Abstract
Recent evaluations of both temporal and spatial precision in bird migration have called for external cues in addition to the inherited programme defining the migratory journey in terms of direction, distance and fuelling behaviour along the route. We used juvenile European robins (Erithacus rubecula) to study whether geomagnetic cues affect fuel deposition in a medium-distance migrant by simulating a migratory journey from southeast Sweden to the wintering area in southern Spain. In the late phase of the onset of autumn migration, robins exposed to the magnetic treatment attained a lower fuel load than control birds exposed to the ambient magnetic field of southeast Sweden. In contrast, robins captured in the early phase of the onset of autumn migration all showed low fuel deposition irrespective of experimental treatment. These results are, as expected, the inverse of what we have found in similar studies in a long-distance migrant, the thrush nightingale (Luscinia luscinia), indicating that the reaction in terms of fuelling behaviour to a simulated southward migration varies depending on the relevance for the species. Furthermore, we suggest that information from the geomagnetic field act as an important external cue overriding the seasonal effect on fuelling behaviour in migratory birds.
Keywords: magnetic cues, bird migration, fuelling decisions, magnetic cues, endogenous time programme
1. Introduction
Seasonal migration is a common life-history trait in birds in response to changes in environmental conditions. Outside the tropics the most reliable predictor of seasonal change is the annual change in day length, photoperiod helping to synchronize migratory movements (Dingle 1996). The energy needed for avian migration is stored as fat accumulated at stopover sites along the route. Carrying fat loads, however, entail costs in terms of impaired escape performance (Kullberg et al. 1996, 2000; Lind et al. 1999) and increased flight costs (Alerstam & Lindström 1990), so birds accumulate fat in a strategic way with lower fuel loads when it is possible to refuel (Alerstam & Lindström 1990) and extreme fuel loads in case of ecological barriers, e.g. Sahara desert (Fry et al. 1970; Finlayson 1981; Fransson et al. 2006). The amount and timing of fuelling during migration has generally been assumed to be governed by circannual rhythm fine tuned by photoperiod (Berthold 1996). Nevertheless environmental conditions will affect the onset of migration, and unpredictable weather and feeding conditions will influence a bird's timing en route. Recent evaluations of both temporal and spatial precision in bird migration have called for external cues in addition to the inherited migratory programme. By comparing data from three migratory species with a computer model of a clock-and-compass system, Thorup & Rabøl (2001) showed that the observed migratory routes of first-year migrants are too precise to be explained by an inherited migratory programme alone. More recently, Fransson et al. (2005) showed that migrants from rather large breeding areas converge in confined areas in the eastern Mediterranean region, which in several cases completely differ between species. This means that birds of the same species have to follow different migratory directions depending on the location of their starting point. Thus, in combination with a clock-and-compass orientation procedure, birds must use some external cues in order to find confined species-specific areas.
Most passerine migrants travel alone during nocturnal flights using a combination of innate information about migratory direction and external information such as celestial information from star patterns (Emlen 1970) and skylight polarization associated with the Sun (Able 1982; Moore 1987; Helbig 1991). Further, numerous studies have shown that animals can use the information from the Earths' magnetic field to find their way during migration (reviewed by Wiltschko & Wiltschko 1995). Experimental changes in the magnetic field have been shown to trigger directional changes in species of many different animal groups (birds: Beck & Wiltschko 1988; Wiltschko & Wiltschko 1992; Fischer et al. 2003; sea turtles: Lohmann et al. 2001, 2004; newts: Phillips et al. 1995; Fischer et al. 2001; lobsters: Boles & Lohmann 2003).
Not only are birds able to use the information from the geomagnetic field for finding their way during migration, but also we have shown in two earlier studies that changes in the magnetic field can cause physiological changes in long-distance migratory thrush nightingales (Luscinia luscinia; Fransson et al. 2001; Kullberg et al. 2003). First-year thrush nightingales exposed to a successive change in the magnetic field simulating a migratory flight from Sweden to northern Egypt, where extensive fuelling would be expected before the trans-Saharan passage, increased fuel deposition rate when compared with control birds. However, whether the effect shown is triggered by a simple latitudinal change or is an evolved response to a specific area where an extensive fuel load is needed remains to be investigated. To study whether geomagnetic cues also affects fuel deposition in medium-distance migrants, where no extensive fuel loads are needed, we here study fuel deposition in juvenile European robins (Erithacus rubecula), a medium-distance European migrant, exposed to a magnetic treatment simulating their migratory journey from southeast Sweden to the wintering area in southern Spain. We would expect robins that experience the magnetic field of their wintering area to reduce fuel deposition compared with control birds still experiencing the magnetic field of Sweden.
2. Material and methods
(a) Subjects
European robins were trapped using mist nets during September and October 2005, in the vicinity of Tovetorp Zoological Research Station, in southeast Sweden (58°56′ N, 17°08′ E). To avoid the effects of age and experience from an earlier migration, only first-year birds were used in the study. Individuals that had completed their post-juvenile moult and had small amounts of visible fat reserves were chosen (fat score 0–2 according to Pettersson & Hasselquist (1985)).
(b) Husbandry
Experiments were performed in four wooden sheds (5 m×5 m×5 m) built of non-magnetic materials and placed 20 m apart. Each shed contained a magnetic coil system enabling manipulation of the magnetic field. Semi-transparent plastic roofs blocked potential celestial cues but allowed some light through. The reduction in light spectra caused by the plastic roof was compensated for using four daylight bulbs in each shed (HP1-T Plus Philips Powertone 400 W) following the natural daylight. In each shed, four birds were housed in separate circular cages (ø: 70 and 70 cm height) constructed of non-magnetic materials with a plastic circular perch. Plastic baffles (0.5 cm thickness) between each cage were used to separate birds visually. Birds were fed a dry food mixture (5 g; Berthold et al. 1990), mealworms (20 g; Tenebrio molitor) and water ad libitum. Fresh food and water was provided daily and the amount of food eaten were recorded by weighing the remaining food. Automatic and continuous registration of body mass was achieved by placing food trays on electronic scales (Precisa 310C or Precisa XB320C) connected to computers. When calculating increase in body mass during the 10 days of the experiment, daily body mass at 16.00 was used.
(c) Treatments
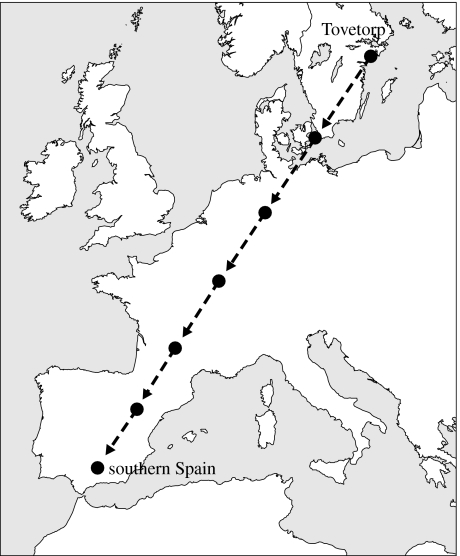
Individual birds were randomly assigned to either an experimental treatment or a control treatment. Two of the sheds were used for control treatment; birds housed in these sheds received no manipulation and experienced the ambient geomagnetic field of Tovetorp during the 10 days of the experiment. In the two other sheds, experimental birds were exposed to a gradual change in the magnetic field during the course of the experiment to simulate the change in the geomagnetic field robins experience during migration. The simulated migration was based on autumn/winter recoveries of robins ringed in Sweden and Finland, showing that many robins from this breeding area winter in southern Spain (Saurola 1983; Pettersson & Hasselquist 1985). At the start of the experiment, morning day 1 (trapping day), both experimental and control birds remained in the ambient magnetic field. For the experimental birds, the magnetic field was changed every evening from day 1 to 6, in successive steps towards southern Spain (table 1; figure 1). The magnetic field of the stopover sites was calculated according to International Geomagnetic Reference Field 10th generation (International Association of Geomagnetism and Aeronomy 2005). The experimental birds remained thereafter in the magnetic field of southern Spain until the experiment ended at day 10 when all birds were released into the wild. This protocol was replicated, once in the middle of September and once in early October. In total, 16 birds were subjected to the magnetic treatment and 16 birds were used as controls. Owing to technical problems, mainly at day 2, we did not get body mass data for all birds on all nights, thus sample size varies from 12 to 14 birds between the different treatments when performing analysis with repeated measurement. The trapping of first-year robins at Ottenby Bird Observatory, Southeast Sweden, shows that the autumn migration starts in the beginning of September and continues to the end of October, with a median date of 30 September (Pettersson 1983). Thus, birds used in the first replicate were trapped in the early phase of the onset of autumn migration while birds used in the second replicate were trapped late in the onset of migration.
Table 1.
Magnetic fields used during the experiment. (The ambient geomagnetic field of Tovetorp, and of the simulated stopover sites for experimental birds. The magnetic field was changed on the evening of day 1–6.)
| day of change | location | position | total intensity (nT) | inclination (°) |
|---|---|---|---|---|
| Tovetorp | 58°56′ N 17°08′ E | 50 900 | 72.3 | |
| 1 | southern Sweden | 55°23′ N 12°49′ E | 49 900 | 69.8 |
| 2 | Germany | 51°50′ N 8°55′ E | 48 900 | 67.0 |
| 3 | northern France | 48°20′ N 5°15′ E | 47 700 | 64.0 |
| 4 | southern France | 44°48′ N 1°50′ E | 46 400 | 60.5 |
| 5 | northern Spain | 41°17′ N 1°22′ W | 45 000 | 56.5 |
| 6 | southern Spain | 37°46′ N 4°25′ W | 43 400 | 52.0 |
Figure 1.
Map showing location of Tovetorp Zoological Research Station and the simulated stop over sites during the experiment.
(d) The magnetic coils
To generate a volume of 1.5 m×1.5 m×0.75 m in which four caged birds could be subjected to a manipulated homogenous magnetic field, each shed contained a magnetic coil system of aluminium profiles (3 m×3 m×3 m). The system consisted of three independent series of four quadratic coils each, arranged to control the X (north–south), Y (east–west) and Z (vertical) components of the magnetic field. The magnetic coil system was calculated using Biot–Savart law. We found, numerically, a configuration of the coils giving an inhomogeneity of lower than 1% in the experimental area, which was verified with a proton magnetometer (GEM Systems). Coils were wound with copper wire (1.5 mm diameter). The coils controlling the X and Y components of the magnetic field were wound with 4 and 9 turns in the two inner and outer coils, respectively, and the coil controlling the Z component of the field were wound with 7 and 18 turns in the inner and outer coils, respectively. Each of the three coils was connected to a power supply (Delta Elektronika ES030-5 for X and Y; and Delta Elektronika ES030-10 for Z) enabling us to change each component of the magnetic field independently (stability of the power supplies: ±0.0011 A). Before the experiment, we set the three components of the magnetic field for the different stopover sites, using a fluxgate magnetometer (Zeiss Jena theodolite with Bartington Instruments fluxgate magnetometer). We also checked that the field of one coil did not affect the others more than expected, showing that the coils were sufficiently oriented.
(e) Orientation
We recorded migratory restlessness behaviour at night for both experimental and control birds. A digital network camera (Axis 221 day & night network camera) and a separate infra-red light source (infrared light-emitting diode, LED, Day & Night) in each shed were positioned over the four cages (1 m above the magnetic coil) and video recordings saved directly to hard disk. As magnetic compass orientation in birds is believed to be light-dependant (Ritz et al. 2000), a LED, 525 nm (green), gave a suitable ambient light source not directly visible to the birds.
(f) Orientation analysis
To determine the directional preference of birds the video films were analysed by placing a transparent acetate sheet divided into sixteen 22.5° sectors onto a computer screen and played back frame-by-frame for each cage. A bird's position (sector) on the wall of the cage was recorded directly after a flight or jump from the perch or cage wall. Video films were sampled for two 1 h periods, at 22.00 and 01.00, respectively. A maximum of 80 positions per hour were recorded giving a possible total of 160 positions per night for each bird. The Rayleigh test (Batschelet 1981) was applied to test for significant directional preferences for each bird on night 9 when birds in the experimental treatment had been in the magnetic position of southern Spain for 3 days. The Mardia–Watson–Wheeler test (Batschelet 1981) was used to compare differences in distribution between treatments.
3. Results
It was warmer in the sheds during the early replicate than in the late replicate (ANOVA with repeated measurement day 2–10: F1,4=10.6, p=0.031; mean noon temperature ±s.e., early replicate, 21.7°C±0.8; late replicate, 18.0°C±0.8), however, there was no difference in temperature between the sheds with different experimental treatments (ANOVA with repeated measurement day 2–10: F1,4=0.3, p=0.59; mean noon temperature ±s.e., control treatment: 19.5°C±0.8; experimental treatment, 20.2°C±0.8).
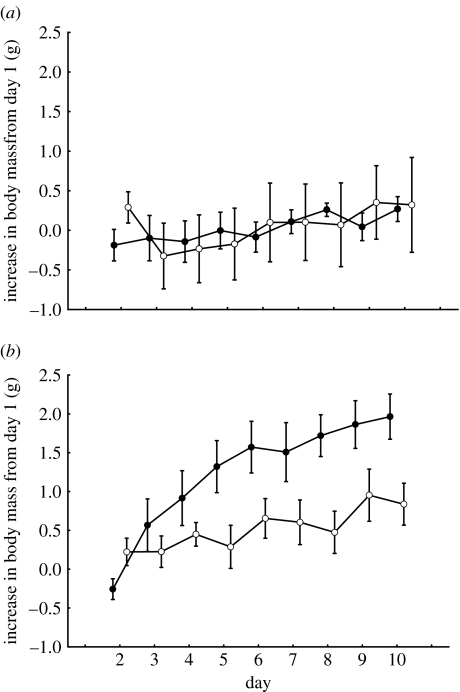
There was no difference in either wing length or initial body mass between replicates (wing length: F1,28=0.01, p=0.9; body mass: F1,28=0.2, p=0.7) or treatments (wing length: F1,28=0.05, p=0.8; body mass: F1,28=0.3, p=0.6). Birds captured in the early phase of the onset of migration showed no difference in cumulative food intake (table 2). For birds captured late, however, control birds tended to increase more in cumulative food intake during the course of the experiment when compared with experimental birds (table 2). Food intake was also reflected by body mass increase of the birds. In the early replicate, there was no difference between treatments, while control birds increased more in body mass than experimental birds in the late replicate (figure 2 and table 3). Control birds in the late replicate increased more in body mass than control birds in the early replicate, while experimental birds in both replicates did not differ in body mass increase (figure 2 and table 4). To disentangle when the difference between treatments appeared in the late replicate, we further compared daily body mass increase each day. Control birds showed higher daily body mass increase compared with experimental birds during day 4, 5 and 10 (Student's t-test: n=8+8, d.f.=14; day 4: p=0.006, t=3.24; day 5: p=0.02, t=2.6; day 10: p=0.059, t=2.05; figure 2b).
Table 2.
Cumulative food intake from day 1. (Statistics from ANOVAs for early and late replicates, respectively. Factors: treatment (experimental or control) and day (repeated measurement factor with nine levels; day 2–10).)
| early replicate | late replicate | |||||
|---|---|---|---|---|---|---|
| d.f. effect, d.f. error | F | p | d.f. effect, d.f. error | F | p | |
| treatment | 1,14 | 0.06 | 0.8 | 1,14 | 0.7 | 0.4 |
| day | 8,112 | 2030 | <0.001 | 8,112 | 1101 | <0.001 |
| treatment×day | 8,112 | 0.1 | 0.9 | 8,112 | 1.9 | 0.057 |
Figure 2.
Effect of magnetic field and time of season on migratory fuelling in robins. Body mass increase from day 1 (mean±s.e.) for the (a) early and (b) late replicate, respectively. Filled circles are control birds and open circles are experimental birds.
Table 3.
Increase in body mass from day 1. (Statistics from ANOVAs for early and late replicates, respectively. Factors: treatment (experimental or control) and day (repeated measurement factor with eight levels; day 3–10).)
| early replicate | late replicate | |||||
|---|---|---|---|---|---|---|
| d.f. effect, d.f. error | F | p | d.f. effect, d.f. error | F | p | |
| treatment | 1,10 | 0.001 | 0.9 | 1,12 | 7.3 | 0.02 |
| day | 7,70 | 4.8 | <0.001 | 7,84 | 9.8 | <0.001 |
| treatment×day | 7,70 | 1.3 | 0.3 | 7,84 | 5.5 | <0.001 |
Table 4.
Increase in body mass from day 1. (Statistics from ANOVAs for control and experimental birds, respectively. Factors: replicate (early or late replicate) and day (repeated measurement factor with eight levels; day 3–10).)
| control birds | experimental birds | |||||
|---|---|---|---|---|---|---|
| factor | d.f. effect, d.f. error | F | p | d.f. effect, d.f. error | F | p |
| replicate | 1,12 | 12.1 | 0.005 | 1,10 | 0.4 | 0.6 |
| day | 7,84 | 12.9 | <0.001 | 7,70 | 3.8 | 0.002 |
| replicate×day | 7,84 | 4.5 | <0.001 | 7,70 | 0.9 | 0.5 |
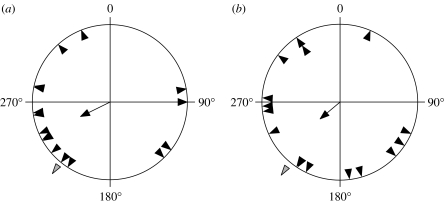
All birds showed directional night activity on night 9, and mean direction for all birds in both replicates was southwest (Rayleigh test: α=238°, r=0.38, p=0.009, n=32) close to the expected migratory direction of 220° (Fransson & Hall-Karlsson in press).
The mean orientation direction for control birds was approximately southwest (Rayleigh test: α=244°, r=0.42, p=0.05, n=16; figure 3a), while experimental birds showed no significant overall mean direction (Rayleigh test: α=230°, r=0.34, p=0.16, n=32; figure 3b). There was, however, no significant difference in scatter between the treatments (Mardia–Watson–Wheelers test: W=1.28, p=0.53, nexp=16, ncontr=16).
Figure 3.
Mean orientation directions of robins on night 9 (black triangles) for the (a) control and (b) experimental treatment, respectively. The arrow shows mean direction of orientation, and the length of the arrow represents concentration (r). The grey triangle represents the mean direction of Swedish robins during first autumn migration (220°, r=0.93, n=358; Fransson & Hall-Karlsson in press).
4. Discussion
All robins were night active and exhibited clear directional preferences, and the mean vector for all birds were close to the expected autumn direction towards southwest, showing that all birds under study were in a migratory mode (Berthold 1996). Our results show that control birds experiencing the ambient magnetic field in southeast Sweden attained higher fuel loads as the season progressed (early replicate versus late replicate). This is in line with the observation that birds tend to increase their fuel load late in the migratory season when they are likely to be more time constrained (Dänhardt & Lindström 2001; Kullberg et al. 2003), and is also verified by the fact that body mass increase in robins as the autumn progresses (trapping data at Tovetorp of juvenile robins that have completed their post-juvenile moult 1994–1997; r2=0.03, p=0.001, b=0.17, n=382; Tovetorp Zoological Research Station 2007, unpublished data). In the late replicate, experimental birds showed a lower fuel deposition rate than control birds, suggesting that robins exposed to a simulated migration to southern Spain did not experience the same time stress as control birds, and thus showed a similar body mass increase as all birds in the early replicate. The fact that fuel deposition rate seems to be affected by food intake of the birds is in line with our findings in thrush nightingales (I. Henshaw et al., unpublished data) Furthermore, body mass increase during premigratory fattening has been shown to be due to an increase in food intake in combination with an increased efficiency of food usage (Bairlein 1985).
It could be argued that robins experiencing the magnetic field of their wintering area in southern Spain, as a response should stop showing directional night activity and reduce their fuel loads instead of continuing to show southwesterly directions and slowly increase in body mass as was the case for experimental birds. The fact that the overall mean direction for experimental birds was not significant, while this was the case for control birds, could reflect their simulated arrival in southern Spain, where the main southwest direction during migration is no longer required. Since the sample size, however, was small for both control and experimental birds, it is uncertain whether this is the case. Furthermore, due to constraints in our experimental set up, for example the simulated fast migration and the fact that the temperature dropped during the experiment, we cannot expect to observe fuel loads and activity patterns that totally resemble the ones in the wild.
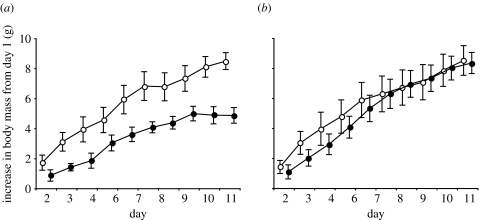
Our results confirm earlier studies on thrush nightingales indicating that migratory birds can use information from the Earth's magnetic field for fuelling decisions (Fransson et al. 2001; Kullberg et al. 2003). Furthermore, by comparing the responses of nightingales and robins in these experiments, we can conclude that a reduction in the magnetic intensity does not trigger a general response in migratory birds increasing fuel deposition in the same way as decreasing day length triggers migratory activity in autumn, as suggested by Freake et al. (2006). Both species experience a reduction in total intensity and inclination of the magnetic field in our experiments; however, they react more or less inversely to the treatment, although, in a biologically relevant way for each species. As predicted, thrush nightingales captured in the early phase of the onset of autumn migration, and exposed to a simulated flight to northern Egypt where extensive fuelling would be expected before the trans-Saharan passage, increased fuel deposition rate when compared with control birds (figure 4; Kullberg et al. 2003). In the present study, however, robins captured in the late phase of the onset of autumn migration and exposed to a successive change in the magnetic field to southern Spain, showed a lower fuel deposition rate when compared with control birds, which is expected since they have no reason to deposit large fuel loads when approaching their wintering area (figure 2b).
Figure 4.
The effect of magnetic field and time of season on migratory fuelling in thrush nightingales. Body mass increase from day 1 (mean±s.e.) for the (a) early and (b) late replicate, respectively. Filled circles are control birds and open circles are experimental birds. Adapted from Kullberg et al. (2003).
Fuel deposition rate is known to be affected by various environmental (e.g. food availability, predation risk and competition), intrinsic (e.g. moult and current body mass) and endogenous factors (e.g. photoperiod; Jenni & Schaub 2003). Jenni & Schaub (2003) pointed out, however, that when food availability is adequate, it is largely unknown to what extent environmental factors and the endogenous time programme interacts and affects fuelling behaviour. Several studies verify that the progress of the season increases fuel deposition rate in migratory birds (Lindström et al. 1994; Fransson 1998; Schaub & Jenni 2000b; Dänhardt & Lindström 2001) and that late birds tend to migrate at higher speed (e.g. Ellergren 1993; Fransson 1995; Schaub & Jenni 2000a,b). The difference in reaction in early and late replicates in nightingales (figure 4; Kullberg et al. 2003) and robins (figure 2) nicely illustrates that geomagnetic cues can affect migratory birds both in the early and late phase of the onset of autumn migration and that the effect of magnetic treatment does not seem to be affected by the endogenous time programme. Early nightingales experiencing the magnetic field of northern Egypt increase their fuel load to be able to pass the Saharan desert. In the late replicate, control nightingales showed just as high fuel deposition rate as experimental birds, probably due to the endogenous time programme causing increased fuel deposition rate also in control birds. Early robins, on the other hand, experiencing a magnetic change towards their wintering area have no reason to change their fuel deposition rate that is already low due to the lack of seasonal time pressure. In robins, we instead see the effect of the magnetic treatment in the late birds. Late control robins, experiencing the ambient magnetic field of southern Sweden, react to the time of season and increase fuel deposition rate to increase their migratory speed, while robins exposed to the magnetic treatment can remain on a low fuel deposition rate, since they experience no time stress. The pattern of fuel deposition rate in our magnetically manipulated robins and nightingales (Kullberg et al. 2003) shows that body mass is affected before the birds experience the goal areas (south Spain and Egypt, respectively) indicating that advancement along the migratory route affects fuelling behaviour. It remains, however, to be investigated if birds use the information from the Earth's magnetic field to navigate using, e.g. a bicoordinate map or if they also can use specific magnetic field values as signposts at special places (Lohmann & Lohmann 2006), such as in front of ecological barriers or at their wintering or breeding areas.
The responses of nightingales and robins to magnetic simulated autumn migration confirm that the relative importance of the endogenous time programme and external cues varies depending on the specific time phase (Jenni & Schaub 2003). Our results show that information from the geomagnetic field overrides the seasonal effect, which should be expected from an external cue helping birds to make the right fuelling decisions along the migratory route. The progress of migration for an individual bird is affected by many unpredictable conditions, e.g. breeding performance, weather and feeding conditions en route, and a bird's position at a given time might therefore vary considerably. This means that migratory birds need an external cue which is unaffected by seasonal effects. Thus, we suggest that geomagnetic cues work together with the endogenous time programme triggering behaviour in specific geographical areas.
Acknowledgments
The study has been done under permission from the Swedish Animal Welfare Agency (permission nr 26-05).
The authors thank Johan Fransson, Thomas Giegold, Lars Kullberg and Ulf Norberg for their technical assistance. We are most grateful for 7000 m copper wire received from Dahréntråd AB. Financial support was received from the Swedish Research Council (to C.K.).
References
- Able K.P. Skylight polarization patterns at dusk influence migratory orientation in birds. Nature. 1982;288:550–551. doi:10.1038/299550a0 [Google Scholar]
- Alerstam T, Lindström Å. Optimal bird migration: the relative importance of time, energy, and safety. In: Gwinner E, editor. Bird migration: the physiology and ecophysiology. Springer; Berlin, Germany: 1990. pp. 331–350. [Google Scholar]
- Bairlein F. Efficiency of food utilization during fat deposition in the long-distance migratory garden warbler, Sylvia borin. Oecologia. 1985;68:118–125. doi: 10.1007/BF00379483. doi:10.1007/BF00379483 [DOI] [PubMed] [Google Scholar]
- Batschelet E. Academic Press; London, UK: 1981. Circular statistics in biology. [Google Scholar]
- Beck W, Wiltschko W. Magnetic factors control the migratory direction of pied flycatchers (Ficedula hypoleuca Pallas) In: Ouellet H, editor. Acta XIX Congr. Int. Ornithology. vol. 2. Springer; Berlin, Germany: 1988. pp. 1955–1962. [Google Scholar]
- Berthold P. Chapman & Hall; London, UK: 1996. Control of bird migration. [Google Scholar]
- Berthold P, Querner U, Schlenker R. Die neue Brehm-Bücherei; Wittenberg Lutherstadt, Germany: 1990. Die Mönchgrasmücke Sylvia atricapilla. [Google Scholar]
- Boles L.C, Lohmann K.J. True navigation and magnetic maps in spiny lobsters. Nature. 2003;421:60–63. doi: 10.1038/nature01226. doi:10.1038/nature01226 [DOI] [PubMed] [Google Scholar]
- Dänhardt J, Lindström Å. Optimal departure decisions of songbirds from an experimental stopover site and the significance of weather. Anim. Behav. 2001;62:235–243. doi:10.1006/anbe.2001.1749 [Google Scholar]
- Dingle K.P. Oxford University Press; Oxford, UK: 1996. Migration—the biology of life on the move. [Google Scholar]
- Ellergren H. Speed of migration and migratory flight lengths of passerine birds ringed during autumn migration in Sweden. Ornis Scand. 1993;24:220–228. doi:10.2307/3676737 [Google Scholar]
- Emlen S.T. Celestial rotation: its importance in the development of migratory orientation. Science. 1970;170:1198–1201. doi: 10.1126/science.170.3963.1198. doi:10.1126/science.170.3963.1198 [DOI] [PubMed] [Google Scholar]
- Finlayson J.C. Seasonal distribution, weights, and fat of passerine migrants at Gibraltar. Ibis. 1981;123:88–95. [Google Scholar]
- Fischer J.H, Freake M.J, Borland S.C, Phillips J.B. Evidence for the use of magnetic map information by an amphibian. Anim. Behav. 2001;62:1–10. doi:10.1006/anbe.2000.1722 [Google Scholar]
- Fischer J.H, Munro U, Phillips J.B. Magnetic navigation by an avian migrant? In: Berthold P, Gwinner E, Sonnenschein E, editors. Avian migration. Springer; Heidelberg, Germany; New York, NY: 2003. pp. 141–154. [Google Scholar]
- Fransson T. Timing and speed of migration in North and West European populations of Sylvia warblers. J. Avian Biol. 1995;26:39–48. doi:10.2307/3677211 [Google Scholar]
- Fransson T. A feeding experiment on migratory fuelling in whitethroats (Sylvia communis) Anim. Behav. 1998;55:153–162. doi: 10.1006/anbe.1997.0573. doi:10.1006/anbe.1997.0573 [DOI] [PubMed] [Google Scholar]
- Fransson, T. & Hall-Karlsson, S. In press. Swedish bird ringing atlas, vol. 3. Stockholm, Sweden: Swedish Museum of Natural History.
- Fransson T, Jakobsson S, Johansson P, Kullberg C, Lind J, Vallin A. Bird migration: magnetic cues trigger extensive refuelling. Nature. 2001;414:35–36. doi: 10.1038/35102115. doi:10.1038/35102115 [DOI] [PubMed] [Google Scholar]
- Fransson T, Jakobsson S, Kullberg C. Non-random distribution of ring recoveries from trans-Saharan migrants indicates species-specific stopover sites. J. Avian Biol. 2005;36:6–11. doi:10.1111/j.0908-8857.2005.03471.x [Google Scholar]
- Fransson T, Jakobsson S, Kullberg C, Mellroth R, Pettersson T. Fuelling in front of the Sahara desert in autumn—an overview of Swedish field studies of migratory birds in the eastern Mediterranean. Ornis Svecica. 2006;16:74–83. [Google Scholar]
- Freake M.J, Muheim R, Phillips J.B. Magnetic maps in animals: a theory comes of age? Q. Rev. Biol. 2006;81:327–347. doi: 10.1086/511528. doi:10.1086/511528 [DOI] [PubMed] [Google Scholar]
- Fry C.H, Ash J.S, Ferguson-Lees I.J. Spring weights of some Palearctic migrants at Lake Chad. Ibis. 1970;112:58–82. [Google Scholar]
- Helbig A.J. Dusk orientation of migratory European robins, Erithacus rubecula—the role of sun-related directional information. Anim. Behav. 1991;41:313–322. doi:10.1016/S0003-3472(05)80483-X [Google Scholar]
- International Association of Geomagnetism and Aeronomy (IAGA) Division V, Working Group VMOD: Geomagnetic Field Modeling. The 10th generation international geomagnetic reference field. Geophys. J. Int. 2005;161:561–565. doi:10.1111/j.1365-246X.2005.02641.x [Google Scholar]
- Jenni L, Schaub M. Behavioural and physiological reactions to environmental variation in bird migration: a review. In: Berthold P, Gwinner E, Sonnenschein E, editors. Avian migration. Springer; Heidelberg, Germany: 2003. [Google Scholar]
- Kullberg C, Fransson T, Jakobsson S. Impaired predator evasion in fat blackcaps (Sylvia atricapilla) Proc. R. Soc. B. 1996;263:1671–1675. doi:10.1098/rspb.1996.0244 [Google Scholar]
- Kullberg C, Jakobsson S, Fransson T. High migratory fuel loads impair predator evasion in sedge warblers. Auk. 2000;117:1034–1038. doi:10.1642/0004-8038(2000)117[1034:HMFLIP]2.0.CO;2 [Google Scholar]
- Kullberg C, Lind J, Fransson T, Jakobsson S, Vallin A. Magnetic cues and time of season affect fuel deposition in migratory thrush nightingales (Luscinia luscinia) Proc. R. Soc. B. 2003;270:373–378. doi: 10.1098/rspb.2002.2273. doi:10.1098/rspb.2002.2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind J, Fransson T, Jakobsson S, Kullberg C. Reduced take-off ability in robins (Erithacus rubecula) due to migratory fuel load. Behav. Ecol. Sociobiol. 1999;46:65–70. doi:10.1007/s002650050593 [Google Scholar]
- Lindström Å, Daan S, Visser H. The conflict between moult and migratory fat deposition: a photoperiodic experiment with bluethroats. Anim. Behav. 1994;48:1173–1171. doi:10.1006/anbe.1994.1349 [Google Scholar]
- Lohmann K.J, Lohmann C.M.F. Sea turtles, lobsters, and oceanic magnetic maps. Mar. Freshw. Behav. Phys. 2006;39:49–64. doi:10.1080/10236240600563230 [Google Scholar]
- Lohmann K.J, Cain S.D, Dodge S.A, Lohmann C.M.F. Regional magnetic fields as navigational markers for sea turtles. Science. 2001;294:364–366. doi: 10.1126/science.1064557. doi:10.1126/science.1064557 [DOI] [PubMed] [Google Scholar]
- Lohmann K.J, Lohmann C.M.F, Ehrhart L.M, Bagley D.A, Swing T. Animal behaviour—geomagnetic map used in sea-turtle navigation. Nature. 2004;428:909–910. doi: 10.1038/428909a. doi:10.1038/428909a [DOI] [PubMed] [Google Scholar]
- Moore F.R. Sunset and the orientation behavior of migratory birds. Biol. Rev. Camb. Philos. Soc. 1987;62:65–86. [Google Scholar]
- Pettersson J. The autumn migration of the robin, Erithacus rubecula, at Ottenby. Vår Fågelvärld. 1983;42:333–342. [In Swedish with English summary] [Google Scholar]
- Pettersson J, Hasselquist D. Fat deposition and migratory capacity of robins Erithacus rubecula and goldcrests Regulus regulus at Ottenby, Sweden. Ringing Mig. 1985;6:66–76. [Google Scholar]
- Phillips J.B, Adler K, Borland S.C. True navigation by an amphibian. Anim. Behav. 1995;50:855–858. doi:10.1016/0003-3472(95)80146-4 [Google Scholar]
- Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurola P. Autumn migration and wintering of robins (Erithacus rubecula) ringed in Finland. Lintumies. 1983;18:108–115. [In Finnish with English summary.] [Google Scholar]
- Schaub M, Jenni L. Body mass of six long-distance migrant passerine species along the autumn migration route. J. Ornithol. 2000a;141:441–460. [Google Scholar]
- Schaub M, Jenni L. Fuel deposition of three passerine bird species along the migratory route. Oecologia. 2000b;122:306–317. doi: 10.1007/s004420050036. doi:10.1007/s004420050036 [DOI] [PubMed] [Google Scholar]
- Thorup K, Rabøl J. The orientation system and migration pattern of long-distance migrants: conflict between model predictions and observed patterns. J. Avian Biol. 2001;32:111–119. doi:10.1034/j.1600-048X.2001.320203.x [Google Scholar]
- Wiltschko R, Wiltschko W. Springer; Berlin, Germany: 1995. Magnetic orientation in animals. [Google Scholar]
- Wiltschko W, Wiltschko R. Migratory orientation: magnetic compass orientation of garden warblers (Sylvia borin) after a simulated crossing of the magnetic equator. Ethology. 1992;91:70–74. [Google Scholar]