Abstract
Background and Objectives
Various methods have been suggested to enhance photon density in biological tissues in an attempt to maximize the efficacy of laser therapy. In this study, the effects of tissue compression, glycerol, and micro-needling methods on the laser beam profile (LBP)were investigated by quantitatively evaluating the spatial distribution of subsurface tissue photon density.
Study Design/Materials and Methods
The LBP in tissue was obtained by imaging the laser beam transmitted through ex vivo porcine skin samples. The independent and combinational effects of tissue compression, glycerol, and micro-needling methods on the LBP were evaluated by quantitatively analyzing the full width at half-maximum (FWHM), maximum intensity, and total intensity at FWHM.
Results
Experimental results indicate the enhancement of the quality of Gaussian beam profile in ex vivo porcine skin. Glycerol and tissue compression resulted in an increase of maximum and total intensity and a decrease of FWHM. Tissue compression in conjunction with glycerol was determined to be the most effective method for enhancing the LBP. The topical application of glycerol in conjunction with micro-needling reduced the time period to optically clear tissue, which resulted in a further increase of subsurface tissue photon density.
Conclusion
Tissue compression, glycerol, and micro-needling methods might be used independently or in combination to effectively enhance the photon density delivered to target chromophores in subsurface tissue, thus improving the LBP quality.
Keywords: laser beam profile, compression, microneedling, glycerol, soft tissue
INTRODUCTION
Cellular components in biological tissue have different refractive indices that contribute to light scattering, which limits the photon density delivered to subsurface target chromophores [1]. Recently, there has been growing interest to enhance the subsurface photon density by changing the light scattering property of the tissue [2,3].
Attempts to alter the light scattering properties of biological tissues have been reported using various physicochemical methods, such as compression, stretching, dehydration, coagulation, UV illumination, heating, and impregnation of hyperosmotic chemical agents (HCAs) [4]. In this study, tissue compression, glycerol, and micro-needling methods were considered as being potentially clinically useful to enhance the subsurface tissue photon density.
Since Vargas et al. [5] first reported optical tissue clearing by glycerol, HCAs have been frequently utilized as an effective method to reduce light scattering of tissue and, therefore, improve optical tissue clearing. HCAs have been shown to induce optical tissue clearing by skin dehydration and collagen dissociation or a combination thereof [6,7]. Recently, Rylander et al. [8] demonstrated that skin dehydration induced by HCAs is the dominant mechanism leading to the reduction of light scattering. In another study, Choi and coworkers [9] demonstrated that the reversible change of collagen structure and size due to glycerol has implications on the reduction of light scattering properties.
A number of physical methods in conjunction with HCAs have been investigated in order to enhance the efficacy of optical tissue clearing [10–15]. Recently, Yoon et al. [16] proposed a new physical method: micro-needling in conjunction with topical application of glycerol. The method uses a micro-needle roller which creates numerous artificial micro-channels that accelerate the tissue permeability of glycerol and, therefore, shortens the time period for optical clearing to occur.
During laser therapy, it is a common clinical practice to press the laser probe firmly against the tissue to minimize any air gaps at the tissue–probe interface and, therefore, increase the transmission of the laser beam [17,18].In 1998, Shangguan et al. [18] reported that the light transmission in tissue can be increased by tissue compression which contributes to minimizing the refractive index mismatch between the scatterers and their surrounding medium.
In many dermatologic laser procedures, it is important to destroy selectively target chromophores while at the same time minimizing thermal damage to adjacent dermal structures. However, it is difficult to focus the laser beam directly on the target chromophores in tissue because light scattering causes dispersion of the laser beam and, therefore, limits the effective subsurface photon density delivered to the target. In order to address this problem, this study described herein was focused on enhancing the laser beam profile (LBP) in soft tissue using glycerol, tissue compression, and micro-needling methods independently or in combination. We evaluated the efficacy of the methods by quantitatively analyzing the intensity profile of the laser beam transmitted through ex vivo porcine skin samples.
MATERIALS AND METHODS
Skin Sample Preparation
Fresh ex vivo abdominal porcine skin samples with a small layer of fat were obtained from a local slaughter-house. In order to investigate the reproducibility of the experimental results, three sets of identical experiments were implemented. Each skin sample was 5×5 cm2 in size.
Experimental Setup
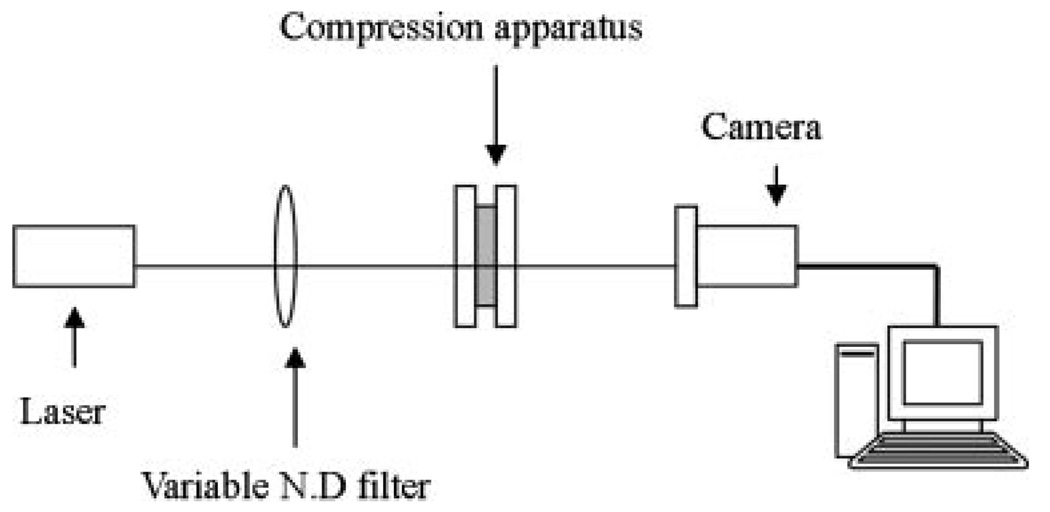
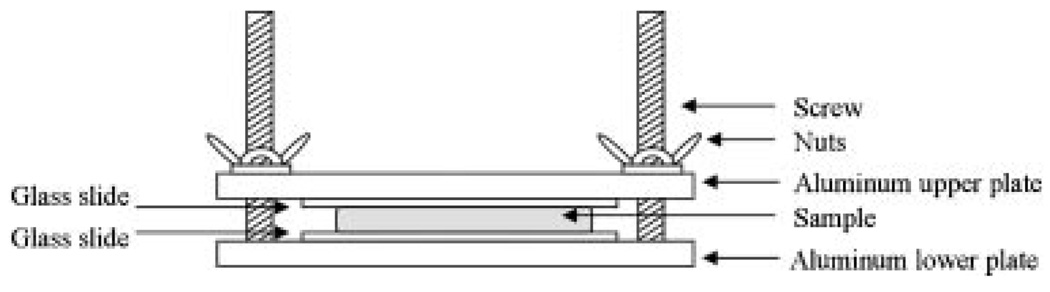
Figure 1 shows a schematic diagram of the optical setup used to image the LBP which consisted of a collimated diode laser (532 nm, 5 mW), neutral density filter, tissue compression apparatus, and monochromatic CCD camera (648×494 pixels). The tissue compression apparatus (Fig. 2) was built to apply uniform compression to the skin samples [17,18]. Samples were placed between two glass slides, and constant compression was applied by adjusting a set of screws and nuts. The amount of compression applied on the samples was indirectly determined by measuring the skin thickness between two glass slides using a micrometer.
Fig. 1.
Optical setup for measurement of the LBP consisting of a collimated laser, neutral density filter, tissue compression apparatus, and monochromatic CCD camera.
Fig. 2.
Schematic diagramof the tissue compression apparatus.
Measurement of the Laser Beam Profile
Images of the LBP were subsequently acquired for the following cases from each sample: (1) the LBP in an intact skin sample was measured as a baseline control; (2) the LBP was measured after the skin sample was compressed to a thickness of 2 mm; (3) the skin sample was decompressed and 0.05 ml of 95%-glycerol was topically applied on the epidermal side of the skin sample. After 1 hour of glycerol application, theLBP was measured after removing any residual glycerol from the skin surface; (4) the skin sample of the case (3) was compressed to a thickness of 2 mm, and the LBP was measured.
The effect of micro-needling on the LBP was investigated only for glycerol application without tissue compression. The test (control) sample was (was not) treated with a micro-needle roller. The control and test samples received a topical application of 0.05 ml of 95%-glycerol on the epidermal skin side, letting the glycerol naturally diffuse into the samples. After 1 hour, the LBP was measured on both samples after removing any residual glycerol from the skin surface.
Quantitative Evaluation of the Laser Beam Profile
The LBP was quantitatively evaluated by analyzing the following three parameters: full width at half-maximum (FWHM), maximum intensity, and total intensity at FWHM. Each of the parameters was computed as follows: (1) the image of 120×100 pixels representing the LBP was extracted from the monochromatic CCD image; (2) two-dimensional (2-D) LBP was obtained by averaging the column pixels of the extracted image in order to minimize the distortion of the LBP due to the morphological variation in the skin samples. The LBP intensity was indexed from 0 to 255.
RESULTS
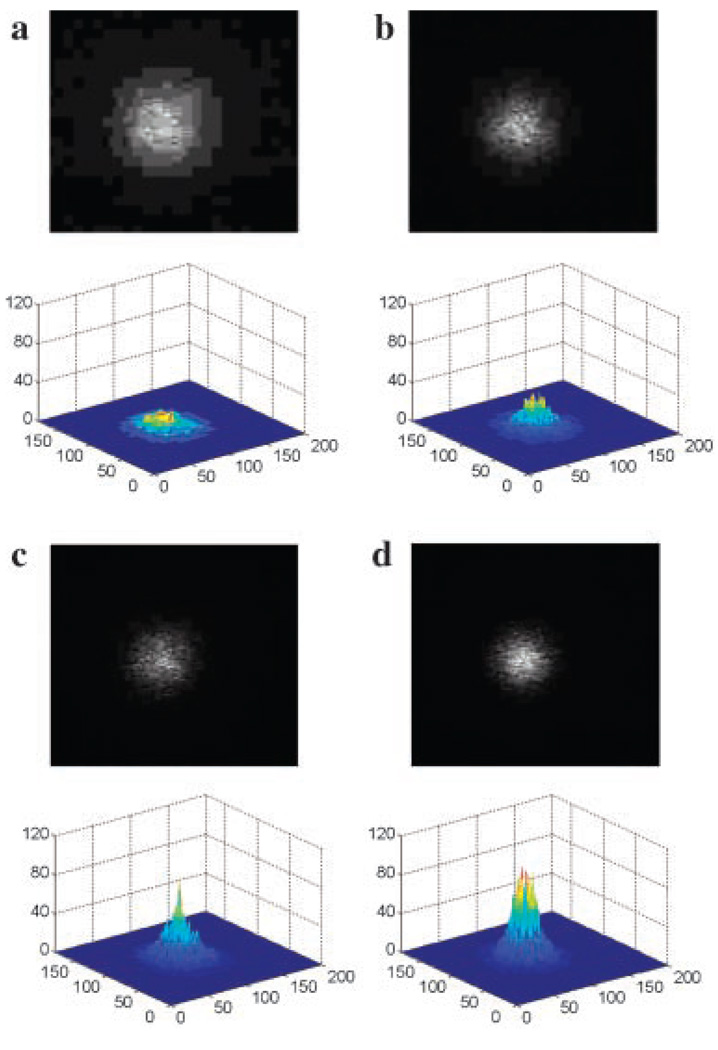
Three sets of identical experiments were performed to evaluate the LBP. Average skin sample thickness was 3.72 mm. Figure 3 shows examples of the LBP in which CCD (upper images) and 3-D images (lower images) were presented for the subsequent four cases described in “Measurement of the Laser Beam Profile Section.”
Fig. 3.
CCD images (upper images) and 3-dimensional LBP (lower images) for the following cases: (1) LBP in an intact skin sample measured as a baseline control; (2) LBP measured after the skin sample was compressed to a thickness of 2 mm; (3) skin sample was decompressed and 0.05 ml of 95%-glycerol was topically applied onto the skin surface. After 1 hour of glycerol application, LBP was measured after removing any residual glycerol from the skin surface; and (4) the skin sample of case (3) was compressed to a thickness of 2mmand the LBP measured.
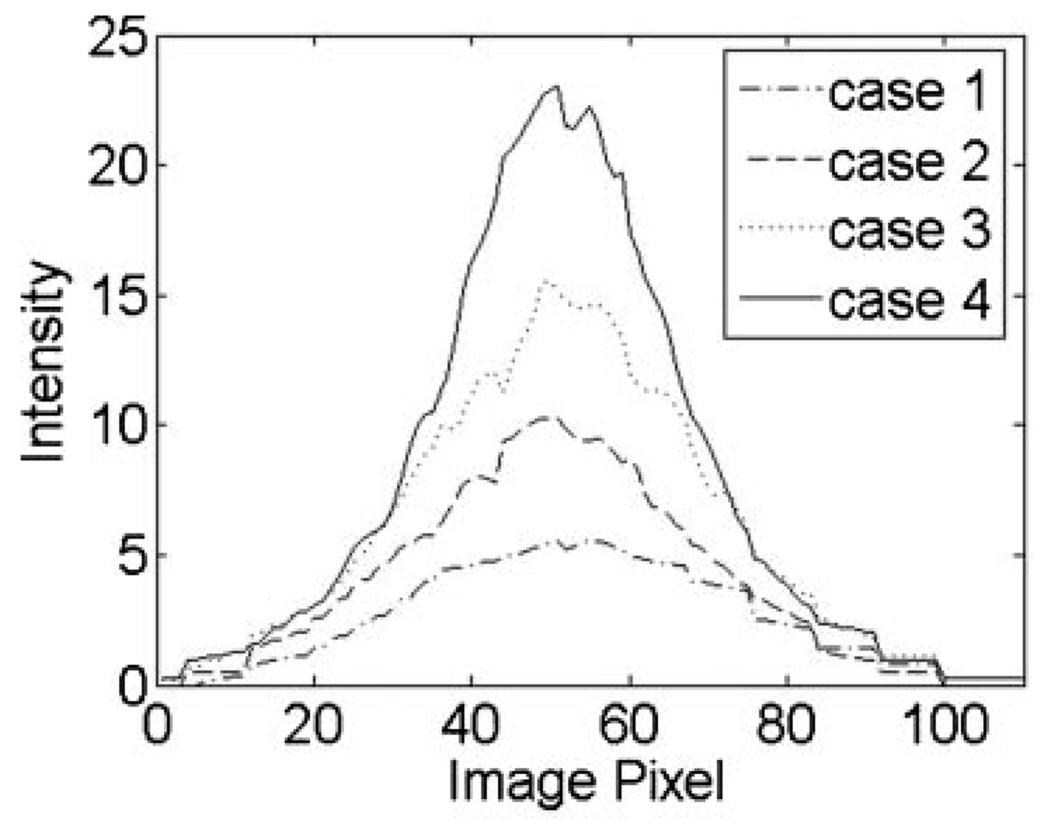
The 3-D images show intuitive visualization of the LBP, which illustrates gradual improvement in the quality of the Gaussian beam profile. The images also qualitatively show a gradual increase of maximum and total intensity and a decrease of FWHM from cases (1) to (4). In order to quantitatively evaluate the LBP, the 2-D LBP was computed for each case. Figure 4 shows the examples of the 2-D LBPs.
Fig. 4.
Examples of 2-dimensional LBP for the four cases described in Figure 3.
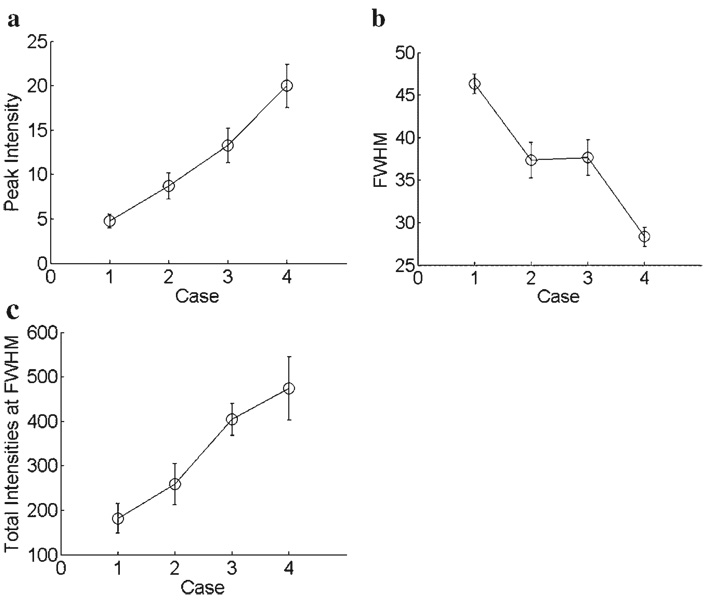
Figure 5 shows the results for the three parameters of LBP. Maximum and total intensity gradually increased from cases (1) to (4) (Fig. 5a and c). Compared to case (1), case (4) resulted in an increase in the maximum intensity by a factor of 4.2 [case (1): 4.7; case (4): 19.9] and an increase in the total intensity by a factor of 2.6 [case (1): 182; case (4): 474]. FWHM decreased when compression and glycerol were applied onto the skin surface. Both cases (2) and (3) resulted in an approximately 0.2-fold decrease in the FWHM (Fig. 5b) when compared to case (1). FWHM was further decreased in case (4), which resulted in an approximately 0.4-fold decrease in the FWHM (Fig. 5b). As a result, the combination of compression and glycerol, namely case (4), was experimentally determined to be the most effective method to enhance the LBP.
Fig. 5.
Evaluation of (a) peak intensity, (b) FWHM, and (c) total intensity at FWHM of the LBP for the four cases described in Figure 3.
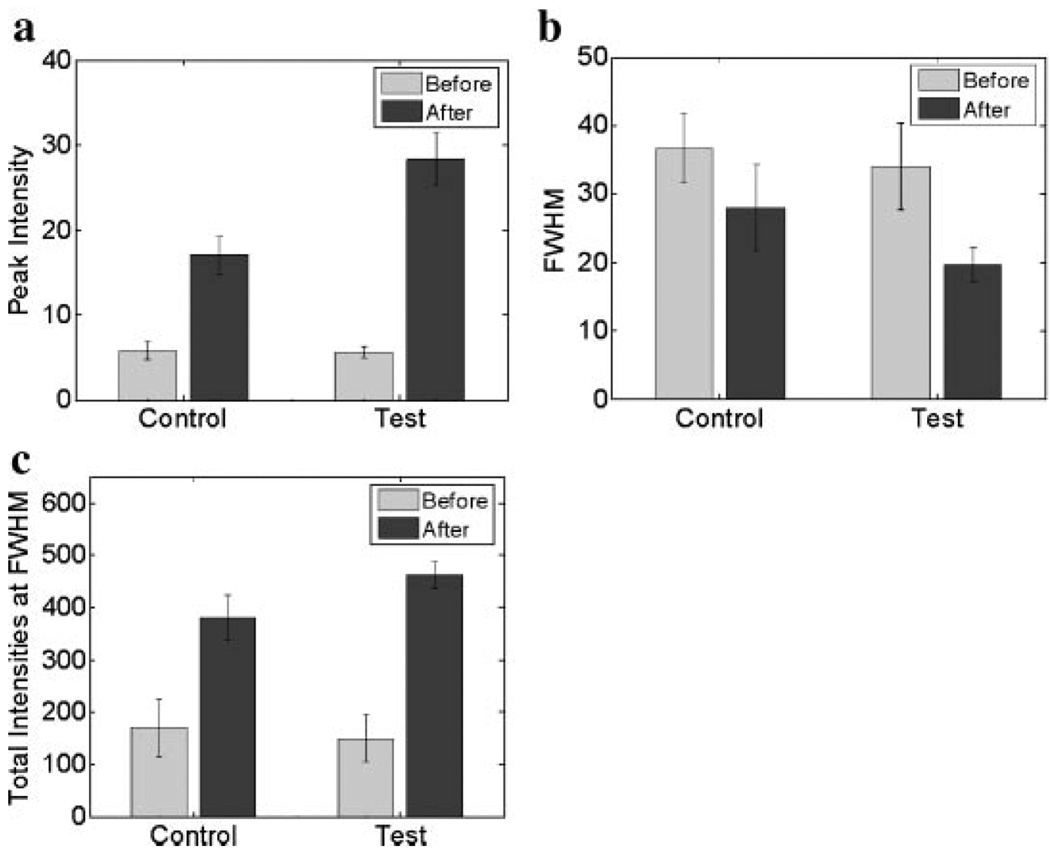
The efficacy of the micro-needling method on LBP was investigated 1 hour after glycerol was applied onto the control and test samples. The average thickness of samples was 3.1 mm. Figure 6 shows the experimental results. FWHM of the test samples was not significantly changed when compared to the control samples. The maximum intensity in the test and control samples increased by a factor of 5.6 and 3.4, respectively. The total intensity in the test and control samples increased by a factor of 3.1 and 2.2, respectively. The results clearly showed that there was an increase in the maximum and total intensity by a factor of 1.5 in the test samples as compared to the control, demonstrating the potential of the micro-needing method to enhance optical clearing of skin.
Fig. 6.
Evaluation of (a) peak intensity, (b) FWHM, and (c) total intensity at FWHM of the LBP on the control (test) samples which were (were not) treated with the micro-needle roller.
DISCUSSION
The quality of the laser beam is important during dermatologic laser procedures. Unlike previous studies, which addressed only the intensity measurement due to glycerol or compression [17,19], this study evaluates the characteristics of the LBP in tissue due to compression, glycerol, and micro-needling methods independently and in combination. The experimental results indicate the potential of the three methods to enhance LBP in tissue.
In Figure 5, case (2) illustrates the potential of tissue compression for the enhancement of LBP in which the maximum and total intensity increased and FWHM decreased with compression. Such results suggest that tissue compression can narrow the FWHM of the laser beam in tissue, increasing the photon density delivered to target chromophores. The results can be partially explained by a previous study in which Chan et al. [17] experimentally demonstrated that overall light transmittance in tissue can be increased by compression. In most studies, compression was uniformly applied over the entire tissue. However, it is general clinical practice to apply a partial tissue compression depending on the size of the laser probe. Therefore, it should be valuable to investigate the effect of partial tissue compression in the LBP [20]. For the study, we are developing a mathematical model and compression controlled optical fiber probe.
In Figure 5, glycerol application [case (3)] resulted in the increase of the maximum and total intensity and the decrease of the FWHM. When compared to the compression result [case (2)], case (3) illustrates that the photon density in tissue can be further increased by glycerol application and that the light scattering reduction by glycerol application resulted in greater transmission of the laser beam than the optical pathlength reduction by tissue compression. On the other hand, the FWHM was similar to that of case (2), potentially suggesting that glycerol application compared to the compression case only affects the intensity. It is well known that glycerol contributes to the reduction of light scattering by minimizing refractive index mismatching between cellular components and their surrounding medium in the dermis and, therefore, the penetration depth of light and the spatial resolution of bioluminescence imaging can be increased [1,21,22].
In Figure 5, case (4) shows the effect of the combination of glycerol and compression. When compared to case (3), the maximum and total intensity of the laser beam were further increased with compression, illustrating that light scattering in tissue pretreated with glycerol can be further reduced by compression. When compared to cases (2) and (3), FWHM was further reduced by combining both methods. Such results show that the compression applied on the tissue pretreated with glycerol can further increase the photon density delivered to target chromophores, even reducing FWHM.
The objective of laser surgical procedures is to deliver an absorbed photon density while confining thermal damage to specific targets. However, high photon density is usually required to produce the desired therapeutic outcome for target chromophores (e.g., port wine stain) in deep tissue layer. In such cases, cryogen spray cooling is frequently utilized to minimize the thermal damage (scarring and dyspigmentation) to the superficial skin layers as a result of the high photon density used [23]. Well-controlled tissue compression might maximize therapeutic outcome by increasing the photon density delivered to target chromophores while minimizing thermal damage to surrounding cellular constituents. With tissue compression, glycerol can be simultaneously applied in order to deliver more photon density to target chromophores.
Glycerol application in conjunction with the micro-needling method was determined to improve the efficacy of optical tissue clearing when compared to application of glycerol only. Shown in Figure 6 after 1 hour of glycerol application, the test sample resulted in an approximately 1.5-fold higher intensity when compared to the control sample. Asimilar result was observed in our previous study [16]. This result means that the combination method can shorten the time required for optical tissue clearing to occur, even resulting in a similar optical tissue clearing effect as in the glycerol application only. Khan et al. [1] clinically demonstrated the potential of a HCA, prepolymer mixture of polypropylene glycol and polyethylene glycol (PPG:PEG) for the treatment of human skin tattoos. In that study, skin was treated with topically applied PPG:PEG for 2 hours prior to laser treatment. In a tattoo removal study done by McNichols et al. [24], glycerol was intradermally injected and transdermally applied using a tape stripping method to a guinea pig tattoo model. However, intradermal glycerol injection resulted in scarring, necrosis, and ulceration after 36–72 hours, and tape stripping transdermal application of glycerol requires a long time period for optical tissue clearing because it depends on natural diffusion of glycerol. The micro-needling method could be employed in conjunction with a HCA in order to shorten the time period that was required for the optical tissue clearing to occur, minimizing side effects found in intradermal glycerol injection. We are currently conducting a study with an in vivo animal tattoo model in order to investigate the clinical usefulness of the micro-needling method in conjunction with glycerol.
The tissue response for laser irradiation depends on wavelength, power density, and pulse duration of the laser, and the optical and thermal properties of the tissue [25]. Our study focused on altering the tissue light scattering properties using three methods (compression, glycerol, and micro-needling). These methods can be utilized independently or in combination to effectively increase the photon density in tissue.
In conclusion, the use of glycerol in conjunction with micro-needling and tissue compression can deliver more photons to deeply located chromophores by effectively improving the LBP in tissue. Therefore, these methods should be considered as important parameters in order to maximize the therapeutic outcome of dermatologic laser procedures.
ACKNOWLEDGMENTS
Contract grant sponsor: Ministry of Knowledge Economy of the Korean Government (Next Generation New Technology Development Program); Contract grant number: 10028424; Contract grant sponsor: National Institutes of Health; Contract grant numbers: AR47751, EB 2495.
This research was supported by a grant from the Next Generation New Technology Development Program (10028424) provided by the Ministry of Knowledge Economy of the Korean Government. JSN was supported by the following grants from the National Institutes of Health (AR47751 and EB 2495).
REFERENCES
- 1.Khan MH, Choi B, Chess S, Kelly KM, McCullough J, Nelson JS. Optical clearing of in vivo human skin: Implications for light-based diagnostic imaging and therapeutics. Laser Surg Med. 2004;34(2):83–85. doi: 10.1002/lsm.20014. [DOI] [PubMed] [Google Scholar]
- 2.Khan MH, Chess S, Choi B, Kelly KM, Nelson JS. Can topically applied optical clearing agents increase the epidermal damage threshold and enhance therapeutic efficacy? Laser Surg Med. 2004;35(2):93–95. doi: 10.1002/lsm.20078. [DOI] [PubMed] [Google Scholar]
- 3.Tuchin VV. A clear vision for laser diagnostics. IEEE J Sel Top Quant Electron. 2007;13:1621–1628. [Google Scholar]
- 4.Tuchin VV, Altshuler GB, Gavrilova AA, Pravdin AB, Tabatadze D, Childs J, Yaroslavsky IV. Optical clearing of skin using flashlamp-induced enhancement of epidermal permeability. Laser Surg Med. 2006;38:824–836. doi: 10.1002/lsm.20392. [DOI] [PubMed] [Google Scholar]
- 5.Vargas G, Chan EK, Barton JK, Rylander HGI, Welch AJ. Use of an agent to reduce scattering in skin. Laser Surg Med. 1999;24(2):133–141. doi: 10.1002/(sici)1096-9101(1999)24:2<133::aid-lsm9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Hirshburg JCB, Nelson JS, Yeh AT. Collagen solubility correlates with skin optical clearing. J Biomed Opt Lett. 2006;11(4):040501. doi: 10.1117/1.2220527. [DOI] [PubMed] [Google Scholar]
- 7.Liu HBB, Kimura M, Chance M. Dependence of tissue optical properties on solute-induced changes in refractive index and osmolarity. J Biomed Opt. 1996;1(2):200–211. doi: 10.1117/12.231370. [DOI] [PubMed] [Google Scholar]
- 8.Rylander CG, Stumpp OF, Milner TE, Kemp NJ, Mendenhall JM, Diller KR, Welch AJ. Dehydration mechanism of optical clearing in tissue. J Biomed Opt. 2006;11(4):041117. doi: 10.1117/1.2343208. [DOI] [PubMed] [Google Scholar]
- 9.Yeh AT, Choi B, Nelson JS, Tromberg BJ. Reversible dissociation of collagen in tissues. J Invest Dermatol. 2003;121(6):1332–1335. doi: 10.1046/j.1523-1747.2003.12634.x. [DOI] [PubMed] [Google Scholar]
- 10.Nelson JS, McCullough JL, Glenn TC, Wright WH, Liaw LH, Jacques SL. Midinfrared laser ablation of stratum corneum enhances in vitro percutaneous transport of drugs. J Invest Dermatol. 1991;97(5):874–879. doi: 10.1111/1523-1747.ep12491600. [DOI] [PubMed] [Google Scholar]
- 11.Henry S, McAllister DV, Allen MG, Prausnitz MR. Micro-fabricated microneedles: A novel approach to transdermal drug delivery. J Pharm Sci. 1998;87(8):922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 12.He Y, Wang RK. Dynamic optical clearing effect of tissue impregnated with hyperosmotic agents and studied with optical coherence tomography. J Biomed Opt. 2004;9(1):200–206. doi: 10.1117/1.1629682. [DOI] [PubMed] [Google Scholar]
- 13.Fang JY, Lee WR, Shen SC, Fang YP, Hu CH. Enhancement of topical 5-aminolaevulinic acid delivery by erbium:YAG laser and microdermabrasion: A comparison with iontophoresis and electroporation. Br J Dermatol. 2004;151(1):132–140. doi: 10.1111/j.1365-2133.2004.06051.x. [DOI] [PubMed] [Google Scholar]
- 14.Stumpp OF, Welch AJ, Milner TE, Neev J. Enhancement of transepidermal skin clearing agent delivery using a 980 nm diode laser. Lasers Surg Med. 2005;37(4):278–285. doi: 10.1002/lsm.20237. [DOI] [PubMed] [Google Scholar]
- 15.Stumpp O, Chen B, Welch AJ. Using sandpaper for noninvasive transepidermal optical skin clearing agent delivery. J Biomed Opt. 2006;11(4):041118. doi: 10.1117/1.2340658. [DOI] [PubMed] [Google Scholar]
- 16.Yoon JH, Son TY, Choi EH, Choi B, Nelson JS, Jung BJ. Enhancement of optical clearing effect of skin using a micro-needle roller. J Biomed Opt. 2008;13(2):021103. doi: 10.1117/1.2907483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan EK, Sorg B, Protsenko D, O’Neil M, Motamedi M, Welch AJ. Effects of compression on soft tissue optical properties. IEEE J Sel Top Quant. 1996;2(4):943–950. [Google Scholar]
- 18.Shangguan H, Prahl SA, Jacques SL, Casperson LW, Gregory KW. Pressure effects on soft tissues monitored by changes in tissue optical properties. Proc SPIE. 1998;3254:366–371. [Google Scholar]
- 19.He Y, Wang RK. Improvement of low-level light imaging performance using optical clearing method. Biosens Bioelectron. 2004;20(3):460–467. doi: 10.1016/j.bios.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Tuchin VV. Optical clearing of tissues and blood. Bellingham: SPIE Press; 2005. [Google Scholar]
- 21.He Y, Wang RK, Xing D. Enhanced sensitivity and spatial resolution for in vitro imaging with low-level light-emitting probes by use of biocompatible chemical agents. Opt Lett. 2003;28(21):2076–2078. doi: 10.1364/ol.28.002076. [DOI] [PubMed] [Google Scholar]
- 22.Jansen ED, Pickett PM, Mackanos MA, Virostko J. Effect of optical tissue clearing on spatial resolution and sensitivity of bioluminescence imaging. J Biomed Opt. 2006;11(4) doi: 10.1117/1.2337651. 041119-1–7. [DOI] [PubMed] [Google Scholar]
- 23.Chang CJ, Nelson JS. Cryogen spray cooling and higher fluence pulsed dye laser treatment improve port-wine stain clearance while minimizing epidermal damage. Dermatol Surg. 1999;25(10):767–772. doi: 10.1046/j.1524-4725.1999.99100.x. [DOI] [PubMed] [Google Scholar]
- 24.McNichols RJ, Fox MA, Gowda A, Tuya S, Bell B, Motamedi M. Temporary dermal scatter reduction: Quantitative assessment and implications for improved laser tattoo removal. Lasers Surg Med. 2005;36:289–296. doi: 10.1002/lsm.20152. [DOI] [PubMed] [Google Scholar]
- 25.Grossweiner LI. Phototherapy light sources. New York: Springer Verlag; 2005. [Google Scholar]