Abstract
Paresh Chaudhari, PharmD, MPH, frames the business case for employers to look beyond the direct medical costs of treatment of RA and consider employee health benefits as an investment.
In a world that emphasizes the bottom line, direct expenses for chronic conditions often receive more attention than indirect costs. But if healthcare benefits are thought of as an investment, then employers would do well to seek ROI data, such as productivity gains.
Any executive can explain return on investment (ROI) analysis and its importance to an organization. ROI is a ratio that determines the return on each dollar spent, measuring how effectively capital is used to generate profit. For decades, ROI analyses have been applied to asset purchases (such as computer systems) and to various programs (e.g., those used for marketing and training purposes) to make business decisions.
So why is it that executives do not consider the health benefits they purchase for their employees an investment? Healthcare consumed 16 percent of the U.S. gross domestic product, or $2.1 trillion, in 2006 (Zhang 2008). As healthcare costs continue to rise, so does the importance of using ROI analysis to assess the financial benefits employers derive from employee healthcare.
In this article, the following common issues concerning rheumatoid arthritis (RA) will be addressed from the perspectives of employers and third-party payers:
What is RA?
How is RA treated?
What are the direct healthcare costs of treating RA patients?
What is the work-related disability in RA patients?
How are work productivity losses associated with RA measured?
How do biologic medications for RA affect work productivity?
What are the indirect health-care costs to employers?
What is the impact of biologic medications on payers?
What is the future of biologics for RA and for the industry?
WHAT IS RA?
RA is a chronic and debilitating disorder that develops in about 3 percent of the world’s population, affecting 2 to 4 times more women than men (Kvien 2004). RA usually strikes people between the ages of 35 and 50 (Burton 2006). To date, the exact cause of RA is unknown, but it is often described as a progressive disease in which components of the immune system attack the synovial fluid between joints, resulting in inflammation that causes swelling, pain, and destruction of the soft tissue lining the joints. Erosion of the synovial fluid, cartilage, bone, and ligaments eventually leads to deformity of the affected joints, disability, and premature death if not treated appropriately (Kvien 2004).

Paresh Chaudhari, PharmD, MPH
MANAGEMENT OF RA
RA is best managed by early diagnosis to reduce the likelihood of irreversible joint damage and other consequences. In addition to the characteristic pattern of symptoms, laboratory indicators, such as rheumatoid factor, a distinctive antibody in the blood of RA patients, and erythrocyte sedimentation rate, a test that measures active inflammation in the body, are used to diagnose and measure the severity of RA.
Treatments range from simple non-pharmacologic measures to medications, and even surgery. The major categories of pharmacologic agents used to manage RA include non-steroidal anti-inflammatory drugs; classic (nonbiologic) disease-modifying antirheumatic drugs (DMARDs), such as methotrexate; slow-acting drugs, such as hydroxychloroquine and auranofin, which compose a special subclass of DMARDs; corticosteroids; and biologic immunosuppressive drugs. At present, six biologics — three tumor necrosis factor-alpha (TNF-α) inhibitors, an interleukin 1 receptor antagonist, a T-lymphocyte activation inhibitor, and a CD20-directed cytolytic antibody that depletes B cells — have received a U.S. Food and Drug Administration indication for the treatment of RA.
American College of Rheumatology (ACR) guidelines recommend that DMARDs, such as methotrexate, hydroxychloroquine, sulfasalazine, and leflunomide, be used as first-line therapy before initiating treatment with one of the biologic agents as an add-on or as monotherapy (ACR 2002). Because of the high cost of bio logics to treat RA, there has been some controversy regarding when during the course of treatment they should be initiated. Two large-scale trials, ASPIRE (Active Controlled Study of Patients Receiving Infliximab for Treatment of Rheumatoid Arthritis of Early Onset) (St. Clair 2004) and the PREMIER study (Breedveld 2006), found that adding a TNF-α blocker to methotrexate significantly improved the signs and symptoms of disease activity, and arrested or even reduced joint erosion in patients with early RA compared with those patients who were treated with methotrexate alone.
DIRECT COSTS
Until recently, relatively low-cost medications have been used to treat RA. Since the advent of biologics, the management of RA has shifted dramatically — from the use of older pharmacologic agents mainly to control symptoms to the more intensive use of biologic treatments earlier in the course of the disease, with the goal of halting disease progression and achieving remission. This approach has increased the utilization of high-cost biologic medications, resulting in an overall increase in the direct costs of RA treatment.
Compared with the U.S. population not afflicted with RA (adjusted for age and gender), RA patients have 3 times the direct healthcare costs, twice the hospitalization rates, and 10 times the work disability rates (ACR 2002). One study found that average total direct costs for a patient treated with a biologic was $19,016 per year compared with $6,164 per year for a patient treated without a biologic (Michaud 2003). Annual medication costs can reach $15,000 to $20,000 per patient treated with a biologic agent (CVS Caremark 2007). Approximately 66 percent of direct costs ($6,324) were attributed to prescription drugs for all RA patients, 25 percent of whom received biologics (Michaud 2003). A review of 15 studies found that, on average, direct medical costs were $5,720 and indirect costs ranged from $1,080 to $37,501 per year (1996 dollars) for RA patients (Cooper 2000).
DISABILITY IN RA PATIENTS
Although direct medical costs for RA result from the utilization of healthcare resources — including medications — indirect costs are attributed, in part, to a reduced ability to perform daily activities both at home and at work due to the crippling effects of the disease. Indirect costs may include lost employee wages, along with low productivity and output levels. Within 2 to 3 years from the onset of RA, approximately 20 to 30 percent of RA patients with a paid job end up work-disabled; work disability has been reported to range from 13 percent at 6 months to 67 percent at 15 years from the onset of RA (Verstappen 2004).
Despite its tremendous effect on work disability, the relative lack of research aimed at early treatment to avoid RA-related work loss should not reflect the greater importance of preventing disability (de Croon 2004). Numerous studies have examined factors that may predict work-related disability in RA patients, but there is an inconsistency in the association of disability with disease duration, impaired body function/structure, financial situation, and gender (de Croon 2004). Some evidence does suggest, however, a relationship between the following factors and the predictability of work disability in RA patients: age, disease activity, disease duration, emotional function, self-reported physical job demands, working hours, education, race, and health assessment questionnaire scores (Holte 2001, Allaire 1996, de Roos 1999, Chorus 2001).
PRODUCTIVITY LOSSES
Understanding that RA-related disability can reduce work productivity is fundamental, especially given that most RA patients are between the ages of 35 and 50 (Burton 2006). But our ability to quantify the associated reduction in monetary terms is rudimentary, and calculating indirect costs is a major challenge. Although absenteeism, disability leave, and workers’ compensation have been the focus of many studies (Lee 1994, Clarke 1997, Fautrel 2002), new research suggests that presenteeism —a decrease in on-the-job performance usually resulting from illness — is the major factor in work productivity losses (Loftland 2004). Costs associated with reduced work productivity, essentially a function of an individual’s wages or compensation, can be quantified by measuring absenteeism and presenteeism using two common approaches — the human capital approach (HCA) and the friction cost approach (FCA).
HCA, the approach used in most studies (Loftland 2001, Liljas 1998), assigns value to lost productivity by estimating expected or potential earnings lost due to a disease or disorder. HCA assigns zero dollars to costs incurred outside of paid work, such as hiring a maid to do housework.
FCA incorporates both the time needed to replace the RA-affected worker and the time needed for the replacement worker to reach the productivity level of the affected worker pre-illness. FCA includes the costs of hiring, replacing, and training a new employee, as well as the lost productivity level prior to the absent worker being replaced and the decreased output associated with any new employee. FCA also assigns zero dollar value to costs incurred outside of paid work. Although FCA tends to yield lower estimates (Liljas 1998), it is probably a better method for calculating work productivity losses from the employer perspective, whereas HCA is more appropriate from a societal perspective (Loftland 2004).
Li (2006) found that 41 percent of RA patients’ work productivity losses were due to reduced performance, and 22 percent were due to absences and abbreviated work hours. It also has been reported that workers with arthritis or back pain have an average productivity loss of 5.2 hours per week (Stewart 2003). Two studies by Yelin (1996, 2007) have shown that half of RA patients stop working 13 years after the onset of symptoms, and that productivity losses for those who remained employed ranged from 22 to 76 percent.
IMPACT OF BIOLOGICS ON PRODUCTIVITY
It should not be surprising that employers want results from (or returns on) their investments, often in the form of work performance. The data they seek are beginning to surface. One study showed the majority of RA patients’ work productivity losses occurred at the onset of disease, and that some of that decline could have been prevented through the use of aggressive treatment earlier in the progression of the disease (Schultz 2007). Although many studies have shown no relationship between the use of classic DMARDs and work disability (Doeglas 1995, Sokka 1999, Young 2002) or work loss (Merkesdal 2001, Ruof 2003), biologics have proven to be superior to all other RA medications, including DMARDs, in terms of reducing joint damage caused by RA (St. Clair 2004, Maini 2004). Studies of patients on biologic regimens have shown fewer lost days from work and increased employability (Kavanaugh 2004), reduced work loss (Bresnihan 2002, Bresnihan 2003), and increased workforce participation (Yelin 2003).
EMPLOYER LOSSES
It has been well documented that the most expensive consequence of RA is work productivity loss (Soderlin 2003, Pugner 2000, Hallert 2004). In the traditional view of employee health benefit packages, most employers focus only on out-of-pocket costs, which consist of group health payments (63 percent), incidental absences (16 percent), workers’ compensation (9 percent), short-term disability (8 percent), and long-term disability (4 percent) (IBI 2004). In that view, the financial impact of lost productivity from absences, which can be captured by FCA, is ignored. One analysis demonstrated that after the inclusion of indirect costs due to absences, 71 percent of the full costs of employee benefits were attributed to lost productivity resulting from absences and 19 percent to group health payments and workers’ compensation (IBI 2004).
More employers now recognize the importance of health and productivity management to their organizations, enabled in part by researchers who are creating tools that will help employers to become better healthcare managers (Kessler 2006, Kessler 2004). Still, only a third of employers view strategies aimed at improving overall health and work productivity as useful (Rosenthal 2007). Though indirect costs may have a larger impact on profit margins, most employers tend to ignore them — mainly due to the lack of a “business case” resulting from measurement challenges — and focus primarily on their direct healthcare costs and insurance premiums (Rosenthal 2007). Thus, it is in the best interest of employers and third-party payers to continue to develop valid and reliable tools for measuring indirect costs, including those that accompany RA (Prasad 2004). This challenge is echoed by the absence of published reports on work-related disability from the employer perspective (Burton 2006).
IMPACT ON PAYERS
It is estimated that by 2020, 37 percent of total drug expenditures in the United States will be attributed to biologics (PCMA 2006). In one survey, 68 percent of managed care medical directors said they consider managing the cost of biologics a high priority (MedPanel 2006).
Arguably, the greatest impact of the high costs of biologics is on un-insured or underinsured patients, especially senior citizens, who comprise approximately 50 percent of RA patients and often cannot afford to pay the entire out-of-pocket cost of RA medications. Even though Medicare now covers several biologics under Part D, copayments, deductibles, premiums, and the “doughnut hole” leave Medicare beneficiaries responsible for about half of their catastrophic medication costs.
Changes in Medicare coverage have increased beneficiary access to medications, but, as expected, they also are leading to increased utilization and overall drug costs (Zhang 2008). If the rate of the rise in expenditures for biologics remains high, total costs for such drugs in 10 years may increase by a factor of nearly 20 — accounting for just under 30 percent of the projected Medicare Part D budget — according to Blue Cross Blue Shield data (Mullins 2005).
IMPACT ON THE INDUSTRY
Arguably, Medicare Part D may serve as one of the contributors to increased utilization of biologics in RA patients, as do the initiation of aggressive treatment earlier and for more patients. Sales of biologic agents grew 20 percent, to $40.3 billion, in 2006 — more than double the rate of traditional pharmaceutical agents (Walgreens 2007). RA was ranked as the number one therapeutic area for biologic sales by class in 2006, and etanercept (Enbrel) was ranked as the number one RA biologic by sales (Table 1, page 39). Biologics comprise approximately half of the current drugs in the pipeline, four of which are expected to gain their first FDA indications this year (Table 2, page 40). As the pipeline of traditional pharmaceuticals dries up, the future of new miracles in medicine lies in biotechnology.
TABLE 1.
SELECTED TOP-SELLING BIOLOGICS AND THEIR PATENT EXPIRATIONS
| Product | Company | Indication | Patent expiration | 2006 revenue (millions) | |
|---|---|---|---|---|---|
| U.S. | Europe | ||||
| etanercept (Enbrel) | Amgen, Wyeth | Rheumatoid arthritis and other inflammatory disorders | 2012 | 2010 | $4,379 |
| darbepoetin alfa (Aranesp) | Amgen | Renal and cancer anemia | 2016 | 2014 | $4,121 |
| rituximab (Rituxan/MabThera) | Genentech, Roche, Biogen Idec | Non-Hodgkin’s lymphoma and rheumatoid arthritis | 2015 | 2013 | $3,912 |
| epoetin alfa (Procrit/Eprex) | Johnson & Johnson | Renal and cancer anemia | 2013 | 2004 | $3,180 |
| pegfilgrastim (Neulasta) | Amgen | Neutropenia | 2015 | 2015 | $2,710 |
| epoetin alfa (Epogen) | Amgen | Renal and cancer anemia | 2013 | 2004 | $2,511 |
| insulin glargine [rDNA] injection (Lantus)* | sanofi aventis | Diabetes | 2014 | 2014 | $2,115 |
| interferon β-1b (Betaferon/Betaseron) | Bayer Schering Pharma | Multiple sclerosis | 2007 | 2008 | $1,273 |
| filgrastim (Neupogen) | Amgen | Neutropenia | 2013 | 2006 | $1,213 |
Lantus revenues converted into U.S. dollars using the average exchange rate in the company’s 2006 annual report.
Sources: CVS 2007, Regent Atlantic Capital 2007
TABLE 2.
KEY PRODUCTS: SPECIALTY DRUG PIPELINE
| Drug | Use | Est. peak sales (millions) | Competitor drugs |
|---|---|---|---|
| 2007 launches | |||
| ambrisentan (Letairis) | Pulmonary arterial hypertension | $500 (global) | bosentan (Tracleer) |
| histrelin (Supprelin LA) | Precocious puberty | $28* | leuprolide (Lupron) |
| lapatinib (Tykerb) | Breast cancer | $1,200 (US) | trastuzumab (Herceptin) |
| nilotinib (Tasigna) | Chronic myeloid leukemia | $400* | dasatinib (Sprycel) |
| 2008 launches | |||
| certolizumab pegol (Cimzia) | Crohn’s disease | $525 (US) | infliximab (Remicade), adalimumab (Humira) |
| 2008 anticipated launches | |||
| icatibant (Firazir) | Hereditary angioedema | $100 (global) | First in class |
| eltrombopag (Promacta) | Idiopathic thrombocytopenia | $2,100* | First in class |
| tetrabenazine (Xenazine) | Huntington’s disease | N/A | First in class |
Market unspecified
Source: CVS 2007
The enormous growth within the biologic industry will stem from the development of follow-on biologics, also known as biosimilars (Genazzani 2007). Biosimilars are not “generic” in the sense of being identical to brand-name biologics, but are similar enough to produce comparable results in patients (Mellstedt 2007). The main differences in the production of biosimilars, compared with traditional pharmaceutical generics, lie in their development and manufacturing methods, with the former being much more complex and difficult to reproduce (Ranke 2008). Even a slight variation in the structure and properties of a biosimilar can cause near-fatal consequences for patients (Ranke 2008). Another challenge is in conducting comprehensive testing, which increases the costs involved in proving that the safety and efficacy of a biosimilar medication are comparable to the original branded version.
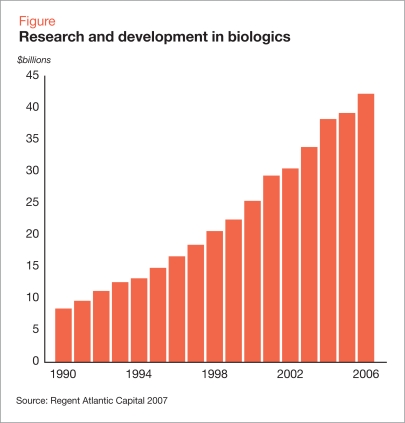
It has been argued that the difficulty in proving biosimilarity will eliminate the majority of potential cost savings (Mellstedt 2007). Nonetheless, employers, third-party payers, and patients have expectations of significant savings, and it has been estimated that biosimilar drugs have the potential to reduce Medicare costs by $14 billion annually (Engel 2007). With a number of high-revenue drugs nearing patent expiration (Table 1), investment in research and development has steadily increased (Figure).
Figure.
Research and development in biologics
Source: Regent Atlantic Capital 2007
Despite disagreement over the benefits of biosimilars, legislators and regulators outside the United States are supporting their development. In 2004, the FDA’s European counterpart, the European Medicines Agency, established regulations governing the development of biosimilars and, in April 2006, approved somatropin (Omnitrope), a biosimilar version of genotropin, a growth hormone (Pavlovic 2008). The U.S. Food and Drug Administration approved the drug the following month, despite lack of a regulatory pathway for biopharmaceuticals in the United States. Since then, legislative proposals have been initiated in Congress to expedite the approval process for biosimilar drugs and to give approval authority to the FDA, but legislation has stalled.
SUMMARY
Despite groundbreaking advances in pharmacologic treatments for RA, particularly from the biotech industry, major net economic benefits from the use of biologics remain to be seen by payers, employers, and society. The business world, including the biotech industry, is aware that ROI usually does not occur overnight, and, in the case of RA, it may take many years to document that return. More than any other group, human resource managers should understand that an organization’s biggest asset is its employees. Employees who suffer with RA significantly affect an organization’s bottom line, whether an employer realizes it or not.
With the help of proper tools, employers are beginning to see the big picture on costly health interventions from an ROI or a full-cost viewpoint. Considering only the costs of various treatment options for coverage decisions is not in the best interest of either employers or third-party payers, both of which tend to focus heavily on short-term direct costs (i.e., prescription medications, physician visits, and hospitalizations). In addition to improving employee health, those individuals and organizations that make decisions regarding employee health benefits from an investment perspective will have a competitive edge compared with those who do not use the full-cost view.
More research is needed on the impact of RA on work-related productivity losses and employers’ other indirect costs. Employers tend to be wary of sharing employee productivity data with researchers or allowing their employees to participate in such studies. However, employers seeking to understand and minimize bottom-line effects of health conditions in their workforces could benefit from collaboration with researchers.
Footnotes
Disclosure
Paresh Chaudhari, PharmD, MPH, reports that he has no financial arrangements or affiliations with manufacturers or products mentioned in this article.
REFERENCES
- Allaire SH, Anderson JJ, Meenan RF. Reducing work disability associated with rheumatoid arthritis: identification of additional risk factors and persons likely to benefit from intervention. Arthritis Care Res. 1996;9:349–357. doi: 10.1002/1529-0131(199610)9:5<349::aid-anr1790090503>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- Bresnihan B. Anakinra as a new therapeutic option in rheumatoid arthritis: clinical results and perspectives. Clin Exp Rheumatol. 2002;20(5 suppl 27):S32–S34. [PubMed] [Google Scholar]
- Bresnihan B, Cobby M. Clinical and radiological effects of anakinra in patients with rheumatoid arthritis. Rheumatology (Oxford) 2003;42(suppl 2):ii22–28. doi: 10.1093/rheumatology/keg329. [DOI] [PubMed] [Google Scholar]
- Burton W, Morrison A, Maclean R, Ruderman E. Systematic review of studies of productivity loss due to rheumatoid arthritis. Occup Med (Lond) 2006;56:18–27. doi: 10.1093/occmed/kqi171. [DOI] [PubMed] [Google Scholar]
- Chorus AM, Miedema HS, Wevers CW, van der Linden S. Work factors and behavioural coping in relation to withdrawal from the labour force in patients with rheumatoid arthritis. Ann Rheum Dis. 2001;60:1025–1032. doi: 10.1136/ard.60.11.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AE, Zowall H, Levinton C, et al. Direct and indirect medical costs incurred by Canadian patients with rheumatoid arthritis: a 12 year study. J Rheumatol. 1997;24:1051–1060. [PubMed] [Google Scholar]
- Cooper NJ. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 2000;39:28–33. doi: 10.1093/rheumatology/39.1.28. [DOI] [PubMed] [Google Scholar]
- CVS Caremark TrendsRx Report 2007«https://www.caremark.com/portal/asset/TrendsRxReport_07.pdf«. Accessed July 16, 2008.
- de Croon EM, Sluiter JK, Nijssen TF, et al. Predictive factors of work disability in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2004;63:1362–1367. doi: 10.1136/ard.2003.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roos AJ, Callahan LF. Differences by sex in correlates of work status in rheumatoid arthritis patients. Arthritis Care Res. 1999;12:381–391. doi: 10.1002/1529-0131(199912)12:6<381::aid-art6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Doeglas D, Suurmeijer T, Krol B, et al. Work disability in early rheumatoid arthritis. Ann Rheum Dis. 1995;54:455–460. doi: 10.1136/ard.54.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel & Novitt LLP Potential savings that might be realized by the Medicare program from enactment of legislation such as the Access To Life-Saving Medicine Act (H.R. 6257/S. 4016) that establishes a new cBLA pathway for follow-on biologics. Jan. 2007. «http://www.pcmanet.org/assets/2008-03-24_Asset_EN%20Paper%20on%20Follow-on%20Biologics%20Jan.%202007.pdf«. Accessed July 16, 2008.
- Fautrel B, Guillemin F. Cost of illness studies in rheumatic diseases. Curr Opin Rheumatol. 2002;14:121–126. doi: 10.1097/00002281-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Genazzani AA, Biggio G, Caputi AP, et al. Biosimilar drugs: concerns and opportunities. BioDrugs. 2007;21:351–356. doi: 10.2165/00063030-200721060-00003. [DOI] [PubMed] [Google Scholar]
- Hallert E, Husberg M, Jonsson D, Skogh T. Rheumatoid arthritis is already expensive during the first year of the disease (the Swedish TIRA project) Rheumatology (Oxford) 2004;43:1374–1382. doi: 10.1093/rheumatology/keh324. [DOI] [PubMed] [Google Scholar]
- Holte HH, Tambs K, Bjerkedal T. Becoming a disability pensioner with rheumatoid arthritis in Norway 1971–1990. J Rheumatol. 2001;28:54–61. [PubMed] [Google Scholar]
- IBI (Integrated Benefits Institute) The business case for managing health and productivity: Results from IBI’s full-cost benchmarking program. 2004. «http://ibiweb.org/do/viewdocument/DocumentDetail?linkId=37837&aId=D732E93E740897CD25C644489EB118BF». Accessed July 16, 2008.
- Kavanaugh A, Han C, Bala M. Functional status and radiographic joint damage are associated with health economic outcomes in patients with rheumatoid arthritis. J Rheumatol. 2004;31:849–855. [PubMed] [Google Scholar]
- Kessler RC, Ames M, Hymel PA, et al. Using the World Health Organization health and work performance questionnaire (HPQ) to evaluate the indirect workplace costs of illness. J Occup Environ Med. 2004;46:S23–S37. doi: 10.1097/01.jom.0000126683.75201.c5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Stang PE.Health and Work Productivity: Making the Business Case for Quality Health CareThe John D. and Catherine T. MacArthur Foundation Series on Mental Health and DevelopmentChicago: University of Chicago Press; 2006 [Google Scholar]
- Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22(2 suppl 1):1–12. doi: 10.2165/00019053-200422001-00002. [DOI] [PubMed] [Google Scholar]
- Lee P. The economic impact of musculoskeletal disorders. Qual Life Res. 1994;3(suppl 1):S85–S91. doi: 10.1007/BF00433381. [DOI] [PubMed] [Google Scholar]
- Li X, Gignac MA, Anis AH. The indirect costs of arthritis resulting from unemployment, reduced performance, and occupational changes while at work. Med Care. 2006;44:304–310. doi: 10.1097/01.mlr.0000204257.25875.04. [DOI] [PubMed] [Google Scholar]
- Liljas B.How to calculate indirect costs in economic evaluations Pharmacoeconomics 199813(1 Pt 1)1–7. [DOI] [PubMed] [Google Scholar]
- Lofland JH, Locklear JC, Frick KD. Different approaches to valuing the lost productivity of patients with migraine. Pharmacoeconomics. 2001;19:917–925. doi: 10.2165/00019053-200119090-00003. [DOI] [PubMed] [Google Scholar]
- Lofland JH, Pizzi L, Frick KD. A review of health-related workplace productivity loss instruments. Pharmacoeconomics. 2004;22:165–184. doi: 10.2165/00019053-200422030-00003. [DOI] [PubMed] [Google Scholar]
- Maini RN, Breedveld FC, Kalden JR, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–1065. doi: 10.1002/art.20159. [DOI] [PubMed] [Google Scholar]
- MedPanel Inc. MedPanel survey of managed care medical directors predicts small rate of increase in MCO medical costs, reveals cost control strategies for 2006. May 2006. «http://mcfpanelintelligence.com/view_pr.asp?file=may092006». Accessed July 16, 2008
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411–419. doi: 10.1093/annonc/mdm345. [DOI] [PubMed] [Google Scholar]
- Merkesdal S, Ruof J, Schöffski O, et al. Indirect medical costs in early rheumatoid arthritis: composition of and changes in indirect costs within the first three years of disease. Arthritis Rheum. 2001;44:528–534. doi: 10.1002/1529-0131(200103)44:3<528::AID-ANR100>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Michaud K, Messer J, Choi HK, Wolfe F. Direct medical costs and their predictors in patients with rheumatoid arthritis: a three-year study of 7,527 patients. Arthritis Rheum. 2003;48:2750–2762. doi: 10.1002/art.11439. [DOI] [PubMed] [Google Scholar]
- Mullins CD, DeVries AR, Hsu VD, et al. Variability and growth in spending for outpatient specialty pharmaceuticals. Health Aff (Millwood) 2005;24:1117–1127. doi: 10.1377/hlthaff.24.4.1117. [DOI] [PubMed] [Google Scholar]
- Pavlovic M, Girardin E, Kapetanovic L, et al. Similar biological medicinal products containing recombinant human growth hormone: European regulation. Horm Res. 2008;69:14–21. doi: 10.1159/000111790. [DOI] [PubMed] [Google Scholar]
- Pharmaceutical Care Management Association. Specialty pharmacy trends and management strategies. April 2006. «http://www.pcmanet.org/assets/2008-03-25_Research_sp_trendsstrategies.pdf». Accessed July 16, 2008.
- Prasad M, Wahlqvist P, Shikiar R, Shih Y. A review of self-report instruments measuring health-related work productivity: a patient-reported outcomes perspective. Pharmacoeconomics. 2004;22:225–244. doi: 10.2165/00019053-200422040-00002. [DOI] [PubMed] [Google Scholar]
- Pugner KM, Scott DI, Holmes JW, Hieke K. The costs of rheumatoid arthritis: an international long-term view. Semin Arthritis Rheum. 2000;29:305–320. doi: 10.1016/s0049-0172(00)80017-7. [DOI] [PubMed] [Google Scholar]
- Ranke MB. New preparations comprising recombinant human growth hormone: Deliberations on the issue of biosimilars. Horm Res. 2008;69:22–28. doi: 10.1159/000111791. [DOI] [PubMed] [Google Scholar]
- Regent Atlantic Capital LLC, Fiduciary Network. The continuing evolution of the pharmaceutical industry: Career challenges and opportunities. December 2007. «http://www.regentatlantic.com/media/pdf/RegentAtlantic-Pharma-Paper-Dec-07.pdf». Accessed July 16, 2008.
- Rosenthal MB, Landon BE, Normand SL, et al. Employers’ use of value-based purchasing strategies. JAMA. 2007;298:2281–2288. doi: 10.1001/jama.298.19.2281. [DOI] [PubMed] [Google Scholar]
- Ruof J, Hülsemann JL, Mittendorf T, et al. Costs of rheumatoid arthritis in Germany: a micro-costing approach based on healthcare payer’s data sources. Ann Rheum Dis. 2003;62:544–550. doi: 10.1136/ard.62.6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz AB, Edington DW. Employee health and presenteeism: a systematic review. J Occup Rehabil. 2007;17:547–579. doi: 10.1007/s10926-007-9096-x. [DOI] [PubMed] [Google Scholar]
- Soderlin MK, Kautiainen H, Jonsson D, et al. The costs of early inflammatory joint disease: a population-based study in southern Sweden. Scand J Rheumatol. 2003;32:216–224. doi: 10.1080/03009740310003703. [DOI] [PubMed] [Google Scholar]
- Sokka T, Kautiainen H, Möttönen T, Hannonen P. Work disability in rheumatoid arthritis 10 years after the diagnosis. J Rheumatol. 1999;26:1681–1685. [PubMed] [Google Scholar]
- St. Clair EW, Van Der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–3443. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain conditions in the US work-force. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- Verstappen SM, Bijlsma JW, Verkleij H, et al. Overview of work disability in rheumatoid arthritis patients as observed in cross-sectional and longitudinal surveys. Arthritis Rheum. 2004;51:488–497. doi: 10.1002/art.20419. [DOI] [PubMed] [Google Scholar]
- Walgreens Specialty Pharmacy. Walgreens specialty pharmacy outlook: State of the industry report-2007. Walgreens Co.; 2007;SP5751-0507. «http://www.walgreenshealth.com/common/pdf/FinalSOI05152007.pdf». Accessed July 16, 2008.
- Yelin E. Work disability in rheumatic diseases. Curr Opin Rheumatol. 2007;19:91–96. doi: 10.1097/BOR.0b013e3280126b66. [DOI] [PubMed] [Google Scholar]
- Yelin E. The costs of rheumatoid arthritis: Absolute, incremental, and marginal estimates. J Rheumatol Suppl. 1996;44:47–51. [PubMed] [Google Scholar]
- Yelin E, Trupin L, Katz P, et al. Association between etanercept use and employment outcomes among patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:3046–3054. doi: 10.1002/art.11285. [DOI] [PubMed] [Google Scholar]
- Young A, Dixey J, Kulinskaya E, et al. Which patients stop working because of rheumatoid arthritis? Results of five years’ follow up in 732 patients from the early RA study (ERAS) Ann Rheum Dis. 2002;61:335–340. doi: 10.1136/ard.61.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Drug benefit fueled Medicare spending The Wall Street Journal OnlineJan. 8, 2008:A2. «http://online.wsj.com/public/article/SB119976460147574133.html?mod=blog». Accessed July 16, 2008.