Abstract
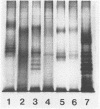
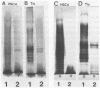
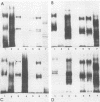
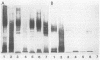
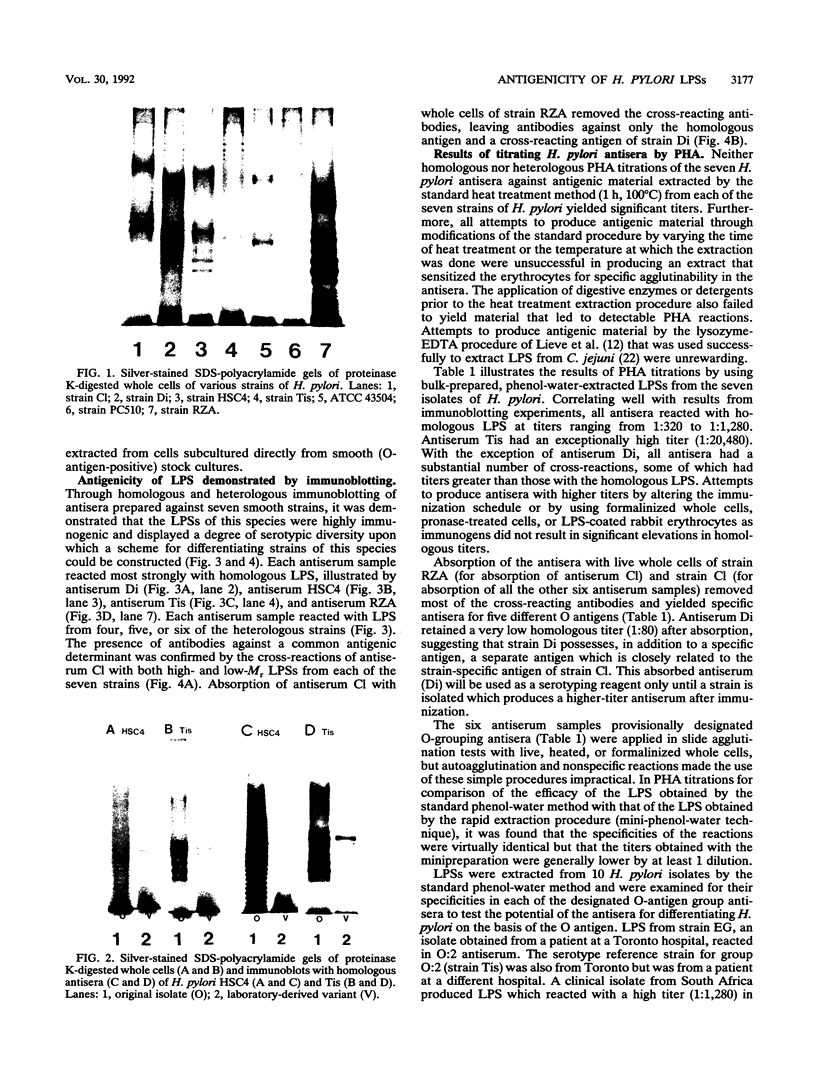
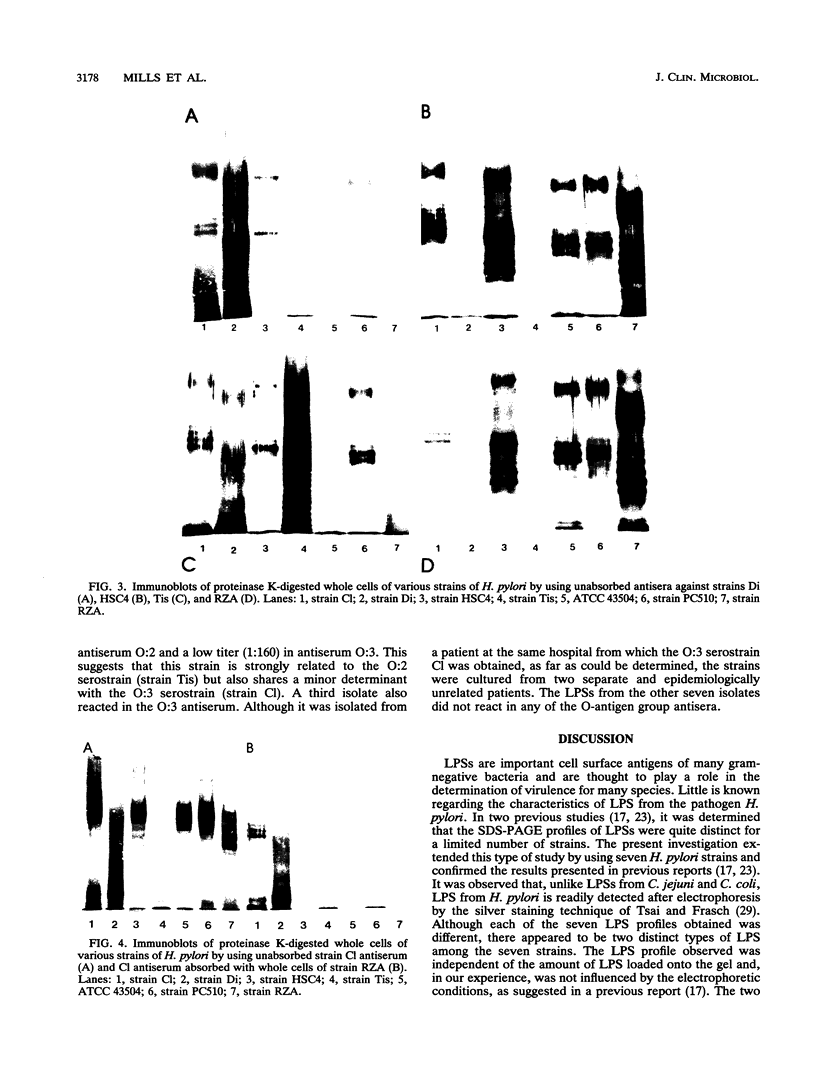
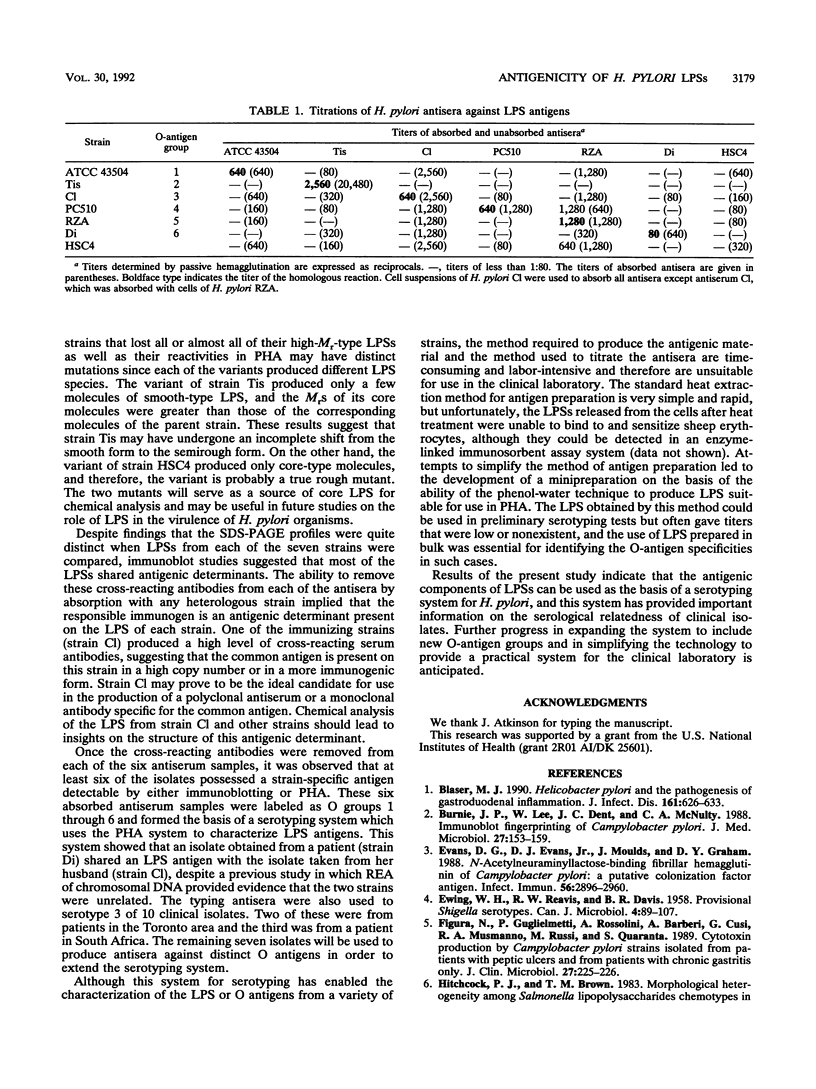
An investigation of the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profiles of lipopolysaccharides (LPSs) extracted from seven strains of Helicobacter pylori revealed that these molecules were silver stainable and exhibited a high degree of variability in their patterns. Two strains synthesized a variety of sizes of LPS molecules such that fractionation by SDS-PAGE resulted in a stepwise gradation of bands which extended from the top to the bottom of the silver-stained gel. The LPSs from the remaining five strains were made up of molecules which were more homogeneous in size and clustered around two separate areas of the gel. Antigenic analyses of phenol-water-extracted LPSs by immunoblotting and the passive hemagglutination assay suggested that, in addition to strain-specific antigens, all of the LPSs carried a common antigen. Antibodies to this common antigen could be removed from antisera by absorption, and the resulting antisera were used to differentiate strains on the basis of their O antigens by the passive hemagglutination assay technique. The finding that LPSs from 3 of 10 clinical isolates reacted specifically in one or two of the typing antisera suggested that the development of a scheme for differentiating H. pylori on the basis of O antigens is feasible.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Burnie J. P., Lee W., Dent J. C., McNulty C. A. Immunoblot fingerprinting of Campylobacter pylori. J Med Microbiol. 1988 Oct;27(2):153–159. doi: 10.1099/00222615-27-2-153. [DOI] [PubMed] [Google Scholar]
- EWING W. H., REAVIS R. W., DAVIS B. R. Provisional Shigella serotypes. Can J Microbiol. 1958 Apr;4(2):89–107. doi: 10.1139/m58-012. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Moulds J. J., Graham D. Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988 Nov;56(11):2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handfield-Jones S. E., Kennedy C. T., Bradfield J. B. Angiosarcoma arising in an angiomatous naevus following irradiation in childhood. Br J Dermatol. 1988 Jan;118(1):109–112. doi: 10.1111/j.1365-2133.1988.tb01758.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Majewski S. I., Goodwin C. S. Restriction endonuclease analysis of the genome of Campylobacter pylori with a rapid extraction method: evidence for considerable genomic variation. J Infect Dis. 1988 Mar;157(3):465–471. doi: 10.1093/infdis/157.3.465. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., McGechie D. B., Rogers P. A., Glancy R. J. Pyloric Campylobacter infection and gastroduodenal disease. Med J Aust. 1985 Apr 15;142(8):439–444. doi: 10.5694/j.1326-5377.1985.tb113444.x. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Megraud F., Bonnet F., Garnier M., Lamouliatte H. Characterization of "Campylobacter pyloridis" by culture, enzymatic profile, and protein content. J Clin Microbiol. 1985 Dec;22(6):1007–1010. doi: 10.1128/jcm.22.6.1007-1010.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A. P., Helander I. M., Kosunen T. U. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J Bacteriol. 1992 Feb;174(4):1370–1377. doi: 10.1128/jb.174.4.1370-1377.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Costas M., Morgan D. D., On S. L., Hill L. R., Pearson A. D., Morgan D. R. Strain variation in Campylobacter pylori detected by numerical analysis of one-dimensional electrophoretic protein patterns. Antonie Van Leeuwenhoek. 1989 Mar;55(3):253–267. doi: 10.1007/BF00393854. [DOI] [PubMed] [Google Scholar]
- Penfold S. S., Lastovica A. J., Elisha B. G. Demonstration of plasmids in Campylobacter pylori. J Infect Dis. 1988 Apr;157(4):850–851. doi: 10.1093/infdis/157.4.850. [DOI] [PubMed] [Google Scholar]
- Penner D. W. An overview of the College of American Pathologists' programs in surgical pathology and cytopathology. Data summary of diagnostic performance in cervical cytopathology. Acta Cytol. 1989 Jul-Aug;33(4):439–442. [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N., Congi R. V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983 Aug;2(4):378–383. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980 Dec;12(6):732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez G. I., Blaser M. J. Conservation and diversity of Campylobacter pyloridis major antigens. Infect Immun. 1987 May;55(5):1256–1263. doi: 10.1128/iai.55.5.1256-1263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakazaki R., Tamura K., Gomez C. Z., Sen R. Serological studies on the cholera group of vibrios. Jpn J Med Sci Biol. 1970 Feb;23(1):13–20. doi: 10.7883/yoken1952.23.13. [DOI] [PubMed] [Google Scholar]
- Simor A. E., Shames B., Drumm B., Sherman P., Low D. E., Penner J. L. Typing of Campylobacter pylori by bacterial DNA restriction endonuclease analysis and determination of plasmid profile. J Clin Microbiol. 1990 Jan;28(1):83–86. doi: 10.1128/jcm.28.1.83-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]