Abstract
We recently showed that resistance exercise and ingestion of essential amino acids with carbohydrate (EAA+CHO) can independently stimulate mammalian target of rapamycin (mTOR) signaling and muscle protein synthesis in humans. Providing an EAA+CHO solution postexercise can further increase muscle protein synthesis. Therefore, we hypothesized that enhanced mTOR signaling might be responsible for the greater muscle protein synthesis when leucine-enriched EAA+CHOs are ingested during postexercise recovery. Sixteen male subjects were randomized to one of two groups (control or EAA+CHO). The EAA+CHO group ingested the nutrient solution 1 h after resistance exercise. mTOR signaling was assessed by immunoblotting from repeated muscle biopsy samples. Mixed muscle fractional synthetic rate (FSR) was measured using stable isotope techniques. Muscle protein synthesis and 4E-BP1 phosphorylation during exercise were significantly reduced (P < 0.05). Postexercise FSR was elevated above baseline in both groups at 1 h but was even further elevated in the EAA+CHO group at 2 h postexercise (P < 0.05). Increased FSR was associated with enhanced phosphorylation of mTOR and S6K1 (P < 0.05). Akt phosphorylation was elevated at 1 h and returned to baseline by 2 h in the control group, but it remained elevated in the EAA+CHO group (P < 0.05). 4E-BP1 phosphorylation returned to baseline during recovery in control but became elevated when EAA+CHO was ingested (P < 0.05). eEF2 phosphorylation decreased at 1 and 2 h postexercise to a similar extent in both groups (P < 0.05). Our data suggest that enhanced activation of the mTOR signaling pathway is playing a role in the greater synthesis of muscle proteins when resistance exercise is followed by EAA+CHO ingestion.
Keywords: muscle protein synthesis, mammalian target of rapamycin, essential amino acids
IT IS NOW WIDELY ACCEPTED that skeletal muscle contraction in the form of resistance-type exercise stimulates muscle protein synthesis during postexercise recovery. The positive effect of resistance exercise on muscle protein synthesis has been measured in as little as 1 h postexercise (18) and may last for up to 24 h in trained (34) and 48 h in untrained subjects (37). Increases in muscle protein synthesis are likely to be mediated, in part, through changes in muscle cell signaling. In particular, resistance exercise appears to elicit its effects by signal transduction through the mammalian target of rapamycin (mTOR) pathway leading to phosphorylation and activation of its down-stream target proteins, the eukaryotic initiation factor 4E-binding protein (4E-BP1) and p70 ribosomal S6 kinase 1 (S6K1) (3, 4, 9, 10).
We (18) recently showed that the mTOR signaling pathway is activated during and after a single bout of resistance exercise, which was associated with a significant increase in muscle protein synthesis. Those data showed that mTOR signaling was stimulated at a time when the fractional synthesis rate (FSR), a direct measure of muscle protein synthesis, of mixed muscle proteins was elevated 41% above baseline after 2 h of postexercise recovery. Others (3, 17, 25) also have shown a positive response to resistance exercise with regard to mTOR signaling, but interpretation of the results and direct comparisons are difficult because of differences in study design such as number of sets and repetitions, differences in mode of contraction (concentric vs. eccentric), and intensity.
Essential amino acids (EAA), particularly leucine, also have been shown to activate the mTOR signaling pathway, which turns on the translational machinery necessary for muscle protein synthesis in both rodent and human models (1, 2, 27). Recently, it was shown that EAA apparently activate mTOR via a unique class 3 phosphatidylinositol 3-kinase (PI3K), hVps34, which stimulates mTOR by an unknown mechanism, bypassing the insulin-induced activation of mTOR through Akt (14, 35). However, mTOR activation due to nutrient intake of both essential amino acids and carbohydrate (EAA+CHO) may be accomplished via the insulin-stimulated signaling pathway through PI3K-Akt-TSC2 as well as the insulin-independent amino acid-induced pathway just described (i.e., hVps34).
More recently, we measured mTOR signaling and muscle protein synthesis after ingestion of a leucine enriched EAA+CHO solution (21). After ingestion of EAA+CHO, muscle protein synthesis increased ∼100% within 1 h (21). The rapid increase in muscle protein synthesis was associated with a significant increase in Akt and mTOR phosphorylation as well as phosphorylation of downstream components S6K1 and 4E-BP1, indicating that translation initiation was enhanced. In addition, eukaryotic elongation factor-2 (eEF2) phosphorylation decreased significantly compared with base-line, suggesting that elongation of translation was also stimulated by this leucine-enriched “anabolic” nutrient solution (21).
Postexercise nutrient ingestion, in the form of EAA alone or in combination with carbohydrate (EAA+CHO) has clearly shown that muscle protein synthesis is elevated above that measured following resistance exercise alone (5, 11–13, 29, 38, 39, 41). However, the mechanisms for the enhanced synthesis of muscle proteins following exercise with ingestion of EAA+CHO have not been determined. Our goal was to explore potential mechanisms for the augmented muscle protein synthesis observed when leucine-enriched EAA+CHO are ingested during postexercise recovery. We hypothesized that ingestion of a leucine-enriched EAA+CHO solution during postexercise recovery would result in enhanced mTOR signaling and greater muscle protein synthesis than recovery without nutrients.
MATERIALS AND METHODS
Subjects
We studied 16 young healthy males who reported that they were not currently engaged in a resistance exercise training program during the screening interview. In addition, all volunteers were asked to refrain from performing vigorous physical activity for 24 h before participating in the study. All subjects gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch (which is in compliance with the Declaration of Helsinki). Screening of subjects was performed with clinical history, physical exam, and laboratory tests including complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance test, hepatitis B and C screening, human immunodeficiency virus test, TSH, lipid profile, urinalysis, drug screening, and ECG. The subjects’ physical characteristics are summarized in Table 1.
Table 1.
Physical characteristics of subjects
| Control | EAA+CHO | |
|---|---|---|
| n | 8 | 8 |
| Age, yr | 27±2 | 30±2 |
| Height, cm | 176±3 | 177±2 |
| Weight, kg | 78±5 | 81±3 |
| Body mass index, kg/m2 | 25±1 | 26±1 |
| Lean body mass,kg | 61±3 | 62±2 |
| Body fat, % | 19±2 | 20±2 |
| Leg lean mass, kg | 10.4±0.7 | 10.8±0.4 |
| OGTT fasting, mg/dl | 81±3 | 87±2 |
| OGTT 1 h, mg/dl | 132±10 | 120±5 |
| OGTT 2 h, mg/dl | 94±5 | 88±6 |
| HOMA-IR | 1.0±0.1 | 1.4±0.1 |
Values are means ± SE. EAA+CHO, essential amino acids with carbohydrate; OGTT, oral glucose tolerance test; HOMA-IR, homeostasis model assessment of insulin resistance (43).
Study design
Details of the cross-sectional study design have been published previously (18). Briefly, each subject’s one repetition maximum (1RM) was determined on two separate occasions on a leg extension machine (Cybex-VR2; Medway, MA), which was located within the General Clinical Research Center’s (GCRC) exercise laboratory and used for each study. The 1RM values obtained were used to determine the starting weight (70% of 1RM) for the resistance exercise portion of our study. In addition, a dual-energy X-ray absorptiometry (DEXA) scan (Hologic QDR 4500W; Bedford, MA) was performed to measure body composition and lean mass.
Each subject was admitted to the GCRC of the University of Texas Medical Branch the day before the exercise study. All subjects were instructed to refrain from physical exercise for 24 h before arriving and to maintain their regular diet before study participation. The subjects were all fed a standardized meal (12 kcal/kg body wt; 60% carbohydrate, 20% fat, and 20% protein) prepared by the Bionutrition Division of the GCRC. Each subject was also offered a snack at 2200 and did not eat again until the study the following day. The snack was provided at 2200 to avoid prolonged fasting during the study the following day, since subjects in the control group would not ingest food until the study was completed (∼18 h).
The morning of the study, polyethylene catheters were inserted into a forearm vein for tracer infusion, in the contralateral hand vein, which was heated, for arterialized blood sampling, and in the femoral artery and vein (retrograde placement) of the leg for blood sampling. The femoral lines were placed in the same leg from which muscle biopsies were obtained. The arterial catheter was also used for the infusion of indocyanine green (ICG; Akorn, Buffalo Grove, IL) to determine blood flow.
After a background blood sample was drawn, a primed continuous infusion of L-[ring-2H5]phenylalanine (Cambridge Isotope Laboratories, Andover, MA) was begun (time 0) and was maintained at a constant rate until the end of the experiment (Fig. 1). The priming dose for the labeled phenylalanine was 2 μmol/kg, and the infusion rate was 0.05 μmol·kg-1·min-1. All studies were begun between 0700 and 0800.
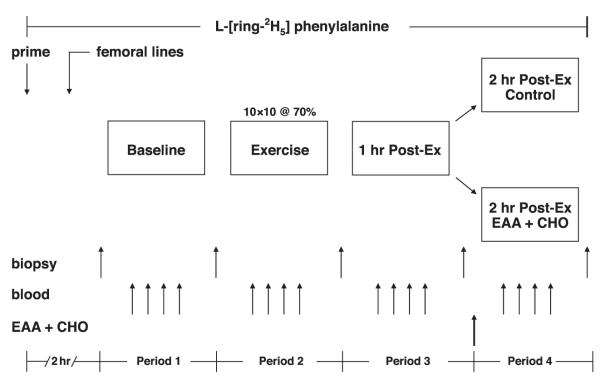
Fig. 1.
Study design and time line. Schematics display the study design used to measure the combined effect of resistance exercise plus leucine-enriched essential amino acids with carbohydrate (EAA+CHO) solution on the regulation of muscle protein synthesis in human subjects. The study design consisted of a basal period (hours 2–3), an exercise period (hours 3–4), and 2 postexercise periods (hours 4–5 and 5–6, respectively). Marking the beginning of the second hour postexercise, an EAA+CHO solution was ingested by each subject. Indocyanine green (ICG) was infused to measure blood flow in each of the periods. Blood samples were collected to measure blood glucose uptake, lactate, and pH. Muscle biopsies were used to measure muscle protein synthesis and components of the mammalian target of rapamycin (mTOR) signaling pathway involved in translation initiation and elongation.
Data were collected from 16 subjects who were studied identically. Data from seven of the control subjects have been previously published (18). The four periods included a basal period (baseline), which was the hour before exercise, an exercise period (exercise), and one hour following exercise (1 h post); the two groups were separated into those not receiving nutrients (2 h post) and those who ingested a leucine-enriched EAA+CHO solution immediately following the first hour of recovery (EAA+CHO group). Thus each group was treated identically through the first hour of postexercise recovery. For those subjects in the EAA+CHO group, the EAA+CHO solution was ingested as a bolus immediately after the fourth biopsy, 1 h following exercise (see Fig. 1). For each period, except during exercise, all subjects rested comfortably in the semirecumbent position. The entirety of the study was conducted in the exercise room at the GCRC.
Marking the beginning of the basal period (baseline) and 2 h after the start of tracer infusion, the first muscle biopsy was obtained from the lateral portion of the vastus lateralis of the leg with the biopsy site between 15 and 25 cm from the midpatella. The biopsy was performed using a 5-mm Bergström biopsy needle under sterile procedure and local anesthesia (1% lidocaine). Once harvested, the muscle tissue was immediately blotted and frozen in liquid nitrogen (within seconds) and stored at −80°C until analysis. Immediately after the first biopsy, a continuous infusion of ICG was started in the femoral artery (0.5 mg/min) and maintained for 50 min. Ten minutes after ICG infusion was started, blood samples were drawn four times, at 10-min intervals, from the femoral vein and the arterialized hand vein to measure ICG concentration (Fig. 1). In addition to the blood obtained for ICG measurement, blood samples were also taken from the femoral artery and vein and from the arterialized hand vein to measure blood pH, glucose and lactate concentrations, amino acid enrichments, and blood flow. At the end of baseline, a second biopsy was obtained; however, the biopsy needle was inclined at a different angle so that the second biopsy was taken ∼5 cm apart from the first.
After the second biopsy, the subjects were seated in a Cybex leg extension machine to perform the exercise portion of the study (exercise period). After a brief warm-up (50 lb. × 10 repetitions), each subject performed 10 sets of 10 repetitions of bilateral leg extension exercises. Each set was separated by 3 min, except during blood collection (performed following sets 3, 6, 8, and 10), which required additional time. As during the baseline period, ICG was continually infused into the femoral artery during exercise to measure leg blood flow. Blood samples were again drawn for blood pH, glucose and lactate concentrations, amino acid enrichments, and blood flow. The third muscle biopsy was immediately preceded by 10 repetitions at 70% of the 1RM and obtained with the subject seated in the Cybex leg extension machine (i.e., within seconds of completing the final muscle contraction). As with the second biopsy, the needle was inserted into the same incision as the first two biopsies; however, the biopsy needle was inclined at a different angle so that the third biopsy was taken ∼5 cm apart from the previous biopsy sampling site. This method has been previously used by us (18, 21) and others (17, 25, 33).
During the third period (1 h post), ICG was again infused continuously (as during the first and second periods) to measure leg blood flow, and blood was drawn for the measurement of blood pH, glucose and lactate concentrations, amino acid enrichments, and blood flow. Samples were obtained every 10 min (as during the first and second periods). At the end of the first hour postexercise, a fourth muscle biopsy was obtained through a new incision site ∼5 cm proximal to the first incision. As during the first period (baseline), subjects were in a semirecumbent position in a hospital bed.
Marking the beginning of the fourth and final period, subjects were assigned to an exercise alone control group (2 h post) or to a leucine-enriched EAA+CHO solution (EAA+CHO) group. The composition of the nutrient solution consisted of eight of the essential amino acids (absent tryptophan) and carbohydrate mixed with a flavored, noncaloric beverage to aid in palatability (see Composition of leucine-enriched EAA+CHO solution). Blood samples were collected in the same manner as during the previous periods. At the end of that hour (2 h post), a final muscle biopsy was collected as described above from the second incision; however, the biopsy needle was again inclined at a different angle so that the muscle sample was obtained from tissue ∼5 cm apart from the prior biopsy. As during the first and third periods, subjects were in a semirecumbent position in a hospital bed. Each biopsy was taken an average of 70 ± 1.4 min apart.
Composition of the leucine-enriched EAA+CHO solution
The leucine enriched EAA+CHO solution consisted of essential amino acids in the following proportions: histidine, 8%; isoleucine, 8%; leucine, 35%; lysine, 12%; methionine, 3%; phenylalanine, 14%; threonine, 10%; and valine, 10%; and has been used by us previously (21). To minimize the potential of tracer dilution with the addition of the amino acids, we added the phenylalanine tracer to the oral EAA solution at 6.5% of the total phenylalanine content. Lean mass (LM) as determined by DEXA was used to calculate the proportion of each EAA (0.35 g·kg-1·LM-1) added to the nutrient solution. Similarly, carbohydrate (sucrose) was added at 0.5 g·kg-1·LM-1 to each nutrient solution. All ingredients (EAA+CHO) were dissolved in a noncaloric, caffeine-free, flavored beverage to increase palatability.
Blood flow, pH, glucose uptake, lactate, and phenylalanine net balance across the leg
Serum ICG concentration for the determination of leg blood flow was measured spectrophotometrically (Beckman Coulter, Fullerton, CA) at λ = 805 nm (24). Plasma glucose and lactate concentrations were measured using an automated glucose and lactate analyzer (YSI, Yellow Springs, OH). Blood pH was measured at the University of Texas Medical Branch core laboratory using standard procedure. Leg glucose utilization was calculated as net glucose uptake across the leg: leg glucose uptake = (CA — CV) × BF, where CA and CV are the blood glucose concentrations in the femoral artery and vein, respectively, and are expressed as micromoles of glucose utilized per minute per kilogram of fat-free mass (FFM) of the leg (μmol·min-1·kg leg FFM-1); BF is blood flow. Net muscle phenylalanine balance across the leg was calculated as (phenylalanine arterial concentration — phenylalanine venous concentration) × blood flow.
Muscle fractional synthetic rate
Muscle tissue samples were ground, and intracellular free amino acids and muscle proteins were extracted as previously described (45). Muscle intracellular free concentration and enrichment of phenylalanine and leucine were determined by gas chromatography-mass spectrometry (GC-MS; 6890 Plus GC, 5973N MSD, 7683 autosampler; Agilent Technologies, Palo Alto, CA) using appropriate internal standards (45). Mixed muscle protein-bound phenylalanine enrichment was analyzed by GC-MS after protein hydrolysis and amino acid extraction (45), using the external standard curve approach (15). We calculated the FSR of mixed muscle proteins by measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEp/t) and using the precursor-product model to calculate the synthesis rate as FSR = (ΔEp/t)/[EM(1) + EM(2)/2] × 60 × 100, where ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two sequential biopsies, and EM(1) and EM(2) are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies. Data are expressed as percent per hour.
SDS PAGE and immunoblotting
Details of the immunoblotting procedures have been previously published (18) with slight modifications for the current study. Aliquots from homogenates were loaded (equal amount of protein) per lane in duplicate and separated by SDS-PAGE. All proteins were run on 7.5% gels (Bio-Rad, Hercules, CA) for 60 min at 150 V except for 4E-BP1, which was run on 15% gels for the same duration. A molecular weight ladder (Bio-Rad; Precision Plus protein standard) and a rodent internal loading control were also included on each gel. Positive control experiments were performed to verify antibody specificity for mTOR/S6K1. After SDS-PAGE, proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Hybond-P; Amersham Biosciences, Piscataway, NJ) at 50 V for 1 h. We confirmed equal loading on each gel and that an equivalent amount of protein was transferred to the membrane by Coomassie and/or Ponceau S staining. Once transferred, PVDF membranes were placed in blocking buffer [5% nonfat dry milk (NFDM) in TBST (Tris-buffered saline and 0.1% Tween 20)] for 1 h. After serial washes, the membranes were incubated with primary antibody in 5% NFDM in TBST overnight at 4°C with constant agitation. The next morning, the blots were washed in TBST twice and incubated with secondary antibody for 1 h in 5% NFDM in TBST at room temperature with constant agitation. After serial washes, the blots were then incubated for 5 min with enhanced chemiluminescence reagent (ECL Plus Western blotting detection system; Amersham Biosciences) to detect horseradish peroxidase activity. Images were obtained with a ChemiDoc XRS imaging system (Bio-Rad). Once the appropriate image was captured, densitometric analysis was performed using Quantity One 1-D Analysis software (version 4.5.2; Bio-Rad). Total protein was determined for each blot and did not change from baseline over the course of the experiment (representative blots in Figs. 3–5). However, data are presented as phosphorylation status relative to an internal loading control in arbitrary units to remain consistent with our previous publications.
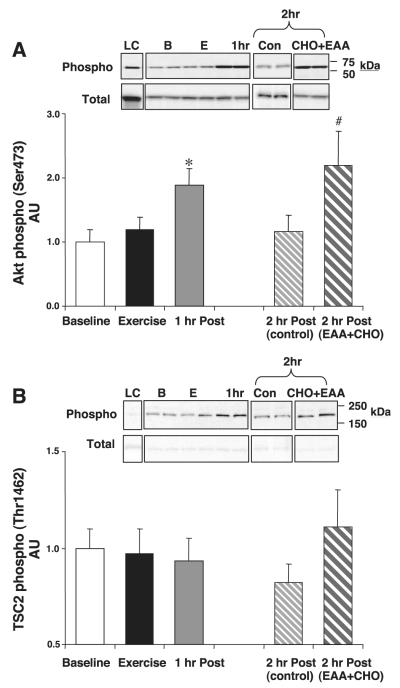
Fig. 3.
Akt and TSC2. All subjects were treated identically during baseline, exercise, and 1 h postexercise. One-half of the subjects ingested a nutrient solution at the end of the first hour of postexercise recovery [2 h post (EAA+CHO)] compared with control subjects [2 h post (control)]. Data are from each biopsy taken at baseline, immediately following resistance exercise (exercise), 1 h following exercise (1 h post), and 2 h following resistance exercise [2 h post (control) and 2 h post (EAA + CHO)]. A: Akt phosphorylation and total Akt. B: TSC2 phosphorylation and total TSC2. Data are means ± SE in arbitrary units (AU). *P < 0.05 vs. baseline. #P < 0.05 vs. 2 h post (control). Insets show representative blots for the loading control (LC) and for each time point. B, baseline; Ex, exercise alone; Con, control.
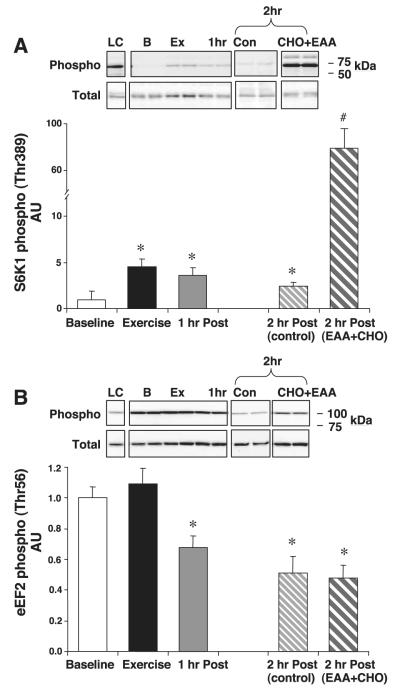
Fig. 5.
p70 ribosomal S6 kinase 1 (S6K1) and eukaryotic elongation factor-2 (eEF2). All subjects were treated identically during baseline, exercise, and 1 h postexercise. One-half of the subjects ingested a leucine-enriched EAA+CHO solution at the end of the first hour of postexercise recovery [2 h post (EAA+CHO)] compared with control subjects [2 h post (control)]. A: S6K1 phosphorylation and total S6K1. B: eEF2 phosphorylation and total eEF2. Data are means ± SE. *P < 0.05 vs. baseline. Insets show representative blots for the loading control and for each time point.
Antibodies
The primary antibodies used were all purchased from Cell Signaling (Beverly, MA): phospho-mTOR (Ser2448; 1:1,000), phospho-p70 S6K1 (Thr389; 1:500), phospho-Akt (Ser473; 1:500), phospho-tuberin/TSC2 (Thr1462; 1:1,000), phospho-4E-BP1 (Thr37/46; 1:1,000), phospho-eEF2 (Thr56; 1:1,000), total mTOR (1:1,000), total S6K1 (1:1,000), total Akt (1:1,000), total TSC2 (1:1,000), total 4E-BP1 (1:1,000), and total eEF2 (1:1,000). Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from Amersham Bioscience (1:2,000).
Statistical analysis
All values are means ± SE. All subjects received the same treatment for the first three periods (baseline, exercise, and 1 h post). Therefore, we did not expect the groups to react differently during those time points and were only interested in the treatment effect at 2 h postexercise. Thus the comparisons for the first three time periods were performed on all subjects (n = 16) using analysis of variance with repeated measures (ANOVA), the effects being subject and time (baseline, exercise, 1 h post). Post hoc testing was performed using Dunnett’s test for multiple comparisons; however, if a test of normality or equal variance failed, then ANOVA on ranks followed by Dunn’s post hoc multiple comparison test was performed. For glucose uptake, mTOR, and S6K1, ANOVAs were run on the natural logarithm of the variables, since the variance was clearly proportional to the means of the groups.
For comparisons to baseline and each group at 2 h postexercise (i.e., 2 h post and EAA+CHO groups), a paired t-test corrected with Bonferroni’s inequalities for the multiple comparisons over time was conducted with baseline. For comparisons between 2 h post and EAA+CHO groups, an independent t-test was conducted (i.e., n = 8 per group). Since we assumed a priori that there would be no differences between treatment groups at baseline, exercise, and 1 h post, we made comparisons between treatment groups at only one time point, i.e., 2 h post. Significance was set at P ≤ 0.05.
RESULTS
Blood flow, glucose uptake, blood glucose, insulin, lactate, and pH
Blood flow was significantly increased during exercise [baseline, 3.9 ± 0.4; and exercise, 13.2 ± 0.9 ml·min-1·100 ml leg volume-1 (P < 0.05)]. Blood flow returned to baseline values during the postexercise recovery [1 h post, 4.8 ± 0.6; 2 h post (control), 4.5 ± 0.7; and 2 h post (EAA+CHO), 4.7 ± 0.6 ml·min-1·100 ml of leg volume-1 (P > 0.05)]. No differences were observed between groups postexercise when EAA+CHO were ingested.
Glucose uptake was significantly increased during exercise [baseline, 3.5 ± 0.5; and exercise, 21.8 ± 2.8 μmol·min-1·kg leg LM-1 (P < 0.05)]. Glucose uptake remained elevated following exercise [1 h post, 8.3 ± 1.5; 2 h post (control); 9.5 ± 2.4, and 2 h post (EAA+CHO), 21.2 ± 4.2 μmol·min-1·kg leg LM-1 (P < 0.05)]. As expected, glucose uptake after EAA+CHO ingestion was significantly greater than in baseline and 2 h post (control) groups (P < 0.05). Blood glucose concentrations were not affected by exercise or recovery in the control group but were significantly elevated at 2 h postexercise in the EAA+CHO group (P < 0.05; Table 2).
Table 2.
Blood amino acid, insulin, and glucose concentrations
| 2 h Post |
|||||
|---|---|---|---|---|---|
| Baseline | Exercise | 1 h Post | Control | EAA+CHO | |
| Leucine, μmo/l | 149±10 | 139±6 | 122±6* | 142±5 | 766±20*# |
| Phenylalanine, μmol/l |
56±3 | 55±2 | 54±1 | 54±1 | 158±11*# |
| Insulin, μU/ml | 5.3±0.5 | 8.0±0.8* | 5.5±0.5 | 3.8±0.6 | 20.5±4.6*# |
| Glucose, mM | 5.2±0.1 | 5.4±0.4 | 5.2±0.1 | 5.0±0.1 | 6.1±0.2*# |
Values are means ± SE.
P < 0.05 vs. baseline.
P < 0.05 vs. 2 h post (control).
Serum insulin was slightly elevated during exercise (P < 0.05; Table 2). Insulin levels returned to baseline during the 1 h of postexercise recovery and remained at baseline values in the control group. In the EAA+CHO group, however, insulin levels increased significantly (P < 0.05; Table 2).
Lactate in the femoral vein was significantly increased during the exercise period and remained elevated for 1 h in the control group [baseline; 0.9 ± 0.1; exercise, 10.7 ± 0.9; 1 h post, 2.3 ± 0.3 (P < 0.05); and 2 h post (control), 1.0 ± 0.1 mM (P > 0.05)]. Lactate values were significantly greater following EAA+CHO ingestion than at 2 h postexercise in the control [2 h post (EAA+CHO), 1.6 ± 0.1 mM (P < 0.05)]. Blood pH was significantly reduced from basal during exercise [baseline, 7.37 ± 0.01; and exercise, 7.19 ± 0.02 (P < 0.05)] but returned to baseline values immediately postexercise with no change observed following leucine-enriched EAA+CHO ingestion [1 h post, 7.36 ± 0.01; 2 h post (control), 7.37 ± 0.01; and 2 h post (EAA+CHO), 7.36 ± 0.01 (P > 0.05)].
Plasma and intracellular amino acid concentrations
Arterial leucine concentrations at 2 h postexercise were significantly elevated in the leucine-enriched EAA+CHO group compared with control (P < 0.05; Table 2). Arterial phenylalanine concentrations at 2 h postexercise were similarly and significantly elevated in the leucine-enriched EAA+CHO group compared with control (P < 0.05; Table 2). Muscle intracellular leucine concentrations at 2 h postexercise were significantly elevated in the leucine-enriched EAA+CHO group compared with control (564 ± 31 vs. 142 ± 16 μM, respectively; P < 0.05). Muscle intracellular phenylalanine concentrations at 2 h postexercise were also significantly elevated in the EAA+CHO group compared with control (179 ± 9 vs. 64 ± 4 μM, respectively; P < 0.05).
Muscle protein synthesis and phenylalanine net balance across the leg
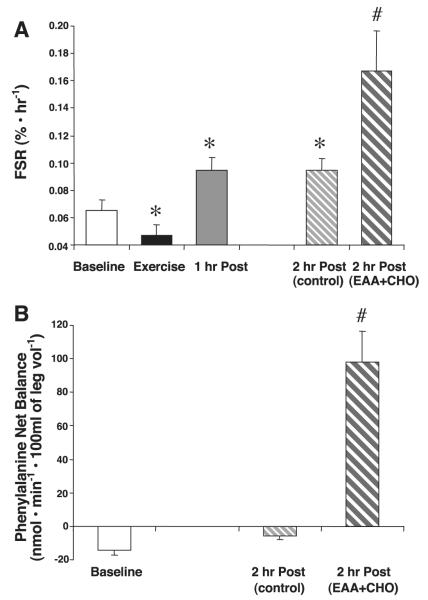
Mixed muscle protein FSR decreased immediately following resistance exercise (P < 0.05; Fig. 2A). FSR was increased (compared with baseline) at 1 h postexercise (P < 0.05) and 2 h postexercise (P < 0.05). FSR in the EAA+CHO group was significantly greater than in control at 2 h postexercise (P < 0.05; Fig. 2A).
Fig. 2.
Muscle protein synthesis (fractional synthesis rate, FSR) and net balance across the leg. All subjects were treated identically during baseline, exercise, and 1 h postexercise (1 h post). One-half of the subjects ingested a nutrient solution at the end of the first hour of postexercise recovery [2 h post (EAA+CHO)] compared with control subjects [2 h post (control)]. A: FSR increased in all subjects following exercise but was further increased with the addition of a leucine-enriched EAA+CHO solution (EAA + CHO). B: net balance across the leg, comparing baseline with control and subjects ingesting the leucine-enriched EAA+CHO solution. Data are means ± SE; n = 13 (baseline), n = 7 (exercise alone), n = 6 (exercise and EAA+CHO). *P < 0.05 vs. baseline. #P < 0.05 vs. 2 h post (control).
Phenylalanine net balance across the leg showed a trend to become less negative in the control group (P > 0.05). In the group ingesting EAA+CHO 1 h following exercise, net balance across the leg became significantly positive (P < 0.05; Fig. 2B).
Akt and TSC2
The phosphorylation of Akt at Ser473 was unchanged immediately following resistance exercise but was significantly elevated at 1 h (P < 0.05; Fig. 3A). In the control group, Akt Ser473 phosphorylation returned to baseline at 2 h postexercise, but it remained significantly elevated in the EAA+CHO group (P < 0.05; Fig. 3A). Total Akt protein content did not change throughout the experiment (P > 0.05).
The phosphorylation of TSC2 at Thr1462 was unchanged immediately postexercise and 1 h following exercise. In addition, a trend for an increased phosphorylation was observed following the ingestion of the leucine-enriched EAA+CHO solution (P = 0.08; Fig. 3B). Total TSC2 protein content did not change throughout the experiment (P > 0.05).
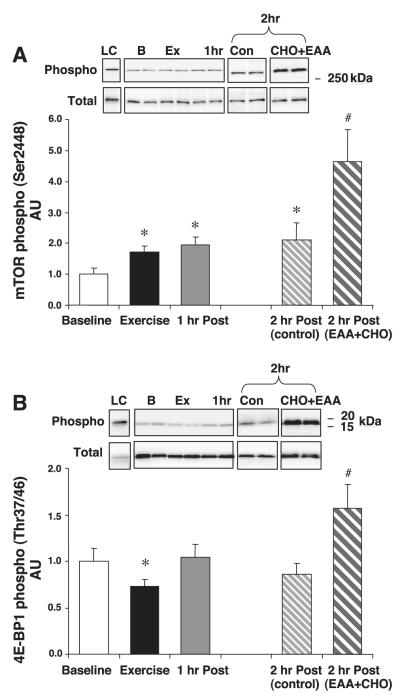
mTOR and 4E-BP1
mTOR phosphorylation at Ser2448 was significantly increased immediately following exercise and remained so for the 2 h of postexercise recovery (P < 0.05; Fig. 4A). However, mTOR phosphorylation was significantly greater at 2 h postexercise in the EAA+CHO group (1 h following leucine-enriched EAA+CHO ingestion) relative to the control group. Total mTOR protein content did not change throughout the experiment (P > 0.05).
Fig. 4.
mTOR and the eukaryotic initiation factor 4E-binding protein (4E-BP1). All subjects were treated identically during baseline, exercise, and 1 h postexercise. One-half of the subjects ingested a nutrient solution at the end of the first hour of postexercise recovery [2 h post (EAA+CHO)] compared with control subjects [2 h post (control)]. A: mTOR phosphorylation and total mTOR. B: 4E-BP1 phosphorylation and total 4E-BP1. Data are means ± SE. *P < 0.05 vs. baseline. #P < 0.05 vs. 2 h post (control). Insets show representative blots for the loading control and for each time point.
The phosphorylation of 4E-BP1 at Thr37/46 was significantly reduced immediately following exercise (P < 0.05) but returned to baseline values over the next 2 h in the control group (Fig. 4B). However, at 2 h postexercise in the EAA+CHO group, 4E-BP1 was phosphorylated to a greater extent (P < 0.05) than in the exercise alone group (Fig. 4B). Total 4E-BP1 protein content did not change throughout the experiment (P > 0.05).
S6K1 and eEF2
The phosphorylation of S6K1 at Thr389 increased significantly immediately following resistance exercise and remained elevated for the 2 h of postexercise recovery (P < 0.05). However, with the addition of the leucine-enriched EAA+CHO solution, S6K1 was significantly more phosphorylated than in the control group at 2 h postexercise (P < 0.05; Fig. 5A). Total S6K1 protein content did not change throughout the experiment (P > 0.05). A positive correlation (r2 = 0.6) was shown between S6K1 phosphorylation, a key indicator of mTOR activity, and FSR, although the correlation was not significant (P > 0.05, power = 30%). With eight subjects per group, we could detect a significant correlation with 80% power if r2 was 0.85 or greater. Therefore, it appears that with only eight subjects per group, we did not have enough power to do correlations within time periods. However, we do have sufficient power to show that FSR and S6K1 are both significantly elevated by EAA+CHO.
Immediately following resistance exercise, eEF2 phosphorylation at Thr56 tended to increase, although not quite reaching significance (P > 0.05; Fig. 5B). The phosphorylation of eEF2 Thr56 at 1 and 2 h postexercise was significantly reduced from baseline levels in both groups (P < 0.05) with no significant difference between control and EAA+CHO groups (P > 0.05). Total eEF2 protein content did not change throughout the experiment (P > 0.05).
DISCUSSION
The primary and novel finding from our study was that leucine-enriched EAA+CHO ingestion following resistance exercise simultaneously enhanced both mTOR signaling and mixed muscle protein synthesis in human subjects. Specifically, we observed a significant increase in S6K1 and 4E-BP1 phosphorylation when the leucine-enriched EAA+CHO solution was ingested following exercise relative to exercise alone. In addition, the leucine-enriched EAA+CHO-induced mTOR activation was associated with a 145% increase in mixed muscle protein synthesis compared with only a 41% increase observed in those subjects performing exercise alone.
Resistance exercise has a potent and acute effect on mTOR signaling (36) and muscle protein synthesis (18). However, our data suggest that signaling through mTOR is substantially increased during the postexercise recovery period when a leucine-enriched EAA+CHO solution is ingested. mTOR phosphorylation increases twofold with exercise alone, whereas phosphorylation increases fivefold when leucine-enriched EAA+CHO are ingested during recovery. However, we acknowledge that our findings are associative and do not establish cause and effect, since alternate signaling pathways such as MAPK or eukaryotic initiation factor-2 (eIF2) may be influencing translation initiation and elongation as well. In addition, glycogen status, which was not measured in this study, prior to or as a consequence of exercise may also be contributing or influencing muscle protein synthesis via GSK3. Further research is needed to accurately assess the potential of each signaling pathway to positively or negatively influence overall rates of muscle protein synthesis.
Downstream targets of mTOR signaling such as 4E-BP1 and S6K1 were also positively affected by the ingestion of the leucine-enriched EAA+CHO solution. In particular, 4E-BP1 phosphorylation following exercise alone had returned to base-line, whereas phosphorylation, when a leucine-enriched EAA+CHO solution was ingested, was significantly elevated above baseline, indicating enhanced translation initiation. In addition, and most remarkably, S6K1 phosphorylation was significantly enhanced above baseline and was measurably greater than the change observed in the exercise alone group. The substantial increase in S6K1 phosphorylation with the ingestion of the leucine-enriched EAA+CHO solution following exercise was even greater than that previously observed by our laboratory (21) when subjects ingested an identical leucine-enriched EAA+CHO solution without any additional exercise stimulus. Evidence for amino acids (leucine in particular) working through a novel class 3 PI3K receptor (hVps34) have been previously reported (14, 35), potentially suggesting two separate points of convergence on mTOR activation (insulin receptor-PI3K-Akt-mTOR pathway and amino acid-hVps34-mTOR pathway).
Previous studies have demonstrated that performing resistance exercise (6, 37) or ingesting amino acids (26, 42) alone stimulates muscle protein synthesis; however, the combined effects of ingesting EAA following exercise appear to be more anabolic than either amino acids or exercise independently (12, 13, 29, 39, 41). Other studies have shown that amino acid ingestion in combination with exercise stimulates components of the mTOR signaling pathway (7, 8, 16, 17, 20, 28, 44). Using a less intense resistance exercise protocol compared with the current study, Karlsson et al. (25) have shown that resistance exercise increases Ser424/Thr421 phosphorylation of S6K1 and that ingestion of branched-chain amino acids further enhances that phosphorylation. The same group (25) also measured S6K1 Thr389 phosphorylation and showed a significant and robust increase postexercise when branched-chain amino acids were ingested but no change with exercise alone. This is in contrast to our data, which show an increase in Thr389 phosphorylation in subjects performing exercise alone. The differences between studies in S6K1 phosphorylation at Thr389 may be partially explained by the difference in the exercise stimulus. For example, in the study by Karlsson et al. (25), the number of sets (4 sets at 80% of a 1RM) was much less than our exercise protocol, which required subjects to perform 10 × 10 sets at 70% of their 1RM. Our data are in agreement with recent data showing an increase in mTOR (Ser2448) and S6K1 (Thr389) phosphorylation and muscle protein synthesis in subjects following ingestion of 10 g of EAA (16). However, when we compare the phosphorylation data from the current study to our previous work (21), it is clear that phosphorylation of mTOR and S6K1 was greater when the nutrients were ingested postexercise compared with ingestion of leucine-enriched EAA+CHO alone. Other groups also have shown a positive response on downstream components of mTOR signaling (4E-BP1 and S6K1 phosphorylation) following amino acid administration (22, 31, 32).
Our data indicate that muscle protein synthesis is upregulated soon after resistance exercise and is further stimulated when a leucine-enriched EAA+CHO solution is ingested. These data are a progression of previous studies from our laboratory showing that FSR (a direct measure of amino acid incorporation into muscle protein) is acutely upregulated (within hours) and in association with components of the mTOR signaling pathway following exercise alone (18) and ingestion of a leucine-enriched EAA+CHO solution (21). These data are in opposition to the data reported by Cuthbertson et al. (17), who showed muscle protein synthesis to be delayed by greater than 3 h following exercise. Although we are unable to explain the blunted response to exercise in that study (17), several differences exist between studies. For example, their exercise protocol had subjects lift 25% of their body weight during repeated stepping exercise, whereas subjects in our study performed heavy resistance exercise (at or near 70% of 1RM). In addition, Cuthbertson et al. (17) provided large doses of essential amino acids (45 g) and carbohydrate (135 g) 2 h before each biopsy. In our study, we provided ∼20 g of “leucine”-enriched essential amino acids and 30 g of carbohydrate. The seemingly contradictory findings in addition to the differences in study design make comparisons difficult. It appears that further research is necessary to elucidate the contribution of nutrients and the timing of ingestion on signaling pathways influencing muscle protein synthesis in relation to exercise.
Although the ingestion of a leucine-enriched EAA+CHO solution following a single bout of resistance exercise had a profound effect on translation initiation (enhanced 4E-BP1 and S6K1 phosphorylation), it appeared to have no additive effect on translation elongation as measured by eEF2 phosphorylation. eEF2 phosphorylation was reduced during the 2 h of postexercise recovery (indicative of increased translation elongation) but was not further affected following the ingestion of the leucine-enriched EAA+CHO solution. This was interesting, because we previously showed that eEF2 phosphorylation was significantly decreased 1 h following ingestion of an identical leucine-enriched EAA+CHO solution without an exercise stimulus (21). This may be due to our study design, given that others have shown that eEF2 phosphorylation changes rapidly (within minutes) and that the activation pattern is biphasic (23).
We have previously shown that mixed muscle protein synthesis increased by 94% following leucine-enriched EAA+CHO ingestion in resting human subjects (21). In the current study, we provided the identical leucine-enriched EAA+CHO solution to subjects 1 h following a single bout of resistance exercise and found that mixed muscle protein synthesis rates increased by 145% above baseline, whereas an increase of only 41% was measured in those subjects that performed the exercise without nutrition. Moreover, the positive change in FSR (a direct measure of muscle protein synthesis) was associated with positive changes in our mTOR signaling data, reflecting increases in translation initiation in those subjects who ingested the leucine-enriched EAA+CHO solution. Indeed, both mTOR and S6K1 phosphorylation was much higher in the subjects ingesting nutrients during postexercise recovery compared with those subjects ingesting nutrients without exercise (21). Although the potential for a greater proportion of the anabolic response may have been due to ingestion of the EAA+CHO alone, our data support the concept that ingesting a leucine-enriched EAA+CHO solution during postexercise recovery potentially has an additive or synergistic effect on the exercise-induced anabolic response during recovery.
Although a great deal of research effort has implicated mTOR signaling to its downstream effectors, 4E-BP1 and S6K1, as a primary mechanism whereby translation initiation and elongation are activated, other signaling pathways exist (30). While the research focus of our laboratory has centered on the mTOR pathway (18, 19, 21), we acknowledge that many alternate pathways both dependent and independent of mTOR may exist and that further research is necessary to fully understand and characterize those pathways that also may be involved with postexercise anabolism associated with leucine and essential amino acids in general.
In summary, our data suggest that a leucine-enriched EAA+CHO solution ingested 1 h following a single bout of resistance exercise enhances muscle protein synthesis beyond exercise alone in association with enhanced mTOR, S6K1, and 4E-BP1 phosphorylation. Whereas others have measured signaling events following exercise and nutrient ingestion, to our knowledge this is the first study to also include direct measures of muscle protein synthesis during the acute phase of postexercise recovery. In addition, as the complexity of the signaling pathways controlling translation initiation and elongation are becoming more clear, we cannot rule out the possibility that other signaling pathways (30) not measured in this study may also play a significant, if not more important, role than that of the mTOR pathway. In conclusion, our data suggest that ingestion of a leucine-enriched EAA+CHO solution 1 h following a single bout of resistance exercise augments human muscle protein synthesis, which may be partially explained by an increase in mTOR signaling.
ACKNOWLEDGMENTS
We thank the nurses and personnel of the General Clinical Research Center of the University of Texas Medical Branch for help with the conduct of the clinical portion of this study. We also thank Ming-Qian Zheng and Shelly Medina for technical assistance.
GRANTS This study was supported by National Institutes of Health Grants R01 AR-049877, S10 RR-16650, M01 RR-00073, and P30 AG-024832. H. Dreyer was supported by National Institute on Disability and Rehabilitation Research, Department of Education Grant H133 P040003.
REFERENCES
- 1.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 2.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 3.Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- 4.Baar K, Esser K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 5.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268:E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- 6.Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- 7.Blomstrand E, Eliasson J, Karlsson HK, Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. 2006;136:269S–273S. doi: 10.1093/jn/136.1.269S. [DOI] [PubMed] [Google Scholar]
- 8.Blomstrand E, Saltin B. BCAA intake affects protein metabolism in muscle after but not during exercise in humans. Am J Physiol Endocrinol Metab. 2001;281:E365–E374. doi: 10.1152/ajpendo.2001.281.2.E365. [DOI] [PubMed] [Google Scholar]
- 9.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/m TOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 10.Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol. 2003;553:213–220. doi: 10.1113/jphysiol.2003.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borsheim E, Aarsland A, Wolfe RR. Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. Int J Sport Nutr Exerc Metab. 2004;14:255–271. doi: 10.1123/ijsnem.14.3.255. [DOI] [PubMed] [Google Scholar]
- 12.Borsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J Appl Physiol. 2004;96:674–678. doi: 10.1152/japplphysiol.00333.2003. [DOI] [PubMed] [Google Scholar]
- 13.Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–E657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- 14.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 15.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–009 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 16.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 17.Cuthbertson DJ, Babraj JA, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie MJ. Anabolic signalling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731–E738. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- 18.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreyer HC, Glynn EL, Lujan HL, Fry CS, Dicarlo SE, Rasmussen BB. Chronic paraplegia-induced muscle atrophy downregulates the mTOR/S6K1 signaling pathway. J Appl Physiol. doi: 10.1152/japplphysiol.00736.2007. First published September 20, 2007; doi:10.1152/japplphysiol.00736.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliasson J, Elfegoun T, Nilsson J, Kohnke R, Ekblom B, Blomstrand E. Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab. 2006;291:E1197–E1205. doi: 10.1152/ajpendo.00141.2006. [DOI] [PubMed] [Google Scholar]
- 21.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greiwe JS, Kwon G, McDaniel ML, Semenkovich CF. Leucine and insulin activate p70 S6 kinase through different pathways in human skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281:E466–E471. doi: 10.1152/ajpendo.2001.281.3.E466. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi AA, Proud CG. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab. 2007;292:E1647–E1655. doi: 10.1152/ajpendo.00674.2006. [DOI] [PubMed] [Google Scholar]
- 24.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287:E1–E7. doi: 10.1152/ajpendo.00430.2003. [DOI] [PubMed] [Google Scholar]
- 26.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 27.Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr. 2006;83:500S–507S. doi: 10.1093/ajcn/83.2.500S. [DOI] [PubMed] [Google Scholar]
- 28.Koopman R, Pennings B, Zorenc AH, van Loon LJ. Protein ingestion further augments S6K1 phosphorylation in skeletal muscle following resistance type exercise in males. J Nutr. 2007;137:1880–1886. doi: 10.1093/jn/137.8.1880. [DOI] [PubMed] [Google Scholar]
- 29.Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288:E645–E653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- 30.Kubica N, Jefferson LS, Kimball SR. Eukaryotic initiation factor 2B and its role in alterations in mRNA translation that occur under a number of pathophysiological and physiological conditions. Prog Nucleic Acid Res Mol Biol. 2006;81:271–296. doi: 10.1016/S0079-6603(06)81007-X. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Jahn LA, Long W, Fryburg DA, Wei L, Barrett EJ. Branched chain amino acids activate messenger ribonucleic acid translation regula-tory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab. 2001;86:2136–2143. doi: 10.1210/jcem.86.5.7481. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Wu Y, Nicklas EW, Jahn LA, Price WJ, Barrett EJ. Unlike insulin, amino acids stimulate p70S6K but not GSK-3 or glycogen synthase in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E523–E528. doi: 10.1152/ajpendo.00146.2003. [DOI] [PubMed] [Google Scholar]
- 33.Louis ES, Raue U, Yang Y, Jemiolo B, Trappe SW. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103:1744–1751. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 34.MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol. 1995;20:480–486. doi: 10.1139/h95-038. [DOI] [PubMed] [Google Scholar]
- 35.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA. 2002;99:9213–9218. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen BB, Phillips SM. Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev. 2003;31:127–31. doi: 10.1097/00003677-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 40.Richter EA, Vistisen B, Maarbjerg SJ, Sajan M, Farese RV, Kiens B. Differential effect of bicycling exercise intensity on activity and phosphorylation of atypical protein kinase C and extracellular signal-regulated protein kinase in skeletal muscle. J Physiol. 2004;560:909–918. doi: 10.1113/jphysiol.2004.071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol Endocrinol Metab. 1999;276:E628–E634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- 42.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 43.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, MacDonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. Principles and Practice of Kinetic Analysis. Wiley-Liss; New York: 1992. [Google Scholar]