Abstract
Mimetic species have evolved to resemble other species to avoid predation (protective mimicry) or gain access to food (aggressive mimicry). Mimicry systems are frequently tripartite interactions involving a mimic, model and ‘signal receiver’. Changes in the strength of the relationship between model and signal receiver, owing to shifting environmental conditions, for example, can affect the success of mimics in protective mimicry systems. Here, we show that an experimentally induced shift in the strength of the relationship between a model (bluestreak cleaner fish, Labroides dimidiatus) and a signal receiver (staghorn damselfish, Amblyglyphidodon curacao) resulted in increased foraging success for an aggressive mimic (bluestriped fangblenny, Plagiotremus rhinorhynchos). When the parasite loads of staghorn damselfish clients were experimentally increased, the attack success of bluestriped fangblenny on damselfish also increased. Enhanced mimic success appeared to be due to relaxation of vigilance by parasitized clients, which sought cleaners more eagerly and had lower overall aggression levels. Signal receivers may therefore be more tolerant of and/or more vulnerable to attacks from aggressive mimics when the net benefit of interacting with their models is high. Changes in environmental conditions that cause shifts in the net benefits accrued by models and signal receivers may have important implications for the persistence of aggressive mimicry systems.
Keywords: aggressive mimicry, coral reef fish, cleaner wrasse, cleaning symbioses
1. Introduction
Mimicry is defined as the adaptive resemblance of one species to another to avoid predation (protective mimicry) or gain access to food (aggressive mimicry; Wickler 1968). Many mimicry systems involve three participants: a model, a mimic and a signal receiver (Wickler 1968); however, the identity of the latter depends on the mimicry system. Batesian mimics are palatable species that resemble unpalatable or otherwise defended models to avoid being attacked by the signal receiver (a predator; Bates 1862). Aggressive mimics are ‘predatory’ species that resemble a model in order to approach and attack the signal receiver (a dupe; Wickler 1965).
In both cases, the strength of the relationship between model and signal receiver may affect the success of mimics. For example, in Batesian mimicry, if the model is extremely toxic (i.e. less beneficial) to the signal receiver, then the mimic is conferred greater protection from the signal receiver (Goodale & Sneddon 1977; Lindström et al. 1997). In contrast, the models and the signal receivers, for example, reproductively receptive females and the males seeking them (Lloyd 1975), and flowers and their pollinators (Wickler 1968), in aggressive mimicry are usually engaged in more cooperative interactions. Therefore, it seems reasonable to assume that if the benefits (e.g. access to fertilizable eggs or food) accrued by a signal receiver when interacting with a model are high, then signal receivers should be willing to incur greater costs (e.g. travel costs, risk of predation) to gain access to the model. For example, if signal receivers that stand to benefit greatly from interacting with specific models reduce their vigilance to access these models more quickly, the success of aggressive mimics could in turn increase.
In the marine environment, cleaner fish and their fangblenny mimics provide a classic case of aggressive mimicry. Client reef fish (the signal receivers) benefit from interacting with bluestreak cleaner fish (Labroides dimidiatus; the models) in terms of a significant reduction in ectoparasites (Grutter 1999). However, when clients have few ectoparasites, the nature of the relationship between cleaner and client can change from mutualism to one closer to parasitism (Cheney & Côté 2005b). The bluestriped fangblenny (Plagiotremus rhinorhynchos) closely mimics the black and neon blue coloration of the juvenile cleaner fish. However, instead of removing parasites from reef fish, they take scales, mucus and dermal tissue (Kuwamara 1981).
In this study, we ask whether the success of aggressive mimics depends on the strength of the relationship between model and signal receiver, using the cleaner-fish–fangblenny mimic system. Specifically, we predict that fangblenny mimics should be more successful in their attacks when the net benefit to clients of interacting with cleaners is large, as clients should be less vigilant and seek cleaners more eagerly (Grutter 1995). The scope for large benefits (i.e. more parasites removed) is greater when client ectoparasite load is high (Cheney & Côté 2005b). In an earlier correlational study, Cheney & Côté (2005a) found no relationship between fangblenny mimic attack success and client ectoparasite load across four reefs. The power of this study was limited by the low number of client species considered and the coarse spatial scale. Here, we use a carefully controlled experimental approach in which we manipulate ectoparasites loads and observe direct interactions between individual models, mimics and signal receivers.
2. Material and methods
(a) Study site and species
The study was conducted at the Lizard Island Research Station, Great Barrier Reef, Australia in November–December 2006. Staghorn damselfish (Amblyglyphidodon curacao), juvenile cleaner fish (L. dimidiatus) and bluestriped fangblennies (P. rhinorhynchos; figure 1) were collected from shallow reefs around the Lizard Island Group hand and barrier nets. In addition to exhibiting a blue and black mimetic colour form, bluestriped fangblennies can also be found in non-mimetic colours (Randall et al. 1997) and have been shown to switch between the colour forms (Côté & Cheney 2005). However, only mimic fangblennies were used in this study, and they maintained this coloration throughout the study period. All fish were held in running seawater aquaria separately (in the case of mimics and cleaners) or by species (clients) and fed pieces of prawn and fish flakes daily for 3–5 days to acclimatize. After experiments were conducted, all fish were released at the point of capture. Staghorn damselfish (figure 1c) are common on reefs at the study site, occupy small home ranges and are frequent visitors to L. dimidiatus cleaning stations.
Figure 1.
The cleaner-fish–fangblenny mimic study system. (a) Model, juvenile L. dimidiatus; (b) mimic, black and blue form of P. rhinorhynchos; (c) signal receiver, A. curacao.
Gnathiid isopod larvae are common coral reef fish ectoparasites (Grutter 1994) and are the main food source for cleaner fish (Grutter 1996, 1997). As juveniles, gnathiids emerge from the benthos and attach to reef fish to feed on blood and tissue fluids (Monod 1926). Gnathiids were obtained from a gnathiid culture maintained at the research station (as per Grutter 2001).
(b) Experimental design
Experimental trials were carried out in glass aquaria (1 m long×0.45 m wide×0.4 m high). Staghorn damselfish were placed individually in the aquarium for at least 16 h prior to the trial to acclimatize. Thirty minutes prior to the trial, a Plexiglas partition was placed in the centre and one juvenile cleaner fish and one fangblenny were added to one half, while the damselfish was confined to the other. Two treatments were conducted on each staghorn damselfish: a ‘parasitized’ and ‘unparasitized’ treatment, the order of which was randomized. To parasitize staghorn damselfish, each fish was confined to a 10 cm wide section of the aquarium by means of a partition. Approximately 10 unfed gnathiid isopods were released in the water adjacent to the fish with a plastic pipette. Some gnathiids were observed to attach to the fish immediately; however, owing to the small size of the parasites (1–2 mm), it was difficult to obtain visually absolute numbers of parasite infection. However, for five fish that were parasitized in this way and then placed in a bucket for 2 h (without a cleaner fish), we collected (mean±s.d.) 6.4±1.7 fed gnathiids. In the field, an average of five to six parasites have been found on A. curacao from this site (Grutter 1995); therefore, we considered our experimentally parasitized fish to carry a representative ectoparasite load. Each fish was left in the small section for 5 min to ensure gnathiid attachment. All partitions were then removed, fish were left to settle for 5 min, and then a 15 min observation began.
After each trial, staghorn damselfish were placed individually in a bucket with an aerator for 2 h. The water was then filtered, and fed gnathiids were collected. We recovered fed gnathiids from all fish from the parasitized trials, confirming that damselfish had been infested during the trial. Although some gnathiids would have been eaten by the cleaner fish, we obtained an average of 2.3±1.1 (mean±s.d.) fed gnathiids per fish. No gnathiids were obtained from fish from the unparasitized trial. Gnathiids usually attach only to feed on fish for 1 h (Grutter 2003); therefore, all treatments were conducted at least 2 h apart to ensure that gnathiids had fed and left their host prior to the beginning of trials.
For each trial, a 15 min observation was conducted, and we recorded the time damselfish spent being cleaned by cleaner fish. In addition we recorded whether the damselfish or cleaner terminated the cleaning interaction, whether the damselfish avoided an approach by a cleaner fish by swimming away, the number of successful and unsuccessful attacks by fangblennies, and whether the staghorn damselfish aggressively chased the fangblenny mimic or cleaner fish before or after an attack. Cleaning interactions were defined as contact made with the body surface of the damselfish by the cleaner fish and were terminated when one of the two interacting fishes actively swam away from the other. The damselfish sometimes clearly avoided an approaching cleaner by altering markedly its swimming trajectory away from the cleaner. An attack was considered to be a dart by the fangblenny towards staghorn damselfish with contact made with the victim's body that usually resulted in a slight jolt by the damselfish. Despite these jolts, no mark or wound was ever noted upon visual inspection. An unsuccessful attack was an attempt by the fangblenny to attack, but with no contact made. Aggressive chases were noted when the staghorn damselfish swam directly and rapidly at the cleaner fish or fangblenny mimic; however, the fish rarely touched. Preliminary trials indicated that 15 min was a suitable observation period as gnathiids can begin to detach 30 min after initial infection (Grutter 2003).
Fourteen paired trials were conducted with a different staghorn damselfish and P. rhinorhynchos used for each trial pair (i.e. parasitized–unparasitized). Juvenile L. dimidiatus were changed every two paired trials owing to difficulties in obtaining them during the study period. Therefore, to compare inspection duration between treatments, we averaged data for each cleaner fish from the two trials.
3. Results
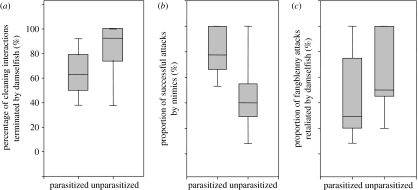
Damselfish were cleaned for longer when parasitized than when unparasitized (mean ±1 s.d.; parasitized: 82.6±25.1 s, unparasitized: 20.1±12.8 s; paired t-test: t6=6.2, p=0.001). They also terminated a smaller proportion of cleaning interactions (Wilcoxon signed-rank test: Z=2.27, p=0.02; figure 2a) and avoided approaching cleaner fish less often when parasitized (parasitized: 2.0±2.4 avoidance events, unparasitized: 4.6±1.9 avoidance events; paired t-test: t13=2.9, p=0.012).
Figure 2.
Box plots showing: (a) percentage of cleaning interactions that were terminated by damselfish, (b) proportion (%) of all attacks by mimics towards damselfish that were successful, and (c) proportion (%) of successful attacks by mimics that led to a retaliatory chase by damselfish. Medians are represented by black line, while grey boxes indicate upper and lower quartiles.
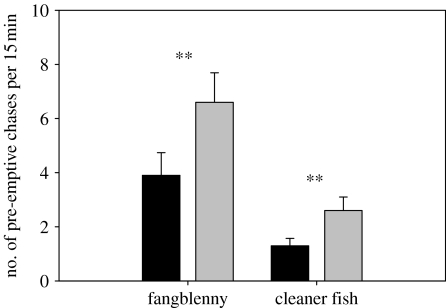
The total number of attacks by fangblenny mimics on parasitized and unparasitized damselfish did not differ (mean ±s.d.; parasitized: 4.9±1.1 attacks 15 min−1, unparasitized: 4.1±0.7 attacks 15 min−1; paired t-test: t13=0.94, p=0.36). However, the proportion of successful attacks was significantly greater on parasitized damselfish (Wilcoxon signed-rank test: Z=2.67, n=14, p=0.04; figure 2b). Parasitized staghorn damselfish retaliated by aggressive chasing to a smaller proportion of successful attacks by mimics than did unparasitized damselfish (Wilcoxon signed-rank test: Z=2.0, p=0.05; figure 2c). Staghorn damselfish were also observed chasing both mimics and cleaner fish prior to any attack by mimics. The rate of pre-emptive chases aimed at fangblennies and cleaner fish was lower for parasitized than unparasitized damselfish (towards fangblenny mimic: paired t-test: t13=4.5, p=0.001; towards cleaner fish; paired t-test: t13=2.7, p=0.02; figure 3).
Figure 3.
Number of pre-emptive chases by parasitized (black bars) and unparasitized (grey bars) staghorn damselfish towards cleaner fish and fangblenny mimics. Bars are mean ±1 s.e. **≤0.01; paired t-test.
4. Discussion
We have shown that the success of aggressive mimics is influenced by the potential benefits obtained by participants in the model–signal receiver interaction. In our cleaner-fish–fangblenny system, we created variation in the scope for staghorn damselfish (the signal receivers) to benefit from being cleaned by altering the number of parasites they carried. The success of mimic fangblennies in attacking damselfish was highest when the potential benefit to damselfish of being cleaned was high, that is when damselfish had more parasites.
The behavioural mechanisms by which mimic-attack success was enhanced when targeting parasitized fish appear to be linked to the eagerness with which parasitized fish sought cleaners. Parasitized damselfish exhibited fewer avoidance responses to approaching cleaner fish, they spent longer being cleaned during which time they remained largely motionless (K.L.C., 2006 personal observations), and they terminated fewer cleaning interactions than unparasitized damselfish. Parasitized damselfish were also less aggressive towards cleaners and mimics, both before and after attacks, perhaps because aggressive chasing is a time-consuming behaviour that precludes engaging in other activities, such as interacting with cleaners. Alternatively, low aggression rates by parasitized damselfish could be due to deleterious effects on ectoparasites on fish health. However, this seems unlikely because pathological consequences of gnathiid infestations are only manifested at very high parasite densities (Mugridge & Stallybrass 1983). In our study, damselfish were only moderately infested with these small ectoparasites (1–10 gnathiids per fish; mean ±s.d., 6.4±1.7), which are similar loads to those found in the field (Grutter 1995). Similarly, low parasite loads have been previously shown to drive clients to seek cleaners more frequently (Cheney & Côté 2001; Sikkel et al. 2004), perhaps because the parasite causes dermal irritation (Limbaugh 1961; Feder 1966). The apparent determination of parasitized damselfish to gain access to and maintain contact with cleaners, combined with their lack of aggression towards mimics, made parasitized fish an easy target for fangblennies.
Our interpretation of the results hinges on the link between ectoparasite availability and the variable strength of the interaction between models and signal receivers in this aggressive mimicry system. This link appears to be strong as there is accumulating evidence that the net benefit to client fish of being cleaned does increase with increasing parasite load. Not only do cleaners remove more parasites when those are available (Cheney & Côté 2005b), they also cheat less frequently by removing fewer scales and less mucus (Bansemer et al. 2002; Cheney & Côté 2005b). In this study, it was not possible for us to verify that cleaners cheated less when inspecting parasitized damselfish because of the paired nature of our experimental design; however, it is probable that this was the case. The behaviour of unparasitized damselfish is consistent with this interpretation. When clients were unparasitized, cleaners still attempted to initiate cleaning interactions because they can feed on mucus if the search for parasites is unfruitful. However, clients quickly terminated these interactions and were generally less tolerant of cleaners than parasitized damselfish.
The (foraging) success of aggressive mimics and their models therefore depends on the strength of the interaction between models and signal receivers. A similar dependence occurs in protective mimicry complexes where the success of mimics and models varies, for example, with the availability of alternative prey (Dill 1975; Nonacs 1985; Slobodchikoff 1987; Kokko et al. 2003). In these cases, an abundance of alternative prey weakens the signal receiver's reliance on models and mimics, thereby increasing the survival of both models and mimics. Changing the interaction strength between models and signal receivers in protective mimicry does not essentially alter the nature of their relationship, that is it remains a predator–prey relationship. However, the equivalent change in the cleaner-fish–fangblenny system can transform the relationship from mutualism to near parasitism. Natural temporal variability in ectoparasite availability appears to ensure that unfavourable conditions for the maintenance of cleaning interactions are never maintained for long (Cheney & Côté 2005b). The same variability may therefore also maintain aggressive mimics in this system.
Acknowledgments
Thank you to A. Grutter for supplying gnathiids, fieldwork equipment and for comments on the manuscript; to P. Mansell, B. Cameron, T. Brewer, L. Curtis, C. Jones for their help in the field; and to the staff at Lizard Island Research Station for their continuing support. This work was funded by the Australian Research Council with a grant and fellowship to K.L.C.
References
- Bansemer C, Grutter A.S, Poulin R. Geographic variation in the behaviour of the cleaner fish Labroides dimidiatus (Labridae) Ethology. 2002;108:353–366. doi:10.1046/j.1439-0310.2002.00777.x [Google Scholar]
- Bates H.W. Contributions to an insect fauna of the Amazon Valley. Lepidoptera: Heliconidae. Trans. Linn. Soc. 1862;23:495–566. [Google Scholar]
- Cheney K.L, Côté I.M. Are Caribbean cleaning symbioses mutualistic? Costs and benefits of visiting cleaning stations to longfin damselfish. Anim. Behav. 2001;62:927–933. doi:10.1006/anbe.2001.1832 [Google Scholar]
- Cheney K.L, Côté I.M. Frequency-dependent success of aggressive mimics in a cleaning symbiosis. Proc. R. Soc. B. 2005a;272:2635–2639. doi: 10.1098/rspb.2005.3256. doi:10.1098/rspb.2005.3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney K.L, Côté I.M. Mutualism or parasitism? The variable outcome of cleaning symbioses. Biol. Lett. 2005b;1:162–165. doi: 10.1098/rsbl.2004.0288. doi:10.1098/rsbl.2004.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté I.M, Cheney K.L. Choosing when to be a cleaner-fish mimic. Nature. 2005;433:211–212. doi: 10.1038/433211a. doi:10.1038/433211a [DOI] [PubMed] [Google Scholar]
- Dill L.M. Calculated risk-taking by predators as a factor in Batesian mimicry. Can. J. Zool. 1975;53:1614–1621. [Google Scholar]
- Feder H.M. Cleaning symbiosis in the marine environment. In: Henry S.M, editor. Symbiosis. Academic Press; New York, NY: 1966. pp. 327–380. [Google Scholar]
- Goodale M.A, Sneddon I. The effect of distastefulness of the model on the predation of artificial Batesian mimics. Anim. Behav. 1977;25:660–665. doi:10.1016/0003-3472(77)90117-8 [Google Scholar]
- Grutter A.S. Spatial and temporal variations of the ectoparasites of 7 reef fish species from Lizard Island and Heron Island, Australia. Mar. Ecol. Prog. Ser. 1994;115:21–30. [Google Scholar]
- Grutter A.S. Relationship between cleaning rates and ectoparasite loads in coral reef fishes. Mar. Ecol. Prog. Ser. 1995;118:51–58. [Google Scholar]
- Grutter A.S. Parasite removal rates by the cleaner wrasse Labroides dimidiatus. Mar. Ecol. Prog. Ser. 1996;130:61–70. [Google Scholar]
- Grutter A.S. Spatiotemporal variation and feeding selectivity in the diet of the cleaner fish Labroides dimidiatus. Copeia. 1997;2:346–355. doi:10.2307/1447754 [Google Scholar]
- Grutter A.S. Cleaner fish really do clean. Nature. 1999;398:672–673. doi:10.1038/19443 [Google Scholar]
- Grutter A.S. Parasite infection rather than tactile stimulation is the proximate cause of cleaning behaviour in reef fish. Proc. R. Soc. B. 2001;268:1361–1365. doi: 10.1098/rspb.2001.1658. doi:10.1098/rspb.2001.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutter A.S. Feeding ecology of the fish ectoparasite Gnathia sp. (Crustacea: Isopoda) from the Great Barrier Reef, and its implications for fish cleaning behaviour. Mar. Ecol. Prog. Ser. 2003;259:295–302. [Google Scholar]
- Kokko H, Mappes J, Lindström L. Alternative prey can change model–mimic dynamics between parasitism and mutualism. Ecol. Lett. 2003;6:1068–1076. doi:10.1046/j.1461-0248.2003.00532.x [Google Scholar]
- Kuwamara T. Mimicry of the cleaner wrasse Labroides dimidiatus by the blennies Aspidontus taeniatus and Plagiotremus rhynorhynchos. Nanki Seibutu. 1981;23:61–70. [Google Scholar]
- Limbaugh C. Cleaning symbiosis. Sci. Am. 1961;205:42–49. [Google Scholar]
- Lindström L, Alatalo R.V, Mappes J. Imperfect Batesian mimicry—the effects of the frequency and the distastefulness of the model. Proc. R. Soc. B. 1997;264:149–153. doi:10.1098/rspb.1997.0022 [Google Scholar]
- Lloyd J.E. Aggressive mimicry in Photuris fireflies—signal repertoires by femmes fatales. Science. 1975;187:452–453. doi: 10.1126/science.187.4175.452. doi:10.1126/science.187.4175.452 [DOI] [PubMed] [Google Scholar]
- Monod T. Les gnathiidae. Essai monographique (morphologie, biologie, systematique) Mem. Soc. Sci. Nat. Maroc. 1926;13:1–661. [Google Scholar]
- Mugridge R.E.R, Stallybrass H.G. A mortality of eels, Anguilla anguilla, attributed to gnathiidae. J. Fish Dis. 1983;6:81–82. doi:10.1111/j.1365-2761.1983.tb00054.x [Google Scholar]
- Nonacs P. Foraging in a dynamic mimicry complex. Am. Nat. 1985;126:165–180. doi:10.1086/284407 [Google Scholar]
- Randall J.E, Allen G.R, Steene R. Crawford House; Bathurst, Canada: 1997. Fishes of the Great Barrier Reef and Coral Sea. [Google Scholar]
- Sikkel P.C, Cheney K.L, Côté I.M. In situ evidence for ectoparasites as a proximate cause of cleaning interactions in reef fish. Anim. Behav. 2004;68:241–247. doi:10.1016/j.anbehav.2003.10.023 [Google Scholar]
- Slobodchikoff C.N. Aversive conditioning in a model–mimic system. Anim. Behav. 1987;35:75–80. doi:10.1016/S0003-3472(87)80212-9 [Google Scholar]
- Wickler W. Mimicry and evolution of animal communication. Nature. 1965;208:519–521. doi:10.1038/208519a0 [Google Scholar]
- Wickler W. McGraw-Hill; New York, NY: 1968. Mimicry in plants and animals. [Google Scholar]