Abstract
Testosterone promotes aggressive behaviour in male vertebrates during the breeding season, but the importance of testosterone in female aggression remains unclear. Testosterone has both beneficial and detrimental effects on behaviour and physiology, prompting the hypothesis that selection favours an association between aggression and testosterone only in certain contexts in which intense or persistent aggression may be beneficial. We tested this hypothesis in a year-round territorial female buff-breasted wrens (Thryothorus leucotis), by exposing free-living females to experimental intrusions in different social (either single female or male, or paired decoys) and seasonal (pre-breeding or breeding) contexts. Females responded more aggressively to intrusions by females and pairs than to males. However, female intrusions elicited stronger responses during pre-breeding, whereas responses to pair intrusions were more intense during breeding. Territorial females had elevated testosterone levels after female intrusions and intermediate levels after pair intrusions during pre-breeding, but the levels of testosterone remained low after these intrusions during breeding. These results demonstrate seasonal differences in circulating testosterone following territorial aggression in female buff-breasted wrens and are suggestive of differences according to social context as well. Context-dependent elevation of testosterone implies that selection acts directly on female vertebrates to shape patterns of testosterone secretion.
Keywords: female, hormones, neotropics, territorial behaviour, testosterone, wren
1. Introduction
Although often assumed to be subtle and inconspicuous, females of many vertebrate species behave aggressively in several of the same contexts that elicit aggressive behaviour in males. Females use aggression to acquire and defend resources (e.g. nest sites and territories; Prosen et al. 2004; Heinsohn et al. 2005), to defend partnerships and protect access to paternal care (e.g. Sandell & Smith 1997) and to defend their offspring (e.g. Gill & Sealy 1996; Wolff & Peterson 1998). Accordingly, aggressive behaviour can profoundly affect the fitness of females (e.g. Koskela et al. 1997; Kokita 2002), their mates (e.g. Sandell 1998) and their offspring (e.g. Groothuis et al. 2005; Dloniak et al. 2006).
Whereas the function of female aggression is being increasingly understood, the physiological mechanisms underlying aggressive behaviour have remained ambiguous (e.g. Woodley & Moore 1999a; Elekonich & Wingfield 2000; Davis & Marler 2003; Goymann & Wingfield 2004; Hay & Pankhurst 2005; Rubenstein & Wikelski 2005). In male vertebrates, the sex steroid hormone testosterone (T) promotes aggression during reproduction (e.g. Wingfield et al. 1990; Hirschenhauser & Oliveira 2006; Hau 2007): T concentrations are elevated early in the breeding season when males establish territories and can increase further following aggressive interactions with other males (but see Caldwell et al. 1984; Moore et al. 2004; Van Duyse et al. 2004). Similar to patterns observed in males, female T concentrations in many species are seasonally elevated shortly after their arrival on the breeding grounds when mates and territories are acquired (e.g. Woodley & Moore 1999a; Ketterson et al. 2005). The few attempts to more directly link T with aggressive behaviour in females have led to conflicting results: circulating T concentrations in females are associated with aggression in some species (e.g. Hegner & Wingfield 1986; Woodley & Moore 1999a; Langmore et al. 2002; Desjardins et al. 2006), but not in others (e.g. Elekonich & Wingfield 2000; Davis & Marler 2003; Hay & Pankhurst 2005; Jawor et al. 2006). However, female hormones have been sampled after a variety of natural and experimental encounters (e.g. naturally occurring interactions, Hegner & Wingfield 1986; Rubenstein & Wikelski 2005; male decoys, Schwabl et al. 2005; female decoys, Woodley & Moore 1999a; Hay & Pankhurst 2005; multiple decoys, Wingfield & Lewis 1993), raising the possibility that T differentially modulates the female aggression depending on the context in which it occurs.
Elevated concentrations of testosterone may have beneficial effects in boosting aggressive behaviour or some other aspect of territorial aggression, such as the persistence of aggression (e.g. Wingfield 1994) or the probability of winning subsequent aggressive encounters (e.g. Oyegbile & Marler 2005). However, elevated circulating T may also negatively affect female reproductive physiology and parental behaviour (e.g. Searcy 1988; Fite et al. 2005; Rutowska et al. 2005). Hence, selective pressures might be expected to favour increased secretion of testosterone in association with aggressive behaviour in females only during certain contexts, and a dissociation of T and aggression in others. Indeed in male vertebrates, territorial aggression is differentially hormonally regulated depending on the seasonal context (breeding versus non-breeding seasons, e.g. Caldwell et al. 1984; Soma et al. 2000; Canoine & Gwinner 2002; Wingfield & Soma 2002; Buck & Barnes 2003; Hau et al. 2004). Evidence is scanty, but different endocrine mechanisms may regulate breeding and non-breeding territorial aggression in females as well (Schwabl 1992; Hau et al. 2004; Scotti et al. 2007).
Tropical birds are ideal systems to examine a possible context dependence in the relationship between aggressive behaviour and testosterone in females because in many species females express robust territorial aggression, including song, throughout the year. We investigated the behavioural and hormonal responses of free-living neotropical buff-breasted wren (Thryothorus leucotis) females by presenting intruders in different social settings (single female or male, or paired male and female conspecifics) during two reproductive stages (pre-breeding and breeding). Like males, female buff-breasted wrens establish territories, are strongly territorial and sing throughout the year (Gill 2003; Gill & Stutchbury 2006). If hormones and aggression vary according to context, we would expect more intense aggressive responses and elevation in circulating T (i) during intrusions by single females as they pose the greatest threat to the status of female territory holders and (ii) during pre-breeding when challenges to territorial status are more probable (Gill & Stutchbury 2006; unpublished data), but not during the breeding period when elevated T may interfere with parental behaviour (Wingfield et al. 2001). This study is one of the few to examine the influence of a combination of social and seasonal situations on hormonal and aggressive responses in a free-living female vertebrate and is unique in testing this question in a neotropical species with a year-round territoriality.
2. Material and methods
We studied a population of buff-breasted wrens around Gamboa, Panama (Gill & Stutchbury 2005) during portions of the pre-breeding (12–29 March 2004, 19 February–21 March, 2005) and breeding (16 June 2004–13 July 2004) seasons. Pre-breeding observations were made approximately four to six weeks prior to the onset of rains, which mark the start of breeding in buff-breasted wrens (S. A. Gill, unpublished data). With regard to territoriality, male and female buff-breasted wrens are behaviourally similar, as both sexes sing (during highly coordinated antiphonal duets or when alone producing solo songs) and establish territories, and lone individuals of both sexes maintain their territories after mate loss. Once they have bred, males and females are highly faithful to their territories and mates (Gill 2003; Gill & Stutchbury 2006).
(a) Simulated territorial intrusions to free-living birds
We simultaneously presented live, caged decoys and song playbacks to free-living buff-breasted wrens in the approximate centre of their territories for 30 min (simulated territorial intrusions, hereafter STIs; female decoy: pre-breeding, n=14; breeding, n=12; male decoy: pre-breeding, n=8; breeding, n=12; paired decoys: n=8 for both stages). Both male and female territory holders were present during these experiments; we present data on male responses elsewhere (S. A. Gill & M. Hau 2004–2005, unpublished data). We used two groups of focal females (one tested with both single male and female decoys, the second tested with paired decoys only), and within each group, we performed STIs on the same pairs in the pre-breeding and breeding seasons when possible (for a maximum of two STIs per pair per season, separated by 9–10 days). Over the course of the study, banded individuals disappeared and accessibility of territories changed between seasons; thus, the number of STIs used in paired comparisons (see below) was less than the total number of experiments performed.
We coupled the presentation of decoys with playbacks of sex-specific solo songs (for male and female decoys) or duets (for paired decoys) using vocalizations recorded in 1998 by SAG. Each playback (n=5 per song type) consisted of a single vocalization from one individual or pair, repeated every 30 s (mean ±s.d. song duration=4.2±0.73 s) for 5 min, with the playback loop repeated six times for a total of 30 min. Before the start of trials, we set two to four mist nets in the area in which the decoys were placed; these nets remained closed during the STIs. We set cage(s) and a speaker near the nets (less than 1 m), and one of us then uncovered the decoy(s) while SAG initiated the playback tape and began behavioural observations. After 30 min of observation, we removed the decoy(s). We then opened the mist nets and attempted to catch focal birds using either the same stimulus tape or one consisting of duet playback, or both. We recorded the time at which the focal birds were caught to calculate total duration of playback to which the birds were exposed (30 min STI plus duration of playback until capture). We did not present heterospecific STIs as controls because they typically do not elicit any behavioural or hormonal responses (e.g. Hau et al. 2004), but based on prior experience with this species and casual observations prior to STIs, the experiments elicited robust aggressive responses from females (S. A. Gill, personal observation). For hormonal responses to STIs, we compared experimental females with females randomly captured at similar times of day in 2004–2005.
Most individuals were not banded at the onset of our experiments in 2004; all received three coloured and one numbered aluminium band after capture. We sexed all individuals based on sex-specific songs (Gill et al. 2005) during observations and on body size once captured; within pairs, males are larger than their mates in wing chord, tarsus length and mass (Gill & Vonhof 2006).
(b) Behavioural responses
We classified buff-breasted wren responses according to previous observations (Gill 2003; Gill et al. 2005). We calculated the latency (s) to first response as the time from the beginning of the playback to the first female song or approach within 10 m of the decoys. We recorded time (s) females spent within 5 m of the decoy cages and time (s) spent on the decoy cages. As individuals differed in response latencies, we corrected for differences by presenting these data as the proportion of time during STIs that females spent within 5 m of the decoys or on the cages. We calculated female song initiation rate as the number of duets initiated by females plus the number of solo songs by females given during an STI. Song initiation rate was expressed as the number per minute to correct for individual differences in latency of response. Responses of buff-breasted wrens were recorded in real time on audiotapes by SAG and transcribed onto data sheets later.
(c) Blood sampling and hormone measurement
We took small blood samples (150–250 μl) from individuals by puncturing the brachial vein with a sterile 26 guage needle within 10 min of capture (mean±s.d.=6.1±5.1 min, n=44). Blood was collected in heparinized microcapillary tubes, expelled into labelled Eppendorf tubes and then stored on ice until return from the field, at which time blood was spun for 5–6 min in a microcentrifuge (within 3 h). The plasma portion of the blood was aspirated using Hamilton syringes and frozen at −20°C. We transported the samples on dry ice from Panama to the USA under permission from Panamanian and US authorities.
We extracted steroids from plasma using 5 ml re-distilled dichloromethane in a single extraction. Unlike other species with which we have worked, we did not observe lipids in the plasma of pre-breeding or breeding females, suggesting that lipid interference was minimal. Steroids were partially purified using column chromatography to separate T from other steroids and T concentrations were determined in two radioimmunoassays, according to Wingfield & Farner (1975) and Wikelski et al. (1999). Samples were positioned randomly within the assays with respect to date and STI type. Overall recoveries were determined by adding 20 μl labelled T to samples prior to extraction and calculating the percentage of labelled T detected after separation; recoveries for the assays were 71.0 and 72.9%. Lower detection limits of the assays were determined by setting up standard curves with serially diluted concentrations of stock T, and then estimating the concentrations of the standard curve using the four-parameter logistic curve fit in ImmunoFit v. 3.0 (Beckman Inc., Fullerton, CA). The lower detection limits of the standard curves was set as the first value outside the 95% CI, and equalled 0.02 and 0.06 ng ml−1 for the two assays. The intra-assay coefficient of variation (CV) averaged 4.3% for the two assays and was determined by including one cold standard of the same concentration at the beginning and end of the RIA. Inter-assay CV was 7.5% and was determined by averaging CVs between assays for each of four cold standards.
For a small subset of females during the pre-breeding period only (n=8), we determined baseline corticosterone concentrations within 3 min of capture. Laboratory procedures were as above, except that we performed a single direct radioimmunoassay after extraction (recovery=88.7%). Owing to the reduced sample size, we cannot analyse whether plasma corticosterone is related to female aggression in the same way as testosterone. However, within this subset of females, there was no relation between plasma T and corticosterone after STIs (log-transformed data, Pearson correlation coefficient, r=0.066, n=8, p=0.9).
(d) Data analysis
Data were analysed using SPSS v. 12.0 (SPSS Inc., Chicago, IL) and presented as mean±s.e. When analysing behavioural data, we used non-parametric tests as assumptions of normality, and equality of variances were not met in the original dataset or after transformation (i.e. log, square root or reciprocal). Within-group, paired comparisons were analysed using Wilcoxon matched pairs tests, whereas comparisons between groups were unpaired and analysed using Mann–Whitney tests.
We used general linear models (GLM) and Bonferroni post hoc tests to analyse plasma T concentrations after log-transforming the data. We could not group hormone data for analyses because we did not capture all females that received STIs; thus, plasma T concentrations were compared among experiments, within seasons using univariate GLM and between seasons using unpaired t-tests. We calculated estimates of power (β) and effect size (ω for ANOVA and r for t-tests, Field 2005) for the hormone analyses owing to low sample sizes. Effect sizes provide a standardized measure of the magnitude of an effect regardless of statistical significance (Field 2005); thus, we used them to assess the relative size of the effect of social and seasonal contexts of female T concentrations. Hormone samples below the limit of detection of our assays were set at the minimum detection limit for statistical analyses.
3. Results
Female territory owners responded to 93.5% of STI trials (n=62). Females approached within 10 m of caged decoys during 96.2% of female STIs (n=26), 85% of male STIs (n=20) and all pair STIs (n=16; Χ22=3.82, p=0.2).
(a) Behavioural responses to male versus female intruders
Females responded to single decoys rather quickly in both reproductive periods (Wilcoxon matched-pairs tests, pre-breeding: Z=1.68, n=8, p=0.09; breeding: Z=1.73, n=12, p=0.08; figure 1). Female territory owners initiated more songs during female than male STIs regardless of the time of year (pre-breeding: Z=2.51, n=8, p=0.012; breeding: Z=2.59, n=12, p=0.01). Moreover, females spent a greater proportion of trials within 5 m of female than male decoys during pre-breeding (Z=2.51, n=8, p=0.012), although when breeding, there was little difference between STIs in close approach (Z=0.71, n=12, p=0.48). During both stages, females perched on cages holding females, at which time they chased and occasionally attempted to attack the decoys. Females neither perched on cages holding males nor attacked male decoys.
Figure 1.
Female buff-breasted wrens were significantly more aggressive during pair and female intrusions than male intrusions during both pre-breeding (grey bars) and breeding (white bars) periods (see text for details and sample sizes). (a) Latency (s) to show first response to intrusions. (b) Song initiation rate (songs min−1). (c) Proportion of trial spent within 5 m of the decoy(s). (d) Proportion of trial spent on the decoy cage(s); females never sat on cages during male STIs. The line separates experiments performed on two different groups of females.
(b) Behavioural responses to single versus paired intruders
During pre-breeding, females responded similarly to female STIs and pair STIs in all aggressive responses (Mann–Whitney tests, Z<1.64, n=22, p>0.1; figure 1). By contrast, females responded more strongly to pair STIs than male STIs during this period, as they spent a greater proportion of trials within 5 m of decoys (Z=3.02, n=16, p=0.001), initiated more songs (Z=3.26, n=16, p=0.0001) and only sat on decoy cages during pair STIs. There was no difference in response time between pair and male STI (Z=1.37, n=16, p=0.2).
When breeding, females spent a greater proportion of trials within 5 m of the decoys during pair STIs than both female (Z=3.37, n=20, p=0.001) and male STIs (Z=3.14, n=20, p=0.002). Whereas females tended to respond more quickly (Z=2.93, n=20, p=0.054) and they initiated more songs (Z=2.01, n=20, p=0.047) during pair than male challenges, there was no difference between pair and female STIs in these responses (Z<1.09, n=20, p>0.3), nor the proportion of trials spent on cages (Z=1.52, n=20, p=0.2).
(c) Effect of breeding stage on responses to intruders
Comparing female STIs between the two stages, females responded more aggressively to female decoys during pre-breeding than breeding, as they spent a greater proportion of trials within 5 m of the decoy (Z=2.63, n=11, p=0.009) and initiated more songs (Z=1.96, n=11, p=0.05) during pre-breeding. Neither the latency of first response (Z=0.18, n=11, p=0.9) nor the proportion of the trial spent on the cage (Z=1.26, n=11, p=0.2) differed between stages.
During male STIs, by contrast, females initiated more songs (Z=2.37, n=8, p=0.02) and tended to respond more quickly (Z=1.82, n=8, p=0.07) during breeding than pre-breeding. There was no difference between stages in the proportion of trials spent within 5 m of male decoys (Z=1.35, n=8, p=0.2).
Finally, females responded more aggressively to paired intruders during breeding than pre-breeding, as they spent more time within 5 m of the decoys (Wilcoxon matched-pairs tests, Z=2.21, n=6, p=0.027), initiated more songs (Z=1.99, n=6, p=0.046) and tended to spend a greater proportion of trials on the cages (Z=1.75, n=6, p=0.08) when breeding (figure 2). There was no difference between stages in the latency to respond to paired challengers (Z=0.73, n=6, p=0.5).
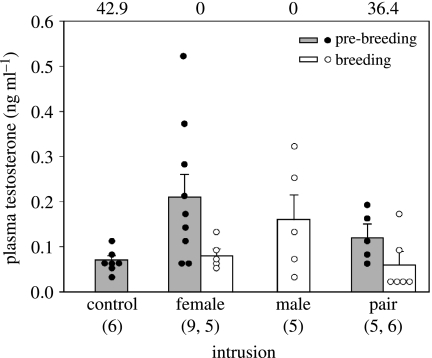
Figure 2.
Concentrations of plasma testosterone (ng ml−1) in female buff-breasted wrens after simulated territorial intrusions during pre-breeding and breeding seasons. Bars refer to mean plasma testosterone concentrations (+s.e.), whereas circles indicate plasma testosterone concentrations of individual females (grey bars and filled circles, pre-breeding; white bars and open circles, breeding). Numbers above the bars refer to the percentage of females exposed to each intrusion type in which testosterone was non-detectable in the assays.
(d) Hormonal responses to STIs
We captured a total of 38 female territory holders randomly (as controls) and after STIs. Plasma T was detectable in 87% of females (n=38). However, detectability differed among females: in over one-third of females caught after pair STIs (36.4%, n=11) and random capture (42.9%, n=7), T levels were non-detectable, whereas all females caught after single female (n=14) or single male (n=6) STIs had detectable T concentrations (Χ2=9.65, p=0.022; figure 2).
Unchallenged (control) females had very low concentrations of circulating T (mean±s.e.=0.06±0.01 ng ml−1, n=6; figure 2). Only one female was captured after male intrusions in the pre-breeding season (plasma T=0.21 ng ml−1; this female was excluded from statistical analyses). Plasma T concentrations differed significantly among females exposed to different types of intrusions during the pre-breeding season (ANOVA, F(2,18)=5.93, p=0.011), with STI type having a large effect on female T levels (ω=0.57). Post hoc tests revealed that pre-breeding females exposed to female intrusions had elevated concentrations of T compared with control females. T titres were intermediate after pair STIs: they did not differ from T titres of females exposed to female intrusions or of control females. During the breeding period, plasma T concentrations were not statistically significantly different among females exposed to different STIs (figure 2; F(3,15)=2.66, p>0.1, β=0.44). However, STI type had a moderate effect on female T levels during breeding (ω=0.41), suggesting that the differences in T levels among experimental females may be biologically relevant, but not statistically significant owing to sample size.
There was a seasonal difference in plasma T levels of female territory owners after STIs. During the pre-breeding season, females had higher T concentrations compared with the breeding season after both female (t=2.56, d.f.=13, p=0.013) and paired decoys (t=2.35, d.f.=9, p=0.043). Seasonal differences in T responses were of moderate effect size for both female (ω=0.28) and pair STIs (ω=0.34).
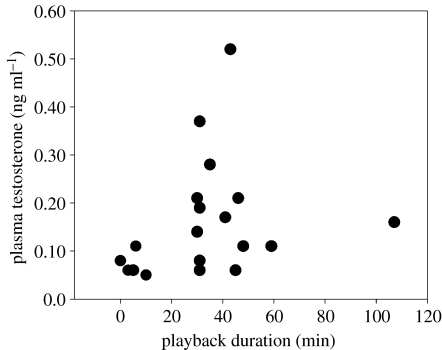
The duration of exposure to territorial intrusions was related to female T concentrations during pre-breeding (figure 3, Spearman rank correlation, ρ=0.43, n=21, p=0.049), but not during breeding (ρ=0.01, n=17, p>0.9). No aggressive behaviour was correlated with female T concentrations during either period (p>0.2).
Figure 3.
During the pre-breeding period, concentrations of plasma testosterone (ng ml−1) in female buff-breasted wrens were positively correlated with the duration of playback to which females were exposed (Spearman rank correlation, ρ=0.4345, n=21, p=0.049).
4. Discussion
We found that aggression and circulating testosterone in female neotropical buff-breasted wrens varied with the context of territorial challenges. Overall, females reacted more aggressively to simulated intrusions by single female and pair decoys than single male decoys. These responses varied seasonally, with more intense aggressive behaviour to female intrusions during pre-breeding, but more intense responses to pair and male intrusions during breeding. After challenges by female intruders during pre-breeding, territorial females had higher circulating T than unchallenged females, whereas T levels of females exposed to pair intrusions at this stage were intermediate between unchallenged females and those that interacted with female intruders. However, the intense aggressive responses by territorial females elicited by pair intrusions at breeding were not accompanied by an elevation in plasma T. These results suggest that T may facilitate aggression in female buff-breasted wrens when challenged by same sex and paired intruders during pre-breeding, but probably not during breeding when other mechanisms may be important. Thus, the hypothesis that female aggressive behaviour is accompanied by an elevation in T only during particular contexts is supported.
(a) Context dependence in aggressive behaviour
Female buff-breasted wrens responded aggressively to all intruders, indicating that same and opposite sex, and paired, intruders were perceived as threats to territorial ownership, partnerships or both. However, females were more aggressive to female and pair intruders than males, particularly during the pre-breeding period. During this time, territory holders are more likely to encounter challenges from prospecting birds than once breeding has commenced because (i) offspring of both sexes that delayed dispersing from their natal territories through the non-breeding period typically do so during pre-breeding (Gill 2004; S. A. Gill & B. J. M. Stutchbury, unpublished data), (ii) territory holders are more likely to switch mates and territories during this time (Gill & Stutchbury 2006), and (iii) territorial vacancies are rapidly replaced during pre-breeding, but not breeding, suggesting that more floaters are prospecting for territories at that time (S. A. Gill & B. J. M. Stutchbury, unpublished data). Thus, female buff-breasted wrens responded more aggressively to female and pair intruders at the time when the likelihood of encountering prospecting females was elevated and the benefits of intense, persistent aggressive responses are likely to be greater. Interestingly, aggression to female and pair intruders was similar during pre-breeding, suggesting that females may have been responding primarily to the presence of the female decoy of the pair.
Male intrusions elicited relatively weaker aggressive responses from females during both periods, mirroring many studies that find greater intra- than intersexual aggression (e.g. Sandell 1998; Mougeot et al. 1999; Boydston et al. 2001; Hau et al. 2004; Hay & Pankhurst 2005). Female aggression towards male intruders may function to defend their mates against eviction by prospecting males, which would be advantageous if staying with current partners confers some benefit, such as increased reproductive success with a familiar mate (e.g. van de Pol et al. 2006). Similar to non-territorial females, encounters with non-territorial males should be more prevalent during pre-breeding, leading to the prediction that female aggression should be more intense then than during breeding. However, females were more aggressive to male challenges at the latter stage. Instead, this finding is consistent with the hypothesis that females respond aggressively to male intruders to maintain access to paternal care, as both parents are required to successfully fledge offspring and individuals that take over territories do not assist in rearing already completed clutches or broods (Gill 2003).
We did not remove males before conducting STIs; thus, it is possible that males may have influenced the behavioural (e.g. Mougeot et al. 1999) as well as the hormonal (Canoine & Gwinner 2005) responses of their partners. Paired birds were typically in close proximity during STIs, and some aggressive responses (e.g. time spent close to the decoys), but not others (e.g. song initiation rates), were consistently highly correlated within pairs (S. A. Gill & M. Hau, unpublished data). However, rather than males influencing the behaviour of their mates, it appears that the reverse may be true, as males responded more intensely to social challenges involving female than male decoys (S. A. Gill & M. Hau, unpublished data). Moreover, males did not closely guard their partners during male compared with other intrusions, nor did we witness within-pair aggression or copulation solicitation during male STIs (S. A. Gill & M. Hau, unpublished data). Thus, males appeared to be defending their mates from usurpation by non-territorial females, rather than guarding them against pursuit by intruding males. The apparent lack of mate defence by males during male intrusions is consistent with findings that buff-breasted wrens exhibit a low incidence of extra-pair paternity even in the absence of paternity guards (Gill et al. 2005).
(b) Context dependence in testosterone response
After female and pair intrusions during pre-breeding, territorial females had elevated T levels relative to unchallenged or breeding females. By contrast, the intense aggressive responses to pair intrusions during breeding were not accompanied by increases in circulating T. Moreover, the duration of challenge to which females were exposed was positively related to circulating T during pre-breeding, but not breeding. These results suggest a seasonal difference in the secretion of T during aggressive encounters in female buff-breasted wrens. T may be involved in mediating high-intensity aggression in females during pre-breeding at which time the likelihood of territorial challenges and potentially territory loss is elevated (Gill 2003; Gill & Stutchbury 2006). During this period, T might act to facilitate the persistence of aggression (Wingfield 1994) or to increase the probability of winning future aggressive encounters (Oyegbile & Marler 2005). Indeed, periods of elevated plasma T coincide with heightened aggression in females of other species (e.g. Woodley & Moore 1999a; Desjardins et al. 2006), although the action of T may be indirect following its conversion to oestradiol (Woodley & Moore 1999b). Experimental manipulation of T levels in female buff-breasted wrens is necessary to determine the function of these increases in T following territorial encounters.
Context-dependent increases in T suggest that alternative hormonal mechanisms support female territorial aggression during the breeding season. If T promotes persistent territorial aggression in female vertebrates, as it does in males (e.g. Wingfield 1994), prolonged territorial encounters might compromise the time females spend on caring for their offspring (Fite et al. 2005, but see Clotfelter et al. 2004). Both short-term and prolonged elevated T levels may also interfere with other aspects of reproduction, such as egg production and brood patch development (Searcy 1988; Clotfelter et al. 2004; Rutowska et al. 2005). This might compromise reproductive success of females and may select against an association between T and aggression during breeding.
Relative to temperate-zone species (Ketterson et al. 2005), circulating levels of T in unchallenged (and challenged) female buff-breasted wrens were low. Although few data exist at present, T levels in female buff-breasted wrens appear on par with T concentrations in females of other tropical species (Wikelski et al. 2003; Goymann & Wingfield 2004; see also Goymann et al. 2004). The function of these low T concentrations in females needs more study, but evidence is emerging that even low T concentrations might exert biological actions. For example, in male neotropical spotted antbirds (Hylophylax naevioides), androgen and oestrogen receptors in brain regions associated with aggressive and reproductive behaviour are upregulated during the non-breeding period, which may increase the sensitivity of the brain to very low concentrations of sex steroids (Canoine et al. 2007). Furthermore, females of sex-role reversed afrotropical black coucals (Centropus grillii) have lower circulating T levels than males despite being more aggressive, but have a higher density of androgen receptors in brain regions associated with aggressive behaviour compared with males (Goymann & Wingfield 2004; Voigt & Goymann 2007).
Recent comparative analyses have suggested that T concentrations of females reflect a correlated response to direct selection on T levels in males, rather than direct selection on females themselves (Ketterson et al. 2005; Mank 2007). Whereas absolute T concentrations in females might be the result of correlated evolution, our present data suggest an association between territorial aggression and T in female birds in some contexts and a dissociation between them in others. These findings may point to natural selection acting directly to shape patterns of testosterone secretion in female vertebrates.
Acknowledgments
This research was performed under the approval of Princeton University's Institutional Committee on Animal Care and Autoridad Nacional del Ambiente (A.N.A.M.) in Panama.
We thank (A.N.A.M.) for permitting us to perform our research in Panama, the Smithsonian Tropical Research Institute for logistical support, and L. Costa and M. Vonhof for their assistance in the field. L. Spinney was invaluable in the laboratory. E. Adkins-Regan, W. Goymann and three anonymous reviewers kindly provided their comments on the manuscript. S.A.G. was supported by a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada and E.D.A. was supported by grants from Princeton University. This research was funded by Integrated Research Challenge grant no. 0212587 from the National Science Foundation to M.H.
References
- Boydston E.E, Morelli T.L, Holekamp K.E. Sex differences in territorial behavior exhibited by the spotted hyena (Hyaenidae, Crocuta crocuta) Ethology. 2001;107:369–385. doi:10.1046/j.1439-0310.2001.00672.x [Google Scholar]
- Buck C.L, Barnes B.M. Androgens in free-living Arctic ground squirrels: seasonal changes and influence of staged male–male aggressive encounters. Horm. Behav. 2003;43:318–326. doi: 10.1016/s0018-506x(02)00050-8. doi:10.1016/S0018-506X(02)00050-8 [DOI] [PubMed] [Google Scholar]
- Caldwell G.S, Glickman S.E, Smith E.R. Seasonal aggression independent of testosterone in wood rats. Proc. Natl Acad. Sci. USA. 1984;81:5255–5257. doi: 10.1073/pnas.81.16.5255. doi:10.1073/pnas.81.16.5255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoine V, Gwinner E. Seasonal differences in the hormonal control of territorial aggression in free-living European stonechats. Horm. Behav. 2002;41:1–8. doi: 10.1006/hbeh.2001.1720. doi:10.1006/hbeh.2001.1720 [DOI] [PubMed] [Google Scholar]
- Canoine V, Gwinner E. The hormonal response of female European Stonechats to a territorial intrusion: the role of the male partner. Horm. Behav. 2005;47:563–568. doi: 10.1016/j.yhbeh.2004.12.007. doi:10.1016/j.yhbeh.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Canoine V, Fusani L, Schlinger B, Hau M. Low sex steroids, high steroid receptors: increasing the sensitivity of the nonreproductive brain. Dev. Neurobiol. 2007;67:57–67. doi: 10.1002/dneu.20296. doi:10.1002/dneu.20296 [DOI] [PubMed] [Google Scholar]
- Clotfelter E.D, O'Neal D.M, Gaudioso J.M, Casto J.M, Parker-Renga I.M, Snajdr E.A, Duffy D.L, Nolan V, Jr, Ketterson E.D. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm. Behav. 2004;46:171–178. doi: 10.1016/j.yhbeh.2004.03.003. doi:10.1016/j.yhbeh.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Davis E.S, Marler C.A. The progesterone challenge: steroid hormone changes following a simulated territorial intrusion in female Peromyscus californicus. Horm. Behav. 2003;44:185–198. doi: 10.1016/s0018-506x(03)00128-4. doi:10.1016/S0018-506X(03)00128-4 [DOI] [PubMed] [Google Scholar]
- Desjardins J.K, Hazelden M.R, Van der Kraak G.J, Balshine S. Male and female cooperatively breeding fish provide support for the “challenge hypothesis”. Behav. Ecol. 2006;17:149–154. doi:10.1093/beheco/arj018 [Google Scholar]
- Dloniak S.M, French J.A, Holekamp K.E. Rank-related maternal effects of androgens on behaviour in wild spotted hyaenas. Nature. 2006;440:1190–1193. doi: 10.1038/nature04540. doi:10.1038/nature04540 [DOI] [PubMed] [Google Scholar]
- Elekonich M.M, Wingfield J.C. Seasonality and hormonal control of territorial aggression in female song sparrows (Passeriformes: Emberizidae: Melospiza melodia) Ethology. 2000;106:493–510. doi:10.1046/j.1439-0310.2000.00555.x [Google Scholar]
- Field A. Discovering statistics using SPSS. 2nd edn. Sage Publication; London, UK: 2005. [Google Scholar]
- Fite J.E, French J.A, Patera K.J, Hopkins E.C, Rukstalis M, Ross C.N. Elevated urinary testosterone excretion and decreased maternal caregiving effort in marmosets when conception occurs during the period of infant dependence. Horm. Behav. 2005;47:39–48. doi: 10.1016/j.yhbeh.2004.08.005. doi:10.1016/j.yhbeh.2004.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. A. 2003 Territory acquisition, divorce, pair formation, and parental care in a neotropical wren, Thryothorus leucotis PhD thesis, York University, Toronto, Canada.
- Gill S.A. First record of cooperative breeding in a Thryothorus wren. Wilson Bull. 2004;116:337–341. doi:10.1676/04-057 [Google Scholar]
- Gill S.A, Sealy S.G. Nest defence by yellow warblers: recognition of a brood parasite and an avian nest predator. Behaviour. 1996;133:263–282. [Google Scholar]
- Gill S.A, Stutchbury B.J.M. Nest building is an indicator of parental quality in a monogamous, tropical wren Thryothorus leucotis. Auk. 2005;122:1169–1181. doi:10.1642/0004-8038(2005)122[1169:NBIAIO]2.0.CO;2 [Google Scholar]
- Gill S.A, Stutchbury B.J.M. Long-term mate and territory fidelity in neotropical buff-breasted wrens (Thryothorus leucotis) Behav. Ecol. Sociobiol. 2006;61:245–253. doi:10.1007/s00265-006-0255-4 [Google Scholar]
- Gill S.A, Vonhof M.J. Sexing monochromatic birds in the field: cryptic sexual size dimorphism in buff-breasted wrens (Thryothorus leucotis) Ornithol. Neotrop. 2006;17:409–418. [Google Scholar]
- Gill S.A, Vonhof M.J, Stutchbury B.J.M, Morton E.S, Quinn J.S. No evidence for acoustic mate guarding in duetting buff-breasted wrens (Thryothorus leucotis) Behav. Ecol. Sociobiol. 2005;57:557–565. doi:10.1007/s00265-004-0893-3 [Google Scholar]
- Goymann W, Wingfield J.C. Competing females and caring males. Sex steroids in African black coucals, Centropus grillii. Anim. Behav. 2004;68:533–540. doi:10.1016/j.anbehav.2003.12.012 [Google Scholar]
- Goymann W, Moore I.T, Scheuerlein A, Hirschenhauser K, Grafen A, Wingfield J.C. Testosterone in tropical birds: effects of environmental and social factors. Am. Nat. 2004;164:327–334. doi: 10.1086/422856. doi:10.1086/422856 [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G, Eising C.M, Dijkstra C, Müller W. Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol. Lett. 2005;1:78–81. doi: 10.1098/rsbl.2004.0233. doi:10.1098/rsbl.2004.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau M. Testosterone and the evolution of vertebrate life-history trade-offs. BioEssays. 2007;29:133–144. doi: 10.1002/bies.20524. doi:10.1002/bies.20524 [DOI] [PubMed] [Google Scholar]
- Hau M, Stoddard S.T, Soma K.K. Territorial aggression and hormones during the non-breeding season in a tropical bird. Horm. Behav. 2004;45:40–49. doi: 10.1016/j.yhbeh.2003.08.002. doi:10.1016/j.yhbeh.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Hay A.C, Pankhurst N.W. Effect of paired encounters on plasma androgens and behaviour in males and females of the spiny damselfish Acanthochromis polyacanthus. Mar. Freshw. Behav. Physiol. 2005;38:127–138. doi:10.1080/10236240500125528 [Google Scholar]
- Hegner R.E, Wingfield J.C. Behavioral and endocrine correlates of multiple brooding in the semicolonial house sparrow Passer domesticus. II. Females. Horm. Behav. 1986;20:313–326. doi: 10.1016/0018-506x(86)90040-1. doi:10.1016/0018-506X(86)90040-1 [DOI] [PubMed] [Google Scholar]
- Heinsohn R, Legge S, Endler J.A. Extreme reversed sexual dichromatism in a bird without sex role reversal. Science. 2005;309:617–619. doi: 10.1126/science.1112774. doi:10.1126/science.1112774 [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Oliveira R.F. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 2006;71:265–277. doi:10.1016/j.anbehav.2005.04.014 [Google Scholar]
- Jawor J.M, Young R, Ketterson E.D. Females competing to reproduce: dominance matters but testosterone may not. Horm. Behav. 2006;49:362–368. doi: 10.1016/j.yhbeh.2005.08.009. doi:10.1016/j.yhbeh.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Ketterson E.D, Nolan V, Jr, Sandell M. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 2005;166:S85–S98. doi: 10.1086/444602. doi:10.1086/444602 [DOI] [PubMed] [Google Scholar]
- Kokita T. The role of female behavior in maintaining monogamy of a coral-reef filefish. Ethology. 2002;108:157–168. doi:10.1046/j.1439-0310.2002.00766.x [Google Scholar]
- Koskela E, Mappes T, Ylönen H. Territorial behaviour and reproductive success of bank vole Clethrionomys glareolus females. J. Anim. Ecol. 1997;66:341–349. doi:10.2307/5980 [Google Scholar]
- Langmore N.E, Cockrem J.F, Candy E.J. Competition for male reproductive investment elevates testosterone levels in female dunnocks, Prunella modularis. Proc. R. Soc. B. 2002;269:2473–2478. doi: 10.1098/rspb.2002.2167. doi:10.1098/rspb.2002.2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank J.E. The evolution of sexually selected traits and antagonistic androgen expression in actinopterygiian fishes. Am. Nat. 2007;169:142–149. doi: 10.1086/510103. doi:10.1086/510103 [DOI] [PubMed] [Google Scholar]
- Moore I.T, Wada H, Perfito N, Busch D.S, Hahn T.P, Wingfield J.C. Territoriality and testosterone in an equatorial population of rufous-collared sparrows, Zonotrichia capensis. Anim. Behav. 2004;67:411–420. doi:10.1016/j.anbehav.2003.03.021 [Google Scholar]
- Mougeot F, Arroyo B.E, Bretagnolle V. Decoy presentations as a means to manipulate the risk of extrapair copulation: an experimental study in a semicolonial raptor, the Montagu's harrier (Circus pygargus) Behav. Ecol. 1999;12:1–7. doi:10.1093/beheco/12.1.1 [Google Scholar]
- Oyegbile T.O, Marler C.A. Winning fights elevates testosterone in California mice and enhances future ability to win fights. Horm. Behav. 2005;48:259–267. doi: 10.1016/j.yhbeh.2005.04.007. doi:10.1016/j.yhbeh.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Prosen E.D, Jaeger R.G, Lee D.R. Sexual coercion in a territorial salamander: females punish socially polygynous male partners. Anim. Behav. 2004;67:85–92. doi:10.1016/j.anbehav.2003.02.005 [Google Scholar]
- Rubenstein D, Wikelski M. Steroid hormones and aggression in female Galápagos marine iguanas. Horm. Behav. 2005;48:329–341. doi: 10.1016/j.yhbeh.2005.04.006. doi:10.1016/j.yhbeh.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Rutowska J, Cichon M, Puerta M, Gil D. Negative effects of elevated testosterone on female fecundity. Horm. Behav. 2005;47:585–591. doi: 10.1016/j.yhbeh.2004.12.006. doi:10.1016/j.yhbeh.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Sandell M.I. Female aggression and the maintenance of monogamy: female behaviour predicts male mating status in European starlings. Proc. R. Soc. B. 1998;265:1307–1311. doi:10.1098/rspb.1998.0434 [Google Scholar]
- Sandell M.I, Smith H.G. Female aggression in the European starling during the breeding season. Anim. Behav. 1997;53:13–23. doi:10.1006/anbe.1996.0274 [Google Scholar]
- Schwabl H. Winter and breeding territorial behaviour and levels of reproductive hormones of migratory European robins. Ornis Scand. 1992;23:271–276. doi:10.2307/3676649 [Google Scholar]
- Schwabl H, Flinks H, Gwinner E. Testosterone, reproductive stage, and territorial behavior of male and female European stonechats Saxicola torquata. Horm. Behav. 2005;47:503–512. doi: 10.1016/j.yhbeh.2004.08.003. doi:10.1016/j.yhbeh.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Scotti, M.-A. L., Place, N. J. & Demas, G. E. 2007 Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus). Horm. Behav (doi:10.1016/j.yhbeh.2007.03.029) [DOI] [PubMed]
- Searcy W.A. Do female red-winged blackbirds limit their own breeding densities? Ecology. 1988;69:85–95. doi:10.2307/1943163 [Google Scholar]
- Soma K.K, Tramontin A.D, Wingfield J.C. Oestrogen regulates male aggression in the non-breeding season. Proc. R. Soc. B. 2000;267:1089–1096. doi: 10.1098/rspb.2000.1113. doi:10.1098/rspb.2000.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pol M, Heg D, Bruinzeel L.W, Kuijper B, Verhulst S. Experimental evidence for a causal effect of pair-bond duration on reproductive performance in oystercatchers. Behav. Ecol. 2006;17:982–991. doi:10.1093/beheco/arl036 [Google Scholar]
- Van Duyse E, Pinxten R, Darras V.M, Arckens L, Eens M. Opposite changes in plasma testosterone and corticosterone levels following a simulated territorial challenge in male great tits. Behaviour. 2004;141:451–467. doi:10.1163/156853904323066739 [Google Scholar]
- Voigt C, Goymann W. Sex-role reversal is reflected in the brain of African black coucals (Centropus grillii) Dev. Neurobiol. 2007 doi: 10.1002/dneu.20528. doi:10.1002/dneu.20528 [DOI] [PubMed] [Google Scholar]
- Wikelski M, Hau M, Wingfield J.C. Social instability increases plasma testosterone in a year-round territorial neotropical bird. Proc. R. Soc. B. 1999;266:551–556. doi:10.1098/rspb.1999.0671 [Google Scholar]
- Wikelski M, Hau M, Robinson W.D, Wingfield J.C. Reproductive seasonality of seven neotropical passerine species. Condor. 2003;105:683–695. doi:10.1650/7251 [Google Scholar]
- Wingfield J.C. Control of territorial aggression in a changing environment. Psychoneuroendocrinology. 1994;19:709–721. doi: 10.1016/0306-4530(94)90052-3. doi:10.1016/0306-4530(94)90052-3 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Farner D.S. The determination of five steroids in avian plasma by radioimmunoassay and competitive-binding protein. Steroids. 1975;26:311–327. doi: 10.1016/0039-128x(75)90077-x. doi:10.1016/0039-128X(75)90077-X [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Lewis D.M. Hormonal and behavioural responses to simulated territorial intrusion in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Anim. Behav. 1993;45:1–11. doi:10.1006/anbe.1993.1001 [Google Scholar]
- Wingfield J.C, Soma K.K. Spring and autumn territoriality in song sparrows: same behavior, different mechanisms? Integr. Comp. Biol. 2002;42:11–20. doi: 10.1093/icb/42.1.11. doi:10.1093/icb/42.1.11 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Hegner R.E, Dufty A.M, Jr, Ball G.F. The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 1990;136:829–846. doi:10.1086/285134 [Google Scholar]
- Wingfield J.C, Lynn S.E, Soma K.K. Avoiding the ‘costs’ of testosterone: ecological bases of hormone–behavior interactions. Brain Behav. Evol. 2001;57:239–251. doi: 10.1159/000047243. doi:10.1159/000047243 [DOI] [PubMed] [Google Scholar]
- Woodley S.K, Moore M.C. Female territorial aggression and steroid hormones in mountain spiny lizards. Anim. Behav. 1999a;57:1083–1089. doi: 10.1006/anbe.1998.1080. doi:10.1006/anbe.1998.1080 [DOI] [PubMed] [Google Scholar]
- Woodley S.K, Moore M.C. Ovarian hormones influence territorial aggression in free-living female spiny mountain lizards. Horm. Behav. 1999b;35:205–214. doi: 10.1006/hbeh.1999.1514. doi:10.1006/hbeh.1999.1514 [DOI] [PubMed] [Google Scholar]
- Wolff J.O, Peterson J.A. An offspring-defense hypothesis for territoriality in female mammals. Ethol. Ecol. Evol. 1998;10:227–239. [Google Scholar]