Abstract
Background
The prevalence of metabolic syndrome is high and is increasing in parallel with increasing incidences of breast and prostate cancers. The combination of soy with tea was shown to have synergistic effects on preventing breast and prostate tumors, but the effects of soy and tea combinations on metabolic syndrome–related elements have not been investigated.
Objective
We aimed to determine the effects of soy and tea components, alone and in combination, on abdominal adipose mass and serum concentrations of adipokines, growth factors, and sex hormones in male and female mice.
Design
Male and female FVB/N mice were treated with soy, tea components, or both. Food intake and body weight were monitored weekly. At the end of the experiment, abdominal white adipose tissue was weighed, and serum concentrations of biomarkers were measured.
Results
Whole teas, but not the tea polyphenol extracts, significantly reduced abdominal white adipose tissue by 43–60% in female mice and by 65–70% in male mice. The combination of soy phytochemical concentrate and green tea reduced serum insulin-like growth factor-I concentrations in both male and female mice in a synergistic manner. The soy phytochemical concentrate and tea combinations reduced serum estrogen concentrations in female mice in a synergistic manner. Soy phytochemical concentrate and teas also significantly reduced serum leptin concentrations in both male and female mice and testosterone concentrations in male mice.
Conclusion
Further research is warranted to investigate whether soy and tea combinations may prevent breast or prostate cancer in a synergistic manner in part by alleviating metabolic disorders.
Keywords: Metabolic syndrome, cancer prevention, tea, soy, insulin-like growth factor-I, IGF-I, leptin, synergy
INTRODUCTION
The metabolic syndrome (MS) is characterized by visceral or intraabdominal obesity, glucose intolerance, hypertension, low serum HDL-cholesterol, and high serum triacylglycerols. The prevalence of MS is high and increases in parallel with increased risks of certain types of cancer, such as breast cancer and prostate cancer (1, 2). Recent epidemiologic studies have shown associations between MS or MS-related elements and increased risks of breast cancer (3–5) and prostate cancer (6–9), which suggests that MS may be an important etiologic risk factor for these cancers.
Despite established epidemiologic associations between MS and increased risks of breast and prostate cancers, little experimental evidence is available to support whether MS plays a direct causal role in breast or prostate cancer. On the other hand, previous experimental research has provided mechanistic plausibility that MS may play an important role in cancer development, progression, or both. MS is associated with altered concentrations of adipokines, growth factors, and sex hormones. Insulin resistance is considered to be the underlying factor for MS. Evidence has linked chronic hyperinsulinemia to greater cancer risks (2). Obesity is associated with higher concentrations of insulin (10–12). Elevated serum insulin concentrations increase the concentration or bioavailability of insulin-like growth factor-I (IGF-I), which also plays a critical role in the development of some breast and prostate cancers (13–16).
Considerable data from animal studies indicate that combinations of agents can be more effective for cancer prevention than any single constituent (17). Research suggests that increased consumption of soybean products or tea contributes to the lower risks of breast and prostate cancers in Asian populations than in industrialized Western nations (18, 19). Asian people consume soy products and tea regularly. It is possible that the effective cancer prevention activity of Asian diets may result at least in part from interactions between soy and tea active components.
We evaluated the effects of soy and tea components on breast and prostate cancers in clinically relevant animal models (20, 21). The combination of soy phytochemical concentrate (SPC) and black tea (BT) synergistically inhibited prostate tumorigenicity, final tumor weight, and metastases to lymph nodes (20). The combinations of soy phytochemicals and tea [BT or green tea (GT)] also inhibited the growth of breast tumors (21) in a synergistic or an additive manner. The combinations also synergistically reduced serum IGF-I concentrations (21) and androgen concentrations (20).
To further define the activity of soy and tea combinations in the prevention of breast and prostate cancers and to determine whether modulation of metabolic elements is in part responsible for the cancer prevention activity of the combination regimens, we evaluated the combined effects of soy and tea components on abdominal white adipose tissue (WAT) and serum concentrations of adipokines (leptin, adiponectin), growth factors (insulin, IGF-I), and sex hormones (androgen in male mice or estrogen in female mice) in mice consuming a normal-fat diet.
MATERIALS AND METHODS
Soy and tea components and experimental diets
Both GT (China Green Tea, Shanghai Tea Import and Export Corporation, Shanghai, China) and BT (Keemun Black Tea, Shanghai Tea Branch, China National Native Produce and Animal By-Products Import and Export Corporation, Shanghai, China) were purchased from a local supermarket. BT extract (BTE) and GT extract (GTE) were prepared by hot water extraction of BT and GT leaves, respectively, followed by freeze-drying. Both BT polyphenols (BTPs) and GT polyphenols (GTPs) were prepared by extraction of tea polyphenols from BTE and GTE, respectively. The tea polyphenols were analyzed by HPLC. The typical compositions of these tea components are shown in Table 1.
TABLE 1.
HPLC analysis of tea polyphenol compositions in typically prepared black tea extract (BTE), black tea polyphenols (BTPs), green tea extract (GTE), and green tea polyphenols (GTPs)1
| Tea component | BTE | BTPs | GTE | GTPs |
|---|---|---|---|---|
| % of Solids | ||||
| EC | 1 | 4.4 | 3.8 | 8.7 |
| EGC | 1 | 1.6 | 8.7 | 13.9 |
| ECG | 2 | 12.7 | 4.3 | 7.6 |
| EGCG | 3 | 20.1 | 15.1 | 47.7 |
| TF | 1 | 4.3 | NA | NA |
| TF-3-gallate | <1 | 4.7 | NA | NA |
| TF-3'-gallate | <1 | 2.5 | NA | NA |
| TF-3,3'-digallate | <1 | 3.8 | NA | NA |
| Gallic acid | 1 | 3.2 | 0.2 | 0.2 |
| Caffeine | 7 | 0.6 | 5.4 | 1.2 |
| Total polyphenols | 12 | 57.3 | 32.1 | 78.1 |
EC, epicatechin; EGC, epigallocatechin; ECG, epigallocatechin gallate; EGCG, epigallocatechin gallate; NA, not analyzed; TF, theaflavin.
Four soybean components that represent commonly used soybean products were evaluated: 1) isoflavone-depleted soy protein isolates (SPIs), representing soy protein and containing 0.002 mg isoflavone aglycones/g material; 2) high-isoflavone-containing SPIs, representing a commonly used soy protein product in food industry and scientific research and containing 2.67 mg isoflavone aglycones/g material; 3) the soy phytochemicals extract SPC, representing the soy phytochemicals profiles commonly consumed in soyfoods and containing 51.9% soy isoflavones by weight (50.8% genistein aglycone equivalents, 40.5% daidzein aglycone equivalents, and 8.7% glycitein aglycone equivalents; other phytochemicals not quantified); and 4) the soy isoflavone genistein, a proposed active component in soy. Both isoflavone-depleted and high-isoflavone-containing SPIs were provided by Solae Co (St Louis, MO),SPCwas provided by Archer Daniels Midland Co (Decatur, IL), and genistein was purchased from LC Laboratories (Woburn, MA).
Diet formulations and treatment groups
The soy and tea components were used to prepared the experimental diets by Research Diets Inc (New Brunswick, NJ) for the following treatment groups: 1) AIN-93G as the control diet; 2) AIN-93 with isoflavone-depleted SPIs in place of casein, 20% by weight; 3) AIN-93 with high-isoflavone-containing SPIs in place of casein, 20% by weight; 4) AIN-93 with the addition of genistein at 0.07% of the diet; 5) AIN-93 with the addition of SPC at 0.5% of the diet; 6) AIN-93 with 1.2% BT infusion in place of drinking water; 7) AIN-93 with 1.2% GT infusion in place of drinking water; 8) AIN-93 with the addition of BTPs at 0.2% of the diet; 9) AIN-93 with addition of GTPs at 0.2% of the diet; 10) AIN-93 with 0.5% SPC and 1.2% BT infusion; and 11) AIN-93 with 0.5% SPC and 1.2% GT infusion.
Animal study 1: effects of soy and tea components on abdominal WAT, adipokines, IGF-I, and estrogen in female mice
Female FVB/N mice (5–6 wk old) were randomly assigned into the experimental groups (n = 12 per group) and were treated with the corresponding experimental diets for 8 wk. Food intake and body weight were measured weekly. At the end of the experiment, 3-h fasting blood samples were collected, the animals were killed, and abdominal WAT and liver were collected and weighed. Serum concentrations of adipokines (leptin, adiponectin), growth factors (insulin and IGF-I), and estrogen were measured by using commercially available radioimmunoassay or enzyme-linked immunosorbent assay kits by following the procedures provided by the manufacturers (Diagnostic Systems Laboratories, Inc, Webster, TX, and Linco Research Inc, St Louis, MO). All procedures with animals were reviewed and approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center according to National Institutes of Health guidelines.
Animal study 2: effects of soy and tea components on abdominal WAT, adipokines, IGF-I, and testosterone in male mice
The animal study was conducted by using the same experimental protocol as in animal study 1 except that male mice, rather than female mice, were used.
Statistical analysis
Measured values are expressed as means ± SEMs. The STAT-VIEW 5.0 program (SAS Institute Inc, Cary, NC) was used to calculate two-sided comparisons among experimental groups through analysis of variance followed by Tukey’s test to determine significance between the treatment group and the control group (22). A P value of < 0.05 was considered significant. The nature of the combined effects of SPC and tea was determined by using the method described by us and other laboratories (20, 21, 23). In brief, the expected value of a combination effect between treatment 1 and treatment 2 is calculated as [(observed treatment 1 value)/(control value)] × [(observed treatment 2 value)/(control value)]×(control value); the combination index is calculated as the ratio (expected value)/(observed value). A ratio of > 1 indicates a synergistic effect, and a ratio of <1 indicates a less than additive effect.
RESULTS
Effects of soy and tea treatments on food intake, body weight, and abdominal WAT mass in mice
The effects of different soy and tea components on food intake, body weight, and WAT weight in both male and female mice are shown in Table 2. Male mice consumed more diets, gained more weight, but had less WAT mass than did female mice. All experimental diets were well consumed by the animals. In fact, the mice in some treatment groups consumed even more food than did the controls. Male mice in the BTP, BT, and GT and SPC combination groups consumed 28.3% (P < 0.0005), 12.9% (P < 0.05), 16.7% (P < 0.005), and 16.1% (P < 0.005) more food, respectively, than did the control group. Female mice in the SPC and GT combination group consumed 13.6% (P < 0.05) more food than did the control group.
TABLE 2.
Effects of soy and tea components on food intake, body weight, and abdominal white adipose tissue mass (WAT) in male or female mice1
| Food intake | Final body weight | WAT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Male | Female | Male | Female | Male | Female | |||
| g/d | g | g | |||||||
| Control | 3.11 ± 0.26 | 2.50 ± 0.21 | 29.7 ± 1.5 | 24.4 ± 2.0 | 0.77 ± 0.23 | 0.90 ± 0.28 | |||
| IDSPIs | 3.32 ± 0.24 | 2.64 ± 0.23 | 30.5 ± 1.3 | 24.2 ± 1.5 | 0.26 ± 0.112 | 0.75 ± 0.21 | |||
| HISPIs | 3.13 ± 0.09 | 2.51 ± 0.20 | 29.7 ± 1.6 | 23.9 ± 2.4 | 0.44 ± 0.152 | 0.71 ± 0.233 | |||
| Genistein | 3.13 ± 0.34 | 2.43 ± 0.22 | 29.2 ± 2.4 | 23.6 ± 1.5 | 0.48 ± 0.314 | 0.71 ± 0.183 | |||
| SPC | 3.00 ± 0.23 | 2.32 ± 0.25 | 28.6 ± 1.8 | 24.0 ± 1.9 | 0.64 ± 0.28 | 0.82 ± 0.22 | |||
| BTPs | 3.99 ± 0.312 | 2.48 ± 0.31 | 29.0 ± 2.6 | 23.0 ± 1.33 | 0.62 ± 0.42 | 0.79 ± 0.19 | |||
| GTPs | 3.49 ± 0.34 | 2.68 ± 0.37 | 31.0 ± 2.6 | 23.7 ± 1.23 | 0.86 ± 0.23 | 0.85 ± 0.22 | |||
| BT | 3.51 ± 0.113 | 2.77 ± 0.41 | 27.4 ± 2.63 | 21.1 ± 1.72 | 0.23 ± 0.102 | 0.36 ± 0.172 | |||
| GT | 3.63 ± 0.104 | 2.72 ± 0.33 | 27.1 ± 2.24 | 22.4 ± 1.64 | 0.27 ± 0.122 | 0.51 ± 0.212 | |||
| BT/SPC | 3.33 ± 0.15 | 2.79 ± 0.18 | 27.0 ± 1.64 | 21.9 ± 1.12 | 0.37 ± 0.142 | 0.24 ± 0.112 | |||
| GT/SPC | 3.61 ± 0.204 | 2.84 ± 0.283 | 26.4 ± 2.92 | 21.8 ± 1.42 | 0.31 ± 0.242 | 0.40 ± 0.162 | |||
All values are x̄ ± SEM. IDSPIs, isoflavone-depleted soy protein isolates; HISPIs, high-isoflavone soy protein isolates; SPC, soy phytochemical concentrate; BTPs, black tea polyphenols; GTPs, green tea polyphenols; BT, black tea; GT, green tea. Values were analyzed by ANOVA followed by Tukey’s test.
Significantly different from control: P < 0.0005
Significantly different from control: P < 0.05
Significantly different from control: P < 0.005.
Soy and tea components showed different effects on food intake and body weight. In general, the soy components did not significantly alter food intake or body weight compared with the control in either male or female mice. Compared with the control, tea components resulted in significantly higher food intake (except the GTPs) in male mice, but significantly lower body weights of both male (except in the BTP and GTP groups) and female mice.
Male mice treated with isoflavone-depleted SPIs, high-isoflavone-containing SPIs, or genistein had significantly lower WAT mass by 65.2% (P < 0.0005), 42.9% (P < 0.0005), and 37.7% (P < 0.005); female mice treated with high-isoflavone-containing SPIs or genistein had significantly lower WAT mass by 21% (P < 0.05), respectively (Table 2). The SPC did not have a significant effect on WAT mass in either male or female mice. Similarly, male or female mice treated with tea polyphenols (BTPs and GTPs) did not have significantly lower WAT mass. Male mice treated with BT or GT had significantly lower WAT mass by 70% (P < 0.0005) and 65% (P < 0.0005), respectively. Female mice treated with BT or GT, on the other hand, had significantly lower WAT mass by 60% (P < 0.0005) and 43% (P < 0.0005), respectively. These results indicate that whole teas have potent effects on reducing abdominal WAT mass and that the effects of tea polyphenols extracts do not have significant effects on WAT and body weight.
Effects of SPC and tea combinations on food intake, body weight, and abdominal WAT mass in mice
The SPC and BT combination and SPC and GT combination were evaluated for their effects on food intake, body weight, and WAT mass. We selected SPC and whole tea combinations because these materials represent the soy and tea phytochemical compositions commonly consumed by humans, and their combinations showed significant and synergistic effects on inhibiting the growth of breast or prostate tumors in vivo. The combinations of SPC and tea did not further alter food intake, body weight, or WAT mass (Table 2).
Effects of SPC and tea combinations on serum concentrations of insulin, leptin, IGF-I, adiponectin, and sex hormones in mice
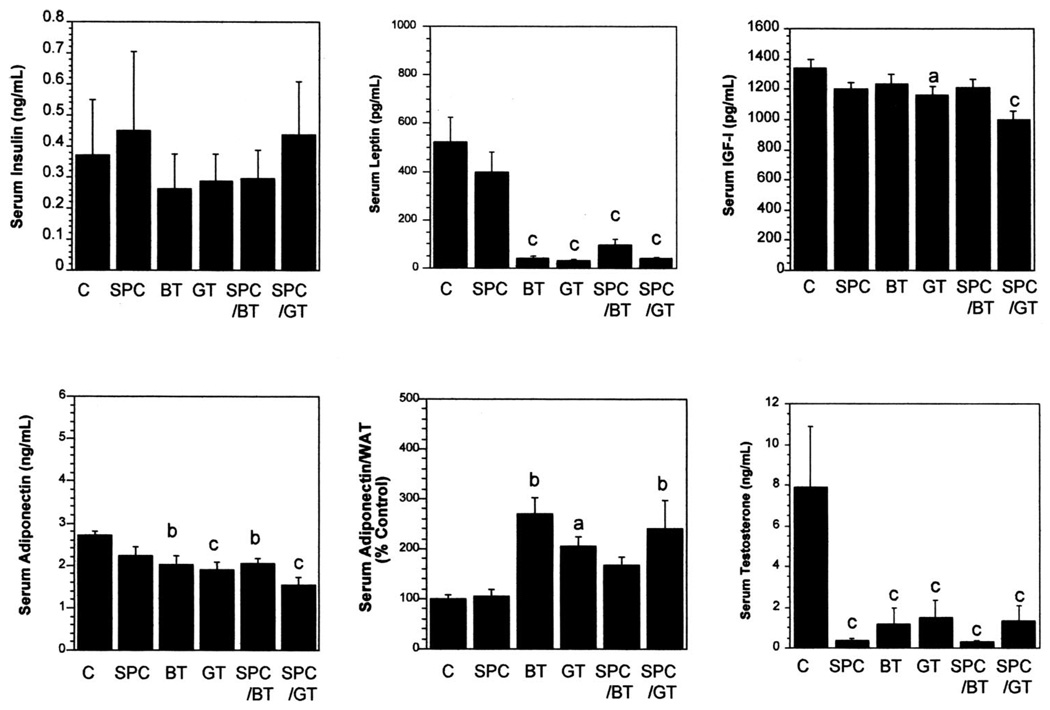
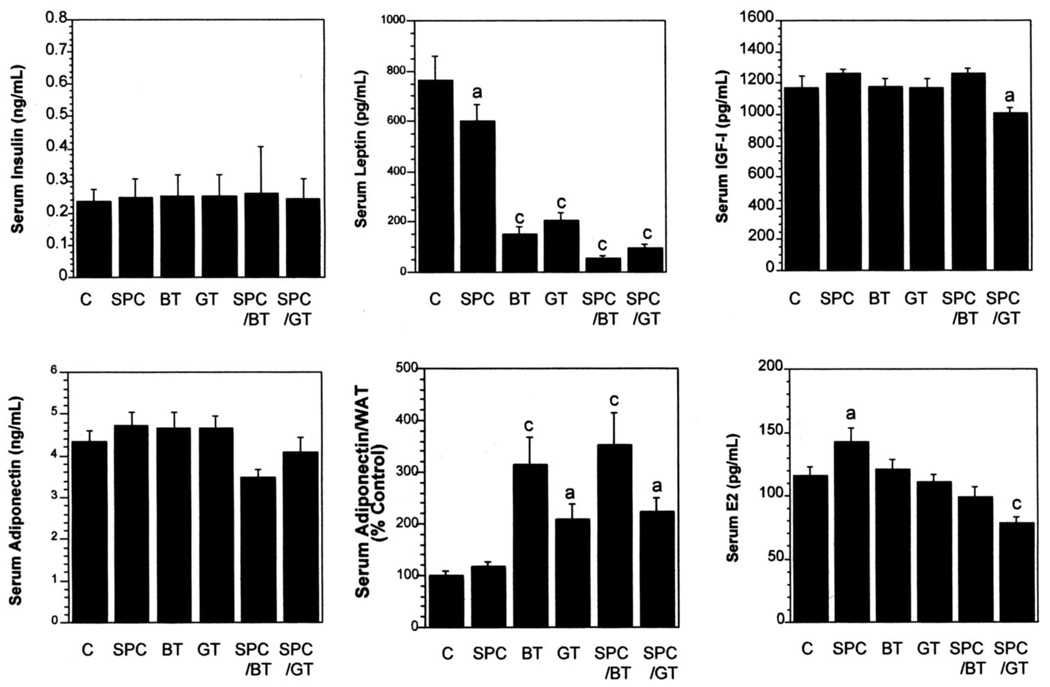
The effects of SPC and tea combinations on modulation of serum concentrations of adipokines (leptin and adiponectin), growth factors (insulin and IGF-I), and sex hormones (testosterone in male mice and 17β-estradiol in female mice) are shown in Figure 1 and Figure 2. The treatments did not significantly alter serum concentrations of insulin in male or female mice. The SPC significantly lowered serum leptin concentrations by 20% in female mice. BT and GT significantly lowered serum concentrations of leptin by 92% (P < 0.0005) and 94% (P < 0.0005), respectively, in male mice and by 80% (P < 0.0005) and 73% (P < 0.0005), respectively, in female mice. The combinations of SPC with BT or GT significantly lowered serum concentrations of leptin by 93% (P < 0.0005) and 87% (P < 0.0005), respectively, in female mice. Analysis showed that the combination of SPC and BT or GT had a synergistic effect (P < 0.05) on lowering leptin concentrations in female mice.
FIGURE 1.
Effects of soy phytochemical concentrate (SPC) and tea combinations on serum concentrations of insulin, leptin, insulin-like growth factor-I (IGF-I), adiponectin, adiponectin corrected for white adipose tissue (WAT), and testosterone in male mice. C, control; BT, black tea; GT, green tea. Values are x̄ ± SEM, n = 12/group, and were analyzed by ANOVA followed by Tukey’s test. Within each panel, the value with a letter is significantly different from the control: aP < 0.05, bP < 0.005, cP < 0.0005.
FIGURE 2.
Effects of soy phytochemical concentrate (SPC) and tea combinations on serum concentrations of insulin, leptin, insulin-like growth factor-I (IGF-I), adiponectin, adiponectin corrected for white adipose tissue (WAT), and estradiol (E2) in female mice. C, control; BT, black tea; GT, green tea. Values are x̄ ± SEM, n = 12/group, and were analyzed by ANOVA followed by Tukey’s test. Within each panel, the value with a letter is significantly different from the control: aP < 0.05, cP < 0.0005.
SPC and BT treatment alone did not significantly lower serum concentrations of IGF-I, whereas GT significantly lowered serum concentrations of IGF-I by 13.3% (P < 0.05) in male mice (Figure 1). The combination of SPC and GT further lowered serum IGF-I by 25.7% (P < 0.0005) in male mice, and the combination effect was synergistic. In female mice, SPC, BT, and GT did not show significant effects on IGF-I concentrations (Figure 2). The combination of SPC and GT, however, significantly lowered IGF-I concentrations by 14% (P < 0.05), and the combination had a synergistic effect (P < 0.05).
The SPC and tea treatments significantly lowered serum concentrations of adiponectin in male mice (except the SPC treatment, Figure 1), but not in female mice (Figure 2), compared with those of the control. Because adiponectin is produced only in adipose tissue, we also expressed adiponectin concentrations on the basis of WAT mass and as percentage of the control amount. SPC did not have a higher WAT-corrected adiponectin concentration compared with that of the control. Other experimental groups had significantly higher ratios (except for the SPC-BT combination in male mice). No synergistic effect was observed.
The experimental treatments significantly lowered the serum testosterone concentration in male mice (Figure 1). No apparent combination effects were observed, maybe in part because of the dramatic effects in individual treatments. In contrast, tea treatments alone did not significantly alter serum estrogen concentrations in female mice (Figure 2), and SPC significantly raised serum estrogen concentrations by 23% (P < 0.05). Interestingly, this estrogen-enhancing activity of SPC was reversed by its combination with BT or GT, and the combinations of SPC with BT or GT lowered serum estrogen concentrations by 14.6% (NS) or 32.5% (P < 0.0005), compared with the control. Calculation of the combination index showed that both the SPC-BT and the SPC-GT combinations had synergistic effects (P < 0.05) on lowering serum estrogen concentrations in female mice.
DISCUSSION
The results of the present study show that tea components significantly lowered body weights without significantly altering food intakes. Whole teas, but not tea polyphenol extracts, significantly lowered WAT mass by 65–70% in male mice and by 43–60% in female mice. SPC and teas, alone and in combination, significantly lowered serum leptin concentrations in both male and female mice. The combination of SPC and GT lowered serum IGF-I concentrations in both male and female mice in a synergistic manner. The SPC and tea combinations also lowered serum estrogen concentrations in female mice in a synergistic manner. Our results suggest that the combinations of soy and tea components may improve metabolic conditions by inhibiting abdominal WAT mass and by modulating certain MS-associated elements such as leptin, IGF-I, and sex hormones.
In this animal study, MS was not induced and the animals were treated with a 10%-fat diet. Even in this situation, the soy and tea components, especially in combinations, showed significant modulation of several MS-related elements, such as leptin, IGF-I, and sex hormones, all favoring cancer-prevention activity. Indeed, the SPC and tea combinations inhibited the growth and progression of both breast and prostate tumors in our preclinical animal models. These findings may have significant effects on the prevention of breast cancer or prostate cancer in a healthy population because the dietary fat was at a “normal” level. Although not conducted in this report, we expect that certain soy and tea combinations may significantly prevent the development of MS and improve metabolic status. To directly establish the cancer-promoting effects of MS, it is also required to determine whether MS enhances the development or progression of breast or prostate cancer in appropriate animal models. Further animal studies are also required to define whether certain soy and tea combinations effectively prevent breast or prostate cancer in part by preventing the development of MS and improving metabolic profiles.
Previous research has primarily focused on the cancer prevention activity of soy or tea components alone (18, 19) but not in combination. Evidence is emerging that soy components, such as soy isoflavones and soy protein, may play a beneficial role in obesity and diabetes. Soy protein associated with isoflavones improves glucose control, lipid profiles, and insulin resistance (24–29) and increases the serum adiponectin concentration (27, 29). Soy isoflavones significantly lower fat mass, plasma glucose in both lean and obese rats (30, 31), and serum concentrations of leptin (32, 33) and improve glucose tolerance (34). Clinical intervention studies showed that soyfood reduces serum concentrations of insulin and leptin (35). Consumption of soy isoflavones is associated with lower body mass indexes and fasting insulin concentrations and higher HDL cholesterol and also lowers the insulin response to an oral glucose load in presumably normal-weight, postmenopausal women (36). Thus, it appears from these studies that soy-based diets may provide potential benefits in conditions associated with impaired glucose tolerance, hyperlipidemia, and reduced insulin sensitivity. On the other hand, some studies did not show significant effects of soy components on metabolic profiles (37, 38).
Similar to the soy studies, previous studies have shown the MS-preventive activity of tea components. Tea components, especially epigallocatechin gallate, had antiobesity activity and improved metabolic disorders via modulation of adipokines and growth factors (39–41), especially suppression of leptin concentrations (39, 41, 42). Besides tea polyphenols, caffeine had antiobesity activity and modulated related adipokines such as leptin (43). GT lowered adipose tissue weight without any change in body weight, other tissue weights, and food and water intakes, and also significantly lowered the plasma concentrations of cholesterol and free fatty acids (41).
Our results suggest that one of the mechanisms by which SPC and tea combinations may have synergistic cancer prevention activity is via synergistic effects on lowering IGF-I concentrations. IGF-I has been shown to play an important role in the development of breast and prostate glands and in carcinogenesis and tumorigenesis. Epidemiologic investigations in general indicated that the increased serum concentrations of IGF-I were significantly associated with breast cancer risk (44–48) and prostate cancer risk (46, 49, 50). On the other hand, the results from epidemiologic investigations on the association between soy components, mostly soy proteins, and serum concentrations of IGF-I are inconsistent, ranging from a positive association (48, 51–54), a negative association (55), to no association (55–59). Our results showed that although SPC and tea alone did not have significant effects on serum IGF-I concentrations, the combination of SPC and GT significantly lowered IGF-I concentrations in a synergistic manner. Our results support further investigation to apply appropriate dietary combination regimens, such as soy and tea combinations, for cancer prevention by targeting IGF-I function.
Our results also showed that SPC and tea combinations had a synergistic effect on lowering the serum estrogen concentration in female mice. Estrogen plays a key role in estrogen-dependent breast cancer development and growth. Obesity has been shown to enhance circulating concentrations of estrogen and thus may favor promotion of breast cancer development. Administration of anti-estrogen tamoxifen is a successful adjuvant therapy for patients with estrogen-dependent breast cancer and significantly improves the survival of those women (60). The results from our study may provide a mechanistic explanation for the finding in our breast cancer study of a synergistic effect of SPC and GT on inhibiting the growth of breast tumors (21). Further research is warranted to investigate the mechanisms by which SPC and tea combinations may reduce circulating estrogen concentrations in a synergistic manner.
Our results also showed that the combination of BT or GT with SPC reduced leptin concentrations in a synergistic manner in female mice. Due to dramatic effects of soy and tea treatment alone, the potentiating effects between soy and tea combinations were not apparent in male mice. Plasma leptin concentrations correlate with fat stores and the volume and number of adipocytes. Epidemiologic and experimental studies in general have shown that leptin promotes the development and progression of breast cancer (61–65) and prostate cancer (66–73). Our findings suggest that one of the mechanisms by which the soy and tea combination may synergistically prevent breast cancer may be through synergistic effects on lowering leptin concentrations in female mice.
In summary, the results of the present study show that the soy and tea combinations lowered abdominal WAT mass, serum concentrations of IGF-I and leptin in both male and female mice, and estrogen concentrations in female mice in a synergistic or an additive manner. The results suggest that the soy and tea combination may prevent MS and improve metabolic profiles, and this MS-preventive activity may be in part responsible for the synergistic effects of the soy and tea combinations on the prevention of breast or prostate cancer observed in the animal studies. The results provide the rationale to support future research to define the causal role of MS in the development and progression of breast or prostate cancer and to apply dietary combination strategies, such as the soy and tea combinations, to cancer prevention by preventing MS.
Footnotes
Presented at the 8th Postgraduate Nutrition Symposium “Metabolic Syndrome and the Onset of Cancer,” held in Boston, MA, March 15–16, 2006.
Supported in part by the National Cancer Institute and the National Center for Complementary and Alternative Medicine, National Institutes of Health (RO1 CA92546, RO1 AT00863, and RO3 CA112640, and RO3 CA112644).
None of the authors had any conflicts of interest to disclose.
REFERENCES
- 1.Stoll BA. Western nutrition and the insulin resistance syndrome: a link to breast cancer. Eur J Clin Nutr. 1999;53:83–87. doi: 10.1038/sj.ejcn.1600700. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 3.Sinagra D, Amato C, Scarpilta AM, et al. Metabolic syndrome and breast cancer risk. Eur Rev Med Pharmacol Sci. 2002;6:55–59. [PubMed] [Google Scholar]
- 4.Okobia MN, Bunker CH, Zmuda JM, et al. Anthropometry and breast cancer risk in Nigerian women. Breast J. 2006;12:462–466. doi: 10.1111/j.1075-122X.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 5.Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119:236–238. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 6.Lund Haheim L, Wisloff TF, Holme I, Nafstad P. Metabolic syndrome predicts prostate cancer in a cohort of middle-aged Norwegian men followed for 27 years. Am J Epidemiol. 2006;164:769–774. doi: 10.1093/aje/kwj284. [DOI] [PubMed] [Google Scholar]
- 7.Hammarsten J, Hogstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005;41:2887–2895. doi: 10.1016/j.ejca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Laukkanen JA, Laaksonen DE, Niskanen L, Pukkala E, Hakkarainen A, Salonen JT. Metabolic syndrome and the risk of prostate cancer in Finnish men: a population-based study. Cancer Epidemiol Biomarkers Prev. 2004;13:1646–1650. [PubMed] [Google Scholar]
- 9.Hammarsten J, Hogstedt B. Clinical, haemodynamic, anthropometric, metabolic and insulin profile of men with high-stage and high-grade clinical prostate cancer. Blood Press. 2004;13:47–55. doi: 10.1080/08037050310025735. [DOI] [PubMed] [Google Scholar]
- 10.Scheen A, Lefebvre P. Insulin action in man. Diabetes Metab. 1996;22:105–110. [PubMed] [Google Scholar]
- 11.Whitelaw D, Gilbey S. Insulin resistance. Ann Clin Biochem. 1998;35:567–583. doi: 10.1177/000456329803500501. [DOI] [PubMed] [Google Scholar]
- 12.Mayer-Davis EJ, Monaco JH, Hoen HM, et al. Dietary fat and insulin sensitivity in a triethnic population: the role of obesity. The Insulin Resistance Atherosclerosis Study (IRAS) Am J Clin Nutr. 1997;65:79–87. doi: 10.1093/ajcn/65.1.79. [DOI] [PubMed] [Google Scholar]
- 13.Bruning PF, Bonfrer JM, van Noord PA, Hart AA, Jong-Bakker M, Nooijen WJ. Insulin resistance and breast-cancer risk. Int J Cancer. 1992;52:511–516. doi: 10.1002/ijc.2910520402. [DOI] [PubMed] [Google Scholar]
- 14.Del Giudice ME, Fantus IG, Ezzat S, McKeown-Eyssen G, Page D, Goodwin PJ. Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res Treat. 1998;47:111–120. doi: 10.1023/a:1005831013718. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Jin F, Shu XO, et al. Insulin-like growth factors and breast cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2002;11:705–712. [PubMed] [Google Scholar]
- 16.Malin A, Dai Q, Yu H, et al. Evaluation of the synergistic effect of insulin resistance and insulin-like growth factors on the risk of breast carcinoma. Cancer. 2004;100:694–700. doi: 10.1002/cncr.20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chemoprevention Working Group. Prevention of cancer in the next millennium: report of the chemoprevention working group to the American Association for Cancer Research. Cancer Res. 1999;59:4743–4758. [PubMed] [Google Scholar]
- 18.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 19.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J-R, Yu L, Zhong Y, Blackburn GL. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J Nutr. 2003;133:516–521. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J-R, Yu L, Mai Z, Blackburn GL. Combined inhibition of estrogen-dependent human breast carcinoma by soy and tea bioactive components in mice. Int J Cancer. 2004;108:8–14. doi: 10.1002/ijc.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steel RGD, Torrie JH. Principles and procedures of statistics: a biometrical approach. 2nd ed. New York, NY: McGraw-Hill Book Company, Inc; 1980. [Google Scholar]
- 23.Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Cancer Res. 2000;60:2190–2196. [PubMed] [Google Scholar]
- 24.Lavigne C, Marette A, Jacques H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol Endocrinol Metab. 2000;278:E491–E500. doi: 10.1152/ajpendo.2000.278.3.E491. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez A, Hubbard RW. Plasma amino acids and the insulin/glucagon ratio as an explanation for the dietary protein modulation of atherosclerosis. Med Hypotheses. 1991;36:27–32. doi: 10.1016/0306-9877(91)90160-z. [DOI] [PubMed] [Google Scholar]
- 26.Sugano M, Ishiwaki N, Nagata Y, Imaizumi K. Effects of arginine and lysine addition to casein and soya-bean protein on serum lipids, apolipoproteins, insulin and glucagon in rats. Br J Nutr. 1982;48:211–221. doi: 10.1079/bjn19820107. [DOI] [PubMed] [Google Scholar]
- 27.Akahoshi A, Koba K, Ichinose F, et al. Dietary protein modulates the effect of CLA on lipid metabolism in rats. Lipids. 2004;39:25–30. doi: 10.1007/s11745-004-1197-3. [DOI] [PubMed] [Google Scholar]
- 28.Nagasawa A, Fukui K, Funahashi T, et al. Effects of soy protein diet on the expression of adipose genes and plasma adiponectin. Horm Metab Res. 2002;34:635–639. doi: 10.1055/s-2002-38254. [DOI] [PubMed] [Google Scholar]
- 29.Nagasawa A, Fukui K, Kojima M, et al. Divergent effects of soy protein diet on the expression of adipocytokines. Biochem Biophys Res Commun. 2003;311:909–914. doi: 10.1016/j.bbrc.2003.10.087. [DOI] [PubMed] [Google Scholar]
- 30.Ali AA, Velasquez MT, Hansen CT, Mohamed AI, Bhathena SJ. Effects of soybean isoflavones, probiotics, and their interactions on lipid metabolism and endocrine system in an animal model of obesity and diabetes. J Nutr Biochem. 2004;15:583–590. doi: 10.1016/j.jnutbio.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Ali AA, Velasquez MT, Hansen CT, Mohamed AI, Bhathena SJ. Modulation of carbohydrate metabolism and peptide hormones by soybean isoflavones and probiotics in obesity and diabetes. J Nutr Biochem. 2005;16:693–699. doi: 10.1016/j.jnutbio.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Bu L, Setchell KD, Lephart ED. Influences of dietary soy isoflavones on metabolism but not nociception and stress hormone responses in ovariectomized female rats. Reprod Biol Endocrinol. 2005;3:58. doi: 10.1186/1477-7827-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szkudelski T, Nogowski L, Pruszynska-Oszmalek E, Kaczmarek P, Szkudelska K. Genistein restricts leptin secretion from rat adipocytes. J Steroid Biochem Mol Biol. 2005;96:301–307. doi: 10.1016/j.jsbmb.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Ae Park S, Choi MS, Cho SY, et al. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006;79:1207–1213. doi: 10.1016/j.lfs.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Wu AH, Stanczyk FZ, Martinez C, et al. A controlled 2-mo dietary fat reduction and soy food supplementation study in postmenopausal women. Am J Clin Nutr. 2005;81:1133–1141. doi: 10.1093/ajcn/81.5.1133. [DOI] [PubMed] [Google Scholar]
- 36.Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr. 2001;131:1202–1206. doi: 10.1093/jn/131.4.1202. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita T, Sasahara T, Pomeroy SE, Collier G, Nestel PJ. Arterial compliance, blood pressure, plasma leptin, and plasma lipids in women are improved with weight reduction equally with a meat-based diet and a plant-based diet. Metabolism. 1998;47:1308–1314. doi: 10.1016/s0026-0495(98)90297-9. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins DJ, Wolever TM, Spiller G, et al. Hypocholesterolemic effect of vegetable protein in a hypocaloric diet. Atherosclerosis. 1989;78:99–107. doi: 10.1016/0021-9150(89)90213-x. [DOI] [PubMed] [Google Scholar]
- 39.Kao YH, Hiipakka RA, Liao S. Modulation of obesity by a green tea catechin. Am J Clin Nutr. 2000;72:1232–1234. doi: 10.1093/ajcn/72.5.1232. [DOI] [PubMed] [Google Scholar]
- 40.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res. 2006;50:176–187. doi: 10.1002/mnfr.200500102. [DOI] [PubMed] [Google Scholar]
- 41.Ashida H, Furuyashiki T, Nagayasu H, et al. Anti-obesity actions of green tea: possible involvements in modulation of the glucose uptake system and suppression of the adipogenesis-related transcription factors. Biofactors. 2004;22:135–140. doi: 10.1002/biof.5520220126. [DOI] [PubMed] [Google Scholar]
- 42.Sayama K, Lin S, Zheng G, Oguni I. Effects of green tea on growth, food utilization and lipid metabolism in mice. In Vivo. 2000;14:481–484. [PubMed] [Google Scholar]
- 43.Westerterp-Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res. 2005;13:1195–1204. doi: 10.1038/oby.2005.142. [DOI] [PubMed] [Google Scholar]
- 44.Agurs-Collins T, Adams-Campbell LL, Kim KS, Cullen KJ. Insulin-like growth factor-1 and breast cancer risk in postmenopausal African-American women. Cancer Detect Prev. 2000;24:199–206. [PubMed] [Google Scholar]
- 45.Allen NE, Roddam AW, Allen DS, et al. A prospective study of serum insulin-like growth factor-I (IGF-I), IGF-II, IGF-binding protein-3 and breast cancer risk. Br J Cancer. 2005;92:1283–1287. doi: 10.1038/sj.bjc.6602471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 47.Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB. Serum levels of insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer. J Clin Endocrinol Metab. 2000;85:4258–4265. doi: 10.1210/jcem.85.11.6990. [DOI] [PubMed] [Google Scholar]
- 48.Probst-Hensch NM, Wang H, Goh VH, Seow A, Lee HP, Yu MC. Determinants of circulating insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations in a cohort of Singapore men and women. Cancer Epidemiol Biomarkers Prev. 2003;12:739–746. [PubMed] [Google Scholar]
- 49.Chan JM, Stampfer MJ, Ma J, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94:1099–1106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 50.Chen C, Lewis SK, Voigt L, Fitzpatrick A, Plymate SR, Weiss NS. Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer. 2005;103:76–84. doi: 10.1002/cncr.20727. [DOI] [PubMed] [Google Scholar]
- 51.Khalil DA, Lucas EA, Juma S, Smith BJ, Payton ME, Arjmandi BH. Soy protein supplementation increases serum insulin-like growth factor-I in young and old men but does not affect markers of bone metabolism. J Nutr. 2002;132:2605–2608. doi: 10.1093/jn/132.9.2605. [DOI] [PubMed] [Google Scholar]
- 52.Gann PH, Kazer R, Chatterton R, et al. Sequential, randomized trial of a low-fat, high-fiber diet and soy supplementation: effects on circulating IGF-I and its binding proteins in premenopausal women. Int J Cancer. 2005;116:297–303. doi: 10.1002/ijc.21042. [DOI] [PubMed] [Google Scholar]
- 53.Arjmandi BH, Lucas EA, Khalil DA, et al. One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women. Nutr J. 2005 Feb 23;:4–8. doi: 10.1186/1475-2891-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woodside JV, Campbell MJ, Denholm EE, et al. Short-term phytoestrogen supplementation alters insulin-like growth factor profile but not lipid or antioxidant status. J Nutr Biochem. 2006;17:211–215. doi: 10.1016/j.jnutbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Sanderson M, Shu XO, Yu H, et al. Insulin-like growth factor-I, soy protein intake, and breast cancer risk. Nutr Cancer. 2004;50:8–15. doi: 10.1207/s15327914nc5001_2. [DOI] [PubMed] [Google Scholar]
- 56.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Dietary soy and fats in relation to serum insulin-like growth factor-1 and insulin-like growth factor-binding protein-3 levels in premenopausal Japanese women. Nutr Cancer. 2003;45:185–189. doi: 10.1207/S15327914NC4502_07. [DOI] [PubMed] [Google Scholar]
- 57.Adams KF, Newton KM, Chen C, et al. Soy isoflavones do not modulate circulating insulin-like growth factor concentrations in an older population in an intervention trial. J Nutr. 2003;133:1316–1319. doi: 10.1093/jn/133.5.1316. [DOI] [PubMed] [Google Scholar]
- 58.Vrieling A, Voskuil DW, Bueno de Mesquita HB, et al. Dietary determinants of circulating insulin-like growth factor (IGF)-I and IGF binding proteins 1,−2 and −3 in women in the Netherlands. Cancer Causes Control. 2004;15:787–796. doi: 10.1023/B:CACO.0000043429.51915.c6. [DOI] [PubMed] [Google Scholar]
- 59.Maskarinec G, Takata Y, Murphy SP, Franke AA, Kaaks R. Insulin-like growth factor-1 and binding protein-3 in a 2-year soya intervention among premenopausal women. Br J Nutr. 2005;94:362–367. doi: 10.1079/bjn20051525. [DOI] [PubMed] [Google Scholar]
- 60.Fisher B, Dignam J, Bryant J, et al. Five vs more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez RR, Cherfils S, Escobar M, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281:26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 62.Sulkowska M, Golaszewska J, Wincewicz A, Koda M, Baltaziak M, Sulkowski S. Leptin—from regulation of fat metabolism to stimulation of breast cancer growth. Pathol Oncol Res. 2006;12:69–72. doi: 10.1007/BF02893446. [DOI] [PubMed] [Google Scholar]
- 63.Garofalo C, Koda M, Cascio S, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 64.Miyoshi Y, Funahashi T, Tanaka S, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118:1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 65.Chen DC, Chung YF, Yeh YT, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 66.Hsing AW, Chua S, Jr, Gao YT, et al. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst. 2001;93:783–789. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 67.Stattin P, Kaaks R, Johansson R, et al. Plasma leptin is not associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:474–475. [PubMed] [Google Scholar]
- 68.Stattin P, Soderberg S, Hallmans G, et al. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J Clin Endocrinol Metab. 2001;86:1341–1345. doi: 10.1210/jcem.86.3.7328. [DOI] [PubMed] [Google Scholar]
- 69.Lagiou P, Signorello LB, Trichopoulos D, Tzonou A, Trichopoulou A, Mantzoros CS. Leptin in relation to prostate cancer and benign prostatic hyperplasia. Int J Cancer. 1998;76:25–28. doi: 10.1002/(sici)1097-0215(19980330)76:1<25::aid-ijc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 70.Baillargeon J, Platz EA, Rose DP, et al. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiol Biomarkers Prev. 2006;15:1331–1335. doi: 10.1158/1055-9965.EPI-06-0082. [DOI] [PubMed] [Google Scholar]
- 71.Gade-Andavolu R, Cone LA, Shu S, Morrow A, Kowshik B, Andavolu MV. Molecular interactions of leptin and prostate cancer. Cancer J. 2006;12:201–206. doi: 10.1097/00130404-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Chang S, Hursting SD, Contois JH, et al. Leptin and prostate cancer. Prostate. 2001;46:62–67. doi: 10.1002/1097-0045(200101)46:1<62::aid-pros1009>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 73.Saglam K, Aydur E, Yilmaz M, Goktas S. Leptin influences cellular differentiation and progression in prostate cancer. J Urol. 2003;169:1308–1311. doi: 10.1097/01.ju.0000055903.18400.25. [DOI] [PubMed] [Google Scholar]