Abstract
Breast cancer is significantly less prevalent among Asian women, whose diets contain high intake of soy products and tea. The objective of our present study was to identify the combined effects of dietary soy phytochemicals and tea components on breast tumor progression in a clinically relevant in vivo model of MCF-7 androgen-dependent human breast tumor in female SCID mice. MCF-7 tumor growth, tumor cell proliferation and apoptosis, microvessel density, and expressions of tumor estrogen receptors were compared in mice treated with genistin-rich soy isoflavones (GSI), soy phytochemical concentrate (SPC), black tea (BT), green tea (GT), SPC/BT combination and SPC/GT combination. GSI and SPC led to dose-dependent inhibition of MCF-7 tumor growth via inhibition of cancer cell proliferation in vivo. GT showed more potent anti-breast tumor activity than BT. GT infusion at 1.5 g tealeaf/100 mL water produced significant (p < 0.05) reductions of 56% in final tumor weight. GT plus SPC at 0.1% of the diet further reduced final tumor weight by 72% (p < 0.005). Analysis of serum and tumor biomarkers showed that the combined effects of SPC and GT inhibited tumor angiogenesis, and reduced estrogen receptor (ER)-α and serum levels of insulin-like growth factor (IGF)-I. Our study suggests that dietary SPC plus GT may be used as a potential effective dietary regimen for inhibiting progression of estrogen-dependent breast cancer.
Keywords: breast cancer, soy phytochemicals, tea
Breast cancer, the third most commonly diagnosed carcinoma in the world, accounts for 21% of all new cancer cases in women.1 Risk of breast cancer varies widely, with about a 6-fold difference between the highest risk, typically seen in women from the United States and Western Europe, and the lowest, usually found in women from China and Japan.2 Asian women born in the U.S. or in Western nations have similar breast cancer incidence rates as women who reside in those areas.3 Japanese women who move to the U.S. tend to have higher breast cancer incidence rates than Japanese women who remain in Japan.3 These data have led researchers to believe that environmental factors, such as diet and chemical exposures, play a large role in the development of breast cancer.
Considerable data from animal studies indicates that combinations of agents can be more effective for cancer prevention than any single constituent.4 Dietary modification has long been considered to be an effective regimen for cancer prevention. Research suggests that increased consumption of soybean products and tea by Japanese and Chinese women contributes to low breast cancer incidence and mortality rates compared to those for women from industrialized Western nations5,6 It is possible that dietary soy and tea components may interact each other to potentiate their anticancer activities. Development of effective anticancer agents for use in humans, however, requires conclusive evidence of efficacy in animal models that closely emulate human disease.7
The estrogen-dependent MCF-7 human breast cancer cell line features molecular and cellular characteristics of estrogen-dependent human breast cancer, e.g., dependence on estrogen for in vivo growth, and expression of both estrogen receptors (ER-α and ER-β). In our study, MCF-7 cells were orthotopically implanted in the mammary fat pads of female severe combined immune deficient (SCID) mice supplemented with estrogen. We applied this clinically-relevant model to evaluate the dose-dependent effects of a dietary soy phytochemical mixture, soy phytochemical concentrate (SPC) and a genistin-rich soy isoflavone mixture (GSI), and the combined effects of SPC and tea components on the progression of MCF-7 breast cancer tumors in vivo.
MATERIAL AND METHODS
Soy isoflavones and soy phytochemical extract
A soy phytochemical extract, SPC, was used as the source of the soybean phytochemical supplement. It contained 51.9% soy isoflavones by weight (50.8% genistein aglycone equivalents, 40.5% daidzein aglycone equivalents, and 8.7% glycitein aglycone equivalents). Other phytochemicals in the SPC were not quantified. A genistin-rich soy isoflavone mixture, GSI, was used as the source of the glycoside form of the soy isoflavone genistein, the proposed major bioactive ingredient in soy. GSI was purified from SPC, and contained 100% soy isoflavones by weight (90.1% genistein aglycone equivalents, 9.1% daidzein aglycone equivalents, and 0.8% glycitein aglycone equivalents). Both SPC and GSI were provided by Archer Daniels Midland Company (Decatur, IL). The company used high performance liquid chromatography to analyze isoflavone levels.
Both green tea (GT) (China Green Tea, Shanghai Tea Import and Export Corporation, Shanghai, China) and black tea (BT) (Keemun Black Tea, Shanghai Tea Branch, China National Native Produce and Animal By-Products Import and Export Corporation, Shanghai, China) were purchased from a local supermarket. Tea infusions were prepared by extracting tea leaves with boiling water (100°C) for 10 min twice. For animal studies, the 1.5% (1.5 g tealeaf/100 ml water) of tea infusions were freshly prepared every Monday, Wednesday and Friday, and were used as the sole source of drinking fluid for tea-treated mice throughout the entire study. The catechins in tea infusions were determined by high performance liquid chromatography by Lipton Tea Co. (Englewood Cliffs, NJ). The compositions of a typical 1.5% black tea infusion and a typical 1.5% green tea infusion are shown in Table I.
TABLE I.
Compositions of Typical 1.5% Black Tea and 1.5% Green Tea Infusions
| 1.5% green tea infusion (ppm) |
1.5% black tea infusion (ppm) |
|
|---|---|---|
| Total catechins | 1,516.4 | 110.2 |
| Epicatechin | 148.7 | 8.02 |
| Epicatechin gallate | 198.5 | 35.9 |
| Epigallocatechin | 353.0 | 11.9 |
| Epigallocatechin gallate | 816.2 | 54.4 |
| Total theaflavins | 1.8 | 29.9 |
| Theaflavin | 0.6 | 3.9 |
| Theaflavin 3-gallate | 0.4 | 9.6 |
| Theaflavin 3′-gallate | 0.2 | 7.3 |
| Theaflavin 3,3′-digallate | 0.6 | 9.1 |
| Total flavonols | 67.7 | 42.6 |
| Kaempferol | 17.2 | 24.8 |
| Quercetin | 31.5 | 15.9 |
| Myricetin | 19.0 | 1.9 |
| Gallic acid | 53.6 | 89.7 |
| Caffeine | 507.4 | 583.7 |
Animal study
Female SCID mice (5- to 8-weeks-old) were purchased from Taconic (Germantown, NY), and housed at the animal facility of Beth Israel Deaconess Medical Center in a pathogen-free environment equipped with laminar flow hoods and standard vinyl cages with air filters. After 1 week of acclimatization, mice were randomized into 1 of 9 experimental groups (in each group, n = 12) that received corresponding dietary treatments 2 weeks before subcutaneous implantation of 17β-estradiol (0.72 mg 17β-estradiol, 90-day release, Innovative Research of America, Sarasota, FL). MCF-7 human breast cancer cells (2 × 106 cells) were then implanted orthotopically into the mammary fat pads of the mice, which continued to receive experimental diets throughout the study.
The experimental groups were: (i) Control: AIN-93M; (ii) 0.1% SPC: AIN-93 with the addition of 0.1% SPC; (iii) 0.5% SPC: AIN-93 with the addition 0.5% SPC; (iv) 0.028% GSI: AIN-93 with GSI at 0.028% of the diet, providing the same amount of genistein equivalent as that in Group ii; (v) 0.14% GSI: AIN-93 with GSI at 0.14% of the diet, providing the same amount of genistein equivalent as that in Group iii; (vi) BT: AIN-93 with BT infusion preparation (1.5 g leaf/100 mL water) in place of drinking water; (vii) GT: AIN-93 with GT preparation (1.5 g leaf/100 mL water) in place of drinking water; (viii) SPC/BT: AIN-93 with 0.1% SPC and BT infusion; and (ix) SPC/GT: AIN-93 with 0.1% SPC and GT infusion. SPC was used because it contains a majority of soy phytochemicals present in soybean. Tea infusions were used because they represent tea compositions that are typically consumed. Food intake and body weight were measured weekly.
Tumor diameters were measured twice weekly by caliper, and tumor volumes were estimated using the following formula: volume (cm)3 = (length (cm) × width (cm)2) × 0.523.8 The experiment was finished 8 weeks after tumor cell implantation, when mean tumor volume in the control mice exceeded 2.0 cm.3.3 At the end of the experiment, the mice were sacrificed, primary tumors were excised and weighed, and blood samples were collected. A tumor slice from each primary tumor tissue was carefully dissected and fixed in 10% buffer-neutralized formalin, paraffin-embedded, and sectioned at 4 µm thickness for histology and immunohistochemistry. Tumor homogenates were prepared for Western blot analysis. All procedures with animals were reviewed and approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center.
In situ detection of apoptotic index
Apoptotic cells were determined by a terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay using the ApopTag in situ Apoptosis Detection System (Oncor Inc., Gaithersburg, MD) according to our previous procedures.8–10 Six representative areas of each section without necrosis were selected, and both apoptotic cells and total nuclei cells were counted under a light microscope at 400-fold magnification. The apoptotic index was expressed as the percentage of positive apoptotic tumor cells to total tumor cells.
Immunohistochemical determination of proliferation index
Proliferating cell nuclear antigen (PCNA) was determined by immunohistochemical staining to quantify proliferation index, as described previously.8–10 Both PCNA-positive proliferating cells and total tumor cells were counted in 3 non-necrotic areas of each section using light microscopy at 400-fold magnification. The proliferation index was calculated as the percentage of PCNA-positive tumor cells to total tumor cells.
Immunohistochemical detection of microvessel density
Microvessel density (MVD) was used as a marker for tumor angiogenesis and quantified by immunohistochemical staining of Factor VIII following a previously described method.8–10 MVD was calculated by counting microvessels on 200× fields under light microscopy at 5 representative sites without necrosis of each section.
Western blot analysis
Western blot analysis was carried out to determine expression of ER-α and ER-β. The housekeeping protein GAPDH was used as the control. Tumor tissue samples frozen at −80°C were thawed and homogenized in a lysis buffer containing 100 mM KCl, 20 mM HEPES (pH 7.9), 10 mM EDTA, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 40 µg/ml leupeptin and 1 µg/ml pepstatin A. After incubation in an ice bath for 30 min, the samples were centrifuged at 10,000 rpm for 20 min at 4°C. The supernatants were collected, and the protein content of the tissue extracts determined by using the Bio-Rad protein assay (Bio-Rad, Hercules, CA) with BSA as a standard.
The proteins were separated on 10% SDS-polyacrylamide gel, transblotted onto nitrocellulose membranes. After incubation with 5% non-fat milk in PBS buffer for 1 hr at room temperature, the membranes were then incubated with a designated specific antibody for 60 minutes, washed and incubated with horseradish peroxidase-conjugated secondary antibody. Resulting protein bands (ER-α, ER-β or GAPDH) were detected by enhanced chemiluminescence carried out according to the manufacturer’s recommendations (Amersham Pharmacia Biotech, Piscataway, NJ), and captured and saved as image files using Chemi Doc device (Bio-Rad, Hercules, CA). The density of the protein band was quantitated using Quantity One software (Bio-Rad, Hercules, CA). To pool data from multiple Western blots, the relative density of each band was normalized against an internal standard (i.e., an assigned density of 100) used on each analyzed blot.
Mouse anti-ER-α monoclonal antibody (1:200, D-12, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-ER-β polyclonal antibody (1:400, H-150, Santa Cruz Biotechnology, Inc.), and mouse anti-GAPDH monoclonal (1:20,000, 6C5, Research Diagnostics Inc., Flanders, NJ) were the primary antibodies used for Western blot analysis. The sheep anti-mouse horseradish peroxidase linked whole antibody (Amersham Life Science, England) was used at 1:10,000, and the monkey anti-rabbit horseradish peroxidase linked whole antibody (Amersham Life Science) was used at 1:5,000.
Determination of serum level of insulin-like growth factor
Serum level of insulin-like growth factor (IGF-I) was determined by enzyme-linked immunosorbent assay following procedures provided by the manufacturer (Diagnostic Systems Laboratory, Inc., Webster, TX).
Statistical analysis
Tumor weight and measured levels of serum and tumor biomarkers were expressed as group means ± SEM. StatView 5.0 program (SAS Institute Inc., Cary, NC) was used to calculate 2-sided comparisons among experimental groups through initial analysis of variance followed by Fisher’s protected least-significant difference.11 A p-value of <0.05 was considered statistically significant.
RESULTS
Effects of soy phytochemicals on tumor volume of estrogen-dependent human breast tumor in mice
Dietary treatments did not significantly alter food intake or final body weight (data not shown). Figure 1 shows the time- and dose-dependent effects of dietary soy components on tumor volume (Fig. 1a) and final tumor weight (Fig. 1b) of MCF-7 breast tumors in mice. To make results easier to interpret, the standard error for each data point (<10% of the mean) was not included in Figure 1a and Figure 2a,c. Soy treatments showed dose-dependent inhibition of tumor volume (Fig. 1a). Compared to control, final tumor weights from mice treated with 0.1% SPC, 0.5% SPC, 0.028% GSI and 0.14% GSI were reduced by 23% (p > 0.05); 62% (p < 0.05); 46.0% (p > 0.05); and 63.4% (p < 0.05), respectively (Fig. 1b).
FIGURE 1.
Dose-dependent effects of GSI and SPC on tumorigenicity and tumor volume in vivo. Mice were treated with experimental diets for 2 weeks, supplemented with 17β-estradiol, implanted orthotopically with MCF-7 cells, and continued on experimental diets throughout the study. Both GSI and SPC showed dose-dependent inhibition on tumor size (a), and final tumor weight (b). Values present mean ± SEM. *p < 0.05 (compared to control).
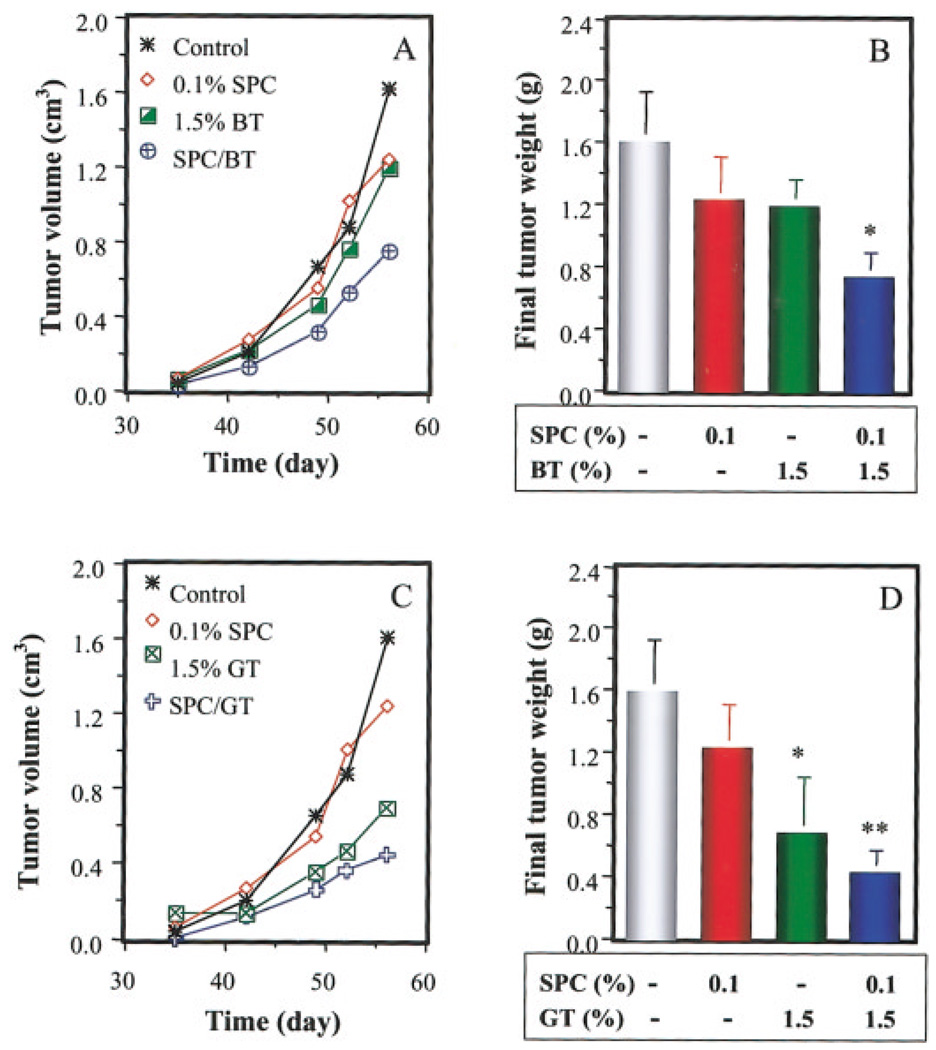
FIGURE 2.
Combined effects between SPC and tea on tumorigenicity, tumor volume and final tumor weight in vivo. Mice were treated with experimental diets for 2 weeks, supplemented with 17β-estradiol, implanted orthotopically with MCF-7 cells, and continued on experimental diets throughout the study. Values present mean ± SEM. *p < 0.05; **p < 0.01 (compared to the control).
Combined effects of soy phytochemicals and tea on tumor volume of estrogen-dependent human breast tumor in mice
The experimental dietary treatments did not significantly alter final body weight (Table III) or food intake (data not shown). Measurement of tea consumption indicated that mice consumed similar amounts of tea drink daily in each tea group (data not shown). GT at 1.5% reduced tumor size (Fig. 2c) and significantly reduced final tumor weight by 56% (p < 0.05) (Fig. 2d), whereas 0.1% SPC or 1.5% BT alone did not show significant effects on tumor size or final tumor weight (Fig. 2a,b).
TABLE III.
Combined Effects Between Soy and Tea Components on Tumor Proliferation Index, Apoptotic Index, Microvessel Density And Expression of Er-A and Serum Level Of Igf-I1
| Final body weight-tumor (g) |
Proliferation index (%) |
Apoptotic index (%) |
MVD (vessels/field) |
ER-α (arbitrary unit) |
IGF-I (ng/mL) | |
|---|---|---|---|---|---|---|
| Control | 23.2 ± 0.8 | 72.2 ± 4.3 | 3.32 ± 0.78 | 6.00 ± 2.65 | 131.1 ± 18.8 | 246.5 ± 31.6 |
| SPC (0.1%) | 24.0 ± 0.7 | 48.7 ± 2.94 | 4.98 ± 0.33 | 4.49 ± 1.29 | 135.7 ± 24.2 | 273.9 ± 21.5 |
| BT | 24.9 ± 0.5 | 48.6 ± 1.54 | 6.05 ± 0.664 | 3.25 ± 0.28 | 83.7 ± 1.12 | 260.2 ± 31.6 |
| GT | 24.8 ± 1.7 | 45.6 ± 0.64 | 5.32 ± 0.304 | 4.50 ± 1.16 | 122.3 ± 14.3 | 199.6 ± 14.8 |
| SPC/BT | 23.2 ± 0.8 | 47.4 ± 3.74 | 6.05 ± 0.654 | 2.51 ± 0.412 | 96.7 ± 20.02 | 256.5 ± 31.6 |
| SPC/GT | 22.6 ± 0.6 | 45.3 ± 3.34 | 5.75 ± 0.323 | 3.13 ± 0.442 | 84.4 ± 9.82 | 158.2 ± 12.82 |
Values are means ± SEM. Within the column, values with superscripts are significantly different from the control value. MVD, microvessel density.
p < 0.05.
p < 0.01.
p < 0.005.
The combination of 0.1% SPC with either 1.5% BT or 1.5% GT further reduced tumor volume (Fig. 2a,c) and final tumor weight (Fig. 2b,d). Compared to control, final tumor weights in mice treated with the 0.1% SPC, 1.5% BT, 1.5% % GT, SPC/BT combination, and SPC/GT combination were reduced by 23% (p > 0.05); 26% (p > 0.05); 56% (p < 0.05); 54% (p < 0.05); and 72% (p < 0.005), respectively (Fig. 2b,d).
The nature of the combined effects of SPC and tea was determined using the method described by Yokoyama et al.,12 and by us.13 In brief, the expected value of combination effect between Treatment 1 and Treatment 2 is calculated as [(observed Treatment 1 value)/(control value)] × [(observed Treatment 2 value)/(control value)] × (control value); and the combination index is calculated as the ratio of (expected value)/(observed value). A ratio of >1 indicates a synergistic effect, and a ratio of <1 indicates a less than additive effect.13 The combined effects of the SPC/BT combination (54%) and the SPC/GT combination (72%) on final tumor weight reduction were greater than the expected additive effects (43% and 66% reduction, respectively), with the ratios of 1.3 and 1.1, respectively. These outcomes suggest that combinations of SPC with either BT or GT may have a synergistic effect on the inhibition of human breast tumor growth.
Effects of soy phytochemicals on tumor cell proliferation, apoptosis and tumor angiogenesis
Tumor cell proliferation indices were measured by immunohistochemical detection of PCNA. Proliferation indices in MCF-7 tumors from mice treated with 0.1% SPC, 0.5% SPC, 0.028% GSI, and 0.14% GSI were reduced by 32.5% (p < 0.0001); 35.9% (p < 0.0001); 34.8% (p < 0.0001); and 37.3% (p < 0.0001), respectively, compared to control (Table II). Regression analysis indicated that the proliferation index was not significantly correlated to final tumor weight (p > 0.05); this suggests that this end-point tumor marker may not be sensitively correlated to tumor growth.
TABLE II.
Dose-Dependent Effects of Soy Phytochemicals and Gsi on Tumor Proliferation Index, Apoptotic Index And Microvessel Density1
| Proliferation index (%) |
Apoptotic index (%) |
MVD (vessels/field) |
|
|---|---|---|---|
| Control | 72.2 ± 4.3 | 3.32 ± 0.78 | 6.00 ± 2.65 |
| SPC (0.1%) | 48.7 ± 2.94 | 4.98 ± 0.33 | 4.49 ± 1.29 |
| SPC (0.5%) | 46.3 ± 3.84 | 4.39 ± 0.51 | 3.73 ± 0.81 |
| GSI (0.028%) | 47.1 ± 2.44 | 4.34 ± 0.66 | 2.43 ± 0.72 |
| GSI (0.14%) | 45.3 ± 0.84 | 4.94 ± 0.51 | 4.45 ± 1.61 |
Values are means ± SEM. Within the column, values with superscript are significantly different from the control value. MVD, microvessel density.
p < 0.05.
p < 0.01.
p < 0.005.
Tumor cell apoptosis and tumor MVD were determined by TUNEL assay and Factor VIII staining, respectively. Although dietary soy treatments tended to induce apoptosis and reduce tumor angiogenesis, the effects were not statistically significant (Table II).
Combined effects of soy phytochemicals and tea on tumor cell proliferation, apoptosis and tumor angiogenesis
Compared to control (Table III), proliferation indices of primary tumors in mice treated with 0.1% SPC, 1.5% BT, 1.5% GT, the SPC/BT combination and the SPC/GT combination were reduced by 32.5% (p < 0.005); 32.7% (p < 0.005); 36.8% (p < 0.005); 34.3% (p < 0.005); and 37.3% (p < 0.005), respectively. There were no apparent synergistic effects between SPC and tea on the tumor proliferation index.
Compared to control, apoptotic indices of primary tumors in mice treated with 0.1% SPC, 1.5% BT, 1.5% GT, the SPC/BT combination and the SPC/GT combination were increased by 50.0% (p > 0.05); 82.2% (p < 0.005); 60.2% (p < 0.05); 82.2% (p < 0.005); and 73.2% (p < 0.01), respectively (Table III). There were no apparent synergistic effects between SPC and tea on the tumor apoptotic index; this again suggests that this end-point tumor marker may not be sensitively correlated to tumor growth.
Compared to control, the MVD of primary tumors in mice treated with 0.1% SPC, 1.5% BT, 1.5% GT, SPC/BT combination and SPC/GT combination were reduced by 25.2% (p > 0.05); 45.8% (p > 0.05); 25.0% (p > 0.05); 58.2% (p < 0.05); and 47.8% (p < 0.05), respectively (Table III). SPC and tea alone had no significant effect on tumor angiogenesis. SPC and tea combinations, however, significantly inhibited tumor angiogenesis. This outcome suggests that one of the possible mechanisms that tea and SPC synergistically inhibit MCF-7 tumor growth is via their combined effects on the inhibition of tumor angiogenesis in vivo.
Combined effects of soy phytochemicals and tea on expression of tumor ERs
SPC and GSI did not significantly modulate expression of ER-α (data not shown). Compared to control, 1.5% BT significantly reduced expression of ER-α in MCF-7 tumors (36.2%, p < 0.05); 1.5% GT did not (Table III). Although 0.1% SPC and 1.5% GT alone did not significantly modulate expression of ER-α, the SPC/GT combination significantly reduced expression of ER-α (35.6%, p < 0.05) (Table III). This suggests that one of the mechanisms by which the SPC /GT combination synergistically inhibits MCF-7 tumors is via combined modulation of ER-α expression in vivo. Expression of ER-β was not significantly modulated by any treatment (data not shown).
Combined effects of soy phytochemicals and tea on serum level of IGF-I
SPC and GSI did not produce any dose-dependent effects on serum levels of IGF-I (data not shown). Compared to control, serum IGF-I levels in mice treated with 0.1% SPC, 1.5% BT, 1.5% GT, and the SPC/BT and SPC/GT combinations were reduced by −11.1% (p > 0.05); −5.5% (p > 0.05); 19.0% (p > 0.05); −4.1% (p > 0.05); and 35.8% (p < 0.05), respectively (Table III). GT alone reduced serum IGF-I by 19.0% (p > 0.05), but the SPC/GT combination did so by 35.8% (p < 0.05). This suggests that one of the mechanisms behind more potent effect of soy/GT combination on breast tumor inhibition is via modulation on IGF-I levels in vivo.
DISCUSSION
Though high doses of single bioactive agents have been shown to have potent anti-cancer effects, the chemopreventive properties of the Asian diet may result from interactions among several components that potentiate the activities of any single constituent. In our study, the combination of soy phytochemicals and tea components showed suggestive synergistic effects on preventing the progression of estrogen-dependent breast tumors in vivo (Fig. 2).
Several in vitro and in vivo studies have shown that green and black tea polyphenols have inhibitory and chemopreventive effects on breast carcinogenesis.14–19 Multiple studies have also demonstrated that components in dietary soy have anti-carcinogenic effects on breast tumors. The chemopreventive properties of the soy isoflavone, genistein, have been the subject of extensive in vitro20–29 and in vivo30–35 research.
Animal studies on genistein and MCF-7 tumor growth have shown conflicting results. Studies using an estrogen-depleted ovariectomized animal model demonstrated that dietary genistein or genistein-containing soy protein stimulated the growth of estrogen-dependent MCF-7 tumors in vivo.34,36–38 Using an estrogen-maintained animal model, Shao et al.35 demonstrated that genistein inhibited the growth of MCF-7 tumors in a dose-dependent manner. We found in this report that genistin-rich soy isoflavones and soy phytochemicals inhibited the growth of MCF-7 tumors (Fig. 1). Both our study and that of Shao et al.35 suggest that soy isoflavone genistein inhibits the growth of estrogen-dependent MCF-7 breast tumors in vivo.
We propose that an animal model with maintained estrogen levels would have greater clinical relevance than one with depleted estrogen levels for evaluating the effects of phytoestrogens (e.g., genistein) on the growth of estrogen-dependent MCF-7 breast tumors in vivo. Estrogen levels in intact female mice are not sufficient to support the growth of estrogen-dependent MCF-7 cells, and the growth of MCF-7 tumors in intact mouse requires estrogen supplements.39 Removal of estrogen pellets stops MCF-7 tumor growth.39 Conversely, estrogen-dependent breast tumors develop and progress in postmenopausal women, suggesting that estrogen levels in postmenopausal women are sufficient to support growth of estrogen-dependent breast tumors. Circulating estrogen levels in animals are higher than that in women. For example, postmenopausal and premenopausal women have blood 17β-estradiol levels of 9.6–53.3 pg/mL40–42 and 50.8–81.2 pg/mL,42,43 respectively. At these concentrations, estrogen-dependent breast tumor develops and grows. We found that mean blood levels of 17β-estradiol in intact female mice were 154.9 ± 4.1 pg/mL at which estrogen-dependent MCF-7 tumor did not grow; mice supplemented with 0.72 mg estradiol pellet (60-day release, Innovative Research of America, providing 300–400 pg/mL 17β-estradiol) had 456.8 ± 70.1 pg/mL of blood 17β-estradiol. We also found that 17β-estradiol supplements at 0.36 mg/pellet (90-day release, Innovative Research of America, providing 100–125 pg/mL) or lower to intact mice markedly reduced tumor-take rate and tumor growth (unpublished data). If this effect indicates that tumor-stimulating estrogen bioactivity in mice might be lower than that in women, then clinically relevant animal models of estrogen-dependent breast cancer should contain estrogen levels adequate to support tumor growth.
Several lines of evidence have demonstrated that angiogenesis is essential for the growth of solid tumors and their metastases.44 Soybean and tea contain diverse bioactive components that may interact to exert anti-cancer properties. Results from our study suggest that soy phytochemicals and tea components may synergistically inhibit growth of MCF-7 breast tumors (Fig. 2b,d), an effect associated, in part, with inhibition of tumor angiogenesis (Table III). Several bioactive components in SPC and tea may contribute to synergistic effects, but soy isoflavones and tea poly-phenols may be the most important. Both genistein and EGCG have shown anti-endothelial cell proliferation and anti-angiogenic activities in vitro, with concentrations of a few µmol producing half-maximal inhibition.45,46 At usual blood levels, genistein and EGCG are <1 µmol10,47 and neither SPC nor tea demonstrated significant anti-angiogenic effects in vivo (Table III). The combination of SPC with either BT or GT, however, significantly inhibited breast tumor angiogenesis, suggesting that one possible mechanism by which soy and tea may synergistically suppress estrogen-dependent breast tumors is via interactions that impede tumor angiogenesis. Further studies on the combined effects of bioactive soy and tea components on modulation of angiogenic (e.g., bFGF and VEGF) and anti-angiogenic factors may provide important information on how soy and tea combinations can inhibit tumor growth and angiogenesis.
IGF-I levels have been associated positively with risk of breast cancer48,49 and stages of breast cancer.50 IGF-I stimulates the proliferation of MCF-7 cells51,52 and inhibits anti-cancer drug-induced MCF-7 cell death53 in vitro. In our study we found that GT was a more potent inhibitor of MCF-7 tumors in vivo than BT, due, in part, to reduce serum levels of IGF-I (Table III). GT reduced serum levels of IGF-I by 19% (p > 0.05, Table III); SPC and BT had no effect. The combination of SPC with GT, but not BT, significantly suppressed IGF-I serum levels by 35.8% (Table III). This result suggests that soy and GT may synergistically inhibit breast tumor growth in part by modulating IGF-I signaling pathway.
Estrogen plays a key role in estrogen-dependent breast cancer development and growth, exerting its effect on estrogen-dependent tumors by binding to ER-α and ER-β and inducing ER-dependent transcriptional expression of estrogen-responsive genes that promote cancer cell proliferation. Iwao et al.54 have demonstrated that ER-α mRNA levels were significantly higher in ER-positive tumors than in ER-negative ones. Conversely, ER-β mRNA levels were significantly lower in ER-positive tumors than in ER-negative ones.54 Anti-estrogen treatment of estrogen-dependent breast tumors significantly reduced ER-α proteins in estrogen-dependent breast cancer cells in vitro55 and in vivo.56 Genistein also reduced ER-α protein levels in MCF-7 cells in vitro.57 We found that the combination of SPC and GT synergistically reduced ER-α protein level in MCF-7 tumors (Table III), an outcome that suggests a possible mechanism for the soy-GT combination’s synergistic effects on estrogen-dependent breast tumors.
It has been reported that genistein has a higher binding affinity to ER-β than that to ER-α in vitro.58 Although we found that the experimental treatments did not significantly modulate ER-β expression in MCF-7 tumors, it is unclear if soy and tea bioactive components or their combination exert any effects on modulating ER-β related signaling pathways in vivo. Further investigation in modulation of ER-β signaling pathways by dietary soy and tea components will be expected to provide important information on understanding the role of soy and tea in prevention of estrogen-dependent breast cancer.
In summary, our in vivo study indicated that soy phytochemicals produced dose-dependent inhibition of MCF-7 breast tumor, an effect associated with reduced tumor cell proliferation; and that SPC combined with GT may synergistically inhibit MCF-7 tumor growth, an outcome associated with inhibited tumor angiogenesis, and reduced ER-α protein and blood IGF-I levels. These different mechanisms of action make the effects of the combined components synergistic. They also make GT a more potent inhibitor of MCF-7 tumor progression than BT. It suggests that the focus of future research should be on combinations of soy and green tea components as dietary supplements for breast cancer chemoprevention and treatment.
ACKNOWLEDGEMENTS
Our study was supported in part by Susan Komen’s Breast Cancer Research Foundation, Massachusetts Department of Public Health Breast Cancer Research Program, and the United States Public Health Service RO1 AT00863. The authors thank R. Buckley, Medical Editor at Center for the Study of Nutrition Medicine, Beth Israel Deaconess Medical Center, for assistance in preparing this manuscript.
Grant sponsor: Susan Komen’s Breast Cancer Research Foundation; Grant number: BCTR2000487; Grant sponsor: Massachusetts Department of Public Health; Grant sponsor: United States Public Health Service; Grant number: RO1 AT00863.
Abbreviations
- BT
black tea
- EGCG
epigallocatechin gallate
- GSI
genistin-rich soy isoflavones
- GT
green tea
- MVD
microvessel density
- IGF
insulin-like growth factor
- PCNA
proliferating cell nuclear antigen
- SCID
severe combined immune deficient
- SPC
soy phytochemical concentrate
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling.
REFERENCES
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Henderson BE, Bernstein L. The international variation in breast cancer rates: an epidemiological assessment. Breast Cancer Res Treat. 1991;18:S11–S17. doi: 10.1007/BF02633520. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AMY, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, Hyer MB. Migration patterns and breast cancer risk in Asian- American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 4.Chemoprevention Working Group. Prevention of cancer in the next millennium: report of the chemoprevention working group to the American Association for Cancer Research. Cancer Res. 1999;59:4743–4758. [PubMed] [Google Scholar]
- 5.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 6.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J-R, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129:1628–1635. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J-R, Mukherjee P, Gugger ET, Tanaka T, Blackburn GL, Clinton SK. The inhibition of murine bladder tumorigenesis by soy isoflavones via alterations in the cell cycle, apoptosis, and angiogenesis. Cancer Res. 1998;58:5231–5238. [PubMed] [Google Scholar]
- 10.Zhou J-R, Yu L, Zhong Y, Nassr RL, Franke AA, Gaston SM, Blackburn GL. Inhibition of orthotopic growth and metastasis of androgen-sensitive human prostate tumors in mice by bioactive soybean components. Prostate. 2002;53:143–153. doi: 10.1002/pros.10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steel RGD, Torrie JH. Principles and procedures of statistics: a biometrical approach. New York: McGraw-Hill Book Company, Inc.; 1980. [Google Scholar]
- 12.Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Cancer Res. 2000;60:2190–2196. [PubMed] [Google Scholar]
- 13.Zhou J-R, Yu L, Zhong Y, Blackburn GL. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J Nutr. 2003;133:516–521. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisburger JH, Rivenson A, Garr K, Aliaga C. Tea, or tea and milk, inhibit mammary gland and colon carcinogenesis in rats. Cancer Lett. 1997;114:323–327. doi: 10.1016/s0304-3835(97)04693-4. [DOI] [PubMed] [Google Scholar]
- 15.Valcic S, Timmermann BN, Alberts DS, Wachter GA, Krutzsch M, Wymer J, Guillen JM. Inhibitory effect of six green tea catechins and caffeine on the growth of four selected human tumor cell lines. Anti Cancer Drugs. 1996;7:461–468. doi: 10.1097/00001813-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995;96:239–243. doi: 10.1016/0304-3835(95)03948-v. [DOI] [PubMed] [Google Scholar]
- 17.Sartippour MR, Heber D, Ma J, Lu Q, Go VL, Nguyen M. Green tea and its catechins inhibit breast cancer xenografts. Nutr Cancer. 2001;40:149–156. doi: 10.1207/S15327914NC402_11. [DOI] [PubMed] [Google Scholar]
- 18.Kavanagh KT, Hafer LJ, Kim DW, Mann KK, Sherr DH, Rogers AE, Sonenshein GE. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem. 2001;82:387–398. doi: 10.1002/jcb.1164. [DOI] [PubMed] [Google Scholar]
- 19.Rogers AE, Hafer LJ, Iskander YS, Yang S. Black tea and mammary gland carcinogenesis by 7,12-dimethylbenz[a]anthracene in rats fed control or high fat diets. Carcinogenesis. 1998;19:1269–1273. doi: 10.1093/carcin/19.7.1269. [DOI] [PubMed] [Google Scholar]
- 20.Monti E, Sinha BK. Antiproliferative effect of genistein and adriamycin against estrogen-dependent and -independent human breast carcinoma cell lines. Anticancer Res. 1994;14:1221–1226. [PubMed] [Google Scholar]
- 21.So FV, Guthrie N, Chambers AF, Carroll KK. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Lett. 1997;112:127–133. doi: 10.1016/s0304-3835(96)04557-0. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman R. Potent inhibition of breast cancer cell lines by the isoflavonoid kievitone: comparison with genistein. Biochem Biophys Res Commun. 1995;211:600–606. doi: 10.1006/bbrc.1995.1855. [DOI] [PubMed] [Google Scholar]
- 23.Peterson G, Barnes S. Genistein inhibits both estrogen and growth factor-stimulated proliferation of human breast cancer cells. Cell Growth Differ. 1996;7:1345–1351. [PubMed] [Google Scholar]
- 24.Shao ZM, Alpaugh ML, Fontana JA, Barsky SH. Genistein inhibits proliferation similarly in estrogen receptor-positive and negative human breast carcinoma cell lines characterized by P21WAF1/CIP1 induction, G2/M arrest, and apoptosis. J Cell Biochem. 1998;69:44–54. [PubMed] [Google Scholar]
- 25.Pagliacci MC, Smacchia M, Migliorati G, Grignani F, Riccardi C, Nicoletti I. Growth-inhibitory effects of the natural phyto-oestrogen genistein in MCF-7 human breast cancer cells. Eur J Cancer. 1994;30A:1675–1682. doi: 10.1016/0959-8049(94)00262-4. [DOI] [PubMed] [Google Scholar]
- 26.Clark JW, Santos-Moore A, Stevenson LE, Frackelton AR., Jr Effects of tyrosine kinase inhibitors on the proliferation of human breast cancer cell lines and proteins important in the ras signaling pathway. Int J Cancer. 1996;65:186–191. doi: 10.1002/(SICI)1097-0215(19960117)65:2<186::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Zava DT, Duwe G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr Cancer. 1997;27:31–40. doi: 10.1080/01635589709514498. [DOI] [PubMed] [Google Scholar]
- 28.Dees C, Foster JS, Ahamed S, Wimalasena J. Dietary estrogens stimulate human breast cells to enter the cell cycle. Environ Health Perspect. 1997;3:633–636. doi: 10.1289/ehp.97105s3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Kurzer MS. Phytoestrogen concentration determines effects on DNA synthesis in human breast cancer cells. Nutr Cancer. 1997;28:236–247. doi: 10.1080/01635589709514582. [DOI] [PubMed] [Google Scholar]
- 30.Constantinou AI, Mehta RG, Vaughan A. Inhibition of n-methyl-n-nitrosourea-induced mammary tumors in rats by the soybean isoflavones. Anticancer Res. 1996;16:3293–3298. [PubMed] [Google Scholar]
- 31.Lamartiniere CA, Murrill WB, Manzolillo PA, Zhang JX, Barnes S, Zhang X, Wei H, Brown NM. Genistein alters the ontogeny of mammary gland development and protects against chemically-induced mammary cancer in rats. Proc Soc Exp Biol Med. 1998;217:358–364. doi: 10.3181/00379727-217-44245. [DOI] [PubMed] [Google Scholar]
- 32.Lamartiniere CA, Moore JB, Brown NM, Thompson R, Hardin MJ, Barnes S. Genistein suppresses mammary cancer in rats. Carcinogenesis. 1995;16:2833–2840. doi: 10.1093/carcin/16.11.2833. [DOI] [PubMed] [Google Scholar]
- 33.Lamartiniere CA, Moore J, Holland M, Barnes S. Neonatal genistein chemoprevents mammary cancer. Proc Soc Exp Biol Med. 1995;208:120–123. doi: 10.3181/00379727-208-43843. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh C-Y, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. [PubMed] [Google Scholar]
- 35.Shao Z-M, Wu J, Shen Z-Z, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998;58:4851–4857. [PubMed] [Google Scholar]
- 36.Ju YH, Allred CD, Allred KF, Karko KL, Doerge DR, Helferich WG. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131:2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- 37.Allred CD, Ju YH, Allred KF, Chang J, Helferich WG. Dietary genistin stimulates growth of estrogen-dependent breast cancer tumors similar to that observed with genistein. Carcinogenesis. 2001;22:1667–1673. doi: 10.1093/carcin/22.10.1667. [DOI] [PubMed] [Google Scholar]
- 38.Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- 39.Soule HD, McGrath CM. Estrogen responsive proliferation of clonal human breast carcinoma cells in athymic mice. Cancer Lett. 1980;10:177–189. doi: 10.1016/0304-3835(80)90042-7. [DOI] [PubMed] [Google Scholar]
- 40.Han KK, Soares JM, Jr, Haidar MA, de Lima GR, Baracat EC. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstet Gynecol. 2002;99:389–394. doi: 10.1016/s0029-7844(01)01744-6. [DOI] [PubMed] [Google Scholar]
- 41.Koh KK, Cardillo C, Bui MN, Hathaway L, Csako G, Waclawiw M, Panza JA, Cannon RO., III Vascular effects of estrogen and cholesterol-lowering therapies in hypercholesterolemic postmenopausal women. Circulation. 1999;99:354–360. doi: 10.1161/01.cir.99.3.354. [DOI] [PubMed] [Google Scholar]
- 42.Petrakis NL, Barnes S, King EB, Lowenstein J, Wiencke J, Lee MM, Miike R, Kirk M, Coward L. Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785–794. [PubMed] [Google Scholar]
- 43.Woods MN, Barnett JB, Spiegelman D, Trail N, Hertzmark E, Long-cope C, Gorbach SL. Hormone levels during dietary changes in premenopausal African-American Women. J Natl Cancer Inst. 1996;88:1369–1374. doi: 10.1093/jnci/88.19.1369. [DOI] [PubMed] [Google Scholar]
- 44.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1989;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 45.Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wahala K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 46.Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 47.Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–354. [PubMed] [Google Scholar]
- 48.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 49.Peyrat J, Bonneterre J, Hecqet B, Vennin P, Louchez M, Fournier C, Lefebvre J, Demaille A. Plasma insulin-like growth factor-I (IGF-I) concentrations in human breast cancer. Eur J Cancer. 1993;29A:492–497. doi: 10.1016/s0959-8049(05)80137-6. [DOI] [PubMed] [Google Scholar]
- 50.Eppler E, Zapf J, Bailer N, Falkmer UG, Falkmer S, Reinecke M. IGF-I in human breast cancer: low differentiation stage is associated with decreased IGF-I content. Eur J Endocrinol. 2002;146:813–821. doi: 10.1530/eje.0.1460813. [DOI] [PubMed] [Google Scholar]
- 51.Bentel JM, Lebwohl DE, Cullen KJ, Rubin MS, Rosen N, Mendelsohn J, Miller WJ. Insulin-like growth factors modulate the growth inhibitory effects of retinoic acid on MCF-7 breast cancer cells. J Cell Physiol. 1995;165:212–221. doi: 10.1002/jcp.1041650124. [DOI] [PubMed] [Google Scholar]
- 52.Costantino A, Vinci C, Mineo R, Frasca F, Pandini G, Milazzo G, Vigneri R, Belfiore A. Interleukin-1 blocks insulin and insulin-like growth factor-stimulated growth in MCF-7 human breast cancer cells by inhibiting receptor tyrosine kinase activity. Endocrinology. 1996;137:4100–4107. doi: 10.1210/endo.137.10.8828463. [DOI] [PubMed] [Google Scholar]
- 53.Geier A, Beery R, Haimsohn M, Karasik A. Insulin-like growth factor-1 inhibits cell death induced by anticancer drugs in the MCF-7 cells: involvement of growth factors in drug resistance. Cancer Invest. 1995;13:480–486. doi: 10.3109/07357909509024911. [DOI] [PubMed] [Google Scholar]
- 54.Iwao K, Miyoshi Y, Egawa C, Ikeda N, Tsukamoto F, Noguchi S. Quantitative analysis of estrogen receptor-alpha and -beta messenger RNA expression in breast carcinoma by real-time polymerase chain reaction. Cancer. 2000;89:1732–1738. doi: 10.1002/1097-0142(20001015)89:8<1732::AID-CNCR13>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 55.Pink JJ, Jordan VC. Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res. 1996;56:2321–2330. [PubMed] [Google Scholar]
- 56.Luo S, Martel C, Gauthier S, Merand Y, Belanger A, Labrie C, Labrie F. Long-term inhibitory effects of a novel anti-estrogen on the growth of ZR-75-1 and MCF-7 human breast cancer tumors in nude mice. Int J Cancer. 1997;73:735–739. doi: 10.1002/(sici)1097-0215(19971127)73:5<735::aid-ijc21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 57.Diel P, Olff S, Schmidt S, Michna H. Molecular identification of potential selective estrogen receptor modulator (SERM) like properties of phytoestrogens in the human breast cancer cell line MCF-7. Planta Med. 2001;67:510–514. doi: 10.1055/s-2001-16474. [DOI] [PubMed] [Google Scholar]
- 58.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JÅ. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:3863–3870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]