Abstract
Overexpression of the proto-oncogene MYC has been implicated in the genesis of diverse human cancers. One explanation for the role of MYC in tumorigenesis has been that this gene might drive cells inappropriately through the division cycle, leading to the relentless proliferation characteristic of the neoplastic phenotype. Herein, we report that the overexpression of MYC alone cannot sustain the division cycle of normal cells but instead leads to their arrest in G2. We used an inducible form of the MYC protein to stimulate normal human and rodent fibroblasts. The stimulated cells passed through G1 and S but arrested in G2 and frequently became aneuploid, presumably as a result of inappropriate reinitiation of DNA synthesis. Absence of the tumor suppressor gene p53 or its downstream effector p21 reduced the frequency of both G2 arrest and aneuploidy, apparently by compromising the G2 checkpoint control. Thus, relaxation of the G2 checkpoint may be an essential early event in tumorigenesis by MYC. The loss of p53 function seems to be one mechanism by which this relaxation commonly occurs. These findings dramatize how multiple genetic events can collaborate to produce neoplastic cells.
The MYC proto-oncogene encodes a transcription factor whose activity has been implicated in diverse cellular functions, including proliferation, differentiation, and apoptosis (1–3). Overexpression of MYC has been found in numerous human tumors and is thought to play a role in tumorigenesis (1). Excessive activity of MYC might contribute to tumorigenesis in at least three ways: by driving cells inappropriately through the division cycle (1, 2), by creating a mutator phenotype consequent to destabilization of the cellular genome (4–8), and by impeding cellular differentiation (1, 2).
The effect of MYC on the cell-division cycle has been examined mainly in established lines of rodent cells, which are readily transformed by oncogenes and presumably carry multiple genetic lesions. In this study, we examined the effect of MYC on the division cycle of normal rodent and human cells. To perform these studies, we used a molecular construct in which MYC is fused to the hormone-binding domain of the human estrogen receptor (MYCER; ref. 9). The chimeric gene product is active only in the presence of estradiol (E2) or hydroxytamoxifen.
Stimulation with MYC caused normal rodent and human cells to traverse the G1 and S phases of the division cycle, but the cells then arrested in G2 and frequently became aneuploid, apparently as a result of endoreduplication. Absence of the tumor suppressor protein p53 or its downstream effector p21 reduced the frequency of both G2 arrest and aneuploidy, presumably by compromising the G2 checkpoint. Thus, relaxation of the G2 checkpoint may be an essential early event in tumorigenesis by MYC. Loss of p53 function is among the most common genetic lesions in cancer cells and represents a means by which such relaxation could occur (10, 11).
Methods
Cell Culture.
Normal human fibroblasts (NHF) were derived from newborn foreskin. Mouse embryonic fibroblasts (MEF) were isolated by using conventional techniques from day 14 embryos. Cells were cultured in DMEM supplemented with 10% (vol/vol) FCS and penicillin/streptomycin. Fibroblasts were infected with preparations of the pBABE-puro retrovirus containing MYCER as described (7).
Induction of MYC Activity.
NHF or MEF with MYCER were treated with E2 at a concentration of 1 μM prepared as a 1,000× stock in 100% (vol/vol) ethanol.
Proliferation Assays.
Cells were plated into 6-well tissue culture plates in tissue culture medium in the absence or presence of E2 (2 × 103). At each time point, wells in triplicate were trypsinized, and the live cells were enumerated by counting the number of Trypan-blue-negative cells.
Cell Death.
Apoptosis was measured by video microscopy as described (12). At least 25 cells were examined per experiment for 60 h in the absence or presence of E2. The cumulative total numbers of cells, numbers of mitoses, and numbers of apoptoses were measured.
DNA Content.
Fluorescence-activated cell sorting analysis of propidium-iodide-stained cells was performed as described (7). Quantitative image analysis of Feulgen-stained cells was performed by plating cells into 4-chamber tissue culture slides (Tissue Tek, Becton Dickinson) in growth medium in the absence or presence of E2 for 2 days. In some cases, cells were first synchronized in G0 by putting the cells in medium containing low serum (0.05%) for 2 days. Cells were fixed in 10% (vol/vol) formalin for 2 h and then stained with Feulgen. Nuclear Feulgen staining was measured in at least 100 cells at a wavelength of 550 nm (AHRENS system, Bargteheide, Hamburg, Germany).
Results
Excess MYC Causes Proliferative Arrest in Normal Cells.
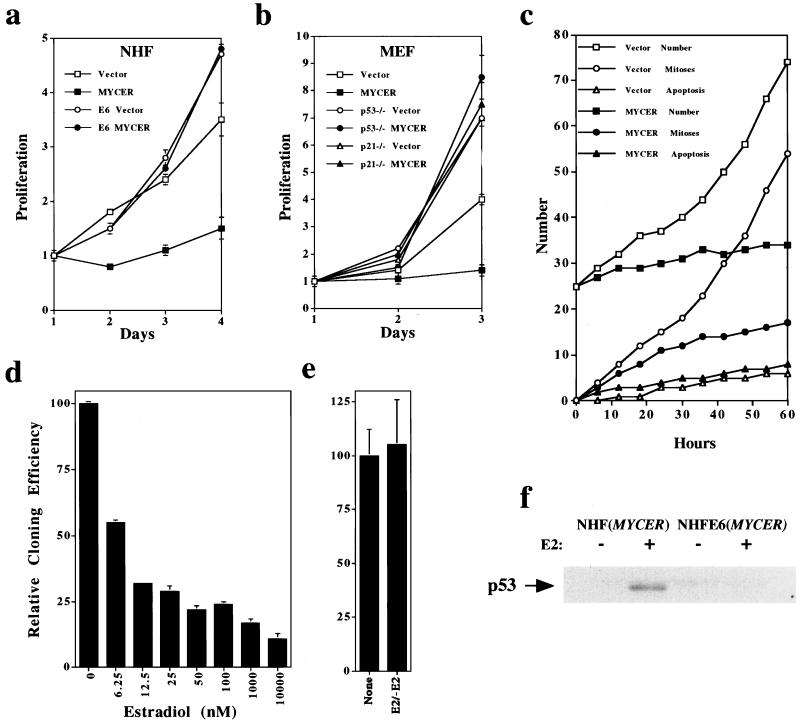
We overexpressed MYCER in NHF and MEF as described (7). Activation of MYCER with E2 immediately suppressed the proliferation of normal cells that had been proliferating asynchronously, whereas MYCER in the absence of E2 had no effect (Fig. 1 a and b). In an additional measure of proliferation, cloning efficiency of NHF was reduced by the sustained activation of MYCER (Fig. 1d). Transient activation of the fusion protein reduced the proliferation of normal cells (data not shown) but did not reduce their cloning efficiency (Fig. 1e). Thus, the inhibition of cellular proliferation by MYCER seems to be reversible.
Figure 1.
Excess of MYC activity inhibits the proliferation of NHF and MEF. To activate MYC activity conditionally, NHF, NHF-E6, or MEF infected with the retroviral vector alone (vector) or the retroviral vector containing MYCER were treated with E2 (1 μM or as otherwise specified). (a) Proliferation of NHF or NHF-E6 in the absence (Vector) or presence (MYCER) of excess of MYC activity. (b) Proliferation of MEF that are wild-type, p53−/−, or p21−/− in the absence (Vector) or presence (MYCER) of excess activity of MYC. For a and b, proliferation is expressed as the number of cells counted after a given time in culture, divided by the initial number of cells plated. (c) Video microscopy of NHF in the absence (Vector) or presence (MYCER) of excess of MYC activity. NHF (n = 25) with vector or MYCER were followed for 60 h after E2 treatment and examined for cumulative total number of cells, mitoses, and apoptosis. (d) Cloning efficiency of NHF in the presence of increasing levels of activity of MYC. NHF (n = 200) with MYCER were plated into 10-cm tissue culture plates and exposed to the indicated concentrations of E2. Colonies of over 50 cells were counted after 1 month of in vitro culture. (e) Cloning efficiency after 2 days of E2 NHF or NHF after 2 days of excess of MYC activity was measured by plating cells into 10-cm tissue culture plates in the absence of E2; colonies of over 50 cells were counted after 1 month. (f) p53 protein expression in NHF or NHF-E6 in the absence (−) or presence (+) of excess activity of MYC. Protein was measured by Western analysis with the monoclonal antibody pAB421 (Calbiochem). (a–e) Samples were obtained in triplicate. Mean values ± SD are shown. One of at least three experiments is shown.
Under certain circumstances, the activation of MYC can lead to cellular apoptosis (3). Therefore, we examined the possibility that apoptosis might account for the failure of NHF and MEF to proliferate in response to activated MYCER. We examined cells for apoptosis by phase microscopy, trypan blue exclusion, fluorescence-activated cell sorting analysis, and video microscopy. We did not detect appreciable apoptosis of either NHF or MEF in response to activation of MYCER (data not shown and Fig. 1c). By video microscopy, we observed that, on activation of MYCER, the number of mitoses did increase during the first 24 h, but cells did not divide. We conclude that MYCER arrests the cell cycle by preventing the completion of cellular division.
Excess MYC Causes Cell-Cycle Arrest in G2.
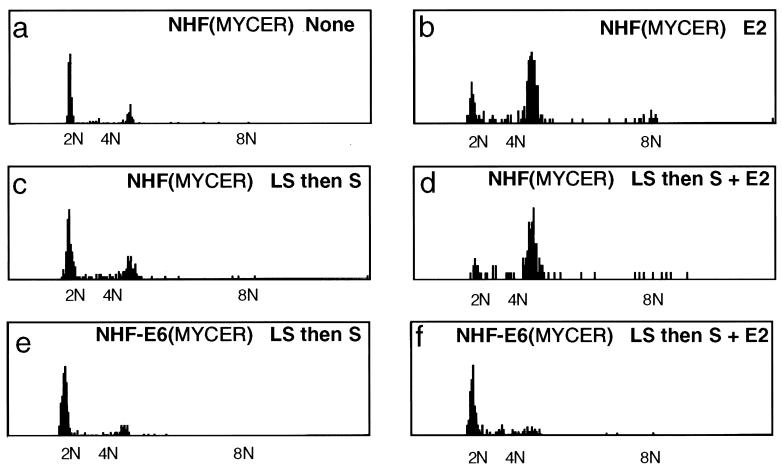
We analyzed at what point the cells had arrested in the cell cycle by measuring their DNA content by fluorescence-activated cell sorting analysis of propidium-iodide-stained cells (data not shown) or by quantitative image analysis of Feulgen-stained cells (Fig. 2). Under normal growth conditions, greater than 80% of the NHF were in G1 (Fig. 2a). In contrast, over 70% of the cells were in G2 after 2 days of sustained activation of MYCER (Fig. 2b).
Figure 2.
Excess of MYC activity causes G2 arrest. NHF or NHF-E6 containing MYCER were either not treated or treated with E2 (1 μM) for 2 days. As outlined below, in some cases, NHF were first synchronized in low serum (0.05%) for 2 days (LS) and then treated with serum (10% vol/vol) (S) or S and E2. The histograms display DNA content as measured by quantitative image analysis of Feulgen-stained preparations of 10% (vol/vol) formalin-fixed cells. (a) Untreated NHF with MYCER. (b) E2-treated NHF with MYCER. (c) NHF with MYCER treated with LS and then S. (d) NHF with MYCER treated with LS and then S and E2. (e) NHF-E6 with MYCER treated with LS and then S. (f) NHF-E6 with MYCER treated with LS and then S and E2. Representative data are shown from one of at least five experiments. Identical results were obtained by measuring DNA content by fluorescence-activated cell sorting analysis of propidium-iodide-stained cells.
Because MYC is thought to stimulate entry into the cell cycle, we speculated that the activation of MYC may have greater consequences if cells were synchronized in G0 through serum starvation. Indeed, we found that when cells were first synchronized in G0, the concurrent addition of serum and activation of MYCER now resulted in over 90% of the cells accumulating in G2 (Fig. 2d). Thus, cells in G0 may be more sensitive to the effects of MYC activity. Note that the serum treatment alone of cells previously synchronized in G0 in low serum for 2 days resulted in less than 20% of cells accumulating in G2 (Fig. 2c). We conclude that MYC caused cells to arrest in G2.
Arrest in G2 Is p53-Dependent.
We presumed that the arrest in G2 could be attributed to activation of a checkpoint control (13). The tumor suppressor protein p53 can play a role in implementing the G2 checkpoint (14–16). We found that the activation of MYCER in NHF led to an increase in the amount of p53 (Fig. 1f).
We pursued the role of p53 in the G2 arrest by expressing the E6 oncogene of the human papilloma virus in NHF. The E6 protein facilitates the proteolytic destruction of p53 (17) and, accordingly, reduced the amounts of p53 in NHF, even when MYCER had been activated (Fig. 1f). The absence of p53 allowed NHF to proliferate normally even when MYCER had been activated with E2 (Fig. 1a). Similarly, activation of MYCER had no effect on the proliferation of MEF that were genetically deficient in either p53 or the cell-cycle kinase inhibitor p21, which is a downstream effector of p53 (ref. 17; Fig. 1b). Accordingly, activation of MYCER in the p53-deficient cells did not cause an abnormal accumulation of cells in G2 (Fig. 2 d versus f). We conclude that p53 is involved in the implementation of the G2 arrest elicited by the activation of MYCER.
Excess MYC Causes Aneuploidy.
Overexpression of MYC causes aneuploidy (4–8). Our results described above suggest a possible mechanism. MYC seems to cause cells to arrest in G2 and then may cause the reinitiation of DNA replication, resulting in endoreduplication. If this supposition were the case, we predicted that if we prevented cells from arresting in G2, then we would reduce the ability of MYC to cause aneuploidy.
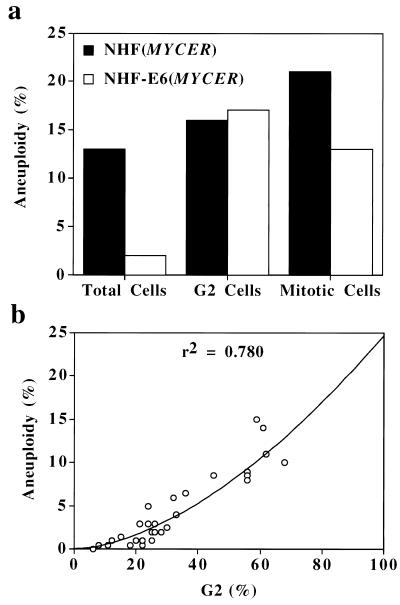
Indeed, in NHF deficient in p53, the activation of MYC failed to arrest the cells in G2 (as described above), and the frequency of aneuploidy was reduced by 10-fold compared with NHF with intact p53 (Fig. 2 d versus f; see below Fig. 4a). However, when we examined mitotic cells, we found that MYC caused a comparable frequency of aneuploidy in NHF intact or deficient in p53 (Figs. 3 and 4a). The frequency of aneuploidy among cells in G2 was also similar regardless of the status of p53 (Fig. 4a). We infer that the absence of p53, rather than preventing MYC from causing aneuploidy, reduces the pool of cells in G2 that could become aneuploid. In fact, the frequency of aneuploidy caused by MYC correlated with the number of cells that were in G2 (Fig. 4b).
Figure 4.
Aneuploidy caused by excess of MYC activity seems to require G2 arrest. To activate MYC, NHF with MYCER or NHF-E6 with MYCER were treated with E2 for 2 days. Aneuploidy was measured as the percentage of the cells with a >4 N DNA content (Total Cells), the percentage of cells with a >4 N DNA content divided by the number of cells in G2 (G2 Cells), or the percentage of metaphases with >50 chromosomes (Mitotic Cells). (a) Aneuploidy caused by MYC in NHF or NHF-E6. (b) Aneuploidy versus the number of cells in G2.
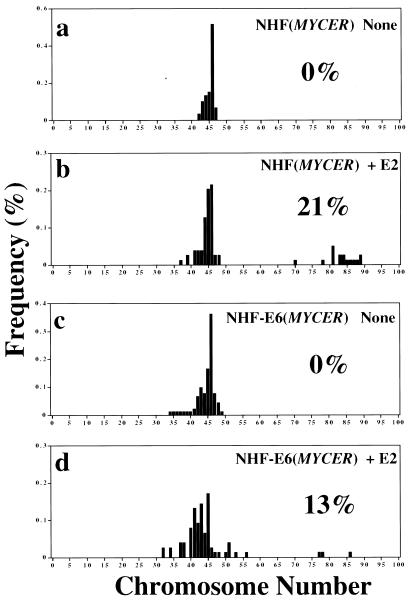
Figure 3.
Aneuploidy caused by excess of MYC activity. To activate MYC conditionally, NHF or NHF-E6 with MYCER were not treated or treated with E2 (1 μM) for 2 days. In each panel, the percentage of aneuploid metaphase spreads with >50 chromosomes is indicated. The histograms reflect the percentage of metaphases found to have a particular number of chromosomes. Metaphases were prepared and analyzed as described (7). (a) NHF with MYCER that were not treated with E2. (b) NHF with MYCER that were treated with E2. (c) NHF-E6 MYCER that were not treated with E2. (d) NHF-E6 with MYCER that were treated with E2.
Discussion
Excess Activation of MYC Prohibits Proliferation.
The overexpression of MYC is generally thought to cause tumorigenesis by constitutively promoting cellular proliferation (1, 2). Our results demonstrate that, instead, excess MYC activation causes the proliferative arrest of NHF and MEF. The overexpression of MYC cannot of itself elicit neoplastic proliferation of otherwise normal cells (1). Our results provide a possible explanation. When a normal cell undergoes a genetic event that causes the overexpression of MYC, the cell may be incapable of further proliferation.
Normal cells express MYC when they transit the cell-division cycle (1, 2). Indeed, MYC has been shown to induce cells to proliferate (1, 2). A possible explanation for the discordance with the results that we report herein is that physiological levels of MYC promote cellular proliferation, whereas high levels of MYC activation cause proliferative arrest. This arrest may not have been appreciated previously in experiments in which MYC was constitutively overexpressed, because cells that expressed high levels of MYC would be incapable of proliferating.
Substantial evidence suggests that MYC may be restrained from causing the malignant transformation of cells, because MYC activation induces apoptosis (3). Our results suggest that the activation of MYC in NHF and MEF causes a proliferative arrest without augmented apoptosis. Whether the activation of MYC causes proliferative arrest or induces apoptosis may depend on the particular cellular lineage, the differentiative state, and the mitogenic stimulation provided by the local microenvironment (16). In this regard, it is notable that the conditional activation of MYC in vivo was not observed to induce apoptosis in the skin (18).
Several reports document that MYC induces telomerase activity, which in turn may contribute to cellular immortalization (19–21). Our results suggest that although MYC may promote immortalization, cells are prevented from further proliferation unless cell-cycle checkpoints are first abrogated.
MYC Causes p53-Dependent G2 Arrest.
MYC activation has been shown to cause genomic destabilization (4–8). Oncogenes may cause genomic damage by accelerating transit through the G1 and S phases of the cell cycle (13). Normal cells may withdraw from the cell-division cycle after the activation of MYC, because they suffer from DNA damage. This damage may trigger a checkpoint response. Cells may arrest in G2 rather than G1, because MYC seems to be capable of overriding the cell-cycle inhibition caused by gene products that normally would be responsible for causing cells to arrest in G1 (7). Apparently, MYC is incapable of overriding a G2 checkpoint response.
We observed that the implementation of the G2 checkpoint requires the activity of p53 as well as its downstream effector p21, because the loss of either gene product permitted the proliferation of normal cells in the presence of excess MYC activity. The absence of p53 function has been shown to permit cells arrested in G2 to adapt and re-enter the cell cycle (14–16).
Tumorigenesis caused by MYC overexpression is greatly accelerated by the loss of p53 (11, 22). The loss of p53 accelerates tumorigenesis associated with MYC activation by preventing apoptosis (10, 11). Our results suggest the additional mechanism that the loss of p53 permits the continued proliferation of cells that are overexpressing MYC.
MYC Causes Aneuploidy.
Previously, we described that MYC activation induces aneuploidy in NHF (7). Our results presented herein provide a possible explanation. MYC activation seems to cause the arrest of cells in G2. Cells arrested in G2 may be enforced by MYC to reinitiate DNA replication resulting in aneuploidy. This model is supported by the observation that the proportion of cells that become aneuploid on MYC activation correlates with the frequency of cells in G2 of the cell cycle (Fig. 3f). Furthermore, when the activation of MYC was prevented from causing G2 arrest through the inactivation of p53, the number of cells that became aneuploid was decreased 10-fold (Fig. 3e).
The loss of the function of p53 causes genomic destabilization (17); thus, it seemed paradoxical that its loss seemed to reduce the frequency of aneuploidy caused by MYC. However, on closer analysis, we found that the absence of p53 did not prevent MYC from causing aneuploidy but rather reduced the pool of cells accumulated in G2/M that could become aneuploid (Fig. 3e). Thus, within the subset of cells that are in the G2 or M phases of the cell cycle, the activation of MYC causes the same proportion of these populations to become aneuploid regardless of the status of p53 function (Fig. 3e). Indeed, because the loss of p53 prevents the arrest of the cell cycle, aneuploid cells would now be capable of proliferatively expanding.
Cell-Cycle Checkpoints as Surveillance Against Oncogenes.
We have shown that MYC overexpression causes the proliferative arrest of normal cells. This arrest may be an important mechanism by which MYC overexpression is prevented from causing tumorigenesis. Several other oncogenes have been shown to induce the arrest of the cell-division cycle. These include RAS, RAF, MAPK, and E2F1 (23–26). In contrast to what we observed with MYC, these oncogenes induce a G1 cell-cycle arrest and cellular senescence. One reason for this difference may be that MYC is capable of overriding the checkpoints that operate during G1 but incapable of overriding the checkpoints during G2.
In addition to monitoring cells for DNA damage, cell-cycle checkpoints may generally function as surveillance mechanisms to prevent a single oncogene from initiating tumorigenesis, as has been suggested (27). Cancer is a multistep process, possibly because cells that acquire individual oncogenic events are suppressed from further malignant progression unless multiple cell-cycle checkpoints are compromised, presumably by other genetic events. Our results specifically suggest that for the activation of MYC to cause tumorigenesis, the G2 checkpoint may first have to be relaxed. The loss of p53 function may be one mechanism by which this relaxation commonly occurs (10, 11).
Acknowledgments
We thank Patrick O'Farrell, David Morgan, Ira Herskowitz, Geoffrey Wahl, and the members of the Bishop laboratory for their many helpful suggestions; Paul Dazin for assistance performing flow cytometry; Alan Bradley for providing p53−/− mice; and Tyler Jacks for providing p21−/− mice. D.W.F. is presently supported by National Cancer Institute Grant K08 CA75967-01 and was supported by a Howard Hughes Medical Institute Postdoctoral Fellowship, a Lymphoma Research Foundation Fellowship, and a Pfizer Postdoctoral Fellowship. This work was supported by National Institutes of Health Grant CA 44338 and the G. W. Hooper Research Foundation.
Abbreviations
- E2
17-β-estradiol
- NHF
normal human fibroblasts
- MEF
mouse embryonic fibroblasts
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190327097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190327097
References
- 1.Marcu K B, Bossone S A, Patel A J. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- 2.Henriksson M, Lüscher B. Adv Cancer Res. 1996;68:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- 3.Harrington E A, Fanidi A, Evan G I. Curr Opin Genet Dev. 1994;4:120–129. doi: 10.1016/0959-437x(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 4.Cerni C, Mougneau E, Zerlin M, Julius M, Marcu K B, Cuzin F. Curr Topics Microbiol Immunol. 1986;132:193–201. doi: 10.1007/978-3-642-71562-4_28. [DOI] [PubMed] [Google Scholar]
- 5.Mai S, Hanley-Hyde J, Fluri M. Oncogene. 1996;12:277–288. [PubMed] [Google Scholar]
- 6.Chernova O B, Chernov M V, Ishizaka Y, Agarwal M L, Stark G R. Mol Cell Biol. 1998;18:536–545. doi: 10.1128/mcb.18.1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felsher D W, Bishop J M. Proc Natl Acad Sci USA. 1999;96:3940–3944. doi: 10.1073/pnas.96.7.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Dang C V. Mol Cell Biol. 1999;19:5339–5351. doi: 10.1128/mcb.19.8.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eilers M, Picard D, Yamamoto K R, Bishop J M. Nature (London) 1989;340:66–68. doi: 10.1038/340066a0. [DOI] [PubMed] [Google Scholar]
- 10.Zindy F, Eischen C M, Randle D H, Kamijo T, Cleveland J L, Sherr C J, Roussel M F. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eischen C M, Weber J D, Roussel M F, Sherr C J, Cleveland J L. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson O, Blegen H, Wejde J, Zetterberg A. Cell Biol Int. 1993;17:565–571. doi: 10.1006/cbir.1993.1100. [DOI] [PubMed] [Google Scholar]
- 13.Paulovich A G, Toczyski D P, Hartwell L H. Cell. 1997;88:315–321. doi: 10.1016/s0092-8674(00)81870-x. [DOI] [PubMed] [Google Scholar]
- 14.Di Leonardo A, Khan S H, Linke S P, Greco V, Seidita G, Wahl G M. Cancer Res. 1997;57:1013–1019. [PubMed] [Google Scholar]
- 15.Lanni J S, Jacks T. Mol Cell Biol. 1998;18:1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 17.Levine A J. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 18.Pelengaris S, Littlewood T, Khan M, Elia G, Evan G. Mol Cell. 1999;3:565–577. doi: 10.1016/s1097-2765(00)80350-0. [DOI] [PubMed] [Google Scholar]
- 19.Wu K J, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, Dalla-Favera R. Nat Genet. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg R A, O'Hagan R C, Deng H, Xiao Q, Hann S R, Adams R R, Lichtsteiner S, Chin L, Morin G B, DePinho R A. Oncogene. 1999;18:1219–1226. doi: 10.1038/sj.onc.1202669. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Xie L Y, Allan S, Beach D, Hannon G J. Genes Dev. 1998;12:1769–1774. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blyth K, Terry A, O'Hara M, Baxter E W, Campbell M, Stewart M, Donehower L A, Onions D E, Neil J C, Cameron E R. Oncogene. 1995;10:1717–1723. [PubMed] [Google Scholar]
- 23.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Woods D, McMahon M, Bishop J M. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimri G P, Itahana K, Acosta M, Campisi J. Mol Cell Biol. 2000;20:273–285. doi: 10.1128/mcb.20.1.273-285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin A W, Barradas M, Stone J C, van Aelst L, Serrano M, Lowe S W. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherr C J. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]