Abstract
Feeding behavior is comprised of both appetitive and consummatory responses to food. Appetitive responses include the motivated acquisition of food. Consummatory responses, including swallowing, are those that move the food from the mouth to the stomach. Intraoral delivery of liquid food bypasses the requirement for appetitive responses and has been used to examine consummatory responses directly in intact rats. In the present study, we administered neuropeptide Y (NPY), agouti-related protein (AGRP) and norepinephrine (NE), into the paraventricular nucleus of the hypothalamus (PVN) or into the fourth cerebral ventricle to examine their effects on the consummatory component of feeding behavior in the rat. To measure consummatory responses, milk (40% lactose free cow’s milk diluted with water) was infused intraorally through a chronic cheek fistula (1 ml/min), using an alternating 5 min on -1 min off schedule, until rejection occurred. We found that both hypothalamic and fourth ventricle injections of NPY, AGRP and NE significantly increased consumption of the intraorally-delivered milk. Our results indicate that the circuitry for modulation of consummatory ingestive responses includes NE, NPY and AGRP receptors operating in both hypothalamic and hindbrain sites.
Keywords: norepinephrine, neuropeptide Y, agouti-related protein, food intake, consummatory feeding responses, appetitive feeding behavior, hindbrain, fourth ventricle, paraventricular nucleus of the hypothalamus
Feeding behavior is comprised of both appetitive and consummatory responses to food. Appetitive responses include those related to the motivated acquisition of food [1]. Consummatory responses are highly stereotyped oromotor responses, such as swallowing, that are required for movement of food from the mouth to the stomach. Anatomical studies [2, 3] and studies using chronically-maintained decerebrate rats [1, 4] have demonstrated that consummatory responses rely on hindbrain neurocircuitry, while appetitive responses require participation of forebrain structures.
During normal ingestive behavior in intact rats, appetitive and consummatory components of feeding are tightly coupled. Consummatory responses occur reflexively only after appetitive responses acquire food and place it in the mouth. Therefore, during normal ingestion, any attempt to examine the effects of neuroactive amines and peptides on consummatory responses is confounded by potential effects on appetitive processes that precede it. However, consummatory responses can be directly assessed in neurologically intact rats using intraoral feeding protocols that eliminate the requirement for appetitive behaviors [5, 6]. Furthermore, in intact rats, consummatory response circuitry is not isolated from forebrain influences, as it is in decerebrate rat preparations. Thus, the modulation of consummatory responses by neural pathways from the forebrain can be studied using this approach.
In the present experiment, we investigated the effects of forebrain and hindbrain injections of neuropeptide Y (NPY), agouti gene-related protein (AGRP) and norepinephrine (NE) on consummatory responding in intact rats using an intraoral feeding protocol. The patterns of co-expression of NPY with either AGRP or epinephrine (E) and NE within the brain define two distinct NPY systems, one originating in the hypothalamus and one originating in the hindbrain. Both NPY systems are responsive to certain ingestive stimuli [7–13] and both innervate distributed brain sites important for feeding behavior, in many cases with overlapping terminal plexuses [14]. AGRP, an endogenous antagonist of melanocortin 3 and 4 (MC3/4) receptors, is exclusively co-expressed by NPY neurons with cell bodies in the arcuate nucleus of the hypothalamus [14]. NPY/AGRP neurons project within the hypothalamus and to extrahypothalamic sites, including a dense projection to the pontine parabrachial nucleus and, possibly, a sparse projection to the dorsal vagal complex [14]. NPY is also co-expressed in a subgroup of hindbrain NE and epinephrine (E) neurons that project locally within the hindbrain and that heavily innervate the arcuate and paraventricular nuclei of the hypothalamus, as well as other forebrain sites [15–18].
NPY, AGRP and NE all appear to have positive actions on appetitive responses during feeding behaviors when they are administered at various sites along the brain’s rostro-caudal axis. NPY evokes appetitive responses when injected into the lateral or fourth ventricle [19] or when injected into the hypothalamus [20, 21]. Administration of AGRP or the synthetic MC3/4 antagonist, SHU9119, into the lateral, third or fourth cerebral ventricle, or into the dorsal vagal complex also evokes feeding in appetitive testing protocols [22–24]. Similarly, NE increases feeding in appetitive testing paradigms when administered intraventricularly [25] or into the hypothalamus [26–28].
Despite the prominent roles of NPY, AGRP and NE in stimulating feeding in appetitive feeding paradigms, the roles of these neurochemicals in mediating consummatory responses is not clear. Selective immunotoxin lesion of NE and E neurons has been shown to abolish both appetitive and consummatory responses to glucoprivation [29], but responses to E or NE have not been directly examined in situations that eliminate the appetitive components of ingestion, such as in intraoral feeding paradigms. Likewise, participation of AGRP in control of food intake in the absence of appetitive behavioral responses has not been examined. Although effects of NPY on intraoral feeding have been examined, NPY’s role in mediating consummatory responses remains ambiguous. Previous reports suggest that injection of NPY into the third [6, 30] or lateral cerebral ventricle [31, 32] does not increase consumption of intraorally-delivered sucrose solution in intact rats, or does so only under certain circumstances [33]. These findings suggest that NPY’s stimulatory effects on food intake may be limited to enhancment of appetitive feeding responses. Alternatively, these results could indicate that NPY injected into the third or lateral ventricle does not reach hindbrain target sites where NPY is capable of influencing consummatory reflexes.
The issue of NPY, AGRP and NE participation in enhancement of consummatory as well as appetitive components of ingestion is critical for understanding the organization of feeding behaviors and their underlying neural circuitry. Therefore, we examined the efficacy of these three substances on the consummatory responses to food using an intraoral feeding protocol. In our experiments we administered NE, AGRP and NPY into the hindbrain (fourth ventricle) or into the hypothalamus in order to determine whether behavioral effects depended on route of administration. In addition, we examined the effects of lateral ventricle NPY injections on consummatory feeding responses. Results of our study indicate that NPY, AGRP and NE all enhance consummatory feeding responses after injection into either hypothalamus or hindbrain, suggesting that both NPY/AGRP and NPY/NE receptive neuronal populations are capable of influencing consummatory feeding responses via actions at multiple levels of the neuroaxis.
Materials and Methods
Animals
Adult male Sprague-Dawley rats, 3 – 5 mo of age and weighing between 320 and 400 g, were obtained from Simonsen Laboratories, Gilroy, CA and Harlan Sprague Dawley, Inc, Madison, WI, for use in these experiments. The rats were housed in individual suspended wire mesh cages in an AAALAC-approved vivarium. The room was maintained at 21°C and was illuminated on a 12:12 light-dark cycle (lights on at 7 am). Rats were permitted ad libitum access to pellet rat chow (Harlan Teklad F6 Rodent Diet W, Madison, WI) and water, except during testing. Tests were conducted between 1400 and 1700 h. All experimental animal protocols were approved by the Washington State University Institutional Animal Care and Use Committee, which conforms to National Institute of Health guidelines.
Preparation of animals
Prior to implantation of cannulas, preliminary studies were conducted to determine the optimal stereotaxic coordinates for cannula placements and the desired injection volumes. Distribution of injectate in cannulated rats was estimated by examining the distribution of fluorogold injected 30 min prior to death to allow for diffusion (since previous work indicated that milk is consumed only during the first 30 min after the start of the intraoral infusion). Fluorogold is retrogradely transported, but after 30 min we assumed that the visible radius would primarily reflect diffusion, rather than transport, and would have the advantage of being stable in situ over the 30-min delay. Fluorogold distribution was readily visualized using fluorescence microscopy. In some rats, black India Ink was injected 5 min prior to death. Intraparenchymal injection of 200 nl of fluorogold 30 min prior to death revealed a microscopically visible diffusion/transport radius of approximately 0.8 mm. Black India ink injections (200 nl) into the 4V revealed ink in the 4V, mainly surrounding the cannula tip, but not in the aqueduct, third ventricle, lateral ventricle or in the subarachnoid space.
For implantation of cheek fistulas and intracranial cannulas, rats were anesthetized by Isoflurane (Webster Veterinary Supply Co.) inhalation. Cheek fistulas, designed and constructed at Washington State University, have been described previously [5]. The fistula was inserted through the skin of the right cheek just lateral and rostral to the first premolar using a 16-gauge needle to pierce the tissue. At this site, the fistula did not compromise the muscles of mastication. When in place, the interior surface of the fistula was located snugly against the inside of the cheek and locked into place by a threaded washer attached to the exterior of the fistula. The fistula was allowed to remain open when not in use. Prior to use, food remnants were cleared from the lumen of the fistula using 26 g stainless steel tubing. An infusion tip was screwed onto the fistula and connected to a syringe pump by silastic tubing. The silastic infusion line was supported above the rat’s head so that the rat could move freely about the cage.
The diet used for intraoral feeding was lactose free cow’s milk, diluted to 40% with tap water. We have used this diet previously for intraoral feeding studies [5]. Following fistula implantation, rats were familiarized with the dilute milk diet, which was presented in home cage drinking tubes during three 2-hr daytime sessions during a 2-wk period. During this same 2-wk period, rats were habituated to the intraoral infusion test apparatus, but were not fed intraorally. Subsequently, they were exposed during 2 training sessions to the intraoral milk infusion protocol with at least 1 day between sessions. All rats readily consumed the milk, regardless of how it was presented, after their first exposure to it.
Following training for intraoral feeding, stainless steel guide cannulas (26 gauge) were implanted unilaterally in anesthetized rats into the PVN, fourth cerebral ventricle (4V) or lateral ventricle (LV). Separate groups of rats were used for each testing site (i.e., each rat was implanted with one cannula only). Due to the extended unavailability of rats from our normal supplier (Simonsen Laboratories, Inc.) at the time of the experiments, only the 4V cannulated rats were obtained from this supplier. The PVH and LV cannulated groups were comprised of rats obtained from Harlan Sprague Dawley, Inc. The stereotaxic co-ordinates for cannula placement were: −1.8 mm caudal and 0.4 mm lateral to bregma, 7.4 mm ventral to the dura mater for PVN; +2.0 mm rostral to the occipital crest, 0.0 mm lateral to midline, and 6.5 mm ventral to dura mater for 4V; −0.4 mm caudal to bregma, 1.5 mm lateral to midline and 3.6 mm ventral to dura mater for LV. A 33 gauge obturator was used to occlude the lumen of the cannula when not in use. Rats were allowed one week of recovery from surgery before experimentation.
Intraoral feeding tests
On test days, solid food was removed from the rats’ cages 60 min before initiation of testing. For intracranial injection of neurochemicals, obturators were removed from the guide cannulas and replaced with 33-gauge stainless steel injectors attached to 10 μL Hamilton microsyringes (Stoelting, Co., Wood Dale, IL) via 30.5-gauge polyethylene tubing. Rats were injected into the PVN or 4V with 200 nl of 0.9% saline control solution or with NPY (78 pmol, Phoenix Pharmaceuticals, Incorp., Belmont, CA), AGRP (200 pmol, Phoenix Pharmaceuticals) or NE (l-norepinephrine bitartrate, 40 nmol, Sigma-Aldrich Co., St. Louis, MO) in 200 nl of 0.9% sterile saline. Doses were chosen from our own and published work. Injectors were left in place for 60 seconds after delivery of neurochemicals to ensure diffusion from the cannula tip. Intraoral infusion lines were then connected and infusions commenced 8 min after NE and NPY injections or two hours after the AGRP injection, consistent with the time courses of the peptides and NE effects on food intake [34, 35]. During the 2-hr interval, AGRP-injected animals remained in their home cages without food. Baseline tests were conducted at corresponding times using the same protocols as for the neurochemical injections. For the PVN-cannulated rats, NPY, AGRP and NE were tested in quasi-random order, with saline tests conducted after each drug test and with 3–5 days intervening between tests. For hindbrain-cannulated rats, the same general procedure was followed for NPY and AGRP, but NE was tested in a separate group of rats. In this latter group, NE effects were tested using the intraoral feeding, as described, and this was followed by separate tests in which the rats consumed the same diet from a drinking tube (see below). The cannulas of a few rats became obstructed during the testing due to breakage or loss of the obturator in the cannula shaft so that not all rats could be used for all tests.
For infusion of liquid food, the intraoral fistulas were connected with silastic tubing (1.57 mm inner diameter. × 3.18 mm outer diameter.) to a 60 cc syringe mounted on a Sage syringe pump (Thermo Electron Corp., Waltham, MA). Intraoral feeding tests were conducted under direct individual observation using an open-topped, 8″ × 8″ × 12″ wire mesh cage where the rat was clearly visible. Paper was placed under the cage to help visualize spillage. During the test, the milk was infused (1 ml/min) in successive 5 min periods separated by 1 min rest periods until the rat allowed the milk to drip out of its mouth. At this point, the infusion was suspended for 1 min and then restarted. If the rat refused the food again within 30 seconds and did so on three successive restarts, the rat was determined to be satiated and the test was terminated.
We could not find a report in the literature in which the effect of 4V NE injection was evaluated in an appetitive feeding test. Since this information was important for our interpretation of the data, we also tested the effects of 4V injection of NE (40 nmol in 200 nl) and saline control on appetitive responding in a test in which 40% lactose free cows milk was presented in a drinking tube affixed to the rat’s home cage for 60 min immediately following the 4V injection. Drinking tube tests were conducted in the same rats tested with NE in the consummatory feeding test, after consummatory feeding tests had been completed.
Because of negative published findings showing no stimulatory effects of NPY on consummatory responses, we tested the generality of our findings with NPY using a different test diet. We conducted two additional experiments using sucrose diets, since sucrose has been used in previous studies of NPY and consummatory feeding responses [6, 32]. These rats were exposed to the sucrose diet and trained for intraoral feeding using protocols described for the milk diet. All rats used for the sucrose tests readily consumed the sucrose solution under basal conditions. One group of rats was implanted with PVN cannulas and cheek fistulas, as described, and tested for effects of NPY (78 pmol) on intake of 0.1 M sucrose. Another group of rats was implanted with lateral ventricle (LV) cannulas and cheek fistulas and tested for effects of LV NPY (1.2 nmol in 3ul) and saline control injection on intake of 0.1 and 1M sucrose, as well as on intake of the 40% milk diet. The higher dose and volume used for LV NPY injections were based on previous intraoral feeding studies using LV or 3V routes of administration for NPY [6, 32]. Saline tests were conducted after the NPY test for each diet. Failure of lateral ventricle administration of NPY to stimulate intraoral feeding have also been reported. Thus, we included a LV cannulated group in our study to determine whether this route of administration would produce an effect on feeding that was similar to that produced by intraparenchymal administration.
Verification of cannula placement
At the conclusion of experimentation, rats were sacrificed by administration of a lethal dose of halothane anesthesia and perfused successively through the heart with 0.1 phosphate buffer and 40% formaldehyde solutions. Brains were removed from the skull, sectioned on a cryostat and stained with cresyl violet for microscopic examination of the cannula sites.
Statistics
Feeding responses were analyzed using SigmaStat statistics software. One-way repeated measures ANOVA was used to test the differences between saline and drug effects. Differences were considered significant if P < 0.05.
Results
Rats recovered rapidly from both intraoral fistula and intracranial cannula surgeries. All rats used for behavioral testing were healthy during the course of testing and continued to show normal weight gain. Histological analysis of cannula placements at the conclusion of testing revealed that intracranial cannulas were located in or immediately adjacent to the PVN, in the 4V or in the tissue ventral to the 4V floor, or in the LV.
Intracranial injections
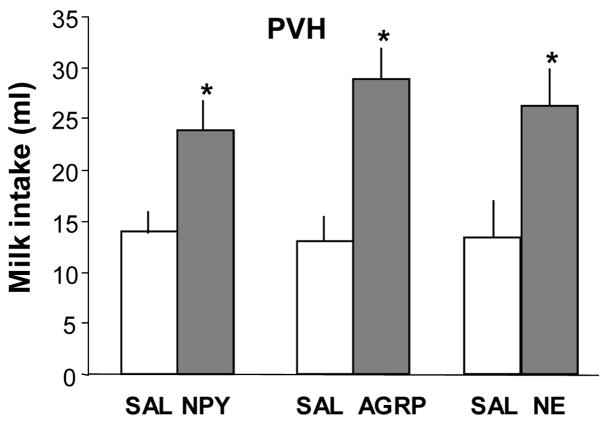
Saline tests conducted 8 min after and 2 hr after injection did not differ significantly and were averaged for data analysis. Figure 1 shows that PVN injections of NPY, AGRP and NE significantly increased intraoral 40% milk intake, compared to saline control injections. Rats consumed 14.0 ± 1.9 ml of milk following PVN saline injection and 23.9 ± 2.77 ml after NPY injection (F= 29.8, P<0.001, n =15). Rats consumed 13.0 ± 1.9 and 29 ± 3.8 ml following saline and AGRP injections, respectively (F= 41.9, P<0.001, n = 8). They consumed 13.4 ± 3.0 and 26.2 ± 3.5 ml after saline and NE injections, respectively (F= 11.5, P < 0.015, n = 7).
Fig. 1.
Consummatory intake of 40% lactose free milk solution infused through an intraoral fistula following PVH injection of 0.9% saline (SAL, 200 nl), neuropeptide Y (NPY, 78 pmol/200 nl), agouti-related protein (AGRP, 200pmol/200 nl), or norepinephrine (NE, 40 nmol/200 nl). Milk intake after NPY, AGRP and NE was significantly increased, compared to intake following PVH saline injections. Data are expressed as mean intraoral intake in ml ± SEM. (*P ≤ 0.01 vs intake after saline).
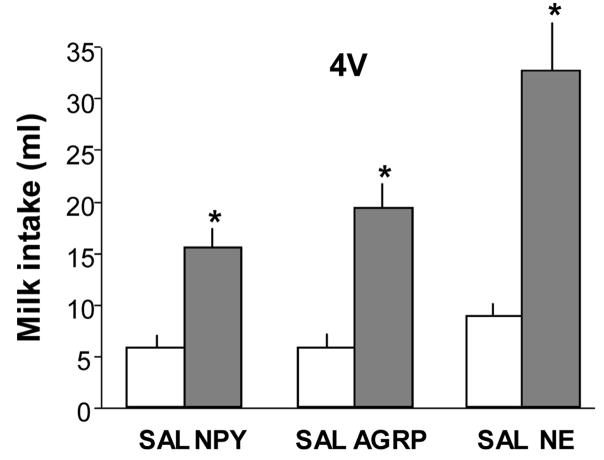
Figure 2 shows that NPY, AGRP and NE also significantly increased intraoral intake of 40% milk solution when administered into the 4V. Rats consumed 5.8 ± 0.82 and 15.6 ± 1.6 ml of milk, respectively, in response to saline and NPY injections (F=30.4, P <.001, n=10). They consumed 5.9 ± 0.9 and 19.5 ± 2.0 ml, respectively, after saline and AGRP (F = 26.4. P < 0.001, n = 9) and 8.8 ± 1.1 and 32.7 ± 4.6 ml, respectively, after saline and NE injections (F = 29.7, P<0.001, n = 8).
Fig. 2.
Consummatory intake of 40% lactose free milk solution infused through an intraoral fistula following 4th ventricular (4V) injection of 0.9% saline (SAL, 200 nl), neuropeptide Y (NPY, 78 pmol/200 nl), agouti-related protein (AGRP, 200 pmol/200 nl), or norepinephrine (NE, 40 nmol/200 nl). NPY, AGRP and NE injections all significantly increased milk consumption, compared to intake following 4V saline injections. Data are expressed as mean intraoral intake in ml ± SEM. (*P < 0.001 vs intake after saline).
PVH injection of NPY significantly increased consumption of 0.1M sucrose solution in the intraoral feeding test (Fig. 3). In these tests, rats consumed 16.8 ml ± 0.7 ml of sucrose following saline control injection and 27.6 ± 1.6 ml in response to NPY (F = 37.9, P < 0.001, n = 13).
Fig. 3.
Consummatory intake of a 0.1M sucrose solution infused through an intraoral fistula after injection of 0.9% saline (SAL, 200 nl) and neuropeptide Y (NPY, 78 pmol/200 nl) into the PVH. Data are expressed as mean intraoral intake in ml ± SEM. NPY injection significantly increased intake of the sucrose solution in comparison to the intake after PVH saline injection (*P < 0.001).
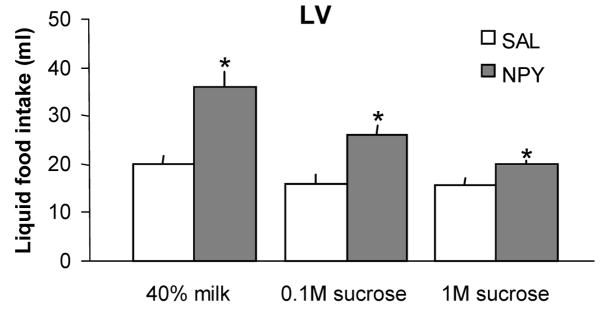
As shown in Figure 4, LV injection of NPY increased intake of the 40% milk and both the 0.1M and the 1M sucrose solutions in the intraoral feeding test. Rats ate 19.8 ± 1.9 and 35.9 ± 3.3 ml of 40% milk after LV saline and NPY injections, respectively (F = 31.0, P < .005, n = 5). They ate 16.1 ± 1.6 and 26.0 ± 1.8 ml of 0.1M sucrose after saline and NPY, respectively (F = 16.3, P < 0.016, n = 5), and 15.7 ± 1.2 and 19.8 ± 1.7 ml of 1M sucrose after saline and NPY, respectively (F = 15.4, P = 0.017, n = 5).
Fig. 4.
Effects of lateral ventricular (LV) injection of NPY (1.2 nmol in 3 μl) or saline (0.9% in 3 μl) on consummatory intake of three test solutions (40% cow’s milk, 0.1M sucrose solution and 1M sucrose solution) infused through an intraoral fistula. Data are expressed as mean intraoral intake in ml ± SEM. Lateral ventricular NPY injections significantly increased consumption of both milk and sucrose solutions, compared to the intake of these diets after LV saline (*P ≤ 0.02).
It is noteworthy that baseline consumption of the dilute milk diet was higher in the PVH cannulated group (Fig. 1) than in the 4V cannulated group (Fig. 2). Baseline intake the dilute milk by LV rats, shown in Figure 4, was also higher than intake of the same diet shown in Figs. 1 and 2. These between-group differences in baseline intakes may be due to the fact that rats in the 4V group were obtained from Simonsen Laboratories, Inc., whereas rats in the other groups were obtained from Harlan. We have noted previously that when maintained under similar conditions, rats from the different commercial suppliers differ significantly in their ad libitum food intake and rates of body weight gain. Whatever the reason for these between-group baseline differences, the differences did not obscure the stimulatory effect of the injected neurochemicals.
Fourth ventricular injection of NE (40 nmol in 200 nl) increased appetitive responses when 40% milk solution was provided from home cage drinking tubes (data not shown) in tests conducted after intraoral feeding experiments were completed. Intake in the 60-min bottle feeding test was 12 ± 1.6 ml following saline injection and 29.1 ± 2.6 ml following 4V NE injection(P<.001).
Discussion
Results of this study show that NPY, AGRP and NE all increased consummatory feeding responses under the conditions of our tests. Thus, physiological activation of either hypothalamic NPY/AGRP co-expressing neurons or hindbrain NPY/NE co-expressing neurons is likely to enhance consummatory feeding responses. Although this conclusion could not be reached on the basis of NPY injections alone, the fact that the substances that are uniquely co-expressed with NPY in each of these two distinct neuronal populations produced effects similar to NPY at each injection site, suggests that at least some neurons in both NPY populations possess this capability. Furthermore, the injection sites effective in increasing consummatory responses are sites where injections of these same neurochemicals have been reported to stimulate appetitive responses [19, 22, 23, 26–28, 36, 37]. Together these results suggest that both the hypothalamus and hindbrain contain NPY-, AGRP- and NE-responsive circuitry capable of enhancing both consummatory and appetitive components of feeding behavior.
The specific sites of action of the injected neurochemicals were not determined by our study. However, the potential site of action of the 4V-injected AGRP is particularly intriquing. The medial and lateral parabrachial areas are sites at which 4V-injected AGRP might influence consummatory feeding responses. These are important integrative sites for gustatory and visceral signals [38]. They receive significant innervation from AGRP/NPY neurons [14] and are heavily interconnected with hindbrain and forebrain sites important for ingestive control [38]. The dorsal vagal complex also is a potential site of action of AGRP. This area is accessible from the 4V, densely expresses MC3/4 receptors [39, 40], and is likely to be a crucial participant in consummatory responses [2, 3]. However, AGRP-ir terminals are sparse in this area [14], raising the possibility that the 4V-injected AGRP could be acting on MC3/4 receptors that are not innervated by AGRP neurons to produce a response not normally associated with AGRP-containing nerve terminals. Additional approaches will be required to determine the specific sites across the neuroaxis that are crucial for NPY AGRP and NE control of consummatory and appetitive responses, including intraparenchymal mapping of sensitive sites using low doses and volumes, detailed attention to distribution of the injected substances, and consideration of overlapping innervation from multiple NPY and NE cell populations.
Previous reports have suggested that NPY does not facilitate consummatory feeding responses [6, 30–32]. The present results directly contradict that conclusion, since we found that NPY stimulated intraoral intake of both milk and sucrose diets and was effective when injected into the lateral ventricle, fourth ventricle or into the PVH. The apparent discrepancy between our study and prior reports may result from differences in the training or testing protocols used for intraoral feeding. Negative effects of lateral ventricular NPY on intraoral feeding have been reported in tests using 1M sucrose solution as the test diet [31, 32]. In our study we found that lateral ventricular NPY was much less effective when 1M sucrose was used than when 0.1 M sucrose was used as the test diet, but the increase in consumption of both concentrations was significant. The 1M sucrose diet may be too concentrated to elicit maximal intake volumes [41], in part because this very concentrated solution could be expected to evoke gastrointestinal osmotic feedback that might limit intake in the intraoral test situation. Negative effects of NPY have also been reported in protocols using 0.1 M sucrose in the feeding test [6]. More recent analysis of the latter effects has suggested that the training protocol used in these studies introduced conditioning effects that interacted with consummatory responding [33] or possibly that training and testing protocols may have encouraged development of high baseline intakes that limited the difference between baseline and peptide-stimulated consummatory responses (i.e., imposed a ceiling effect). Our training and testing protocols avoided ceiling effects and minimized conditioning effects by using intermittent, rather than daily training and testing sessions.
It is evident that modulatory control of consummatory ingestive responses arises from multiple sources. Accordingly, recent work has revealed modulation of consummatory responses by distinct ingestive stimuli and has identified contributions of a number of neurochemical pathways. For example, selective immunotoxin-induced lesions have shown that hindbrain catecholamine neurons are essential for stimulation of consummatory responses during systemic glucose deficit, but are not required for enhancement of consummatory responses to systemic blockade of fat oxidation or overnight food deprivation [5]. In addition, studies in decerebrate rats have shown that the enhanced response to palatability produced by chlordiazepoxide is mediated by hindbrain chlordiazepoxide binding sites that stimulate consummatory responses [42]. Hindbrain urocortin 1 [43] and serotonin 2C/1B [44] receptors that reduce consummatory responses also have been identified. These neurochemically distinct pathways capable of influencing consummatory responses indicate that the control of consummatory response circuitry is likely to be complex.
Previously published work has shown that feeding responses that include an appetitive component can be evoked by hypothalamic [26–28] or 4V (this paper) injection of NE, NPY [19, 36, 37] or MC3/4 receptor antagonists [22, 23]. These findings, together with the present data, suggest that AGRP/NPY or NE/NPY neurons may stimulate both appetitive and consummatory responses to specific ingestive stimuli by their collateral innervation of multiple sites distributed along the neuroaxis. This pattern of neural organization has been proposed previously for hindbrain catecholamine neurons [5] involved in glucoprivic feeding. The latter proposal was based on the fact that selective retrograde immunotoxin destruction of hypothalamically-projecting hindbrain catecholamine neurons abolishes both the appetitive and the consummatory feeding responses to glucoprivic challenge specifically [5,29], whereas decerebration, which transects the rostral projections of these neurons without destroying the neurons themselves or their hindbrain terminals, does not abolish the consummatory feeding response to glucoprivation [45]. Thus, the consummatory responses to glucoprivation may be elicited by the hindbrain terminations of these catecholamine neurons, while their rostral projections activate forebrain appetitive circuitry. This interpretation of the previous data is supported by the present findings showing that consummatory responses can be elicited by 4V injection of NE. Although catecholamine/NPY and AGRP/NPY neurons originate in different brain regions and are likely to be responsive to different ingestive signals, the present results suggest that these two populations of orexigenic NPY neurons may utilize similar strategies for simultaneous arousal of both consummatory and appetitive feeding responses.
Acknowledgments
This work was done in partial fulfillment of requirements for an undergraduate honors thesis (K. Carter and E. Lester) in the Programs for Neuroscience at Washington State University and was supported by PHS grant DK 40498 and NS045520 to S. Ritter. We thank Shannon Rowland for her excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grill HJ. Caudal brainstem contributions to the integrated neural control of energy homeostasis. In: Ritter RC, Ritter S, Barnes CD, editors. Feeding behavior: Neural and humoral controls. Orlando: Academic Press; 1986. pp. 102–29. [Google Scholar]
- 2.Cunningham ET, Jr, Sawchenko PE. Dorsal medullary pathways subserving oromotor reflexes in the rat: Implications for the central neural control of swallowing. J Comp Neurol. 2000;417:448–66. [PubMed] [Google Scholar]
- 3.Amirali A, Tsai G, Schrader N, Weisz D, Sanders I. Mapping of brain stem neuronal circuitry active during swallowing. Ann Otol Rhinol Laryngol. 2001;110:502–13. doi: 10.1177/000348940111000603. [DOI] [PubMed] [Google Scholar]
- 4.Grill HJ. Production and regulation of ingestive consummatory behavior in the chronic decerebrate rat. Brain Res Bull. 1980;5:79–87. [Google Scholar]
- 5.Hudson B, Ritter S. Hindbrain catecholamine neurons mediate consummatory responses to glucoprivation. Physiol Behav. 2004;82:241–50. doi: 10.1016/j.physbeh.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Seeley RJ, Payne CJ, Woods SC. Neuropeptide y fails to increase intraoral intake in rats. Am J Physiol. 1995;268:R423–7. doi: 10.1152/ajpregu.1995.268.2.R423. [DOI] [PubMed] [Google Scholar]
- 7.Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol Behav. 2001;74:703–8. doi: 10.1016/s0031-9384(01)00613-8. [DOI] [PubMed] [Google Scholar]
- 8.Fraley GS, Ritter S. Immunolesion of norepinephrine and epinephrine afferents to medial hypothalamus alters basal and 2-deoxy-d-glucose-induced neuropeptide y and agouti gene-related protein messenger ribonucleic acid expression in the arcuate nucleus. Endocrinology. 2003;144:75–83. doi: 10.1210/en.2002-220659. [DOI] [PubMed] [Google Scholar]
- 9.Akabayashi A, Zaia CT, Silva I, Chae HJ, Leibowitz SF. Neuropeptide Y in the arcuate nucleus is modulated by alterations in glucose utilization. Brain Res. 1993;621:343–8. doi: 10.1016/0006-8993(93)90125-7. [DOI] [PubMed] [Google Scholar]
- 10.Akabayashi A, Levin N, Paez X, Alexander JT, Leibowitz SF. Hypothalamic neuropeptide y and its gene expression: Relation to light/dark cycle and circulating corticosterone. Mol Cell Neurosci. 1994;5:210–8. doi: 10.1006/mcne.1994.1025. [DOI] [PubMed] [Google Scholar]
- 11.Sergeyev V, Broberger C, Gorbatyuk O, Hokfelt T. Effect of 2-mercaptoacetate and 2-deoxy-d-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neuroreport. 2000;11:117–21. doi: 10.1097/00001756-200001170-00023. [DOI] [PubMed] [Google Scholar]
- 12.Li AJ, Ritter S. Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur J Neurosci. 2004;19:2147–54. doi: 10.1111/j.1460-9568.2004.03287.x. [DOI] [PubMed] [Google Scholar]
- 13.Li AJ, Wang Q, Ritter S. Differential responsiveness of dopamine-beta-hydroxylase gene expression to glucoprivation in different catecholamine cell groups. Endocrinology. 2006;147:3428–34. doi: 10.1210/en.2006-0235. [DOI] [PubMed] [Google Scholar]
- 14.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–8. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham ET, Jr, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–67. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- 16.Kalia M, Fuxe K, Goldstein M. Rat medulla oblongata. II. Dopaminergic, noradrenergic (a1 and a2) and adrenergic neurons, nerve fibers, and presumptive terminal processes. J Comp Neurol. 1985;233:308–32. doi: 10.1002/cne.902330303. [DOI] [PubMed] [Google Scholar]
- 17.Hokfelt T, Johansson O, Goldstein M. Central catecholamine neurons as revealed by immunohistochemistry with special reference to adrenaline neurons. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Vol. 2. Amsterdam: Elsevier; 1984. pp. 157–276. [Google Scholar]
- 18.Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovacs KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996;107:201–22. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- 19.Corp ES, Melville LD, Greenberg D, Gibbs J, Smith GP. Effect of fourth ventricular neuropeptide Y and peptide YY on ingestive and other behaviors. Am J Physiol. 1990;259:R317–23. doi: 10.1152/ajpregu.1990.259.2.R317. [DOI] [PubMed] [Google Scholar]
- 20.Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: A powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7:1189–92. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- 21.Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF. The perifornical area: The major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s) Brain Res. 1993;604:304–17. doi: 10.1016/0006-8993(93)90382-w. [DOI] [PubMed] [Google Scholar]
- 22.Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AGRP-(83---132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;279:R47–52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- 23.Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–7. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- 24.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic pomc projections. Am J Physiol Regul Integr Comp Physiol. 2005;289:R247–58. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]
- 25.Ritter S, Wise D, Stein L. Neurochemical regulation of feeding in the rat: facilitation by alpha-noradrenergic, but not dopaminergic, receptor stimulants. J Comp Physiol Psychol. 1975;88:778–84. doi: 10.1037/h0076402. [DOI] [PubMed] [Google Scholar]
- 26.Leibowitz SF. Central adrenergic receptors and the regulation of hunger and thirst. Res Publ Assoc Res Nerv Ment Dis. 1972;50:327–58. [PubMed] [Google Scholar]
- 27.Leibowitz SF, Sladek C, Spencer L, Tempel D. Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: Stimulation of feeding and the release of corticosterone, vasopressin and glucose. Brain Res Bull. 1988;21:905–12. doi: 10.1016/0361-9230(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 28.Ritter RC, Epstein AN. Control of meal size by central noradrenergic action. Proc Natl Acad Sci U S A. 1975;72:3740–3. doi: 10.1073/pnas.72.9.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432:197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- 30.Woods SC, Figlewicz DP, Madden L, Porte D, Jr, Sipols AJ, Seeley RJ. NPY and food intake: Discrepancies in the model. Regul Pept. 1998;75–76:403–8. doi: 10.1016/s0167-0115(98)00095-0. [DOI] [PubMed] [Google Scholar]
- 31.Ammar AA, Sederholm F, Saito TR, Scheurink AJ, Johnson AE, Sodersten P. NPY-leptin: Opposing effects on appetitive and consummatory ingestive behavior and sexual behavior. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1627–33. doi: 10.1152/ajpregu.2000.278.6.R1627. [DOI] [PubMed] [Google Scholar]
- 32.Ammar AA, Nergardh R, Fredholm BB, Brodin U, Sodersten P. Intake inhibition by NPY and CCK-8: A challenge of the notion of NPY as an “orexigen”. Behav Brain Res. 2005;161:82–7. doi: 10.1016/j.bbr.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Benoit SC, Clegg DJ, Woods SC, Seeley RJ. The role of previous exposure in the appetitive and consummatory effects of orexigenic neuropeptides. Peptides. 2005;26:751–7. doi: 10.1016/j.peptides.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Lu XY, Nicholson JR, Akil H, Watson SJ. Time course of short-term and long-term orexigenic effects of agouti-related protein (86–132) Neuroreport. 2001;12:1281–4. doi: 10.1097/00001756-200105080-00045. [DOI] [PubMed] [Google Scholar]
- 35.Stanley BG, Thomas WJ. Feeding responses to perifornical hypothalamic injection of neuropeptide Y in relation to circadian rhythms of eating behavior. Peptides. 1993;14:475–81. doi: 10.1016/0196-9781(93)90135-4. [DOI] [PubMed] [Google Scholar]
- 36.Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide y: Evidence for a hypothalamic site(s) of action. Brain Res Bull. 1985;14:521–4. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 37.Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF. Feeding responses to perifornical hypothalamic injection of neuropeptide y in relation to circadian rhythms of eating behavior. Peptides. 1993;14:475–81. doi: 10.1016/0196-9781(93)90135-4. [DOI] [PubMed] [Google Scholar]
- 38.Hermann GE, Rogers RC. Convergence of vagal and gustatory afferent input within the parabrachial nucleus of the rat. J Auton Nerv Syst. 1985;13:1–17. doi: 10.1016/0165-1838(85)90002-5. [DOI] [PubMed] [Google Scholar]
- 39.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 40.Tatro JB, Entwistle ML. Distribution of melanocortin receptors in the lower brainstem of the rat. Ann N Y Acad Sci. 1994;739:311–4. doi: 10.1111/j.1749-6632.1994.tb19833.x. [DOI] [PubMed] [Google Scholar]
- 41.Flynn FW, Grill HJ. Intraoral intake and taste reactivity responses elicited by sucrose and sodium chloride in chronic decerebrate rats. Behav Neurosci. 1988;102:934–41. doi: 10.1037//0735-7044.102.6.934. [DOI] [PubMed] [Google Scholar]
- 42.Berridge KC. Brainstem systems mediate the enhancement of palatability by chlordiazepoxide. Brain Res. 1988;447:262–8. doi: 10.1016/0006-8993(88)91128-6. [DOI] [PubMed] [Google Scholar]
- 43.Daniels D, Markison S, Grill HJ, Kaplan JM. Central structures necessary and sufficient for ingestive and glycemic responses to urocortin 1 administration. J Neurosci. 2004;24:11457–62. doi: 10.1523/JNEUROSCI.2702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan JM, Song S, Grill HJ. Serotonin receptors in the caudal brainstem are necessary and sufficient for the anorectic effect of peripherally administered mCPP. Psychopharmacology (Berl) 1998;137:43–9. doi: 10.1007/s002130050591. [DOI] [PubMed] [Google Scholar]
- 45.Flynn FW, Grill HJ. Insulin elicits ingestion in decerebrate rats. Science. 1983;221:188–90. doi: 10.1126/science.6344221. [DOI] [PubMed] [Google Scholar]