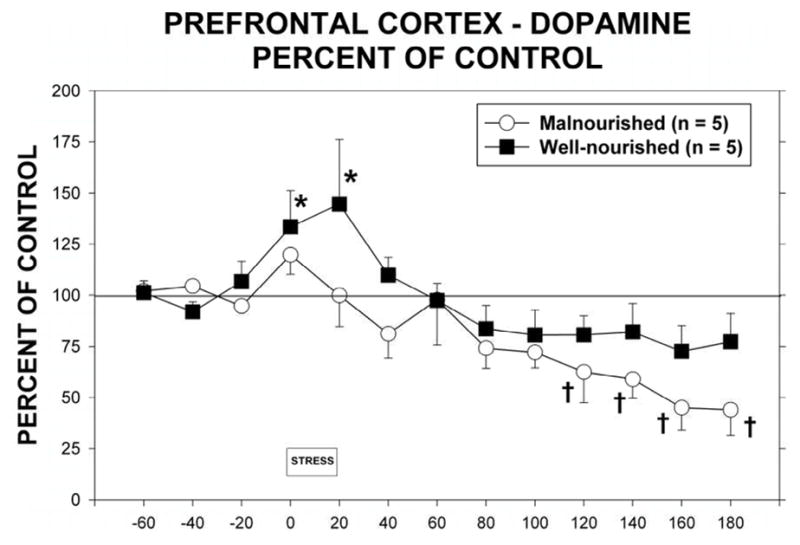
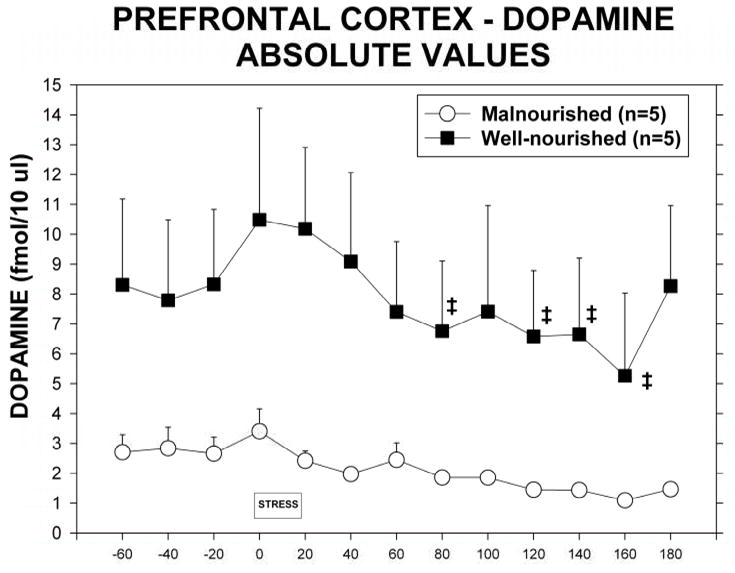
Extracellular dopamine in the mPFC in malnourished (open circles, n = 5) and well-nourished (closed squares, n = 7) rats prior to, during and following 20 minutes of restraint stress. A. Extracellular dopamine expressed as a percent of control as determined by basal extracellular dopamine during periods from −60 to −20. *Significantly different from −20 control period, †Significantly different from stress period, p<0.05. See Results for details of statistical analysis. B. Extracellular dopamine shown as absolute concentrations of dopamine in dialysate. ‡Significantly different from stress period, p<0.05. See Results for details of statistical analysis.
Because of the differences in basal dopamine between the two groups we also analyzed extracellular dopamine in terms of absolute extracellular concentrations of dopamine. Figure 4B shows the differences between well-nourished and malnourished animals and the response to stress. There was a significant difference between nutritional groups (F(1,8) = 5.55, p<0.05) as well as a significant time effect (F(12,96) = 3.78, p < 0.05).
Stress also significantly increased extracellular 5-HT in the mPFC (Fig 5, F(1,12) = 3.079, p<0.05). There were no significant differences in extracellular 5-HT between malnourished and well-nourished animals (F(1,10) = 0.0019).
Figure 5.
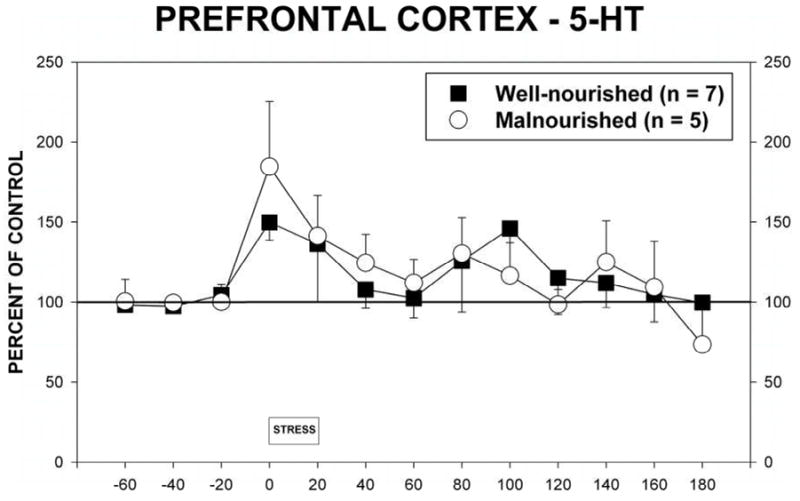
Extracellular 5-HT in the mPFC in malnourished (open circles, n = 5) and well-nourished (closed squares, n = 7) rats prior to, during and following 20 minutes of restraint stress. See Results for details of statistical analysis.
Stress also induced changes in extracellular 5-HT in the hippocampus (Figure 6). Although there were no significant differences between nutritional groups (F(1,10)=0.019) or over time (F1,12)=0.89, there was a significant interaction between the two factors (F(1,110)=3.39, p<0.05). This interaction is best explained by the differences between the two nutritional groups in response to the stress. Thus, stress in the malnourished animal produces a different response than in well-nourished animals.
Figure 6.
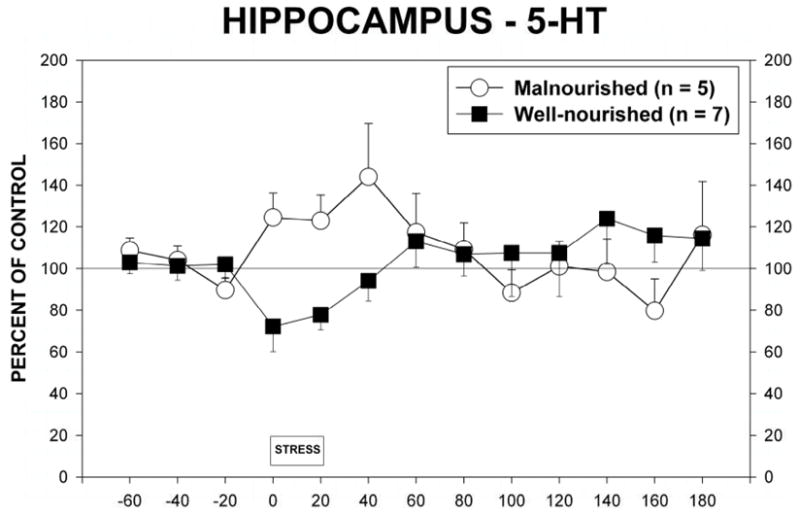
Extracellular 5-HT in the dorsal hippocampus of malnourished (open circles, n = 5) and well-nourished (closed squares, n = 7) rats prior to, during and following 20 minutes of restraint stress. See Results for details of statistical analysis.
3. DISCUSSION
The medial prefrontal cortex (mPFC) has recently been the topic of considerable research due to its role in a number of executive functions. We have begun to examine the mPFC of malnourished animals due to recent reports of human population studies showing the effects of protein malnutrition on behaviors that involve the prefrontal cortex, namely ADHD (Liu et al., 2004; Neugebauer et al., 1999), depression (Susser et al., 1998) and schizophrenia (St Clair et al., 2005). Of particular note was a recent study, which showed an increase in dopamine transporters in the prefrontal cortex of individuals with ADD/ADHD (Arnsten, 2006; Sullivan and Brake, 2003)
A novel finding in the present study is the significant decrease in basal extracellular dopamine in the mPFC in malnourished animals. This decrease suggests either an alteration in the dynamics of dopamine release or decreases in dopamine neurons in malnourished animals. Previous studies have shown that tyrosine levels in the striatum of malnourished rats are decreased which would lead to a decrease in synthesis of dopamine (Chen et al., 1997). Studies examining tyrosine hydroxylase and dopamine transporters in these cortical areas are planned to determine if there is evidence of changes in the dopamine innervation of the mPFC of malnourished animals. Previous studies by our group have reported changes in 5-HT terminal density in the hippocampal formation in malnourished animals (Blatt et al., 1994) thus raising the possibility of a similar change in the dopaminergic system as well.
A decrease in extracellular dopamine in the mPFC may have a significant impact on behavior. Not only are basal extracellular levels of dopamine decreased in the mPFC of malnourished animals but, in response to stress, there is a subsequent further decline in extracellular dopamine. Studies into the neuropathology of attentional deficit disorder have shown a decrease in extracellular dopamine in the mPFC, which has been attributed to an increase in dopamine transporters (Davids et al., 2003; Sullivan and Brake, 2003). Low extracellular dopamine in the mPFC has also been attributed to attentional, and learning and memory problems in animal studies (Davids et al., 2003; Mizoguchi et al., 2000). Malnourished animals have been shown to have deficits in learning and memory tasks (Shumsky et al., 2002; Tonkiss et al., 2000a; Tonkiss et al., 2000b; Tonkiss and Galler, 1990). Thus, the present findings point to a deficit in mPFC dopamine, which would be predicted to have an impact on attention in malnourished animals.
Stress results in an increased dialysate dopamine in the mPFC during the stress period and for 1 hour after stress in well-nourished animals. This is in agreement with numerous other studies that have reported a similar effect of stress on dopamine release (Abercrombie et al., 1989; Deutch and Roth, 1990; Feenstra et al., 1995; Feenstra, 2000; Miura et al., 2002; Pehek et al., 2006; Sorg and Kalivas, 1993; Sullivan, 2004). The small, but significant, increase in dopamine would suggest that this restraint stress is a mild stress to these rats (Sorg and Kalivas, 1993; Vermetten and Bremner, 2002). Malnourished animals did not show an increase in extracellular dopamine in the mPFC following this stressor. The inability of malnourished animals to mount a stress response may suggest that these animals, and possibly humans exposed to prenatal protein malnutrition would respond inappropriately to stress and may lead to the increased risk of behavioral and psychiatric disorders observed in clinical studies of humans exposed to prenatal protein malnutrition (Liu et al., 2004; McClellan et al., 2006; Neugebauer et al., 1999; St Clair et al., 2005).
Stress has been shown to increase extracellular 5-HT in the mPFC and in the hippocampal formation (Matuszewich et al., 2002; Storey et al., 2006). In other studies, stress-induced changes in extracellular 5-HT in the hippocampus have been reported to be either increased or decreased (Chaouloff et al., 1999). Storey et al. (2006) have shown that there are differences between the mPFC response to stress and the hippocampal response to stress in terms of 5-HT release. Both acute and chronic stress increase 5-HT in the mPFC but only chronic stress increases 5-HT in the hippocampus (Storey et al., 2006). Our current results are of interest in that we found a decrease in hippocampal extracellular 5-HT in well-nourished rats and increase in extracellular 5-HT in malnourished rats following stress. The present findings that extracellular 5-HT increase following stress in malnourished animals suggests that these animals may have been exposed to chronic stress (Storey et al., 2006) as a result of exposure to prenatal protein malnutrition. This indicates a change produced by prenatal protein malnutrition in the serotonergic response to stress in the mPFC.
Mechanisms that may contribute to differences in 5-HT release in the hippocampus following stress include the actions of glucocorticoids on the dorsal raphé nucleus (Heim and Nemeroff, 2002). The changes in the serotonergic system that we have reported in malnourished rats may reflect changes in the actions of glucocorticoids at the level of the raphé nuclei. Rosene et al. (2004) have reported, using the same period of restraint stress, that plasma corticosterone levels do not increase in malnourished animals while well-nourished animals show an expected increase in plasma corticosterone. This finding is again suggestive of a blunted stress response in malnourished animals. Of particular note is that in the hippocampus, there are differences in the effect of an acute stress on extracellular 5-HT while no differences are apparent in the medial prefrontal cortex. Hence, since the dorsal hippocampus receives its heaviest innervation from the median raphé nucleus and the mPFC receives its heaviest serotonergic innervation from the dorsal raphé nucleus (Molliver, 1987; Vertes, 1991; Vertes et al., 1999), our findings would suggest a difference in the impact of prenatal protein malnutrition on these rostral raphé nuclei. Related to this, Diaz-Cintra et al. (1981) have shown marked alterations in the dorsal raphé nucleus but to date no studies are available on malnutrition effects on median raphé.
Of particular relevance to the present study is current research examining the interrelationships between the mPFC and the hippocampus. Jay and co-workers (Jay et al., 2005) have shown that increases in dopamine in the mPFC increases the strength of long-term potentiation (LTP) at hippocampal-mPFC synapses whereas depletion of dopamine in the mPFC decreases this LTP. Furthermore, Bronzino et al. (1997) have shown that prenatal protein malnutrition produces a decrease in the strength of LTP in the hippocampal formation throughout the life of the rat. Taken together with the decrease in basal dopamine in the mPFC reported in the present study, these data suggest a mechanism for the deficits in memory and learning seen in animals exposed to prenatal protein malnutrition.
Previous studies of malnourished animals using a diet of 6% casein have shown increases in extracellular 5-HT in the dorsal hippocampus as determined by in vivo microdialysis (Mokler et al., 1999; Mokler et al., 2003). In the current study we did not find a difference between malnourished and well-nourished rats in extracellular 5-HT in either the dorsal hippocampus or the mPFC. Previous studies of malnourished rats by Chen et al. (1992) and Blatt et al. (1994) have also shown no differences between malnourished rats and well-nourished rats in the tissue levels of 5-HT in the dorsal hippocampus or the cerebral cortex, although other studies using a similar prenatal protein restriction have shown increases in 5-HT during development from birth to postnatal day 300 (Resnick and Morgane, 1984; Stern et al., 1975). The present results may be explained by the fact that in the present study animals were handled extensively and thus may not have been stressed initially in the beginning of the dialysis experiment. This would be in keeping with our findings that the increased extracellular 5-HT in the dorsal hippocampus of malnourished rats is decreased to levels of well-nourished controls by administration of chlordiazepoxide (Mokler et al., 2003).
This is the first report of extracellular 5-HT in the mPFC of malnourished animals. Previous studies have shown either increases or no change in 5-HT in the brains of animals exposed to prenatal protein malnutrition (Blatt et al., 1994; Chen et al., 1992; Mokler et al., 1999; Mokler et al., 2003), see introduction). As suggested by the results of the current study on dialysate 5-HT in the hippocampus, these differences in findings may be related to the level of stress in the animals.
In the present study we have shown that malnourished animals show an altered response to stress in the hippocampus and a blunted response to stress in the mPFC. In the hippocampal formation, extracellular 5-HT rises in response to an acute stressor in malnourished animals as compared with a decrease in extracellular 5-HT in the hippocampus of well-nourished rats. Furthermore, basal extracellular dopamine is significantly decreased in the mPFC of animals exposed to prenatal protein malnutrition. There is also a blunted response to an acute stressor in the medial prefrontal cortex. This agrees with previous work in our group that has shown that malnourished animals have a blunted response to stress compared to well-nourished animals (King et al., 2004; Trzcinska et al., 1999). Thus, the present study extends the pathology of prenatal protein malnutrition into the dopaminergic system of the mPFC. This has significant implications for the widespread life-long changes in the limbic system that may result due to prenatal protein malnutrition.
4. EXPERIMENTAL PROCEDURES
4.1 Nutritional Treatment
Virgin female Sprague-Dawley rats (Charles River Laboratories, Kingston, MA) were fed either an adequate protein diet (25% casein, Teklad Laboratories) or an iso-caloric low protein diet (6% casein, Teklad Laboratories, Madison WI) five weeks prior to mating and throughout pregnancy (Almeida et al., 1996; Morgane et al., 1993). All females were mated with males that had been acclimated to the diet for 1 week. Throughout pregnancy and until weaning, females were housed individually in polycarbonate breeding cages (Lab Products, Maywood NJ). At birth, litters were culled to 8 pups (6 males and 2 females) and the entire litter cross-fostered to well-nourished (25% casein) females that had given birth during the same 24-hour period. At 21 days of age (p21) rats were weaned from the dams and housed with same-sex littermates. Animals were then maintained ad libitum on a standard laboratory chow diet (Purina Mills, Richmond, IN; Formula 5001) and water. The vivarium was kept on a reversed light/dark cycle (lights on 1900, lights off 0700). All protocols conform to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Boston University IACUC.
Due to the limited number of animals available from the malnourished studies, the time course of extracellular dopamine and 5-HT in the mPFC following probe implantation was done in a separate group of male Sprague-Dawley rats (Charles River Laboratories, Kingston, MA) weighing between 250–300 g was used. These animals were maintained ad libitum on standard laboratory chow (containing 25% casein) and water and housed as described above. The surgery and microdialysis experiments were performed as indicated below.
4.2 Subjects
One male adult animal (90–120 days of age) from each litter was used for the studies of extracellular 5-HT and dopamine in the mPFC and dorsal hippocampus in well-nourished and malnourished pups.
4.3 Stereotaxic surgery
Adult animals (90 – 120 days of age) were anesthetized with pentobarbital (50 mg/kg ip). Guide cannulae (CMA 12, CMA/Microdialysis AB, Acton, Mass.) were implanted into dorsal hippocampus or mPFC using the coordinates of Paxinos and Watson (Paxinos and Watson, 2005). The coordinates for the tips of the guide cannulae for the dorsal hippocampus used were AP: −3.30 mm; L: 1.6 mm; DV: 2.4 mm with reference to bregma and the coordinates for the mPFC were AP: 3.2 mm; L: 0.8mm; DV: 2mm with reference to bregma. Guide cannulae were affixed to the skull with dental acrylic and three stainless steel screws.
4.4 In Vivo Microdialysis
Following 3 days for recovery, 3 mm CMA 12 probes (CMA/Microdialysis AB, Acton, MA) were slowly lowered through the guide cannula into the dorsal hippocampus and mPFC while the animal was either under gentle hand restraint or moving unrestrained about the dialysis chamber. The probe was perfused with artificial cerebrospinal fluid (artCSF) at a rate of 1.0 μl/min. The artCSF consisted of 147 mM NaCl, 1.26 mM CaCl2, 2.5 mM KCl, and 1.18 mM MgCl in sterile water. The animal was placed in a large Plexiglas bowl with a collar attached by a guide wire to a suspension arm. ArtCSF was perfused through the probe using a CMA/Microdialysis Syringe pump and a 1.0 ml syringe. The awake animal system, syringe pump and fraction collector were purchased from CMA/Microdialysis (N. Chelmsford, MA).
Basal samples were collected every 20 min for 3 hrs. All experiments were started at 0800–0830 with lights off at 0700 (reverse light cycle). Experiments were carried out in low red-filtered lighting. After 3 hrs of baseline determinations the animals were exposed to immobilization stress by being wrapped tightly in plastic mesh secured with Velcro straps for 20 min. The animal was then released from the restraint and samples were collected at 20 min intervals for another 3 hrs.
In studies of the time course of the release of 5-HT and dopamine from the mPFC after probe implantation the animals were not handled throughout the experiment.
4.5 Analysis of 5-HT and Dopamine
Twenty μl microdialysis samples were divided in half and 10 μl samples were analyzed immediately by HPLC with electrochemical detection (HPLC-EC) for 5-HT and dopamine. The system was an ESA Choulochem II using a 3 μM 4.6mm × 100mm C-18 column (Microsorb MV, Varian, Walnut Creek, CA). The mobile phase consisted of 0.1 M disodium phosphate, 15% acetonitrile, 10 mM EDTA, and 1.5 mM octanesulfonic acid at a pH of 5.4 at a flow rate of 1 ml/min. Electrode potentials were set at: guard cell +300 mV, E1 −150 mV, and E2 +175 mV. This allowed for the measurement of 5-HT with a sensitivity of 5.0 fmol/10μl sample. The area under the curve for samples was compared using computer software Chromperfect (Justice Laboratory Software, Palo Alto, CA) with a regression analysis of AUC for three authentic standards (10μl of 10−9, 5×10−10, 10−10 M authentic dopamine and 5-HT) injected onto the column at the beginning of each experimental day to determine the levels of 5-HT and dopamine. 5-HT and dopamine peaks were verified by examining the voltammogram for standards against that determined using microdialysis samples. All standard curves were fitted to a linear regression model and had an r-squared value (correlation coefficient) of greater than 0.97.
4.6 Histology
At the completion of each study, the animal was perfused transcardially with neutral buffered formalin under pentobarbital anesthesia and the brain removed. The brain was sectioned at 40 μm in the frontal plane and Nissl stained for verification of the probe placement (see Figure 1).
4.7 Statistics
Statistical analysis was done using Sigmastat v1.0. Basal extracellular concentrations of dopamine and 5-HT in the mPFC and dorsal hippocampus between groups were compared using a t-test of values determined as time points of −60, −40 and −20 min prior to the beginning of stress. The time courses of changes in extracellular dopamine and 5-HT in the mPFC and dorsal hippocampus before, during and after stress were compared using a two way ANOVA with repeated measures. Statistical significance was determined when p<0.05.
Acknowledgments
This study was supported by NIH Grant HD 22539-15.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Almeida SS, Tonkiss J, Galler JR. Malnutrition and reactivity to drugs acting in the central nervous system. Neurosci Biobehav Rev. 1996;20:389–402. doi: 10.1016/0149-7634(95)00054-2. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67:7–12. [PubMed] [Google Scholar]
- Blatt GJ, Chen JC, Rosene DL, Volicer L, Galler JR. Prenatal protein malnutrition effects on the serotonergic system in the hippocampal formation: An immunocytochemical, ligand binding, and neurochemical study. Brain Res Bull. 1994;34:507–518. doi: 10.1016/0361-9230(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Bronzino JD, Austin-LaFrance RJ, Mokler DJ, Morgane PJ. Effects of prenatal protein malnutrition on hippocampal long-term potentiation in freely moving rats. Exper Neurol. 1997;148:317–323. doi: 10.1006/exnr.1997.6653. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharm. 1999;21(Suppl):S32. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Chen JC, Tonkiss J, Galler JR, Volicer L. Prenatal protein malnutrition in rats enhances serotonin release from hippocampus. J Nutr. 1992;122:2138–2143. doi: 10.1093/jn/122.11.2138. [DOI] [PubMed] [Google Scholar]
- Chen JC, Turiak G, Galler J, Volicer L. Postnatal changes of brain monoamine levels in prenatally malnourished and control rats. Int J Dev Neurosci. 1997;15:257–263. doi: 10.1016/s0736-5748(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Cintra L, Granados L, Aguilar A, Kemper T, DeBassio W, Galler J, Morgane PJ, Durán P, Díaz-Cintra S. Effects of prenatal protein malnutrition on mossy fibers of the hippocampal formation in rats of four age groups. Hippocampus. 1997;7:184–191. doi: 10.1002/(SICI)1098-1063(1997)7:2<184::AID-HIPO5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. BrainRes BrainRes Rev. 2003;42:1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. In: Uylings HBM, VanEden CG, DeBruin JPC, Corner MA, Feenstra MGP, editors. The Prefrontal Cortex: Its Structure, Function and Pathology. Elsevier; Amsterdam: 1990. pp. 367–403. [DOI] [PubMed] [Google Scholar]
- Díaz-Cintra S, Cintra L, Kemper T, Resnick O, Morgane PJ. The effects of protein deprivation on the nucleus raphe dorsalis: A morphometric Golgi study in rats of three age groups. Brain Res. 1981;221:243–255. doi: 10.1016/0006-8993(81)90775-7. [DOI] [PubMed] [Google Scholar]
- Duran P, Galler JR, Cintra L, Tonkiss J. Prenatal malnutrition and sleep states in adult rats: Effects of restraint stress. Physiol Behav. 2006;89:156–163. doi: 10.1016/j.physbeh.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Feenstra MG. Dopamine and noradrenaline release in the prefrontal cortex in relation to unconditioned and conditioned stress and reward. Prog BrainRes. 2000;126:133–163. doi: 10.1016/S0079-6123(00)26012-3. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, vanUlum JF. Novelty-induced increase in dopamine release in the rat prefrontal cortex in vivo: inhibition by diazepam. Neurosci Lett. 1995;189:81–84. doi: 10.1016/0304-3940(95)11456-7. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey F. A follow-up study of the influence of early malnutrition on development: behavior at home and at school. J Am Acad Child Adolesc Psychiatry. 1989;28:254–261. doi: 10.1097/00004583-198903000-00018. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey F, Solimano G, Lowell WE. The influence of early malnutrition on subsequent behavioral development. II Classroom behavior. J Am Acad Child Adolesc Psychiatry. 2006;22:16–22. doi: 10.1097/00004583-198301000-00003. [DOI] [PubMed] [Google Scholar]
- Galler JR, Shumsky JS, Morgane PJ. Malnutrition and brain development. In: Walker WA, Watkins JB, editors. Nutrition and Pediatrics: Basic Science and Clinical Applications. Decker Europe; Neuilly-sure-Seine, France: 1996. pp. 194–210. [Google Scholar]
- Galler JR, Waber D, Harrison R, Ramsey F. Behavioral effects of childhood malnutrition. Am J Psychiatry. 2005;162:1760–1761. doi: 10.1176/appi.ajp.162.9.1760-b. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. Neurobiology of early life stress: Clinical studies. Semin Clin Neuropsychiatry. 2002;7:147–159. doi: 10.1053/scnp.2002.33127. [DOI] [PubMed] [Google Scholar]
- Jay TM, Rocher C, Hotte M, Naudon L, Gurden H, Speeding M. Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: importance for psychiatric disease. Neurotox Res. 2005;6:233–244. doi: 10.1007/BF03033225. [DOI] [PubMed] [Google Scholar]
- King RS, DeBassio WA, Kemper TL, Rosene DL, Tonkiss J, Galler JR, Blatt GJ. Effects of prenatal protein malnutrition and acute postnatal stress on granule cell genesis in the fascia dentata of neonatal and juvenile rats. Dev Brain Res. 2004;150:9–15. doi: 10.1016/j.devbrainres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Liu J, Raine A, Venables PH, Mednick SA. Malnutrition at age 3 years and externalizing behavior problems at ages 8, 11, and 17 years. Am J Psychiatry. 2004;161:2005–2013. doi: 10.1176/appi.ajp.161.11.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewich L, Filon ME, Finn DA, Yamamoto BK. Altered forebrain neurotransmitter responses to immobilization stress following 3,4-methylenedioxymethamphetamine. Neuroscience. 2002;110:41–48. doi: 10.1016/s0306-4522(01)00539-5. [DOI] [PubMed] [Google Scholar]
- McClellan JM, Susser E, King MC. Maternal famine, de novo mutations, and schizophrenia. JAMA. 2006;296:582–584. doi: 10.1001/jama.296.5.582. [DOI] [PubMed] [Google Scholar]
- Miller M, Leahy JP, McConville F, Morgane PJ, Resnick O. Effects of developmental protein malnutrition on tryptophan utilization in brain and peripheral tissues. Brain Res Bull. 1977;2:347–353. doi: 10.1016/0361-9230(77)90068-5. [DOI] [PubMed] [Google Scholar]
- Miura H, Qiao H, Ohta T. Attenuating effects of the isolated rearing condition on increased brain serotonin and dopamine turnover elicited by novelty stress. Brain Res. 2002;926:10–17. doi: 10.1016/s0006-8993(01)03201-2. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–897. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokler DJ, Bronzino JD, Galler JR, Morgane PJ. Effects of median raphé electrical stimulation on serotonin release in the dorsal hippocampal formation of prenatally malnourished rats. Brain Res. 1999;838:95–103. doi: 10.1016/s0006-8993(99)01677-7. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Galler JR, Morgane PJ. Modulation of 5-HT release in the hippocampus of 30-day-old rats exposed in utero to protein malnutrition. Dev Brain Res. 2003;142:203–208. doi: 10.1016/s0165-3806(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Molliver ME. Serotonergic neuronal systems: What their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- Morgane PJ, Austin-LaFrance RJ, Bronzino J, Tonkiss J, Díaz-Cintra S, Cintra L, Kemper T, Galler JR. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev. 1993;17:91–128. doi: 10.1016/s0149-7634(05)80234-9. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Bronzino J, Austin-LaFrance RJ, Galler JR. Malnutrition, central nervous system effects. Encyclopedia of Neuroscience 1999 [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Mokler DJ. The limbic brain: Continuing resolution. Neurosci Biobehav Rev. 2006;30:119–125. doi: 10.1016/j.neubiorev.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev. 2002;26:471–484. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Neugebauer R, Hoek HW, Susser E. Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA. 1999;282:455–462. doi: 10.1001/jama.282.5.455. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; Amsterdam: 2005. [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharm. 2006;31:265–277. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- Resnick O, Morgane PJ. Ontogeny of the levels of serotonin in various parts of the brain in severely protein malnourished rats. Brain Res. 1984;303:163–170. doi: 10.1016/0006-8993(84)90224-5. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Lister JP, Schwagerl AL, Tonkiss J, McCormick CM, Galler JR. Prenatal protein malnutrition in rats alters the c-Fos response of neurons in the anterior cingulate and medial prefrontal region to behavioral stress. Nutr Neurosci. 2004;7:281–289. doi: 10.1080/10284150400015573. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Shumsky JS, Shultz PL, Galler JR, Tonkiss J. Differential efffects of prenatal protein malnutrition and prenatal cocaine on radial arm maze performance in adult male rats. Nutr Neurosci. 2002;2:113–122. doi: 10.1080/1028415X.1999.11747269. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex. Neuroscience. 1993;53:695–703. doi: 10.1016/0306-4522(93)90617-o. [DOI] [PubMed] [Google Scholar]
- St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, Zheng X, Gu N, Feng G, Sham P, He L. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- Stern WC, Miller M, Forbes WB, Morgane PJ, Resnick O. Ontogeny of the levels of biogenic amines in various parts of the brain and in peripheral tissues in normal and protein malnourished rats. Exper Neurol. 1975;49:314–326. doi: 10.1016/0014-4886(75)90214-9. [DOI] [PubMed] [Google Scholar]
- Storey JD, Robertson DA, Beattie JE, Reid IC, Mitchell SN, Balfour DJ. Behavioural and neurochemical responses evoked by repeated exposure to an elevated open platform. Behav Brain Res. 2006;166:220–229. doi: 10.1016/j.bbr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7:131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Brake WG. What the rodent prefrontal cortex can teach us about attention-deficit/hyperactivity disorder: the critical role of early developmental events on prefrontal function. Behav Brain Res. 2003;146:43–55. doi: 10.1016/j.bbr.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: The story of the Dutch famine study. Am J Epidemiol. 1998;147:213–216. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Galler J, Morgane PJ, Bronzino JD, Austin-LaFrance RJ. Prenatal protein malnutrition and postnatal brain function. Ann N Y Acad Sci. 1993;678:215–227. doi: 10.1111/j.1749-6632.1993.tb26124.x. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Galler JR. Prenatal protein malnutrition and working memory performance in adult rats. Behav Brain Res. 1990;40:95–107. doi: 10.1016/0166-4328(90)90002-v. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Shultz PL, Shumsky JS, Fiacco TTA, Vincitore M, Rosene DL, Galler JR. Chlordiazepoxide-induced spatial learning deficits: dose-dependent differences following prenatal malnutrition. Pharmacol Biochem Behav. 2000a;65:105–116. doi: 10.1016/s0091-3057(99)00182-3. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Trzcinska MM, Shultz PL, Vincitore M, Galler JR. Prenatally protein malnourished rats are less sensitive to the amnestic effects of medial septal infusions of chlordiazepoxide. Behav Pharmacol. 2000b;11:437–446. doi: 10.1097/00008877-200009000-00001. [DOI] [PubMed] [Google Scholar]
- Trzcinska MM, Tonkiss J, Galler JR. Influence of prenatal protein malnutrition on behavioral reactivity to stress in adult rats. Stress. 1999;3:71–83. doi: 10.3109/10253899909001113. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. I Preclinical studies. Depress Anxiety. 2002;15:126–147. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphé nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]