Abstract
Purpose of review
Central nervous system (CNS) neurovascular units are multicellular complexes consisting of neurons and astrocytes, vascular endothelial cells and pericytes, as well as an assortment of growth factors and extracellular matrix (ECM) proteins. Here, I will discuss the current knowledge of signaling networks essential for the development and physiology of CNS neurovascular units, particularly in the brain.
Recent findings
Molecular genetic studies have identified various signaling proteins that regulate the formation and function of CNS neurovascular units. These include members of the integrin family of ECM adhesion receptors, ECM proteins such as Wnts and latent transforming growth factor βs, and various transcriptional regulators, including β-catenin and the inhibitors of DNA binding (Ids).
Summary
Neurovascular units are the cellular and molecular interfaces between the circulatory system and the CNS. Recent molecular genetic analyses in mice and other model organisms have revealed the first mechanisms underlying bidirectional communication between neural and vascular components. In particular, ECM-mediated adhesion and signaling pathways have been identified as essential for neurovascular development and physiology. Understanding how these various gene products normally control neurovascular unit formation and function will lend new insights into the causes and possible treatments of debilitating neurovascular-related diseases such as birth defects, stroke, and age-related dementia.
Keywords: angiogenesis, band 4.1 proteins, β8 integrin, neurogenesis, transforming growth factor β, vascular niche
Introduction
The mammalian circulatory system is composed of an elaborate array of arteries, veins, and capillaries that transport cells and blood through mostly every organ and tissue of the body. The traditional view of the circulatory system as a mundane, static network of blood vessels has been challenged in recent years by the discovery that both vascular cells and vascular-derived molecules dynamically modulate cell growth, survival, and differentiation in a diverse array of embryonic and postnatal organs [1–3,4•]. Here, I will focus exclusively on the roles of blood vessels in regulating neural cell functions in the vertebrate central nervous system (CNS). A specific emphasis will be placed on mechanisms of bidirectional neural–vascular cell adhesion and communication in the embryonic brain.
The human CNS, consisting of hundreds of billions of neurons and glia and their myriad connections, is interlaced with a complex web of blood vessels [5,6]. In addition to providing vital oxygen and nutrients to meet the high metabolic demands of the CNS, blood vessels dynamically communicate with neurons and glia, and these events are essential for normal CNS formation and function [7,8]. In turn, neural cells and neural-derived cues influence many vascular functions, including modulation of blood vessel dilation and constriction [9•,10], as well as homeostatic regulation of the blood–brain barrier (BBB) [11,12]. At the cellular and molecular levels, communication between the circulatory system and the CNS occurs within integrated, multicellular structures, termed neurovascular units [13]. The major objective of this review is to summarize the current knowledge of signaling pathways necessary for the development and physiology of neurovascular units as well as to relate some of these findings to CNS neurovascular disorders.
Neural stem and progenitor cells regulate neurovascular development during embryogenesis
Neovascularization of the mammalian CNS occurs entirely via angiogenesis [14]. Bidirectional communication between neural cells and endothelial cells initiates at early stages of CNS development [15–17], when angiogenic blood vessels invade the CNS parenchyma in response to a gradient of neural-derived vascular endothelial growth factor (VEGF) [18,19]. Upon entering the CNS parenchyma, blood vessels migrate along a preformed latticework of neuroepithelia and radial glia [20,21], which are neural stem and progenitor cells that give rise to differentiated neurons and astrocytes in the CNS [22]. Cell–cell communication between blood vessels and radial glia occurs primarily via intervening vascular basement membranes that contain a milieu of growth factors and extracellular matrix (ECM) proteins [4•,23] (Fig. 1). Recent molecular genetic analyses in mice have uncovered critical insights about neural and vascular communication within neurovascular units, particularly related to cell–ECM adhesion and signaling. Some examples of these experimental findings are summarized below.
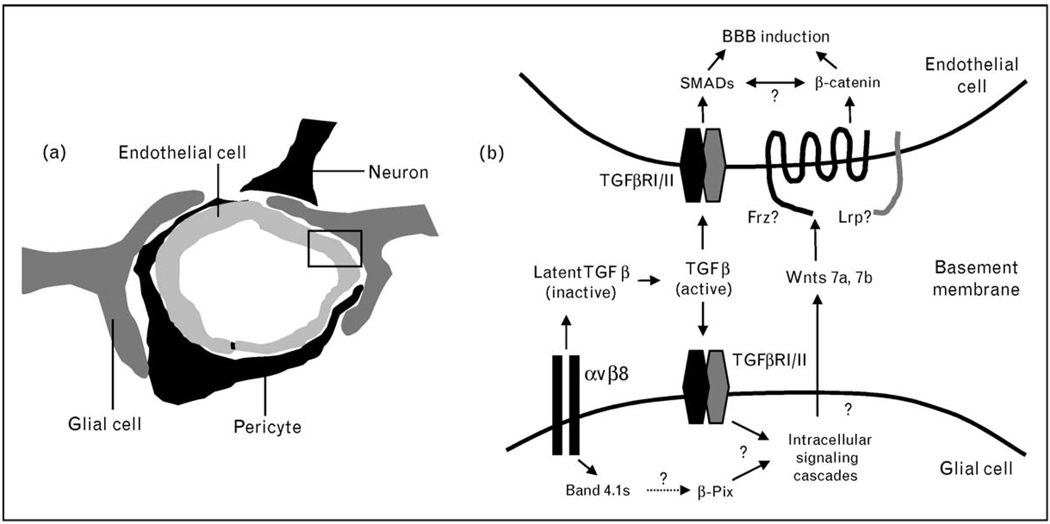
Figure 1. A summary of adhesion and signaling pathways in neurovascular units.
(a) The multicellular composition of a central nervous system (CNS) neurovascular unit composed of vascular endothelial cells and pericytes, neurons, and glia. Extracellular matrix (ECM)-rich basement membranes separate the various neural and vascular cell types. (b) A more detailed view of the boxed area in (a) showing a vascular endothelial cell and perivascular glial cell separated by a vascular basement membrane. The αvβ8 integrin–transforming growth factor (TGF)β adhesion and signaling axis is essential for proper linkage between perivascular glial cells and vascular endothelial cells within neurovascular units. Paracrine TGFβ signaling events in endothelial cells likely involve the SMADs. In perivascular cells, αvβ8 integrin interacts with the Band 4.1s, which may link this integrin with multiple intracellular signaling effectors, including β-Pix. Wnt7a and Wnt7b are ECM-associated proteins expressed by neural cells of the CNS parenchyma that regulate the development of the neurovascular unit. Wnt-mediated signaling pathways require β-catenin in vascular endothelial cells. BBB, blood–brain barrier.
Wnts are lipid-modified, ECM-associated proteins that modulate intracellular signaling cascades via their Frizzled and low-density lipoprotein receptor-related protein (LRP) cell surface receptors [24]. Canonical Wnt signaling involves stabilization and activation of the transcription factor β-catenin, which in the absence of Wnt receptor engagement is targeted for degradation by the proteasome. Stenman et al. [25••] have shown that ablation of both the Wnt7a and Wnt7b genes in mouse CNS neural stem and progenitor cells leads to lethal neurovascular phenotypes, including abnormal BBB maturation and CNS-specific hemorrhage. Liebner et al. [26••] have also shown that Wnt stimulation induces BBB-like properties in cultured endothelial cells, and these events are dependent on canonical β-catenin signaling. Genetic ablation of β-catenin in vascular endothelial cells also induces CNS neurovascular disorders; however, these phenotypes appear more severe than those in the Wnt7a and Wnt7b double knockouts [25••], suggesting that Wnt-independent β-catenin signaling pathways are also important in endothelial cells [27]. Collectively, these findings reveal that canonical Wnt signaling pathways, in part, mediate neurovascular development during mouse embryogenesis.
Other molecular genetic studies in mice have identified transcriptional regulators as essential for proper neurovascular unit development during embryogenesis. For example, combined deletion of the Id1 and Id3 transcription factors causes CNS-specific neurovascular abnormalities and embryonic lethality [28]. Id1/Id3 mutants display premature differentiation of CNS radial glial cells, leading to increased numbers of neurons. This raises the interesting possibility that radial glial cells, which also serve as scaffolds for angiogenic blood vessels in the CNS, may be progressively depleted in Id1/Id3 mutant mice, thus contributing to abnormal blood vessel morphogenesis and hemorrhage. However, Id1 and Id3 likely provide additional functions in CNS neurovascular development, as induction of abnormal radial glial differentiation via other pathways, such as Notch1 activation, is not reported to induce obvious neurovascular disorders [29].
Zebrafish genetics have also proven to be a powerful tool for uncovering mechanisms of neurovascular unit development [30]. Liu et al. [31••] have characterized signaling defects in the zebrafish bubblehead mutant, which develop embryonic CNS-specific neurovascular phenotypes. These phenotypes are due to mutations in the β-Pix gene, leading to defective interactions between β-Pix protein, a GTP exchange factor, and the p21-activated kinase 2a (Pak2a). As a result, Pak2a is mislocalized within the cell and does not effectively activate the Rac and Cdc42 small GTPases at the plasma membrane. It remains to be determined whether the signaling defects in bubblehead mutants occur in vascular cells or perivascular neural cells. Furthermore, it will be interesting to identify upstream effectors of the β-Pix and Pak2a signaling pathway.
The αvβ8 integrin – transforming growth factor β signaling axis in neurovascular development
Integrins are heterodimeric cell surface proteins consisting of α and β subunits that serve as receptors for many ECM proteins [32]. Many integrins play critical roles in adult CNS neurovascular units [33]; however, various molecular genetic data reveal that αvβ8 integrin and its ECM ligands, the latent transforming growth factor (TGF)βs, are absolutely essential for embryonic neurovascular development [34]. β8 integrin is reported to pair only with the αv subunit [35,36], and mouse embryos harboring ablated αυ or β8 integrin genes in all cells develop CNS-specific neurovascular disorders that include abnormal blood vessel morphogenesis and hemorrhage [37,38]. Cre-mediated ablation of αυ or β8 integrin genes in CNS neural stem and progenitor cells also leads to similar CNS-specific neurovascular phenotypes [39,40]. In contrast, deletion of the αυ or β8 integrin genes in vascular endothelial cells does not cause obvious CNS vascular disorders [39,40]; however, these mice develop autoimmunity and cancer due to dendritic cell dysfunction [41•,42•]. The neurovascular phenotypes in αv and β8 integrin mutant embryos are mostly resolved in the postnatal period, although adult mutant mice develop progressive neurological abnormalities likely because of the prior neurovascular damage. Interestingly, repair of these severe neurovascular disorders occurs within a postnatal period when CNS blood vessels transition from an angiogenic to a quiescent status [14], suggesting that αvβ8 integrin provides essential roles in modulating active phases of CNS angiogenesis similar to those that occur during embryonic development.
αvβ8 integrin is reported to bind to multiple ECM ligands [43–45]; however, the neurovascular disorders in αv and β8 integrin mutant mice are due primarily to deficient integrin-mediated TGFβ activation [46]. TGFβs are produced by cells as inactive, ECM-associated complexes [47]. Dissociation of the bioactive portions, TGFβs, from latency-associated peptides (LAPs) leads to release from the ECM, cell surface receptor engagement, and activation of intracellular signaling cascades via the canonical SMADs as well as other noncanonical effectors [48]. LAPs for TGFβ1 and TGFβ3 contain arginine–glycine–aspartic acid (RGD) peptide sequences that serve as integrin-binding motifs. Although all five αv-containing integrins can adhere to LAP–TGFβs via these RGD sequences, only αvβ6 and αvβ8 integrin have been reported to activate TGFβs [49,50]. The functional significance of integrin-mediated TGFβ activation has been demonstrated using gene ‘knock-in’ technology, in which the RGD sequence within the endogenous TGFβ1 gene was mutated to RGE, resulting in inhibition of integrin adhesion. These mutant mice develop lethal phenotypes that are identical to those observed in TGFβ1−/− mice, revealing that integrins play central roles in TGFβ activation during embryogenesis [51••]. Furthermore, mice deficient for both active TGFβ1 and genetically null for TGFβ3 develop CNS-specific neurovascular phenotypes that are nearly identical to those observed in αv and β8 integrin knockout mice [52••]. TGFβs modulate intracellular signal transduction pathways primarily via cell transmembrane receptor serine/threonine kinases [53]; however, neuropilin-1 (NRP1), a receptor for semaphorins and VEGF [54], was recently reported to bind to latent and bioactive forms of TGFβ1 [55••]. This is intriguing, because genetic ablation of the NRP1 gene in mice leads to CNS neurovascular phenotypes that are similar to phenotypes in αvβ8 integrin and TGFβ1/TGFβ3 mutants [56,57]. It will be fascinating to determine whether integrin-activated TGFβs regulate neurovascular development via only the canonical TGFβ receptors or whether NRP1 also plays a role in these processes.
Neurovascular phenotypes that develop in mutant mice lacking components of the αvβ8 integrin and TGFβ signaling axis are quite similar to those reported for the various mouse and zebrafish mutants described above, suggesting possible functional links between these pathways (Fig. 1). For example, intracellular cross-talk may take place between TGFβ and Wnt-mediated signaling pathways within cerebral endothelial cells, as has been shown in other cell types [58]. The Band 4.1s are a family of cytoskeletal adaptor proteins that directly interact with the β8 integrin cytoplasmic tail [59,60].
Although the functional significance of the β8 integrin–Band 4.1 associations have not been determined, it is enticing to speculate that Band 4.1s, which contain FERM domains that mediate protein–protein interactions [61], may serve to couple β8 integrin with other signaling effectors. This is particularly intriguing for the βPix, Pak2a, and Rac signaling pathway identified in the zebrafish bubblehead mutant [31••], as β8 integrin has been reported to activate Rac signaling [62]. An exciting future challenge will be to decipher how these different signaling pathways are functionally connected during neurovascular unit development and physiology in the CNS.
Neurovascular units are niches for neural stem and progenitor cells in the adult brain
Neurogenesis in the adult mammalian brain occurs in two specific regions: the subgranular layer (SGL) of the hippocampal dentate gyrus and the subventricular zone (SVZ) of the lateral ventricle. Neural stem cells within these regions display many radial glial-like properties [63] and intimately associate with specialized neurovascular structures, termed vascular niches [64,65]. Neural stem cell vascular niches were first discovered in the SGL by Palmer et al. [66] during efforts to quantify adult stem cell self-renewal and differentiation. These studies revealed DNA synthesis in both SGL neural stem cells as well as capillary endothelial cells; furthermore, these authors found that most neural stem cells within the SGL were preferentially clustered within 10 µm of angiogenic capillaries. These data suggested an important functional relationship between neural stem cells and cerebral blood vessels, with vascular-derived factors likely influencing stem cell growth and fate determination. Indeed, Lie et al. [67] subsequently showed that Wnts, possibly secreted by vascular cells in the SGL niche, induce canonical signaling cascades within neural stem cells that are essential for proper self-renewal and differentiation.
More recent studies have shown that neural stem cells also localize to vascular niches within the SVZ. Tavazoie et al. [68••] have reported the intriguing finding that SVZ neural stem cells preferentially associate with capillary regions displaying enhanced BBB permeability, suggesting cells or factors from the circulation are important for modulating neural stem cell behaviors. It will be fascinating to determine what these factors are and the mechanisms underlying how their access to neural stem cells is regulated at the temporal and spatial levels. Ultrastructural analyses by Mercier et al. [69] first showed that blood vessels within the SVZ extend specialized basal laminar structures, termed fractones, that are enriched in heparin sulfate proteoglycans, laminins, and probably other ECM proteins. These authors also reported that fractones directly contact neural stem cells and postulated that these interactions regulate cell behaviors via ECM adhesion as well as presentation of growth factors such as basic fibroblast growth factor [70••]. Shen et al. [71••] have shown that SVZ neural stem cells utilize α6β1 integrin to bind to laminins in vascular basement membranes and fractones. Inhibition of α6β1 integrin-mediated adhesion and signaling in vivo promotes neural stem cell proliferation, indicating that stem cell interactions with laminins in vascular niches suppress neural stem cell expansion. α6β1 is also utilized by embryonic neural stem cells to adhere to laminins, suggesting shared regulatory mechanisms between embryonic and adult stem cell niches [72•,73].
My laboratory has recently identified β8 integrin as a critical regulator of neurovascular homeostasis and neurogenesis in the adult mouse brain [74]. β8 integrin is expressed in cultured SVZ neural stem cells, and ablation of β8 gene expression in vivo results in reduced SVZ cell proliferation and survival, likely owing to uncoupling of neural stem cells from ECM components in the vascular niche. As described above, αvβ8 integrin regulates blood vessel morphogenesis and neurovascular development in the embryonic brain, and these processes are dependent on integrin-mediated TGFβ activation. It will be interesting to determine how β8 integrin-mediated TGFβ activation is involved in regulating neural stem cell functions in vascular niches of the adult brain.
Conclusion
Neurovascular units are the cellular and molecular interfaces between the circulatory system and CNS. In recent years, various gene knockout strategies in mice – most of which were not initially aimed at studying neurovascular biology – have yielded the first insights about pathways important for neurovascular development and homeostasis. Large-scale mutagenesis screens in mice [75,76] will be necessary for identifying gene networks involved in neurovascular formation and function. Genomic screens to identify neurovascular mutants in the zebrafish should also provide important mechanistic insights. Gaining a comprehensive understanding of the molecular pathways underlying neurovascular unit development will provide novel insights into the causes and possible treatments of debilitating human neurovascular-related diseases such as birth defects, stroke, cancer, and age-related neurodegeneration.
Acknowledgements
This research was supported by grants awarded to J.H.M. from the Ellison Medical Foundation (AG-NS-0324-06), the National Institute of Neurological Diseases and Stroke (R01NS059876-01A2), and the University Cancer Foundation at the University of Texas, M.D. Anderson Cancer Center. Lastly, because of the space limitations, I must apologize to many colleagues whose published work was not cited in the references.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 231).
- 1.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 2.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 4. Nikolova G, Strilic B, Lammert E. The vascular niche and its basement membrane. Trends Cell Biol. 2007;17:19–25. doi: 10.1016/j.tcb.2006.11.005. An excellent review on ECM-mediated regulation of cell behaviors in vascular niches.
- 5.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 6.Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 8.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 9. Gordon GR, Choi HB, Rungta RL, et al. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. This study characterizes calcium-mediated signaling mechanisms in astrocytes during regulation of cerebral blood flow.
- 10.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 12.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 13.McCarty JH. Cell biology of the neurovascular unit: implications for drug delivery across the blood–brain barrier. Assay Drug Dev Technol. 2005;3:89–95. doi: 10.1089/adt.2005.3.89. [DOI] [PubMed] [Google Scholar]
- 14.Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58:313–320. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 15.McCarty JH, Monahan-Earley RA, Brown LF, Keller ML, et al. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noctor SC, Flint AC, Weissman TA, et al. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 17.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 18.Risau W, Wolburg H. Development of the blood–brain barrier. Trends Neurosci. 1990;13:174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- 19.Ruhrberg C, Gerhardt H, Golding M, et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin-Padilla M. Early vascularization of the embryonic cerebral cortex: Golgi and electron microscopic studies. J Comp Neurol. 1985;241:237–249. doi: 10.1002/cne.902410210. [DOI] [PubMed] [Google Scholar]
- 21.Virgintino D, Maiorano E, Errede M, et al. Astroglia-microvessel relationship in the developing human telencephalon. Int J Dev Biol. 1998;42:1165–1168. [PubMed] [Google Scholar]
- 22.Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- 23.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 24.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 25. Stenman JM, Rajagopal J, Carroll TJ, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. This study demonstrates that Wnt signaling pathways are essential for neurovascular development in the embryonic CNS.
- 26. Liebner S, Corada M, Bangsow T, et al. Wnt/beta-catenin signaling controls development of the blood–brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. This study reveals the involvement of Wnt-mediated β-catenin signaling in BBB formation.
- 27.Cattelino A, Liebner S, Gallini R, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyden D, Young AZ, Zagzag D, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 29.Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein B. Vascular cell biology in vivo: a new piscine paradigm? Trends Cell Biol. 2002;12:439–445. doi: 10.1016/s0962-8924(02)02358-9. [DOI] [PubMed] [Google Scholar]
- 31. Liu J, Fraser SD, Faloon PW, et al. A betaPix Pak2a signaling pathway regulates cerebral vascular stability in zebrafish. Proc Natl Acad Sci U S A. 2007;104:13990–13995. doi: 10.1073/pnas.0700825104. This study describes one of the first neurovascular mutants in zebrafish and demonstrates involvement of the β-Pix gene in neurovascular development.
- 32.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 33.del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- 34.Hynes RO, Lively JC, McCarty JH, et al. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb Symp Quant Biol. 2002;67:143–153. doi: 10.1101/sqb.2002.67.143. [DOI] [PubMed] [Google Scholar]
- 35.Moyle M, Napier MA, McLean JW. Cloning and expression of a divergent integrin subunit beta 8. J Biol Chem. 1991;266:19650–19658. [PubMed] [Google Scholar]
- 36.Nishimura SL, Boylen KP, Einheber S, et al. Synaptic and glial localization of the integrin alphavbeta8 in mouse and rat brain. Brain Res. 1998;791:271–282. doi: 10.1016/s0006-8993(98)00118-8. [DOI] [PubMed] [Google Scholar]
- 37.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Motejlek K, Wang D, Zang K, et al. Beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarty JH, Lacy-Hulbert A, Charest A, et al. Selective ablation of αv integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 40.Proctor JM, Zang K, Wang D, et al. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lacy-Hulbert A, Smith AM, Tissire H, et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. In this study, αvβ8 integrin, expressed in mouse dendritic cells, is shown to be a critical regulator of intestinal homeostasis, as genetic ablation of αv integrin results in colitis and autoimmunity.
- 42. Travis MA, Reizis B, Melton AC, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. This study demonstrates that genetic deletion of β8 integrin results in intestinal autoimmunity and colitis in mice.
- 43.Chernousov MA, Carey DJ. alphaVbeta8 integrin is a Schwann cell receptor for fibrin. Exp Cell Res. 2003;291:514–524. doi: 10.1016/s0014-4827(03)00409-9. [DOI] [PubMed] [Google Scholar]
- 44.Milner R, Huang X, Wu J, et al. Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. J Cell Sci. 1999;112(Pt 23):4271–4279. doi: 10.1242/jcs.112.23.4271. [DOI] [PubMed] [Google Scholar]
- 45.Venstrom K, Reichardt L. Beta 8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV, and fibronectin. Mol Biol Cell. 1995;6:419–431. doi: 10.1091/mbc.6.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cambier S, Gline S, Mu D, et al. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 48.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mu D, Cambier S, Fjellbirkeland L, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGFbeta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munger JS, Huang X, Kawakatsu H, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 51. Yang Z, Mu Z, Dabovic B, et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. This study elegantly demonstrates that integrins are the primary in-vivo regulators of TGFβ1 activation during embryogenesis.
- 52. Mu Z, Yang Z, Yu D, et al. TGFbeta1 and TGFbeta3 are partially redundant effectors in brain vascular morphogenesis. Mech Dev. 2008;125:508–516. doi: 10.1016/j.mod.2008.01.003. This study shows essential roles for TGFβs in neurovascular development and reveals strong phenotyptic similarities with αv and β8 integrin knockout embryos.
- 53.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 54.Vieira JM, Schwarz Q, Ruhrberg C. Role of the neuropilin ligands VEGF164 and SEMA3A in neuronal and vascular patterning in the mouse. Novartis Found Symp. 2007;283:230–235. doi: 10.1002/9780470319413.ch18. discussion 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Glinka Y, Prud’homme GJ. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008;84:302–310. doi: 10.1189/jlb.0208090. The first study to identify NRP-1 as a cell surface receptor for TGFβs, suggesting a possible link between integrins and neuropilins in neurovascular units.
- 56.Gerhardt H, Ruhrberg C, Abramsson A, et al. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 57.Gu C, Rodriguez ER, Reimert DV, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Attisano L, Labbe E. TGFbeta and Wnt pathway cross-talk. Cancer Metastasis Rev. 2004;23:53–61. doi: 10.1023/a:1025811012690. [DOI] [PubMed] [Google Scholar]
- 59.McCarty JH, Cook AA, Hynes RO. An interaction between {alpha}v{beta}8 integrin and Band 4.1B via a highly conserved region of the Band 4.1 C-terminal domain. Proc Natl Acad Sci U S A. 2005;102:13479–13483. doi: 10.1073/pnas.0506068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yi C, McCarty JH, Troutman SA, et al. Loss of the putative tumor suppressor band 4.1B/Dal1 gene is dispensable for normal development and does not predispose to cancer. Mol Cell Biol. 2005;25:10052–10059. doi: 10.1128/MCB.25.22.10052-10059.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun CX, Robb VA, Gutmann DH. Protein 4.1 tumor suppressors: getting a FERM grip on growth regulation. J Cell Sci. 2002;115:3991–4000. doi: 10.1242/jcs.00094. [DOI] [PubMed] [Google Scholar]
- 62.Lakhe-Reddy S, Khan S, Konieczkowski M, et al. Beta8 integrin binds Rho GDP dissociation inhibitor-1 and activates Rac1 to inhibit mesangial cell myofibroblast differentiation. J Biol Chem. 2006;281:19688–19699. doi: 10.1074/jbc.M601110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 64.Currle DS, Gilbertson RJ. The niche revealed. Cell Stem Cell. 2008;3:234–236. doi: 10.1016/j.stem.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 65.Palmer TD. Adult neurogenesis and the vascular Nietzsche. Neuron. 2002;34:856–858. doi: 10.1016/s0896-6273(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 66.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 67.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 68. Tavazoie M, Van der Veken L, Silva-Vargas V, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. This study shows increased BBB permeability in the adult SVZ, implicating the circulatory system in modulating neural stem cell behaviors.
- 69.Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- 70. Kerever A, Schnack J, Vellinga D, et al. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. This study demonstrates that ECM and growth factor components in fractones are involved in modulating neural stem cell behaviors in vascular niches.
- 71. Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell–cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. This study identifies α6β1 in neural stem cells as necessary for interaction with laminin in vascular niches.
- 72. Lathia JD, Patton B, Eckley DM, et al. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J Comp Neurol. 2007;505:630–643. doi: 10.1002/cne.21520. This study reveals important functions for laminins during embryonic CNS neurogenesis.
- 73.Lathia JD, Rao MS, Mattson MP, Ffrench-Constant C. The microenvironment of the embryonic neural stem cell: lessons from adult niches? Dev Dyn. 2007;236:3267–3282. doi: 10.1002/dvdy.21319. [DOI] [PubMed] [Google Scholar]
- 74.Mobley AK, Tchaicha JH, Shin J, et al. beta-8 Integrin regulates homeostasis in the adult brain. J Cell Science. doi: 10.1242/jcs.043257. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ding S, Wu X, Li G, Han M, et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 76.Wu S, Ying G, Wu Q, Capecchi MR. Toward simpler and faster genome-wide mutagenesis in mice. Nat Genet. 2007;39:922–930. doi: 10.1038/ng2060. [DOI] [PubMed] [Google Scholar]