Abstract
Envenomations by the Southern Pacific Rattlesnake (Crotalus oreganus helleri) are the most common snakebite accidents in southern California. Intraspecies venom variation may lead to unresponsiveness of antivenom therapy. Even in a known species, venom toxins are recognized as diverse in conformity with interpopulational, seasonal, ontogenetic and individual factors. Five venoms of individual C. o. helleri located in Riverside and San Bernardino counties of southern California were studied for their variation in their hemostasis activity. The results demonstrated that Riverside 2 and San Bernardino 1 venoms presented the highest lethal activity without hemorrhagic activity. In contrast, San Bernardino 2 and 3 venoms had the highest hemorrhagic and fibrinolytic activities with low lethal and coagulant activities. Riverside 1, Riverside 2 and San Bernardino 1 venoms presented a significant thrombin-like activity. San Bernardino 2 and 3 venoms presented an insignificant thrombin-like activity. In relation to the fibrinolytic activity, San Bernardino 3 venom was the most active on fibrin plates, which was in turn neutralized by metal chelating inhibitors. These results demonstrate the differences amongst C. o helleri venoms from close localities. A metalloproteinase, hellerase, was purified by anionic and cationic exchange chromatography from San Bernardino 3 venom. Hellerase exhibited the ability to break fibrin clots in vitro, which can be of biomedically importance in the treatment of heart attacks and strokes.
Keywords: Crotalus oreganus helleri, fibrinolytic activity, hemostasis, platelet aggregation, Southern Pacific Rattlesnakes, venom variation
1. Introduction
A significant number of snakebites are reported yearly throughout the United States as well as worldwide (Rudolph et al., 1995; Ribeiro et al., 1999; Brooks et al., 2002; Johnston et al., 2002). From 2001–2005, the American Association of Poison Control Centers reported an annual average of about 7,000 venomous snakebites, including exotic specimens, in the United States resulting in 16 deaths during this period (Langely, 2008).
The Southern Pacific Rattlesnake is a frequent and epidemiologically important rattlesnake which co-exists with humans of southwestern California (Fig. 1). This snake lives in different geographic landscapes such as seaside, chaparral spots at piedmonts and woodland areas, broadening to North Baja California, Mexico (Klauber, 1956).
Figure 1.
Geographical locations of C. o. helleri venoms used in this study. Counties of southern California. SB: San Bernardino county; RS: Riverside county.
After envenomation, it takes only a matter of seconds before symptoms become visible. Consistent with many snake species, the most common symptoms presented by patients who have suffered bites from Viperidae and Colubridae snakes were pain and swelling (Ribeiro et al., 1999; Rodriguez-Acosta et al., 2006). Snake venoms are composed of many polypeptides and proteins that perform different functions. For example, snake venoms contain disintegrins, phospholipases, serine proteases, and metalloproteinases (Bjarnason and Tu, 1978; Bajwa et al., 1981; Komori et al., 1985; Estevao-Costa et al., 2000). Hence, it has been recognized for some time that snake venoms may contain compounds that can modulate the blood clotting cascade, and therefore may be of biomedical importance. Snake venom metalloproteinases from Crotalidae and Viperidae venoms are known to be hemorrhagic and/or fibrinolytic (Bjarnason and Fox, 1994; Gold et al., 2002).
Fibrinolytic enzymes isolated from snake venom can digest fibrin clots in vitro suggesting that these fibrinolytic enzymes have potential application for treatment of strokes and heart attacks (Didisheim and Lewis, 1956; Mori et al., 1987; Stocker, 1990). At present there are two products derived from snake venom that are approved for the treatment of ischemic stroke, Aggrastat® or Tirofiban HCl (Merck & Co., 1998) and Viprinex™ or Ancrod (Dempfle et al., 2000), with another snake venom fibrinolytic enzyme, Alfimeprase currently known as Fibrolase, undergoing clinical trials (Guan et al., 1991; Toombs, 2001a, 2001b; Moll et al., 2006). There are also fibrinolytic enzymes that have been isolated from various snake venoms whose clinical potential has not been fully investigated (Willis and Tu, 1988; Rodrigues et al., 2000; Bello et al., 2006).
Crotalus oreganus helleri venom likely contains fibrinolytic enzymes, but little research has been done to isolate these potentially important therapeutic enzymes. In this study, a metalloproteinase, hellerase, has been isolated from the venom of an individual Southern Pacific Rattlesnake that demonstrated the most fibrinolytic activity of the five venoms tested. Furthermore, venom variations of hemostasis and lethal activity in individual specimens have been demonstrated which provides further evidence of the importance of selecting venoms from snakes in wide geographical locations for the production of antivenoms.
2. Materials and methods
2.1 Snakes and venom
Five different C. o. helleri crude venom samples [Avid # 058-806-546-Riverside Co., CA (Riverside 1), 059-009-599-Riverside Co., CA (Riverside 2), 058-819-883-San Bernardino Co., CA (San Bernardino 1), 058-893-793-San Bernardino Co., CA (San Bernardino 2) and 058-359-257-San Bernardino Co., CA (San Bernardino 3)] were purchased from specimens held at the Natural Toxins Research Center at Texas A&M University, Kingsville. The venoms were lyophilized with a Labconco Freeze drying system and stored at −90 °C. The venoms were reconstituted in 0.02 M Tris-HCl buffer at pH 8.0, and filtered using a Millipore Millex HV 0.45 μm filter unit prior to high performance liquid chromatography (HPLC).
2.2 Protein Concentration
The protein concentration of the venoms was measured by a Beckman DU 700 spectrophotometer (Beckman Coulter Inc., Fullerton, CA, USA) at an absorbance of 280nm using the method of D’Suze et al. (1996).
2.3 Lethal dose
Five groups of eight mice for each venom were housed in cages and observed throughout the experiments. Venoms were dissolved in 0.85% saline at the highest test dose per mouse. The lethal toxicity was determined by injecting 0.2 mL of venom containing varying dosages into the tail veins of 18–20 g female BALB/c mice. Saline controls were used. The LD50 was calculated by the Spearman-Karber (1978) method for each pool of venom after a 48 h experimental period.
2.4 Hemolytic activity
The minimal hemolytic activity of the crude venoms was determined with modifications as described by Habermann and Hardt (1972). Briefly, 0.3 mL of packed human erythrocytes were washed five times with saline solution, and 0.3 mL of fresh egg yolk diluted 1:4 with saline solution and 0.25 mL of a 0.01M CaCl2 solution were added to 25 mL of 0.8% agarose dissolved in phosphate-buffered saline (PBS) solution, pH 8.1. The mixture was poured into plastic petri dishes (135 × 80 mm) and allowed to solidify. After the mixture was solidified, wells were prepared and filled with 10 μL of crude venom solution at various concentrations. The plates were incubated at 37°C for 5 h and the diameters of the hemolytic haloes were measured. Saline solution and PBS were used as controls. The minimal hemolytic dose is defined as the amount of venom protein that causes a 10 mm halo spot.
2.5 Hemorrhagic activity
A modified hemorrhagic assay described by Omori-Satoh et al. (1972) was used to determine the hemorrhagic activity of the crude venom and its fractions.
2.6 Amidolytic activity
Amidolytic activity of crude venoms and fractions was measured by a micromethod of Guerrero and Arocha-Piñango (1992) using chromogenic substrate kits (Chromogenix- Instrumentation Laboratory, Milano, Italy). Briefly, in 96 wells polystyrene plates a mixture of 80 μL of the recommended buffer for each substrate, 10 μL of the venom sample (0.1 to 1 mg/mL) and 10 μL of chromogenic substrate were placed in each well. The final concentrations for the substrates were 0.60 mM S-2238 (thrombin), 0.80 mM S-2222 (factor Xa), 0.80 mM S-2251 (plasmin), 1.20 mM S-2288 (t-PA), 0.16 mM S-2444 (urokinase), 1 mM S-2302 (kallikrein) and 0.16 mM S-2586 (chymotrypsin). Bovine thrombin, human factor Xa, plasmin, single chain tissue-plasminogen activor (sct-PA), and two chain urokinase-plasminogen activator (tcu-PA) were used as positive controls. After incubation at 37° C for 5 or 15 min depending on the activity, the absorbance at 405 nm was measured. One unit of amidolytic activity was expressed as ΔA 405 UA/min. Specific activity was calculated as UA/min/μg.
2.7 Coagulant activity
The thrombin-like coagulant activity of crude venoms and fractions of San Bernardino 3 venom was assayed by the modified methods of Austen and Rhymes (1975) and Salazar et al. (2007). Briefly, 0.1 mL of 0.05 M Tris-HCl buffer, pH 7.4 (coagulation buffer) plus 0.1 mL thrombin solution (0.5 to 15 IU/mL) or 0.1 mL venom sample (0.1 to 1 mg/mL) was incubated in a borosilicate tube at 37 °C. Then 0.1 mL of fresh citrated human plasma or 0.3 % human fibrinogen solution in coagulation buffer was added. The solution was thoroughly mixed manually in a 37°C water bath and the clotting time recorded when the appearance of a clot was visually detected. Tests were performed four times and the mean clotting time calculated. The results were expressed in thrombin-like units by plotting the clotting times against a calibration curve prepared with a standard thrombin (National Institute for Biological Standards and Control, London, England).
2.8 Fibrinolytic activity
Fibrinolytic activity of crude venoms and fractions from both anionic and cationic exchange chromatography was studied by the fibrin plate method as proposed by Marsh and Arocha-Piñango (1972). Briefly, fibrin plates were prepared using 3-cm diameter Petri dishes: 1.5 mL of a 0.1% plasminogen-rich fibrinogen (10 % plasminogen as contaminant) in 5 mM imidazole saline buffer, pH 7.4 was clotted by adding 75 μL bovine thrombin (10 IU/mL, in 0.025 M CaCl2). The mixture was incubated at room temperature for 30 min. Then, 10 μL of crude venoms or fractions of different concentrations were applied over the fibrin film, and after 24 h incubation at 37° C the diameters of the lysed areas were measured. Fibrinolytic activity was also evaluated in presence of protease inhibitors using as a pool of serine protease inhibitors (SPI) of 50 μg/mL SBTI, 10 mM PMSF, 10 mM benzamidine/HCl, and 100 IU/mL aprotinin (final concentrations). Metalloproteinase inhibitors were used as a mixture of 10 mM EGTA-Na and 10 mM 1,10 phenanthroline (final concentrations). Fibrinolytic activity was expressed as the diameter of the lysed area per μg of protein (mm2/μg). Human plasmin, sct-PA and tcu-PA were used as positive controls.
2.9 Chromatographic profiles of crude venoms
A total of 200 μL of crude venom (40 mg/mL) from each local was injected into a Waters™ Protein Pak™ DEAE 5PW (7.5 × 7.5 mm) column. The buffer used was 0.02M Tris-HCl, pH 8.0 over 60 min with a flow rate of 1 mL/min using Waters™ 1525 binary pump system. Proteins were detected at 280 nm using a 2487 dual absorbance detector. Waters™ Breeze software v.3.3 was used for storage and acquisition of data. The fractions were tested for proteolytic activities.
2.10 Venom fractionation
Isolation of fibrinolytic activity present in C. o. helleri venom was achieved by two chromatographic steps. First, a total of 200 μL of crude venom (40 mg/mL) from different locations were run in a preparative procedure using anionic exchange chromatography into a Waters Protein Pak™ DEAE 5PW (7.5 × 75 mm) column. The equilibrating buffer used was 0.02M Tris-HCl, pH 8.0. The elution was carried out over 60 min with a flow rate of 1 mL/min, with a linear gradient of 0 to 0.5 M NaCl, in the equilibrium buffer and proteins were detected at 280 nm using a 2487 dual absorbance detector. Acquisition of data and Waters™ 1525 binary pump system was control was through Waters™ Breeze software v.3.3. Fibrinolytic activity was tested for each fraction.
For the second stage of purification, a total of 125 μL of San Bernardino 3 venom fraction 1 (4.5 mg/mL), the most fibrinolytic venom, was injected into a Waters Protein Pak™ SP 5PW (7.5 × 75 mm) column. The equilibrating buffer used was 0.02M Tris-HCl, pH 8.0. In some fractionations, the eluted cationic fractions were collected in the presence of EDTA. The elution was performed with a linear gradient of 0 to 0.5 M NaCl, in same buffer, over 60 min with a flow rate of 1 mL/min and proteins were detected at 280 nm using a 484 tunable detector. Acquisition of data and 510 pump control was through Waters’ Breeze software v.3.3. Fibrinolytic activity was tested for each fraction.
2.11 SDS PAGE
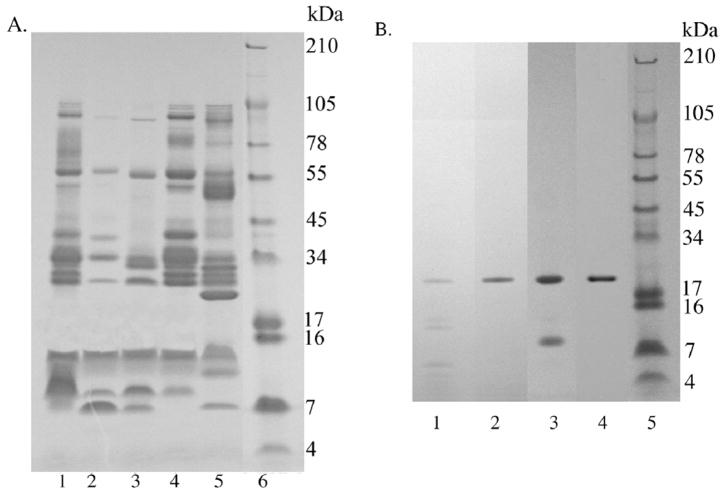
Venoms (250 μg) were run under non-reducing conditions on a 10–20% Tricine SDS PAGE (Invitrogen) gel (Schägger and von Jagow, 1987). A XCell SureLock™ system with Tricine SDS running buffer (10X) diluted to 1X at a voltage of 125 for 90 min using a Bio-Rad PowerPac Basic was used. SeeBlue Plus2 markers ranging from 3–250 kDa were used as standards.
Fraction V with and without 10 mM EDTA (final concentration) was also run under non-reduced and reduced conditions on a 10–20% Tricine gel (Fig. 2B). The molecular weights of the bands were determined by plotting the log of the molecular weights versus the relative mobility.
Figure 2.
SDS-PAGE on 10–20% Tricine gels. A) C. o. helleri crude venoms. A total of 250 μg of venom were run at 125V for 90 min. Lane 1: Riverside 1; Lane 2: Riverside 2; Lane 3: San Bernardino 1; Lane 4: San Bernardino 2; Lane 5: San Bernardino 3; Lane 6: SeeBlue Plus2 (Invitrogen™). B) Cationic exchange fraction FV. A total of 5 μg of fraction FV collected with and without EDTA, under reduced and non-reduced conditions were run at 125V for 90 min. Lane 1: FV collected without 10 mM EDTA and run under reducing conditions; Lane 2: FV collected in 10 mM EDTA and run under reducing conditions; Lane 3: FV collected without EDTA and run under non-reducing conditions; Lane 4: FV collected in EDTA and run under non-reducing conditions; Lane 5: SeeBlue Plus2 Markers (Invitrogen™). Gels were stained with RapidStain.
2.12 N-Terminal Sequence
The five fractions collected from cationic exchange chromatography (Fig. 3) were run on a 10–20% Tricine NuPage gel (Fig. 5) and transferred onto an Immobilon™-p transfer membrane (Millipore). After transfer blot the membrane was stained with 0.02% Coomassie R-250 without acetic acid for 5 min. Bands 1–3 (Fig. 6) were sent to the Protein Facility at Iowa State University for N-terminal sequencing.
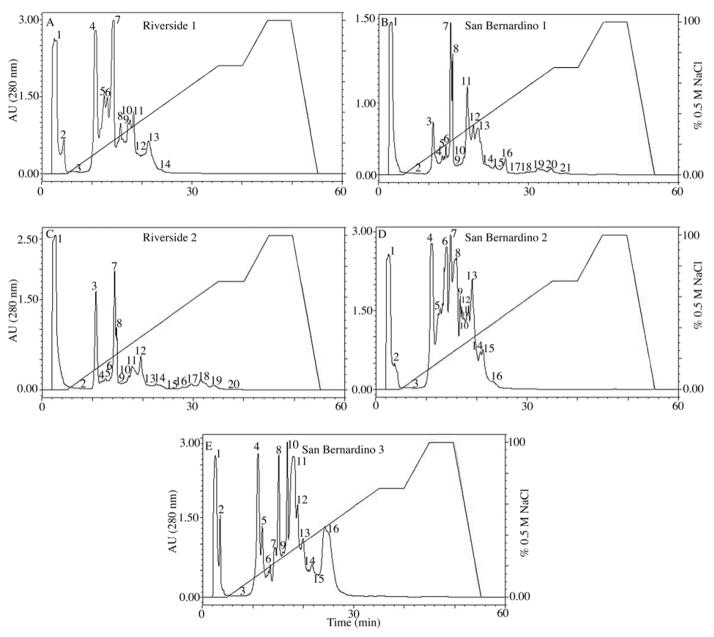
Figure 3.
Anion exchange chromatography crude C. o. helleri venoms. A total of 200 μL of venom sample (40 mg/mL) was injected into a Waters Protein Pak™ DEAE 5PW (7.5 × 7.5 mm) column. The equilibrium buffer used was 0.02M Tris-HCl, pH 8.0. The elution was carried out over 60 min with a flow rate of 1 mL/min, with a linear gradient of 0 to 0.5 M NaCl. Proteins were detected at 280 nm.
Figure 5.
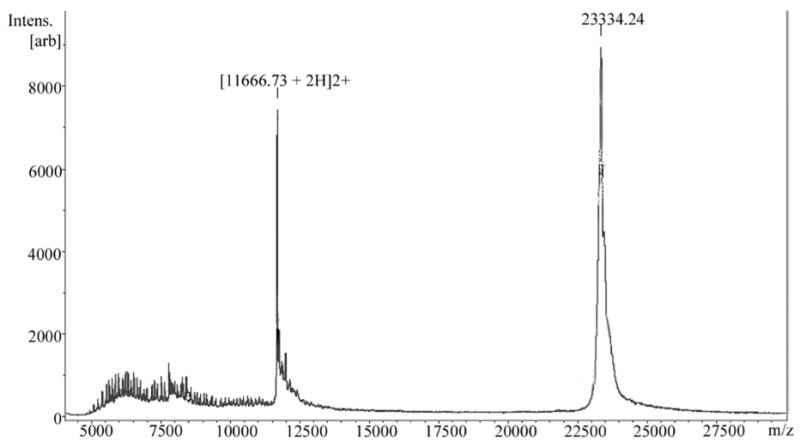
Intact mass spectrometry analysis of fraction FV obtained by cationic exchange chromatography. Fraction FV (hellerase) was cleaned using C18 zip tips (MilliPore). A total of 0.5 μL of fraction was mixed with 0.5 μL of sDHB matrix (Bruker Daltonics) and a total of 1 μL of mixture was spotted on a MALDI target plate. The sample was run in a Bruker Daltonics UltraFlex II MALDI-TOF-TOF in linear mode at 10 Hz using a laser power of 55 %. A total of 490 shots were given.
Figure 6.
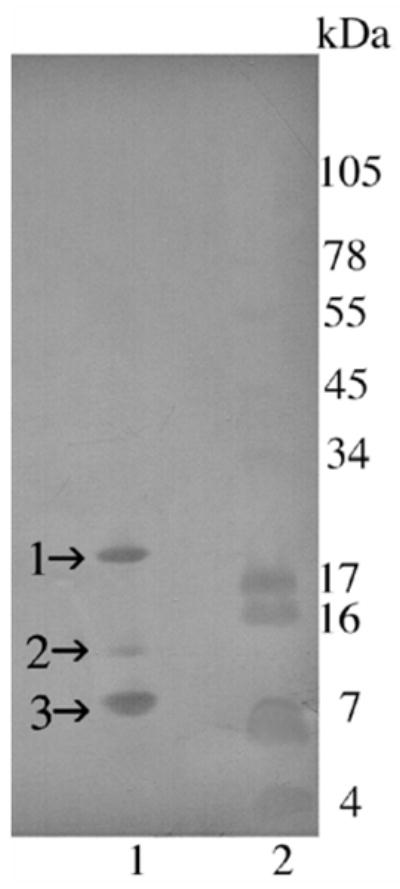
Transferblot of SDS-PAGE of C. o. helleri cationic exchange fraction V (hellerase) (Fig. 4B). A total of 5 μg of venom fractions were run on a 10–20% Tricine gel. The gel was over laid on an Immobilon-p transfer membrane and proteins were transferred using a Bio-Rad Trans-Blot Semi-Dry transfer cell at 100 mA for 1 h. Lane 1: Fraction FV collected without EDTA and run under non-reducing conditions; Lane 2: SeeBlue Plus2 Markers (Invitrogen™). The membrane was stained with 0.02% Coomassie R125 without acetic acid for 5 min. N-terminal sequence was determined for bands 1–3 in lane 1.
2.13 Mass Spectrometry Analysis (MALDI-TOF-TOF)
Mass spectrometry was used to determine the molecular weight and sequence of the purified fibrinolytic enzyme. The isolated and lyophilized fibrinolytic fractions were redissolved in 10 μL of 0.1% of Trifluroacetic acid (TFA) and desalted using a C18 ZipTip (Millipore, Bedford, MA, USA). One microliter of the resulting eluant was mixed with 10 mg/mL of 2, 5-dihydroxybenzoic acid, and spotted onto the matrix associated laser desorption ionization (MALDI) plate. MALDI analysis was performed on the AUTOFLEX II TOF/TOF Mass Spectrometer, Bruker Daltonics. The instrument was operated in linear mode using external standards in order to determine the monoisotopic mass of the intact protein.
2.14 Protein Sequence by LC-MS/MS
a) In gel digestion
Proteins were excised from a gel and cut into approximately 1mm cubes and placed in an eppendorf tube. One hundred microliters of H20 was added to the tube and incubated for 15 min at room temperature. The H20 was removed and 100 μL of 50 % CH3CN solution was added for an additional 15 min. The solution was removed and 40 μL of 100% CH3CN was added for 15 min, or until the gel pieces turned white. The solution was removed and 40 μL of 100 mM NH4CO3, pH 8.0 was added for 5 min. The procedure was repeated 3 times or until the gel pieces were destained. The gel pieces were dried in a vacuum for 15 min. One hundred microliters of 100 mM NH4CO3, pH 8.0/10 mM DTT solution was added, and the sample was incubated for 1h at 56°C. The solution was allowed to cool to room temperature and then removed. One hundred microliters of 100 mM NH4CO3/55mM iodacetamide was added and incubated for 1 h in the dark at room temperature. The gel pieces were washed with 100 μL of 100 mM NH4CO3, pH 8.0 for 5 min. Equal volume of 100 % CH3CN was added to the sample and incubated for 15 min. The solution was then removed. The sample was dried in vacuum for 15 min. Trypsin was added to 50 mM NH4CO3, pH 8.0 at a concentration of 12.5 ng/μL and 40 μL was added to the sample and incubated for 45 min at 4°C. The sample was next incubated for 37°C overnight. The supernatant was collected and the gel pieces were further incubated with 20 mM NH4CO3, pH 8.0 for 10 min, followed by 2 times volume of 100 % CH3CN for 15 min. The supernatant was collected again, and the gel pieces were incubated with 100 μL 5 % acetic acid for 15 min, followed by 2 times the volume of 100 % CH3CN. All supernatants were pooled and dried. The sample was reconstituted in 8 μL of 0.1 % HCOOH.
b) Nanoflow LC-MS/MS
Eight microliters were injected in an Agilent 1100 HPLC system using reverse phase C18 liquid chromatography. The reverse phase C18 was performed using a Prepacked C18 IntegraFrit (50 cm × 75 mm) capillary column (New Objectives) packed with 5 μm C18 Magic beads (Michrom; 75 μm i.d. and 12 cm of bed length) on an 1100 Agilent HPLC. The buffer used was 0.1 % HCOOH with the eluting buffer of 100 % CH3CN run over a shallow linear gradient over 60 min with a flow rate of 0.3 μL/min. The electrospray ionization emitter tip was generated with a laser puller (Model P-2000, Sutter Instrument Co.). The Agilent 1100 HPLC system was coupled online with an LTQ linear ion trap mass spectrometer (Thermoelectron, San Jose, CA, USA). The mass spectrometer was operated in the data-dependent mode, in which a full scan MS was followed by MS/MS scans of the 10 most abundant ions with +2 to +3 charge states. The mass exclusion time was 180 s.
c) Peptide and protein identification
The MS/MS data were converted to a mzXML format using the open-source Trans-Peptide Pipeline (TPP) software (Version 2.9.4), and the resulting mzXML files were searched against Swiss Protein database (Version 55.4 with 385721 entries) using the SEQUEST algorithm on the Sorcerer IDA server (Software Version 2.5.6; SageN, Inc, San Jose, CA, USA). Peptide mass tolerance was set at 3.0 amu, and MS/MS tolerance was set internally by the software with the values varying from 0 up to 1 amu. Search criteria included methionine oxidation (16 Da). Searches were performed with semi-tryptic digestion and allowed maximum 2 missed cleavages on the peptides analyzed from the sequence database. Minimum protein and peptide probabilities were set to 1.0.
2.15 Statistical analysis
All experiments, with the exception of the LD50, were repeated three or four times. Results were expressed as the mean ± standard deviation, and analyzed using the two-tailed Student’s t test for samples with equal variances. Differences were statistically significant if p was less than 0.05.
3. Results
3.1 Activities in crude venoms
a) Lethal
The lethal activities of the five crude venoms ranged between 0.6 to 5.7 mg/kg body mass (Table 1). The non-hemorrhagic venoms, Riverside 2 and San Bernardino 1, had very potent lethal activities of 0.6 and 0.7 mg/kg, respectively.
Table 1.
Comparison of activities of C. o. helleri venoms from 5 geographical locations.
| Activities |
|||||
|---|---|---|---|---|---|
| Crude Venom Localities | LD50 (mg/kg) | MHD (μg/mouse) | Hemolytic (mm2/μg) | Coagulant (Human Plasma) IU thrombin- like/mg | Fibrinolytic (Fibrin Plate- Pg+ mm2/μg) |
| Riverside 1 | 2.5 | 8.1 ± 1.3 | 24.4 ± 3.7 | 7.0 ± 0.1 | 1. 4 ± 0.3 |
| Riverside 2 | 0.6 | No Hemorrhage | 13.3 ± 0.1 | 3.8 ± 0.1 | 0 |
| San Bernardino 1 | 0.7 | No Hemorrhage | 16.5 ± 1.9 | 12.2 ± 0.2 | 0 |
| San Bernardino 2 | 3.0 | 2.8 ± 0.5 | 22.6 ± 2.8 | 0 | 1.6 ± 0.1 |
| San Bernardino 3 | 5.7 | 2.5 ± 0.8 | 14.3 ± 2.1 | < 1.0 | 6.7 ± 0.2 |
LD50 = The amount of venom protein that will kill 50% of a mouse population.
MHD = Minimal hemorrhagic dose; the minimal amount of venom protein that will produced a 10 mm hemorrhagic spot.
Hemolytic = Indirect activity.
Pg+ = Fibrinogen containing plasminogen.
b) Hemorrhagic
Three venoms, Riverside 1, San Bernardino 2 and 3, contained hemorrhagic activity with minimal hemorrhagic doses (MHD) of 8.1, 2.8, and 2.5 μg/mouse, respectively (Table 1). The remaining two venoms, Riverside 2 and San Bernardino 1, did not contain hemorrhagic activity at doses as high as 140 μg/mouse.
c) Hemolytic
All five venoms contained hemolytic activity ranging from 13.3 to 24.4 mm2/μg. The most hemolytic venoms were Riverside 1 and San Bernardino 2 with hemolytic activities of 24.4 and 22.6 mm2/μg, respectively (Table 1).
d) Coagulant
The results in Tables 1 and 2 show thrombin-like activity under fibrinogen and plasma. Three of the five venoms Riverside 1, Riverside 2 and San Bernardino 1 showed a significant coagulant activity. This activity was higher with fibrinogen in comparison to plasma. San Bernardino 3 showed traces of activity.
Table 2.
Coagulation activity of crude C. o. helleri venoms.
| Amidolytic Activity (S-2238) | Coagulant Activity | ||
|---|---|---|---|
| Crude Venom Localities | Human Plasma | Purified Fibrinogen | |
| IU thrombin-like/mg | |||
| Riverside 1 | 1.5 ± 0.2 | 7.0 ± 0.1 | 10.6 ± 0.6 |
| Riverside 2 | 8.1 ± 0.4 | 3.8 ± 0.1 | 11.0 ± 0.5 |
| San Bernardino 1 | 2.0 ± 0.4 | 12.2 ± 0.2 | 26.1 ± 0.3 |
| San Bernardino 2 | 1.6 ± 0.2 | 0 | 0 |
| San Bernardino 3 | 0.7 ± 0.2 | < 1.0 | 2.9 ± 0.1 |
All venoms, except San Bernardino 2 showed significant thrombin-like amidolytic activity. San Bernardino 2 was not able to clot purified fibrinogen or human plasma, however contained very low activity (Table 2). Furthermore, none of the venoms presented significant factor Xa-like or chymotrypsin-like activities.
When a comparative analysis of venoms was carried out (Table 2), it was observed that the San Bernardino 1 venom contained the highest coagulant activity for both human plasma and purified fibrinogen with 26.1 and 12.2 thrombin-like IU/mg, respectively. In contrast, the thrombin-like amidolytic activity was higher (8.1 ± 0.4) in Riverside 2 venom compared with the other four venoms (p< 0.01).
e) Fibrinolytic
The results in Table 3 demonstrate that the highest amidolytic activity was kallikrein-like, followed by low plasmin-like and t-PA-like activities. When a comparative analysis of the five venoms from different regions was carried out, it was observed that kallikrein-like activity was higher (p< 0.01) in Riverside 1 and San Bernardino 1 venoms. This amidolytic activity was neutralized by a pool of SPI in all the venoms.
Table 3.
Fibrinolytic activity of crude C. o. helleri venoms.
| Crude Venom Localities | Fibrin Plate Method (50 μg sample) mm2 | Amidolytic Method UA/min/μg | ||||
|---|---|---|---|---|---|---|
| Pg+ | Pg− | S-2251 Plasmin | S-2288 t-PA | S-2444 Urokinase | S-2302 Kallikrein | |
| Riverside 1 | 67 ± 13 | 84 ± 14 | 32 ± 0.8 | 35 ± 0.8 | 4.8 ± 0.6 | 611 ± 29 |
| Riverside 2 | 0 | 0 | 37 ± 1.4 | 30 ± 1.0 | 29 ± 1.7 | 120 ± 23 |
| San Bernardino 1 | 0 | 0 | 34 ± 1.3 | 31± 1.7 | 6.4 ± 3.0 | 320 ± 25 |
| San Bernardino 2 | 78 ± 5 | 70 ± 12 | 34 ± 1.8 | 29 ± 1.6 | 4.7 ± 1.2 | 207 ± 53 |
| San Bernardino 3 | 336 ± 10 | 427 ± 13 | 20 ± 0.7 | 16 ± 0.8 | 3.1 ± 1.2 | 142 ± 20 |
Pg + = Fibrinogen containing plasminogen.
Pg − = Fibrinogen without plasminogen.
The results on fibrin plate (Tables 1 and 3) evidenced that the venoms Riverside 1, San Bernardino 2 and San Bernardino 3 were active in presence or absence of plasminogen. This activity was inhibited by EDTA, EGTA and 1,10 phenantroline. The non-hemorrhagic venoms, Riverside 2 and San Bernardino 1 did not contain fibrinolytic activity on fibrin plate. Venom San Bernardino 3 contained the highest fibrinolytic activity in presence or absence of plasminogen, at a dose of 10 μg the activity expressed in mm2/μg was of 18.2 and 19.1 mm2/μg, respectively (data not shown). At a dose of 50 μg with and without plasminogen, the activity was lower (6.72 and 8.53, respectively).
3.2 Venom fractionation
Five different crude venom samples of C. o. helleri were initially fractionated by anionic exchange chromatography. The number of fractions ranged from 14–21 (Fig. 3). Riverside 1 venom yield 14 fractions, while San Bernardino 2 and San Bernardino 3 venoms yield 16 fractions. San Bernardino 1 and Riverside 2 venoms yield 21 and 20 fractions, respectively. Three of the venoms, Riverside 1, San Bernardino 2 and 3, had two fractions in the void volume (Fig. 3A, D and E), while the remaining two contained only one fraction in the void volume (Fig. 3B and C).
The evaluation of fibrinolytic activity in anionic fractions showed that Riverside 1 and San Bernardino 1 venoms contained one active fraction (F1) on fibrin plate. Riverside 2 venom had no fractions containing fibrinolytic activity. San Bernardino 2 venom had two active fractions (F1 and F4).
Additionally, San Bernardino 3 venom with the most fibrinolytic activity presented three fractions (F1, F9 and F16) with fibrinolytic activity on anionic exchange chromatography. Fibrinolytic activity assayed by amidolytic method in San Bernardo 3 venom resulted in kallikrein-like activity in several anionic fractions, being higher in the fractions eluted between 20 and 50% saline concentration. In contrast, thrombin-like activity was evidenced in the fractions F4 to F8, F12 and F13 (Fig. 4A).
Figure 4.
A) Fibrinolytic (F1, 9, 16), kallikrein-like (F2, 6–16), and thrombin-like (F4 to F8, F12 and F13) activities presented in anion exchange chromatography fractions obtained from San Bernardino 3 venom. B) Cationic exchange chromatography of fraction 1 collected from crude C. o. helleri venom (Fig. 4A). A total of 125 μL (4.5 mg/mL) of fraction 1 was injected into a Waters Protein Pak™ SP 5PW (7.5 × 75 mm) column. The equilibrium buffer was 0.02M Tris-HCl, pH 8.0. The elution was performed with a linear gradient of 0 to 0.5 M NaCl over 60 min with a flow rate of 1 mL/min. Proteins were detected at 280 nm. Black shaded areas indicate active fractions.
Due to its high fibrinolytic activity, fraction 1 obtained from San Bernardino 3 venom through anionic exchange chromatography was further fractionated by cationic exchange chromatography (Fig. 4B). A total of five fractions were collected (FI to FV). Fraction I came off the void volume, while fractions FII to FV came off between 17 to 22% of a 0.5 M NaCl gradient. Cationic fractions FI and FV were the only ones that contained fibrinolytic activity on both plasminogen and plasminogen free fibrinogen with activities on fibrin plates of 31 and 87 mm2/μg, respectively. However, fraction FV contained the highest activity. This fibrinolytic protein present in FV fraction was designated as hellerase.
3.3 SDS-PAGE electrophoresis
a) Crude venoms
Five different crude venom samples of C. o. helleri were run on a 10–20% Tricine NuPage gel under non-reduced conditions (Fig. 2A). All venoms contained distinct protein bands. The hemorrhagic venoms, Riverside 1, San Bernardino 2 and 3 (lanes 1, 4 and 5) contained more bands than the neurotoxic venoms (Riverside 2 and San Bernardino 1 venoms). Although neurotoxicity was not directly tested, recent studies have proven through DNA analysis that venoms containing low LD50 and no or very little hemorrhagic activity is an indication of the presence of neurotoxins (Sánchez et al., 2005). San Bernardino 3 venom (lane 5) had two strong protein bands at ~23 and ~46 kDa that were not present in the other four venoms. Riverside 2, San Bernardino 1, and San Bernardino 3 venoms contained an additional protein band at 7 kDa.
b) Venom cationic fractions
The five fractions (FI to FV) collected from cationic exchange chromatography originated from anionic fraction 1 of San Bernardino 3 venom were run on a 10–20% Tricine NuPage gel under reduced conditions (Fig. 2B). Fraction I contained 3 bands at 19, 12, and 9.4 kDa (data not shown). Fraction II had a ~ 23 kDa band. Fractions III and IV contained one visible band at ~ 46 kDa (data not shown). Fraction V without EDTA contained 6 bands (line 1) between 19 to 4 kDa and 3 bands (line 3) at ~19, 12, and 9.4 kDa under reduced and non-reduced conditions, respectively. In contrast, fraction FV when collected in presence of EDTA demonstrated a single protein band at ~19 kDa under both reduced and non-reduced conditions (Fig. 2B; lines 2 and 4).
3.4 Mass Spectrometry Analysis (MALDI-TOF-TOF)
Intact mass spectrometry analysis of cationic fraction FV of San Bernardino 3 venom, which contained high fibrinolytic activity, revealed peaks at 23 and 11 kDa (Fig. 5).
3.5 N-Terminal Sequence
Bands 1–3 obtained by Trans-Blot of cationic fibrinolytic fraction FV of San Bernardino 3 venom (Fig. 6) were sequence at the N-terminal end providing a maximum, for each band, of 15 amino acids. Band 2 (12 kDa) had a sequence of KNTLHXGXE (X = no amino acid was determined), and band 3 (9.4 kDa) had a sequence of LTAIVLDDDTLGLAY. Due to the low signal generated by Edmand degradation of band 1, the maximum amino acids (KLGFKEI) could not be determined; and thus Blast search gave insignificant results.
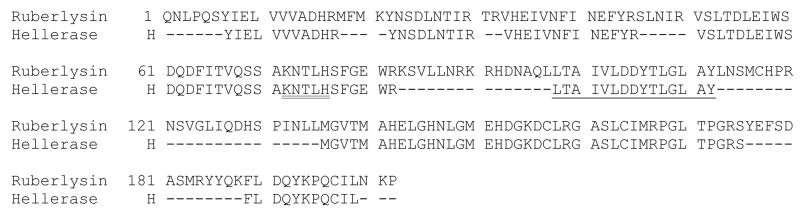
3.6 Partial amino acid sequence of hellerase and comparison with ruberlysin
A total of 57 % of the amino acids of hellerase determined by LC-MS/MS were similar to those of ruberlysin, a metalloproteinase from the venom of C. ruber ruber (Takeya et al., 1990).
4. Discussion
Biological activities with a focus on hemostasis activities of venoms from five specimens of C. o. helleri snakes from close geographical locations are described in this study. Even though the amount of individual samples was limited to five, it could be demonstrated that venoms from the same species in very close proximities vary, both qualitative and quantitatively in their activities. In C.o. helleri venom, the differences in hemorrhagic, neurotoxic, and fibrinolytic activities indicate venom variations in these species, in such that some indeed possess neurotoxic and/or fibrinolytic actions and others do not (Glenn et al., 1983; Sánchez et al, 2005). Many rattlesnake venoms, including C.o. helleri, are considerably more lethal than others mainly because they have neurotoxins (Glenn et al., 1983; French et al., 2004; Sánchez et al., 2005; Jurado et al., 2007).
Crotalus oreganus helleri venoms from Riverside 1, Riverside 2 and San Bernardino 1 presented significant thrombin-like activity, which by the coagulant method was significantly higher in San Bernardino 1 venom. In contrast, by the amidolytic method, Riverside 2 venom was more active (Table 2). This difference may be explained by the presence, in San Bernardino 1 venom, of molecules with thrombin-like activity containing some exosites for the recognition of their natural substrate (fibrinogen), which favors its catalysis as it occurs with the thrombin enzyme (Bock et al., 2007). The highest amidolytic thrombin-like activity in Riverside 2 venom can be explained by other serine proteases containing trypsin-like activity with actions on S-2238 (Friberger, 1982). San Bernardino 2 and 3 venoms were inactive or presented an insignificant thrombin-like activity. These results demonstrate the differences amongst C. o helleri venoms from close localities.
The comparison of activities presented in C.o. helleri venoms a North American rattlesnake with South American rattlesnakes B. atrox and C. durissus cumanensis venoms showed that in general the South American venoms are more procoagulants (Salazar et al., 2007; Aguilar et al., 2007), which could be due to the high presence of thrombin-like activity in these venoms.
Crotalus oreganus helleri venoms also contain fibrinolytic enzymes, which are distinct in the different specimens. The San Bernardino 3 venom showed the highest activity on fibrin plates, in presence or absence of plasminogen, which was inhibited by EGTA, EDTA and 1,10 phenantroline, which have been demonstrated to be specific inhibitors to metal-dependent enzymes classified as metalloproteinases (Salvesen and Nagase, 1989; Fox and Bjarnason, 1998; Salazar et al., 2008). These results demonstrate that fibrinolytic activity is associated with metalloproteinases that act directly on fibrin, similar to fibrinolytic enzymes identified in other snake venoms (Matsui et al., 2000; Swenson and Markland, 2005). In addition, the fibrinolytic activity in this venom, expressed in mm2/μg was lower when 50 μg, in relation to 10 μg, was assayed, indicating the possible presence of plasmin inhibitors. Similar results have been demonstrated in B. atrox venoms (Salazar et al., 2007). This discovery is of great interest since plasmin inhibitors could be applied therapeutically in invasive processes such as viral, parasitic infections and tumor metastasis in which the plasmin activates growth factors, metalloproteinases and degrades of extracellular matrix proteins (Sharma and Sharma, 2007; Verma and Hansch, 2007). Furthermore, the amidolytic activity assays demonstrated that all venoms contain some kallikrein-like activity (Pućkowska, et al., 2008; Petersen, et al., 1990). However, Riverside 1 venom showed the highest kallikrein-like activity, which was neutralized by SPI, indicating the presence of serine proteases that could probably in part activate the plasminogen and thus showing fibrinolytic activity. In relation to the hemolytic activity, the results demonstrate that the Riverside 1 and San Bernardino 2 venoms were the most active, which indicates their high phospholipase concentrations (Condrea and De Vries 1964; Gutiérrez et al., 2005).
Comparing the diverse activities evaluated in these venoms, it was observed that Riverside 2 and San Bernardino 1 venoms showed the highest thrombin-like on plasma and/or purified fibrinogen and lethal activities. Nevertheless, they neither presented hemorrhagic nor fibrinolytic activities. Crotalus oreganus helleri venom is known to contain a neurotoxin similar to that of mojave toxin (French et al., 2004; Mackessy, 1988, 2008). However, visual observation of mice injected with Riverside 2 and San Bernardino 1 venoms showed the possible presence of a crotamine-like toxin (Maeda et al., 1979) due to the spastic paralysis of the hind limbs (Gonçalves, 1956; Cheymol et al., 1971; Chang and Tseng, 1978) that we observed in the mice. Riverside 1 venom also induced such paralysis but in a milder form, which indicates the quantitative differences in toxins amongst these venoms, which is also apparent in the LD50 (Table 1). Therefore, it is possible that a combination of both mojave and crotamine-like toxins are responsible for its potent lethality in these two venoms. In addition, the coagulant activity in concert with these neurotoxins present in some of these venoms can also be responsible for the potent lethality.
San Bernardino 2 and 3 venoms, with the lowest coagulant and lethal activities, presented the highest fibrinolytic and hemorrhagic activities. These activities, as discussed above, may be associated with metalloproteinases. These venoms can induce severe hemorrhagic complications in snake bitten patients due to their high hemorrhagic and/or primary fibrinolytic activities (Matsui et al., 2000; Swenson and Markland, 2005). Currently, the presence of neurotoxins in these two venoms cannot be ruled out unless DNA analysis is performed; however, it can be stated that the quantity of neurotoxins, if present, are significantly lower in comparison with the other venoms.
In the exploration of new components having therapeutic and diagnostic potentials, the San Bernardino 3 venom, which showed the highest fibrinolytic activity, was fractionated in a preparative manner by anionic exchange chromatography on DEAE-5PW column followed by a cationic separation on an SP-5PW column. Anionic fractionation produced a fraction (F1) with very potent fibrinolytic activity (Fig. 4A), which was inhibited by metalloproteinase inhibitors (MPI). This fraction also contained hemorrhagic activity and it lacked amidolytic activities of thrombin- and kallikrein-like. These results support the presence of metalloproteinases with activity against fibrin, a criteria that allowed further separation in order to isolate possible components having therapeutic and diagnostic potentials. Fibrinolytic activity is important because of its potential use as a thrombolytic drug (Chen and Rael, 1997). Most of the snake venom fibrinolytic enzymes are metalloproteinases (Mori et al., 1987; Markland, 1991, 2005; Swenson and Markland, 2005), hence they are less affected by serine protease inhibitors (SERPINS). On the other hand, t-PA, urokinase and plasmin, physiological fibrinolytic enzymes, are serine proteases that can be inhibited by SERPINS (White, 2005).
Fraction 1 of San Bernardino 3 venom (Fig. 4A) was further fractionated by a cationic exchange chromatography and 5 additional fractions were collected (FI to FV; Fig. 4B) and tested for hemorrhagic and fibrinolytic activities. Fractions I and V were active with FV containing the highest activity leaning towards possible metalloproteinases. By observing the highly symmetrical, base line separation of FV (Fig. 4B), it could be assumed a purified protein. However, an SDS 10–20% Tricine gel revealed 3 (non-reducing conditions) to 6 (reducing conditions) additional protein bands for fraction FV at experimental values at 19 kDa and lower (Fig. 2B; lanes 1 and 3). MALDI TOF-TOF revealed a major peak at 23.334 kDa and its double ionized (2H+) partner at 11.666 kDa. The 23 kDa obtained by MALDI is most likely the ~19 kDa obtained by SDS PAGE. The protein bands (1–3) from fraction FV run on SDS gel (Fig. 2B; lane 3) were transferred to a membrane (Fig. 6) for sequencing in order to determine if FV contains various proteins or if it is a product of auto-proteolysis.
The N-terminal sequence for protein band 2 (12 kDa) was KNTLHXGXE, which gave the highest BLAST search identity with a hemorrhagic metalloproteinase HT-2 (ruberlysin) of 23.320 kDa from the venom of C. ruber ruber (Takeya et al., 1990), and the second highest search with a metalloproteinase precursor of 46.644 kDa from the venom of C. molossus molossus. The N-terminal sequence for protein band 3 (9.4 kDa) gave the highest identity match with the C. m. molossus metalloproteinase precursor that was found for band 2. Previous studies have indicated that small proteins (4–14 kDa) isolated from snake venom are disintegrins, phospholipases and/or myotoxins (Mackessy, 2008), which was not the case for band 3. Therefore, it is assumed that cationic fraction FV (Fig. 4B) is one purified protein that is auto-proteolysing into different fragments that are seen in the reduced and non reduced SDS gels (Fig. 2B; lanes 1 and 3). Due to low signal detection during Edman degradation, band 1 rendered only seven amino acids, which were not sufficient to provide an accurate Blast search identity (Altschul, et al, 1997).
In order to further verify auto-proteolysis, FV of cationic exchange was collected in 10 mM EDTA and run on a reduced and non-reduced Tricine SDS gel yielding only one band at ~19 kDa (Fig. 2B; lanes 2 and 4) and 23 kDa by mass spectrometry (data not shown). An in-gel trypsin digestion was done on the hellerase SDS band and subjected to LC-MS/MS, which resulted in 57% sequence similarity to ruberlysin (Fig. 7). Therefore, including the results obtained by Edman degradation, a total of 64% sequence similarity with ruberlysin was achieved. In light of these results, FV could be assumed a single protein, hellerase, which will be subjected to further detailed characterization.
Figure 7.
Amino acid comparison of ruberlysin (Accession #: P20897) with hellerase. Numbered amino acids represent ruberlysin. The letter H represents amino acids from hellerase obtained by LC-MS/MS. Underlined amino acids were obtained by Edman degradation. Double underlined amino acids were obtained by both Edman degradation and LC-MS/MS. The dashes (−) represent no matching amino acids with ruberlysin.
Venom differences have severe inference for snakebite treatment, since diagnosis may perhaps be confused by intraspecies differences in clinical manifestations, and antivenom prepared against venom of a certain species may not be effective against envenomations of specimens of the same species (Chippaux et al., 1991). In conclusion, the hemostatic variations in the venoms of the Southern Pacific Rattlesnakes contribute to our understanding of substrate specificities involved in the hemostasis pathway and further confirm the complex nature of venoms.
Acknowledgments
Financial support was obtained from the Science and Technology Fund (FONACIT) programs (PG- 2005000400; F-2005000212) and IVIC grant (Venezuela), Caracas, Venezuela, and grants to the NTRC at Texas A&M University-Kingsville: NIH/NCRR #1 P40 RR018300-05. We thank Lucy Arispe and Juan Salinas for venom extractions done at the NTRC. We also thank MSc. Zoila Carvajal, Mrs. Amparo Gil, Nora Diaz De Leon, and Angela Wyro for their technical assistance. We are grateful to Drs. Sean P. Bush and William Hayes from Loma Linda University, Loma Linda, CA. for snake donations. Photo of C. o. helleri by Juan C. Lopez-Johnston.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar I, Guerrero B, Salazar AM, Girón ME, Pérez JC, Sánchez EE, Rodríguez-Acosta A. Individual venom variability in the South American rattlesnake Crotalus durissus cumanensis. Toxicon. 2007;50:214–224. doi: 10.1016/j.toxicon.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389 – 3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen D, Rhymes I. A Laboratory Manual of blood coagulation. Oxford: Blackwell Scientific Publications; 1975. [Google Scholar]
- Bajwa S, Markland FS, Russell FE. Fibrinolytic and fibrinogen clotting enzymes present in the venoms of western diamondback rattlesnake, Crotalus atrox, eastern diamondback rattlesnake, Crotalus adamanteus, and southern pacific rattlesnake, Crotalus viridis helleri. Toxicon. 1981;19:53–59. doi: 10.1016/0041-0101(81)90117-3. [DOI] [PubMed] [Google Scholar]
- Bello C, Hermogenes AL, Magalhaes A, Veiga SS, Gremski LH, Richardson M, Sanchez EF. Isolation and biochemical characterization of a fibrinolytic proteinase from Bothrops leucurus (white-tailed jararaca) snake venom. Biochimie. 2006;88:189–200. doi: 10.1016/j.biochi.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Bjarnason J, Tu AT. Hemorrhagic toxins from western diamondback rattlesnake (Crotalus atrox) venom: Isolation and characterization of five toxins and the role of zinc in hemorrhagic toxin e. Biochemistry. 1978;17:3395–3404. doi: 10.1021/bi00609a033. [DOI] [PubMed] [Google Scholar]
- Bjarnason J, Fox JW. Hemorrhagic metalloproteases from snake venoms. Pharmac Ther. 1994;62:325–372. doi: 10.1016/0163-7258(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Bock P, Panizzi P, Verhamme IM. Exosites in the substrate specificity of blood coagulation reactions. J Thromb Haemost. 2007;5(Suppl 1):81–94. doi: 10.1111/j.1538-7836.2007.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D, Graeme KA, Ruha AM, Tanen DA. Respiratory compromise in patients with rattlesnake envenomation. J Emerg Med. 2002;23:329–332. doi: 10.1016/s0736-4679(02)00573-5. [DOI] [PubMed] [Google Scholar]
- Chang C, Tseng KH. Effect of crotamine, a toxin of South American rattlesnake venom, on the sodium channel of murine skeletal muscle. Brit J Pharmacol. 1978;63:551–559. doi: 10.1111/j.1476-5381.1978.tb07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Rael ED. Purification of M5, a fibrinolytic proteinase from Crotalus molossus molossus venom that attacks complement. Int J Biochem Cell Biol. 1997;29:789–799. doi: 10.1016/s1357-2725(96)00139-2. [DOI] [PubMed] [Google Scholar]
- Cheymol J, Gonçalves JM, Bourillet F, Roch-Arveiller M. Action neuromusculaire comparée de la crotamine et du venin de Crotalus durissus terrificus var crotaminus. /I./sur préparations neuromusculaire in situ. Toxicon. 1971;9:279–286. doi: 10.1016/0041-0101(71)90081-x. [DOI] [PubMed] [Google Scholar]
- Chippaux J, Williams V, White J. Snake venom variability: methods of study, results and interpretation. Toxicon. 1991;29:1279–1303. doi: 10.1016/0041-0101(91)90116-9. [DOI] [PubMed] [Google Scholar]
- Condrea E, De Vries A. Hemolysis and splitting of human erythrocyte phospholipids by snake venoms. Biochim Biophys Acta. 1964;84:60–73. doi: 10.1016/0926-6542(64)90101-5. [DOI] [PubMed] [Google Scholar]
- Dempfle C, Argiriou S, Kucher K, Müller-Peltzer H, Rübsamen K, Heene DL. Analysis of fibrin formation and proteolysis during intravenous administration of ancord. Blood. 2000;96:2793–2802. [PubMed] [Google Scholar]
- Didisheim P, Lewis JH. Fibrinolytic and coagulant activities of certain snake venoms and proteases. Proc Soc Exp Biol Med. 1956;93:10–13. doi: 10.3181/00379727-93-22647. [DOI] [PubMed] [Google Scholar]
- D’Suze G, Corona F, Possani LD, Sevcik C. HPLC purification and amino acid sequence of toxins from the muscarinic fraction of Tityus discrepans scorpion venom. Toxicon. 1996;34:591–598. doi: 10.1016/0041-0101(95)00156-5. [DOI] [PubMed] [Google Scholar]
- Estevao-Costa M, Diniz CR, Magalhaes A, Markland FS, Sanchez EF. Action of metalloproteases mutalysis I and II on several components of the hemostatic and fibrinolytic systems. Thromb Res. 2000;99:363–376. doi: 10.1016/s0049-3848(00)00259-0. [DOI] [PubMed] [Google Scholar]
- Fox JW, Bjarnason JB. Metalloproteinase inhibitors. In: Bailey GS, editor. Enzymes from Snake Venoms. Alaken Inc; Fort Collins, CO: 1998. pp. 599–632. [Google Scholar]
- French W, Hayes WK, Bush SP, Cardwell MD, Bader JO, Rael ED. Mojave toxin in venom of Crotalus helleri (Southern Pacific Rattlesnake): molecular and geographic characterization. Toxicon. 2004;44:781–791. doi: 10.1016/j.toxicon.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Friberger P. Chromogenic peptide substrates. Their use for the assay of factors in the fibrinolytic and the plasma kallikrein-kinin systems. Scand J Clin Lab Invest Suppl. 1982;162:1–298. [PubMed] [Google Scholar]
- Glenn J, Straight RC, Wolfe MC, Hardy DL. Geographical variation in Crotalus scutulatus scutulatus (Mojave rattlesnake) venom properties. Toxicon. 1983;21:119–130. doi: 10.1016/0041-0101(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Gold B, Barish RA, Dart RC, Barish RA. Bites of venomous snakes. New Eng J Med. 2002;347:347–356. doi: 10.1056/NEJMra013477. [DOI] [PubMed] [Google Scholar]
- Gonçalves J. Estudos sobre venenos de serpents brasileiras. II-Crotalus terrificus crotaminicus, subespécie biológica. An Acad Bras Cienc. 1956;28:365–367. [Google Scholar]
- Guan A, Retzios AD, Henderson GN, Markland FS., Jr Purification and characterization of a fibrinolytic enzyme from venom of the southern copperhead snake (Agkistrodon contortrix contortrix) Arch Biochem Biophys. 1991;289:197–207. doi: 10.1016/0003-9861(91)90462-r. [DOI] [PubMed] [Google Scholar]
- Guerrero B, Arocha-Piñango CL. Activation of human prothrombin by the venom of Lonomia achelous (Cramer) caterpillars. Thromb Res. 1992;66:169–177. doi: 10.1016/0049-3848(92)90187-f. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Rucavado A, Escalante T, Díaz C. Hemorrhage induced by snake venom metalloproteinases: biochemical and biophysical mechanisms involved in microvessel damage. Toxicon. 2005;45:997–1011. doi: 10.1016/j.toxicon.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Habermann E, Hardt KL. A sensitive and specific plate test for the quantitation of phospholipases. Anal Biochem. 1972;50:163–173. doi: 10.1016/0003-2697(72)90495-2. [DOI] [PubMed] [Google Scholar]
- Johnston M, Fativich DM, Haig AD, Dalys FFS. Successful resuscitation after cardiac arrest following massive brown snake envenomation. Med J Aust. 2002;177:646–649. doi: 10.5694/j.1326-5377.2002.tb04997.x. [DOI] [PubMed] [Google Scholar]
- Jurado J, Rael ED, Lieb CS, Nakayasu E, Hayes WK, Bush SP, Ross JA. Complement inactivating proteins and intraspecies venom variation in Crotalus oreganus helleri. Toxicon. 2007;49:339–350. doi: 10.1016/j.toxicon.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Klauber LR. Their Habits, Life Histories, and Influence on Mankind. University of California Press; Berkeley: 1956. [Google Scholar]; Rattlesnakes, Their Habits, Life Histories, and Influence on Mankind. Berkeley: University of California Press; [Google Scholar]
- Komori Y, Hagihara S, Tu AT. Specificity of hemorrhagic proteinase from Crotalus atrox (western diamondback rattlesnake) venom. Biochim Biophys Acta. 1985;829:127–130. doi: 10.1016/0167-4838(85)90076-7. [DOI] [PubMed] [Google Scholar]
- Langely R. Animal bites and stings reported by United States Poison Control Centers, 2001–2005. Wilderness Environ Med. 2008;19:7–14. doi: 10.1580/07-WEME-OR-111.1. [DOI] [PubMed] [Google Scholar]
- Matsui T, Fujimura Y, Titani K. Snake venom proteases affecting hemostasis and thrombosis. Biochim Biophys Acta. 2000;1477:146–156. doi: 10.1016/s0167-4838(99)00268-x. [DOI] [PubMed] [Google Scholar]
- Moll S, Kenyon P, Bertoli L, De Maio J, Homesley H, Deitcher SR. Phase II trial of alfimeprase, a novel-acting fibrin degradation agent, for occluded central venous access devices. J Clin Oncol. 2006;24:3056–3060. doi: 10.1200/JCO.2006.05.8438. [DOI] [PubMed] [Google Scholar]
- Mori N, Nikai T, Sugihara H, Tu AT. Biochemical characterization of hemorrhagic toxins with fibrinogenase activity isolated from Crotalus ruber ruber venom. Arch Biochem Biophys. 1987;253:108–121. doi: 10.1016/0003-9861(87)90643-6. [DOI] [PubMed] [Google Scholar]
- Mackessy S. Venom Ontogeny in the Pacific Rattlesnakes Crotalus viridis helleri and C. v. oreganus. Copeia. 1988;1988:92–101. [Google Scholar]
- Mackessy S. Venom composition in rattlesnakes: trends and biological significance. Loma Linda: Loma Linda University Press; 2008. [Google Scholar]
- Maeda N, Tamiya N, Pattabhiraman TR, Russell FE. Some chemical properties of the venom of the rattlesnake, Crotalus viridis helleri. Toxicon. 1979;16:431–441. doi: 10.1016/0041-0101(78)90140-x. [DOI] [PubMed] [Google Scholar]
- Markland FJ. Inventory of alpha- and beta- fibrinogenases from snake venom. For the subcommittee on nomenclature of exogenous hemostatic factor of the scientific and standardization committee of the international society on thrombosis and hemostasis. Thromb Haemost. 1991;65:438–443. [PubMed] [Google Scholar]
- Markland F. Snake venom and the hemostatic system. Toxicon. 1998;36:1749–1800. doi: 10.1016/s0041-0101(98)00126-3. [DOI] [PubMed] [Google Scholar]
- Marsh N, Arocha-Piñango CL. Evaluation of the fibrin plate method for estimating plasminogen activators. Thromb Diath Haemorrh. 1972;28:75–88. [PubMed] [Google Scholar]
- MERCK & CO. I. 1998. AGGRASTAT® (Tirofiban hydrochloride injection premixed), AGGRASTAT® (Tirofiban hydrochloride injection). 9123301.
- Omori-Satoh T, Sadahiro S, Ohsaka A, Murata R. Purification and characterization of an antihemorrhagic factor in the serum of Trimeresurus flavoviridis, a crotalid. Biochim Biophys Acta. 1972;285:414–426. doi: 10.1016/0005-2795(72)90328-5. [DOI] [PubMed] [Google Scholar]
- Petersen LC, Boel E, Johannessen M, Foster D. Quenching of the amidolytic activity of one-chain tissue-type plasminogen activator by mutation of lysine-416. Biochemistry. 1990;29:3451–3457. doi: 10.1021/bi00466a005. [DOI] [PubMed] [Google Scholar]
- Pućkowska A, Midura-Nowaczek K, Bruzgo I. Effects of netropsin and pentamidine amino analogues on the amidolytic activity of plasmin, trypsin and urokinase. Acta Pol Pharm. 2008;65:213–215. [PubMed] [Google Scholar]
- Ribeiro L, Puorto G, Jorge MT. Bites by the colubrid snakes Philodryas olfersii: A clinical and epidemiological study of 43 cases. Toxicon. 1999;37:943–948. doi: 10.1016/s0041-0101(98)00191-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues V, Soares AM, Guerra-Sa R, Rodrigues V, Fontes MR, Giglio JR. Structural and functional characterization of neuwiedase, a nonhemorrhagic fibrino(geno)lytic metalloproteinase from Bothrops neuwiedi snake venom. Arch Biochem Biophys. 2000;381:213–224. doi: 10.1006/abbi.2000.1958. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Acosta A, Lemoine K, Navarrete LF, Girón ME, Aguilar I. Experimental ophitoxemia produced by the opisthoglyphous Lora snake (Philodryas olfersii) (Serpentes: Colubridae) venom. Rev Soc Bras Med Trop. 2006;39:193–197. doi: 10.1590/s0037-86822006000200012. [DOI] [PubMed] [Google Scholar]
- Rudolph R, Neal GE, Williams JS, McMahan AP. Snakebite treatment at a Southeastern regional referral center. Am Surg. 1995;61:767–772. [PubMed] [Google Scholar]
- Salazar A, Rodríguez-Acosta A, Girón ME, Aguilar I, Guerrero B. A comparative analysis of the clotting and fibrinolytic activities of the mapanare (Bothrops atrox) snake venom from different geographical areas in Venezuela. Thromb Res. 2007;120:95–104. doi: 10.1016/j.thromres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Aguilar I, Guerrero B, Girón ME, Lucena S, Sanchez EE, Rodríguez -Acosta A. Intraspecies differences in hemostatic venom activities of the South American rattlesnakes, Crotalus durissus cumanensis, as revealed by a range of protease inhibitors. Blood Coagul Fibrinolysis. 2008;19:525–530. doi: 10.1097/MBC.0b013e328304e02e. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Galán JA, Powell RL, Reyes SR, Soto JG, Russell WK, Russell DH, Pérez JC. Disintegrin, hemorrhagic, and proteolytic activities of Mohave rattlesnake, Crotalus scutulatus scutulatus venoms lacking Mojave toxin. Comp Biochem Physiol C. 2005;141:124–132. doi: 10.1016/j.cca.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sharma M, Sharma M. The role of annexin II in angiogenesis and tumor progression: a potential therapeutic target. Curr Pharm Des. 2007;13:3568–3575. doi: 10.2174/138161207782794167. [DOI] [PubMed] [Google Scholar]
- Spearman-Karber R. Alternative methods of analysis for quantal responses. London: Charles Griffin; 1978. [Google Scholar]
- Stocker K. Snake venom proteins affecting hemostasis and fibrinolysis. Boca Raton: CRC Press; 1990. [Google Scholar]
- Swenson S, Markland FS., Jr Snake venom fibrin(ogen)olytic enzymes. Toxicon. 2005;45:1021–1039. doi: 10.1016/j.toxicon.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Takeya H, Onikura A, Nikai T, Sugihara H, Iwanaga S. Primary structure of a hemorrhagic metalloproteinase, HT-2, isolated from the venom of Crotalus ruber ruber. J Biochem. 1990;108:711–719. doi: 10.1093/oxfordjournals.jbchem.a123270. [DOI] [PubMed] [Google Scholar]
- Toombs C. Alfimeprase: Pharmacology of a novel fibrinolytic metalloproteinase for thrombolysis. Hemostasis. 2001a;31:141–147. doi: 10.1159/000048057. [DOI] [PubMed] [Google Scholar]
- Toombs C. New directions in thrombolytic therapy. Curr Opin Pharmacol. 2001b;1:164–168. doi: 10.1016/s1471-4892(01)00030-3. [DOI] [PubMed] [Google Scholar]
- Verma R, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15:2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- White J. Snake venoms and coagulopathy. Toxicon. 2005;45:951–967. doi: 10.1016/j.toxicon.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Willis T, Tu AT. Purification and biochemical characterization of atroxase, a nonhemorrhagic fibrinolytic protease from western diamondback rattlesnake venom. Biochemistry. 1988;27:4769–4777. doi: 10.1021/bi00413a028. [DOI] [PubMed] [Google Scholar]