Abstract
The waxy layer of the cuticle has been shown to play a fundamental role in recognition systems of insects. The biparental burying beetle Nicrophorus vespilloides is known to have the ability to discriminate between breeding and non-breeding conspecifics and also here cuticular substances could function as recognition cue. However, it has not yet been demonstrated that the pattern of cuticular lipids can reflect the breeding status of a beetle or of any other insect. With chemical analysis using coupled gas chromatography–mass spectrometry, we showed that the chemical signature of N. vespilloides males and females is highly complex and changes its feature with breeding status. Parental beetles were characterized by a higher amount of some unusual unsaturated hydrocarbons than beetles which are not caring for larvae. The striking correlation between cuticular profiles and breeding status suggests that cuticular hydrocarbons inform the beetles about parental state and thus enable them to discriminate between their breeding partner and a conspecific intruder. Furthermore, we found evidence that nutritional conditions also influence the cuticular profile and discuss the possibility that the diet provides the precursors for the unsaturated hydrocarbons observed in parental beetles. Our study underlines the fact that the cuticular pattern is rich of information and plays a central role in the burying beetles' communication systems.
Keywords: burying beetles, Nicrophorus, recognition of reproductive status, chemical cues, cuticular hydrocarbons, polyenes
1. Introduction
Aside from its essential function as a water barrier (e.g. Neville 1975; Jackson & Blomquist 1976), the insect cuticle plays an important role in communication systems of insects (Singer 1998; Howard & Blomquist 2005). The chemical composition of the waxy layer is highly complex and is used in species, mate, kin, nest-mate or caste recognition. In addition, the cuticular pattern is not a steady feature, but can change in the course of an insect's life due to environmental or physiological factors; it is known to be affected by age (e.g. Wakonigg et al. 2000; Cuvillier-Hot et al. 2001), ovarian activity (Dillwith et al. 1983), nutritional condition (Liang & Silverman 2000) or habitat (e.g. due to hydrocarbon uptake from substrate; Pan et al. 2006). In social insects like bees, wasps, ants and termites, the impact of extrinsic and intrinsic factors on the chemical composition of an individual's surface has received high attention. Here, the chemical signature may be affected by nesting material (Gamboa et al. 1986; Crosland 1989; Heinze et al. 1996), comb wax (Breed et al. 1995, 1998; D'Ettorre et al. 2006), contact to nest-mates (Smith & Breed 1995) or by acquirement of prey-derived hydrocarbons (Obin & Vandermeer 1988; Florane et al. 2004; Buczkowski et al. 2005) and informs thereby about colony membership. In addition, the cuticular profile appears to play a key role in regulating reproduction and worker policing (e.g. Bonavita-Cougourdan et al. 1991; Ayasse et al. 1995; Peeters et al. 1999; Cuvillier-Hot et al. 2001; De Biseau et al. 2004; Sledge et al. 2004; Hartmann et al. 2005). In non-social insects, however, the effects of diet and physiological condition on the chemical signature have received little attention and it is possible that the cuticular composition represents a communication channel for which importance may be far beyond what we thought. So far, most investigations on the cuticular compounds of non-social insects focused on species and sex specificity. Only few studies have paid attention to ontogenetic variations of the cuticular profile in non-social insects and even fewer have addressed their potential function in chemical communication. Some studies found differences between the chemical patterns of different development stages (Baker et al. 1979; De Renobales & Blomquist 1983; Bartelt et al. 1986), less examined the effect of age in mature adults (e.g. Tregenza et al. 2000). Perhaps the best-studied examples for variations in the cuticular composition which are linked to specific physiological conditions come from some dipteran species. In the eye gnat Hippelates collusor and the housefly Musca domestica, the onset of vitellogenesis parallels the synthesis of hydrocarbons that function as sex pheromones (Adams & Mulla 1968; Dillwith et al. 1983) and in Drosophila mated females in the presence of males produce 7-tricosene, a hydrocarbon which acts as an anti-aphrodisiac (Scott 1986; Scott & Jackson 1990).
In the biparental burying beetle Nicrophorus vespilloides, contact pheromones are involved in recognition and gender is communicated through cuticular compounds (Whitlow et al. 2002). Müller et al. (2003) found that a breeding burying beetle rearing a brood on a buried carcass is able to discriminate between its breeding partner and an intruding conspecific. The beetles do not recognize their individual partner, but their breeding status, i.e. the fact that they have been on a carcass caring for larvae; brood caring individuals of the opposite sex are generally accepted as nest-mates whereas non-breeding individuals are attacked. The experiments reported by Müller et al. (2003) strongly suggest that information about the breeding status is mediated by the profile of the beetle's cuticular lipids. In spite of these findings, nobody has demonstrated yet that the chemical pattern of a N. vespilloides beetle or of any other insect is influenced by the parental state.
Burying beetles exhibit elaborate parental care and raise their young on small vertebrate carcasses which serve as the sole food source for the developing larvae (Pukowski 1933). Usually, a pair of beetles cooperates in feeding and defending the brood. However, even after a carcass is buried, conspecific competitors sometimes take over the carcass and kill the residents' brood (Scott 1990; Trumbo 1990a,b). Residents thus require a mechanism that allows them to discriminate between their original mate and an intruder. The ability for individual recognition is not necessary to defend the larvae, as all beetles which are in parental state do not kill larvae (Müller & Eggert 1990). Moreover, recognition of reproductive status should be favoured owing to the occurrence of joint breeding where more than two beetles reproduce together on a carcass (Müller et al. 2007). Learning the chemical signature of more than one beetle is likely to be a more complicated mechanism than simply to discriminate breeding status.
Our present study was conducted primarily to investigate the role of cuticular lipids in the nest-mate recognition system of burying beetles, but the broader purpose was to demonstrate a cuticular plasticity in a non-social insect's life that reflects physiological conditions. We first identified the chemical substances present on the cuticle of male and female N. vespilloides and then assessed possible effects of the parental state by comparing the cuticular pattern of parental and non-parental beetles. In our laboratory procedures, non-breeding beetles are maintained on mealworms alone whereas parental beetles can feed on the carcass they bury for reproduction. To test for a possible effect of diet on the chemical profiles, we established an additional non-breeding group in which beetles were fed carrion for several days.
2. Material and methods
(a) Collection and maintenance of beetles
Experimental animals were F1 offspring of N. vespilloides beetles trapped in carrion-baited pitfall traps during July and August 2004 in a deciduous forest near Freiburg, Germany (48°00′ N, 07°51′ E). The breeding method applied allowed us to generate beetles free of nematodes and mites that are typically found on field-caught beetles (see Eggert et al. (1998) for detailed description of the breeding protocol). Consequently, we could avoid variation in chemical composition of surface extracts due to parasites. Before the beginning of the experiments, the beetles were maintained in small transparent plastic containers (10×10×7 cm) at 20°C on a 16 : 8 h light : dark cycle and were provided with decapitated mealworms twice a week. All experimental males and females used were sexually mature, aged between 20 and 50 days and of similar size.
(b) Chemical analyses
(i) Experimental units
Male and female burying beetles were randomly assigned with regard to age and body size to three different treatment groups: ‘parental’; ‘non-parental, vertebrate diet’; and ‘non-parental, insect diet’ (for each group and each sex, n=18). Pairs of beetles from the parental group (one male, one female) were placed into plastic containers filled with moist peat and provided with a 10 g mouse carcass. Once the carcass had been buried, the containers were kept in darkness and all following manipulations were done using dim red light. After 48 h, each pair was transferred to a new box along with its carcass. The previous containers, which contained the eggs were checked regularly for the presence of newly hatched larvae. Once we observed larvae, we placed 10 first-instar larvae on the carcass with the respective pair. This standardization required the occasional use of larvae that were not the caring pair's offspring, this is of no consequence to the present study because parents do not distinguish between own and foreign larvae when the larvae appear on the carcass at the same time (Müller & Eggert 1990). To standardize the parental beetles' breeding condition, pairs were left to care for the larvae for a constant period of time (18–26 h). For chemical analysis, we used only beetles that were present on the carcass after the standardized period of parental care. Beetles hiding in the peat were excluded from the analysis as it could not be ruled out that they were not involved in parental care. In a preliminary experiment, we tested whether beetles in such a standardized breeding condition were accepted as nest-mates by other parental beetles. In all cases, males (n=16) and females (n=16) which cared for larvae between 18 and 26 h were tolerated by parental beetles of the opposite sex, whereas non-breeding males (n=10) and females (n=10) were never accepted (for both sexes: Χ2=26.00, p<0.0001).
Beetles of the two non-parental groups were housed separately, each individual in a separate box, and, in contrast to the parental group, did not have access to a carcass suitable for reproduction. They received two different diets to further examine the possible nutritional effects on the cuticular profile: insect carrion (decapitated mealworms, Tenebrio molitor) or vertebrate carrion (small pieces of pinkie mice with a mass of less than 0.5 g). Both diets were provided ad libitum. The beetles from the non-parental, vertebrate diet were fed carrion for a period similar in duration to the parental beetles' stay on the carcass at the time of testing (approx. 4 days). We think that in their natural environment, burying beetles feed mostly on vertebrate carrion. Therefore, when we speak about the general composition of the cuticular profile of an N. vespilloides individual, we always refer to non-parental males and females on a vertebrate diet.
(ii) Sampling and analysis of cuticular compounds
The beetles were killed by placing them in a freezer at −27°C for 15 min and were then left to thaw at room temperature for 30 min. They were soaked individually in 3 ml n-pentane (more than 99%, Fluka, Switzerland) for 15 min on an oscillator. The extract obtained was transferred to a clean vial and reduced by evaporation using a stream of gaseous nitrogen until approximately 0.1 ml remained. To determine the absolute amount of compounds extracted, an internal standard (n-eicosane) was added, which is not detectable on the cuticle of N. vespilloides (Whitlow 2003). The extracts were analysed by GC or GC–MS (full description of chemical analysis are provided in the electronic supplementary material). The chromatograms were manually integrated and the peak areas were converted to percentages of the total hydrocarbon fraction.
(c) Statistical analysis
Statistical analyses were performed using SPSS v. 14.0 and Statistica v. 5. A discriminant analysis (DA) was performed to determine whether the six predefined groups of males and females on an insect diet and on a vertebrate carrion diet, and parental males and females could be discriminated on the basis of their chemical profiles. To evaluate the quality of the DA, Wilks' lambda and the percentage of correct assignments of individuals to the respective group were used. In addition, we report the percentage of correct classification in cross validation (leave-one-out cross validation). The squared Mahalanobis distances, which are a measure how far the clouds of points are apart from each other, were calculated between the groups. The structure coefficients (correlations between the discriminating variables and the discriminant functions) were used to assess the importance of individual compounds in discriminating the groups. To avoid limitations inherent to the analysis of compositional data, each peak area was transformed according to the formula of Aitchinson (1986) prior to DA
where Zi,j is the transformed area of peak i for beetle j; Yi,j is the area of the peak i for beetle j; and g(Yj) is the geometric mean of the areas of all peaks for beetle j. To apply the transformation formula also on profiles with non-detectable components, the constant 0.01 (one-tenth of smallest area measured) was added to the peak areas (Aitchinson 1986; Steiner et al. 2007). For multivariate statistics like PCA and DA, a sample size of at least three times the number of variables is recommended. From the 91 peaks that reoccurred regularly in the samples, we selected 33 for the statistical analysis of our total sample of 100 individuals. Most of the studies reduce the number of substances by excluding peaks with small relative amounts (e.g. Peeters et al. 1999; Liebig et al. 2000). However, also small peaks can carry essential information and thus we think that the relative amount of substances is not an appropriate selection criterion. Instead of selecting the largest peaks, we included the peaks with the highest variation between the groups. Our selection of variables prior to DA was based on the Kruskal–Wallis test statistic H (e.g. Zar 1984) which is a measure for the inhomogeneity of the different groups. H was calculated for each compound and subsequently the 33 substances which had the highest H-value and thus showed the highest intergroup variability were chosen and included in DA.
Differences between the treatment groups in the relative amount of polyenes and the absolute amounts of all chemical substances found on the surfaces were analysed using a one-way ANOVA with a post hoc sequential Bonferroni analysis (Holm 1979).
3. Results
(a) The general cuticular pattern of N. vespilloides
In total, 88 substances out of 91 regularly occurring peaks could be identified in the cuticular extracts from N. vespilloides beetles (table 1). Hydrocarbons were the major compounds in the pentane extracts (over 98%). Although chain length ranged from C18 to C31, C25 (43% of the cuticular profile) and C27 (27% of the cuticular profile) hydrocarbons dominated the cuticular pattern; substances with an even-numbered carbon chain made up only a small proportion of the total cuticular extract. The hydrocarbons were represented by linear alkanes (29%), olefins (35%) and methyl-branched alkanes (36%). Monoenes were the most abundant group of unsaturated hydrocarbons, but we also found dienes, trienes, tetraenes, pentaenes and even hexaenes. The fraction of methyl-branched alkanes consisted predominantly of mono- and dimethylalkanes; trimethylalkanes were only found in very small amounts. Only a small fraction of substances detected in the N. vespilloides extracts did not belong to the group of hydrocarbons. We were able to identify some short-chained aldehydes, a ketone and cholesterol. Cholesterol was also detected in high amounts in the oral and anal secretions, a fact which led us to conclude that its presence is most likely a contamination stemming from the beetles' secretions (methods for analysing oral and anal secretions are provided in the electronic supplementary material). In the secretions as well as in the surface extracts, we could additionally identify fatty acids like n-hexadecanoic and 9-octadecenoic acid. The quantitative variation of acids in the surface extracts was extremely high, most likely because some of the beetles tended to release a large amount of secretion as they were placed in the freezer while others released only negligible amounts. Therefore, we excluded fatty acids in any further analysis of surface extracts.
Table 1.
Relative contribution (%) of specific compounds separated by GC (mean relative peak area) to the cuticular extract of N. vespilloides individuals from different treatment groups. (MM, males fed mealworms; MF, females fed mealworms; CM, males fed vertebrate carrion; CF, females fed vertebrate carrion; PM, parental males; PF, parental females; RI, Retention Index. (Asterisk, substances which were used as variables in the DA.)
| peak number | retention index | compound | MM (n=17) | MF (n=18) | CM (n=15) | CF (n=18) | PM (n=17) | PF (n=15) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1798 | hexadecanal | 0.16 | 0.28 | 0.17 | 0.26 | 0.16 | 0.15 |
| 2 | 1800 | n-C18 | 0.20 | 0.26 | 0.16 | 0.19 | 0.15 | 0.12 |
| 3 | 1900 | n-C19 | 0.16 | 0.22 | 0.12 | 0.13 | 0.09 | 0.09 |
| 4 | 1971 | octadecenal | 0.06 | 0.08 | 0.05 | 0.06 | 0.30 | 0.05 |
| 5 | 2100 | n-C21 | 2.09 | 1.08 | 2.34 | 1.37 | 2.92 | 3.79 |
| 6 | 2151 | 5-MeC21 | 0.17 | 0.36 | 0.16 | 0.02 | 0.09 | 0.06 |
| 7 | 2174 | 3-MeC21 | 0.43 | 0.30 | 0.24 | 0.17 | 0.11 | 0.10 |
| 8 | 2200 | n-C22 | 0.30 | 0.25 | 0.31 | 0.27 | 0.56 | 0.64 |
| 9 | 2211 | 3,7-/3,9-diMeC21 | 0.55 | 0.32 | 0.45 | 0.49 | 0.09 | 0.08 |
| 10 | 2259 | 4-MeC22 | 0.09 | 0.16 | 0.09 | 0.11 | 0.07 | 0.06 |
| 11 | 2264 | 6,9-C23diene | 0.37 | 0.09 | 0.14 | 0.07 | 0.06 | 0.07 |
| 12 | 2274 | 9-C23ene | 0.31 | 0.09 | 0.25 | 0.10 | 0.22 | 0.25 |
| 13* | 2280 | 7-C23ene | 0.07 | 0.01 | 0.10 | 0.02 | 0.09 | 0.11 |
| 14 | 2288 | 2-heneicosanone | 0.08 | 0.12 | 0.09 | 0.11 | 0.09 | 0.08 |
| 15 | 2292 | 4,8-diMeC22 | 0.04 | 0.09 | 0.04 | 0.08 | 0.02 | 0.02 |
| 16 | 2300 | n-C23 | 7.63 | 6.43 | 8.50 | 8.47 | 12.57 | 12.14 |
| 17 | 2306 | unknown | 0.44 | 0.20 | 0.26 | 0.11 | 0.58 | 0.82 |
| 18 | 2336 | 11-MeC23 | 0.04 | 0.04 | 0.04 | 0.04 | 0.07 | 0.07 |
| 19 | 2343 | 7-MeC23 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 |
| 20 | 2351 | 5-MeC23 | 0.21 | 0.38 | 0.26 | 0.33 | 0.41 | 0.40 |
| 21* | 2364 | Δ6,9-C24diene | 0.03 | 0.02 | 0.04 | 0.03 | 0.09 | 0.12 |
| 22 | 2374 | 3-MeC23 | 1.22 | 1.13 | 1.56 | 1.39 | 2.66 | 2.52 |
| 23 | 2385 | 5,9-diMeC23 | 0.08 | 0.12 | 0.08 | 0.14 | 0.20 | 0.17 |
| 24 | 2400 | n-C24 | 0.74 | 0.60 | 0.77 | 0.56 | 0.88 | 0.61 |
| 25 | 2411 | 3,7-/3,9-diMeC23 | 0.51 | 0.77 | 0.73 | 0.79 | 1.90 | 1.26 |
| 26* | 2420 | 6,9,12,15 C25tetraene | 0.02 | 0.03 | 0.05 | 0.04 | 0.70 | 0.94 |
| 27* | 2425 | 3,6,9,12,15 C25pentaene | 0.01 | 0.01 | 0.01 | 0.01 | 0.17 | 0.29 |
| 28* | 2430 | C25triene | — | — | — | — | 0.02 | 0.03 |
| 29* | 2436 | 10-/11-/12-MeC24+C25triene | 0.03 | 0.03 | 0.03 | 0.03 | 0.11 | 0.12 |
| 30* | 2441 | 8-MeC24+C25triene | 0.04 | 0.07 | 0.08 | 0.07 | 0.23 | 0.27 |
| 31 | 2446 | 6-MeC24+C25diene | 0.04 | 0.09 | 0.05 | 0.10 | 0.09 | 0.13 |
| 32 | 2459 | 4-MeC24 | 0.45 | 0.55 | 0.66 | 0.63 | 0.57 | 0.38 |
| 33* | 2464 | 6,9-C25diene | 1.88 | 1.37 | 1.75 | 1.56 | 4.03 | 4.71 |
| 34* | 2474 | 9-C25ene | 9.13 | 4.80 | 11.13 | 7.47 | 17.04 | 19.99 |
| 35* | 2480 | 7-C25ene | 2.09 | 0.54 | 4.71 | 1.57 | 2.45 | 2.99 |
| 36 | 2491 | 4,8-/4,10-/4,12-diMeC24 | 0.23 | 0.37 | 0.31 | 0.40 | 0.43 | 0.33 |
| 37 | 2500 | n-C25 | 14.61 | 11.96 | 14.29 | 11.62 | 12.01 | 7.06 |
| 38 | 2506 | unknown | 1.10 | 1.05 | 0.57 | 0.56 | 0.58 | 0.72 |
| 39 | 2534 | 11/13-diMeC25 | 0.47 | 0.40 | 0.35 | 0.32 | 0.43 | 0.44 |
| 40 | 2537 | 9-MeC25 | 0.39 | 0.39 | 0.54 | 0.54 | 0.55 | 0.54 |
| 41 | 2543 | 7-MeC25 | 0.24 | 0.25 | 0.27 | 0.30 | 0.38 | 0.35 |
| 42 | 2551 | 5-MeC25 | 0.54 | 0.63 | 0.54 | 0.67 | 0.76 | 0.68 |
| 43 | 2565 | 11/15-diMeC25 | 0.19 | 0.19 | 0.17 | 0.18 | 0.32 | 0.29 |
| 44 | 2574 | 3-MeC25 | 6.03 | 5.92 | 6.52 | 6.80 | 5.10 | 4.85 |
| 45 | 2584 | 5,9-/5,11-diMeC25 | 0.41 | 0.35 | 0.48 | 0.59 | 0.36 | 0.37 |
| 46 | 2600 | n-C26 | 0.35 | 0.35 | 0.31 | 0.29 | 0.26 | 0.20 |
| 47* | 2605 | C27hexaene | — | — | tr | tr | 0.28 | 0.50 |
| 48 | 2611 | 3,7-/3,9-diMeC25 | 4.78 | 7.09 | 5.92 | 7.99 | 5.75 | 4.31 |
| 49* | 2620 | 6,9,12,15-C27tetraene | 0.18 | 0.19 | 1.32 | 1.75 | 3.01 | 4.36 |
| 50* | 2625 | 3,6,9,12,15-C27pentaene | 0.12 | 0.17 | 0.29 | 0.41 | 0.95 | 1.43 |
| 51 | 2636 | 10-MeC26+C27triene | 0.24 | 0.30 | 0.25 | 0.27 | 0.16 | 0.32 |
| 52* | 2641 | 8,12-diMeC26+C27triene | 0.29 | 0.42 | 0.39 | 0.63 | 0.74 | 1.04 |
| 53 | 2646 | 6-MeC26+C27diene | 0.06 | 0.16 | 0.15 | 0.19 | 0.12 | 0.23 |
| 54* | 2659 | 4-MeC26 | 0.23 | 0.44 | 0.24 | 0.39 | 0.10 | 0.12 |
| 55* | 2664 | 6,9-C27diene | 3.78 | 4.44 | 2.75 | 4.03 | 1.27 | 1.80 |
| 56 | 2674 | 9-C27ene | 13.75 | 14.57 | 10.73 | 11.66 | 5.27 | 6.49 |
| 57 | 2680 | 7-C27ene | 0.42 | 0.37 | 0.76 | 0.78 | 0.19 | 0.23 |
| 58* | 2691 | 4,8 -/4,10-diMeC26 | 0.32 | 0.54 | 0.41 | 0.64 | 0.16 | 0.16 |
| 59 | 2700 | n-C27 | 2.09 | 2.05 | 1.71 | 1.70 | 1.13 | 0.70 |
| 60 | 2706 | unknown | 0.81 | 1.33 | 0.28 | 0.42 | 0.10 | 0.12 |
| 61* | 2734 | 11-/13-MeC27 | 0.81 | 0.76 | 0.52 | 0.58 | 0.20 | 0.22 |
| 62 | 2737 | 9-MeC27 | 0.32 | 0.24 | 0.25 | 0.26 | 0.13 | 0.16 |
| 63 | 2743 | 7-MeC27 | 0.75 | 0.93 | 0.46 | 0.58 | 0.32 | 0.28 |
| 64* | 2751 | 5-MeC27 | 0.16 | 0.26 | 0.10 | 0.13 | 0.04 | 0.06 |
| 65 | 2765 | 11,15-diMeC27 | 0.24 | 0.24 | 0.18 | 0.19 | 0.18 | 0.18 |
| 66* | 2774 | 3-MeC27 | 1.36 | 1.47 | 1.14 | 1.21 | 0.73 | 0.54 |
| 67* | 2784 | 5,9-diMeC27 | 0.14 | 0.22 | 0.13 | 0.18 | 0.07 | 0.08 |
| 68* | 2800 | n-C28 | 0.16 | 0.24 | 0.15 | 0.17 | 0.12 | 0.08 |
| 69* | 2805 | C29-hexaene | — | — | 0.10 | 0.12 | 0.12 | 0.26 |
| 70* | 2811 | 3,9-diMeC27 | 3.06 | 4.82 | 2.97 | 4.71 | 1.35 | 1.31 |
| 71* | 2820 | 6,9,12,15-C29tetraene | 0.18 | 0.26 | 0.66 | 1.22 | 0.16 | 0.33 |
| 72* | 2825 | 3,6,9,12,15-C29pentaene | 0.05 | 0.10 | 0.27 | 0.50 | 0.05 | 0.14 |
| 73 | 2840 | 3,7,11-triMeC27 | 0.40 | 0.59 | 0.32 | 0.60 | 0.19 | 0.24 |
| 74 | 2864 | 6,9-C29diene | 0.73 | 1.60 | 0.53 | 0.80 | 0.10 | 0.14 |
| 75 | 2874 | 9-C29ene | 0.89 | 1.30 | 0.52 | 0.65 | 0.22 | 0.27 |
| 76 | 2880 | 7-C29ene | 0.07 | 0.13 | 0.11 | 0.20 | 0.02 | 0.02 |
| 77 | 2891 | 4,8-/4,10-diMeC28 | 0.16 | 0.38 | 0.23 | 0.31 | 0.36 | 0.27 |
| 78 | 2900 | n-C29 | 1.62 | 1.69 | 1.56 | 1.65 | 1.10 | 0.69 |
| 79* | 2934 | 11-/13-/15-MeC29 | 1.03 | 1.14 | 0.31 | 0.44 | 0.10 | 0.12 |
| 80* | 2937 | 9-MeC29 | 0.23 | 0.28 | 0.10 | 0.14 | 0.07 | 0.08 |
| 81* | 2943 | 7-MeC29 | 0.40 | 0.52 | 0.18 | 0.24 | 0.05 | 0.06 |
| 82 | 2965 | 11,15-diMeC29 | 0.92 | 1.03 | 0.41 | 0.60 | 0.18 | 0.19 |
| 83 | 2968 | 9,17-/9,19-diMeC29 | 0.45 | 0.71 | 0.27 | 0.40 | 0.10 | 0.10 |
| 84* | 2975 | 7,17-diMeC29 | 0.70 | 0.99 | 0.32 | 0.47 | 0.25 | 0.14 |
| 85 | 2984 | 5,9-diMeC29 | 0.11 | 0.20 | 0.10 | 0.12 | 0.05 | 0.03 |
| 86 | 2990 | 11,15,19-triMeC29 | 0.15 | 0.26 | 0.08 | 0.13 | 0.07 | 0.05 |
| 87 | 3000 | n-C30 | 0.07 | 0.18 | 0.06 | 0.10 | 0.10 | 0.04 |
| 88 | 3011 | 3,9-diMeC29 | 0.59 | 0.95 | 0.55 | 0.85 | 0.49 | 0.36 |
| 89 | 3098 | cholest-5-en-3-ol | 0.30 | 0.54 | 1.51 | 1.19 | 2.42 | 1.88 |
| 90* | 3134 | 11-/13-MeC31 | 1.12 | 1.23 | 0.38 | 0.45 | 0.15 | 0.16 |
| 91* | 3165 | 11,19-diMeC31 | 1.54 | 1.91 | 0.54 | 0.88 | 0.28 | 0.26 |
(b) The effect of gender, nutritional condition and breeding status on the cuticular pattern
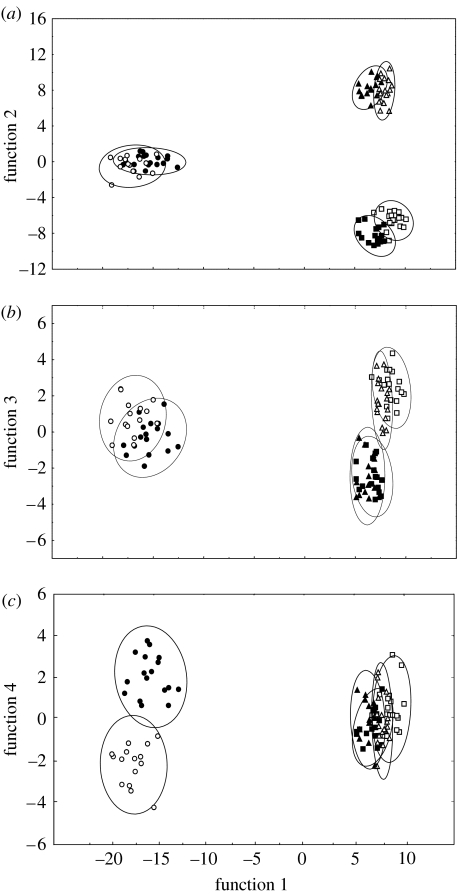
The DA performed on 33 compounds (marked with an asterisk in table 1) significantly differentiated the chemical profiles of N. vespilloides beetles of different sexes and different nutritional and reproductive status (Wilk's λ<0.0001, Χ2=920.90, p<0.0001). Four discriminant functions add significantly to the discrimination between groups (figure 1). The model correctly assigned 99% of the beetles to their predefined group, 85% were correctly classified in cross validation. All misclassification occurred between males and females of the same treatment group: in both non-parental groups as well as in the parental one, beetles were assigned to the wrong sex. Function 1, which accounted for 72.6% of the total variance, clearly separated parental from non-parental beetles, whereas function 2, explaining 24.0% of the variance, discriminated most clearly between non-parental individuals on different diets (figure 1a). The sexes of non-parental beetles were separated by function 3 (figure 1b) where the males fall below the centre line whereas the females tend to gather above; for the parental males and females, function 4 (figure 1c) apparently produced a better separation. The squared Mahalanobis distances between the group centroids, which were statistically significant for all distances (p<0.001), were lowest between males and females from the same treatment groups (table 2 in the electronic supplementary material), indicating that chemical profiles were similar and highest between parental beetles and non-parental ones, indicating the greatest difference in chemical profiles. It is also worthwhile to point out that the group of beetles that had been fed vertebrate carrion for 4 days appeared to be slightly closer to the parental beetles than were beetles on a strict insect diet (table 2 in the electronic supplementary material).
Figure 1.
Discriminant analysis (DA) of 100 N. vespilloides males and females in different nutritional and reproductive conditions on the basis of cuticular compounds. Scatter plots of function 1 versus (a) function 2, (b) function 3 and (c) function 4 are presented. Envelopes represent the 95% confidence ellipses. (filled squares, males fed mealworms; open squares, females fed mealworms; filled triangles, males fed vertebrate carrion; open triangles, females fed vertebrate carrion; filled circles, parental males; open circles, parental females).
(i) Breeding status
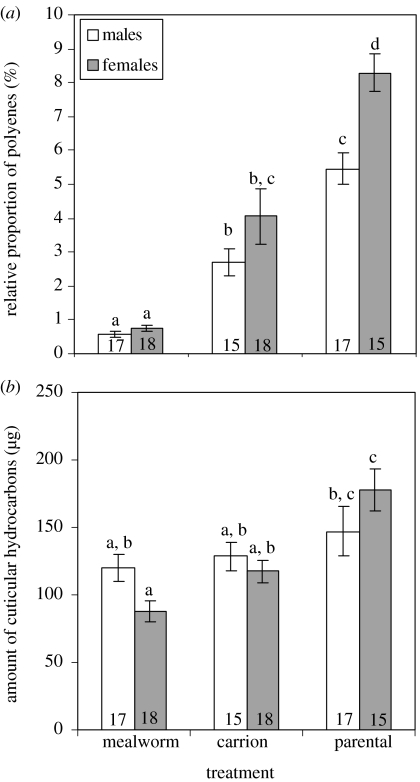
The DA revealed a very good discrimination according to the beetles' breeding status (function 1, figure 1a) and polyenes appear to play an important role in differentiation. Brood-caring individuals are characterized by the occurrence of the C25triene (peak number 28, table 1) and the C27hexaene (47), substances that were found in mere trace quantities or were altogether missing in non-breeding beetles (table 1). Parental beetles had also higher amounts of Δ6,9,12,15-C25tetraene (26), Δ3,6,9,12,15-C25pentaene (27) and C29hexaene (69) than did beetles not caring for larvae. In general, focusing on the total fraction of hydrocarbons with more than two double bonds, it becomes apparent that parental beetles had a significantly higher proportion of this fraction than both non-parental groups (ANOVA, post hoc Bonferroni, males: parental/mealworms, p<0.001, parental/vertebrate carrion, p<0.01; females: parental/mealworms, p<0.001, parental/vertebrate carrion, p<0.001; figure 2a). The beetles provided with a vertebrate carrion diet held an intermediate position: although they are characterized by a smaller proportion of these polyenes than parental beetles, they still had a significantly higher amounts of these compounds when compared with individuals fed mealworms (post hoc Bonferroni, males: p<0.01; females: p<0.001). It should also be mentioned that within the parental group, sex-specific differences in the relative amount of polyenes could be detected (post hoc Bonferroni, p<0.01). Unsaturated hydrocarbons with at least three double bonds could be found in a higher proportion in breeding females (figure 2a). However, breeding status affected not only the relative but also the absolute amount of cuticular hydrocarbons found in females (figure 2b). An increased total amount of substances was found in the pentane extract of parental females compared with non-parental ones (ANOVA post hoc Bonferroni, parental/mealworms p<0.001 parental/vertebrate carrion p<0.01). This finding could be confirmed by the method of solid-phase microextraction (Berlardi & Pawliszyn 1989; Arthur et al. 1992; Malosse et al. 1995; Monnin et al. 1998) which revealed that the uptake of chemicals from the surface of the same female was always higher when the female was caring for larvae (S. Steiger 2005, unpublished data). Although no such significant increase of substances could be found in pentane-extracted males, the absolute amounts of chemicals did not differ between parental males and females (post hoc Bonferroni, p=0.329).
Figure 2.
Mean ±s.e. (a) of relative amount of polyenes with more than two double bonds and (b) absolute amount of all hydrocarbons found in the extract of N. vespilloides males and females in different nutritional and reproductive condition. Sample sizes are shown at the base of the bar graphs. Different letters above the bars indicate statistically significant differences.
(ii) Nutrition
Diet significantly affected the composition of the cuticle volatiles (function 2, figure 1a). The substance which contributed most importantly to the separation of individuals on different diets was C29hexaene (69) which was found in beetles on a vertebrate carrion diet, but could not be detected in beetles fed with mealworms. A vertebrate carrion diet also had a positive effect on the proportion of other polyenes like Δ6,9,12,15-C27tetraene (49), Δ6,9,12,15-C29tetraene (71) and Δ3,6,9,12,15-C29pentaene (72).
(iii) Gender
The two substances which contributed mostly to the differentiation of the sexes of non-breeding individuals were 7-C23en (13) and 7-C25en (35). Males had a higher proportion of the monounsaturated alkenes than females. In contrast, females are characterized by higher amounts of some methyl-branched alkanes like 4-MeC26 (54), 3,9-diMeC27 (70) and 4,8-/4,10-diMeC26 (58). Interestingly, the difference of the cuticular pattern between the two sexes was profoundly affected by an individual's breeding status (function 3 and 4, figure 1b,c) to the point that parental males and females could hardly be differentiated based on the alkenes and methyl-branched alkanes that were responsible for discrimination of the sexes of non-breeding beetles. The amounts of these hydrocarbons found in parental males and females were very similar (table 1). Examination of the components contributing to the differentiation of brood-caring males and females revealed that C29hexaene (69) and Δ3,6,9,12,15-C29pentaene (72) were important in discriminating the sexes; parental females were characterized by higher proportions of the two polyenes. Nevertheless, the squared Mahalanobis distance between the breeding sexes was smaller than all other distances measured (table 2 in the electronic supplementary material).
4. Discussion
Our chemical analyses revealed that the cuticular pattern of N. vespilloides is highly complex and influenced by at least three factors: the individual's gender, immediate nutritional history and parental state. The two latter features imply that the chemical composition is a plastic trait which changes with the beetle's physiological condition. Several investigations have previously demonstrated such plasticity of the cuticular pattern, but never before has the composition of chemicals on the cuticle been found to be contingent on the individual's breeding status. In female eusocial insects, a relationship between the ovarian activity and the production of cuticular hydrocarbons has already been shown (ants: Monnin et al. 1998, Peeters et al. 1999, Liebig et al. 2000, Cuvillier-Hot et al. 2001; bees: Ayasse et al. 1995; wasps: Sledge et al. 2004), but in the case of N. vespilloides the waxy layer of males undergoes changes similar to that of females when breeding. Although this means that vitellogenesis is not required for the altered hydrocarbon pattern, hormones are the prime candidates for the regulation of cuticular composition in these beetles as well as in social insects. Juvenile hormone (JH) is typically considered as a gonadotrophic hormone with a role in the regulation of ovarian development and its concentration is frequently elevated during vitellogenesis (Koeppe et al. 1985). In some insects, it was also demonstrated that JH is responsible for regulating the biosynthesis of volatile as well as contact pheromones (see Tillman et al. (1999) for review). This is an interesting aspect as the JH titres in both male and female burying beetles rise rapidly after the discovery of a carcass and again after the arrival of larvae (Scott et al. 2001). JH may play a role as a dual-function behavioural pacemaker (Robinson & Vargo 1997) mediating the transition from infanticidal to parental behaviour as well as the changes in the cuticular profile. Such a dual function of JH would also ensure the reliability of the cuticular indicators of breeding status.
Our analysis revealed unambiguous differences between parental and non-breeding individuals and several long chain polyunsaturated hydrocarbons were responsible for the clear separation. While long chain alkenes and alkadienes have been reported as major or secondary sex pheromone components for many insect species (e.g. Antony & Jallon 1982; Peschke & Metzler 1987; Syvertsen et al. 1995; Krokos et al. 2001; Ginzel et al. 2003), long chain polyunsaturated hydrocarbons with more than two double bonds are not as widespread. Trienes and tetraenes with chain lengths from 17 to 23 carbons have been found as sex pheromone components in some Lepidoptera (Millar 2000). However, the finding of hydrocarbons with five and even six double bonds and a chain length between 25 and 29 carbons is very rare (e.g. Leal et al. 2005; Millar et al. 2005).
Polyene hydrocarbons have been found to be synthesized de novo from acetate as is usually the case for alkanes, but there are also many examples for a different synthetic pathway in which olefins are products of diet-derived unsaturated fatty acids (Howard 1982; Lockey 1988; Howard & Blomquist 2005). Our results indicate that also in N. vespilloides diet-derived polyunsaturated fatty acids may play a role as precursors for the olefins found. However, a vertebrate carrion diet may be the basis for the forming of the olefins, but it is not sufficient to explain the changes in the hydrocarbon pattern of breeding burying beetles. In our study, we could still find a clear difference between the chemical profile of parental individuals and non-parental beetles fed with vertebrate carrion. It is possible that a surge in JH concentration is required for the production of critical substances from the carrion-derived precursors. The alternative explanation, that the differences found are only contamination from external resources and hydrocarbons are acquired through contact with larvae or carcass, can be excluded by an additional experiment we have conducted (the details about methods and results of this experiment are provided in the electronic supplementary material).
An interesting aspect of our results is the finding that the sex differences that were typical of non-breeding beetles almost disappeared in the parental specimens. One possible explanation may be an exchange of chemicals when beetles are housed together (Harris & Moore 2005; Ivy et al. 2005). However, when males and females were kept together without a carcass, no changes in the sex-specific pattern could be observed (S. Steiger 2005, unpublished results). Another possible explanation may be associated with the recognition mechanism involved which could be based on self-reference. Breeding beetles only accept counterparts if they are in the same state as themselves (Müller et al. 2003) and the underlying mechanism may be a comparison of the own cuticular pattern with that of the encountered beetle. If any difference in the profile caused aggression, we would assume that the cuticular pattern of breeding males and females should be very similar, so that rejection of the own breeding partner is a rare event. The results that the sex differences in the chemical signature disappear once the beetles care for larvae accords with the finding that in the half of the cases, tested parental beetles were not aggressive against breeding individuals of the same sex when encountering them (S. Steiger 2005, unpublished data). Though the self-referent hypothesis sounds feasible, the mechanism seems to be more complicated than stated. While the sex differences found in the profiles of non-breeding beetles vanish when the beetles get in a parental state, different sex differences appeared. Breeding females were characterized by a profile which contains more polyenes than males. If the self-reference hypothesis was as simple as suggested, females should always reject their breeding partner. Nevertheless, it is probable that the selection pressure for the recognition process is different for breeding males and females. Trumbo (2006) has demonstrated that male burying beetles are task specialists, having both a greater tendency as well as a greater ability to guard the brood than females. This may involve parental males inspecting the cuticular pattern of conspecifics more closely or rather that they have a higher acceptance threshold than females (see Sherman et al. (1997) for theory) which could lead to the fact that even a small amount of missing chemicals on the surface of the encountered beetle evokes aggressive behaviour in males. If the amount of polyenes reflects the parental state, breeding females should always produce more of them than males to assure not being rejected by their own partner. To summarize, the self-reference hypothesis can still apply, but with the modification that the acceptance threshold are different for breeding males and females.
Burying beetles are able to distinguish between their breeding partner and a conspecific intruder based on the individual's breeding status. Here, we have demonstrated that the cuticular pattern of hydrocarbons changes with breeding status and thus provides reliable information about a beetle's physiological state. The amount or proportion of one or several unsaturated hydrocarbons may be the cue used in this recognition system, but without specific bioassays we cannot attribute communicative functions to any of the compounds. Overall, our study supports the idea of a variable cuticular profile which may reliably reflect a physiological state or nutritional condition. Moreover, the fact that the waxy layer of one and the same beetle encodes information about species, age (both, Whitlow 2003), gender, nutritional condition and breeding state is striking and emphasizes the potential importance of the cuticular pattern in communication systems. A cuticular profile which is influenced by the quantity and quality of nutrition, for example, might be used as honest signals in mate choice (cp. Fisher & Rosenthal 2006). We hope that our study encourages further studies into the variability of cuticular patterns and its implications for communication in insects.
Acknowledgments
We thank Sonia Whitlow for the transmission of data and information, Sven Geiselhardt and Thomas Schmitt for their assistance concerning chemical analyses and Marc Spelleken for technical assistance. Furthermore, we would like to thank Anne-Katrin Eggert, Patrizia D'Ettorre and Allen J. Moore for their helpful comments on the manuscript. The study was supported by a PhD grant from the German National Academic Foundation to S.S.
Supplementary Material
Supplementary methods and results
References
- Adams T.S, Mulla M.S. Ovarian development, pheromone production, and mating in eye gnat Hippelates collusor. J. Insect Physiol. 1968;14:627–635. doi:10.1016/0022-1910(68)90223-0 [Google Scholar]
- Aitchinson J. Chapmann and Hall; London, UK: 1986. The statistical analysis of compositional data: monographs in statistics and applied probability. [Google Scholar]
- Antony C, Jallon J.M. The chemical basis for sex recognition in Drosophila melanogaster. J. Insect Physiol. 1982;28:873–880. doi:10.1016/0022-1910(82)90101-9 [Google Scholar]
- Arthur C.L, Potter D.W, Buchholz K.D, Motlagh S, Pawliszyn J. Solid-phase microextraction for the direct analysis of water—theory and practice. Lc Gc-Mag. Separat. Sci. 1992;10:656. [Google Scholar]
- Ayasse M, Marlovits T, Tengo J, Taghizadeh T, Francke W. Are there pheromonal dominance signals in the bumblebee Bombus hypnorum L (Hymenoptera Apidae) Apidologie. 1995;26:163–180. [Google Scholar]
- Baker J.E, Sukkestad D.R, Nelson D.R, Fatland C.L. Cuticular lipids of larvae and adults of the cigarette beetle, Lasioderma serricorne. Insect Biochem. 1979;9:603–611. doi:10.1016/0020-1790(79)90099-4 [Google Scholar]
- Bartelt R.J, Armold M.T, Schaner A.M, Jackson L.L. Comparative analysis of cuticular hydrocarbons in the Drosophila virilis species group. Comp. Biochem. Physiol. B. 1986;83:731–742. doi:10.1016/0305-0491(86)90138-0 [Google Scholar]
- Berlardi R, Pawliszyn J. The application of chemically modified fused silicia fibers in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Pollut. Res. J. Can. 1989;24:179. [Google Scholar]
- Bonavita-Cougourdan A, Theraulaz G, Bagneres A.G, Roux M, Pratte M, Provost E, Clement J.L. Cuticular hydrocarbons, social organization and ovarian development in a polistine wasp—Polistes dominulus Christ. Comp. Biochem. Physiol. B. 1991;100:667–680. doi:10.1016/0305-0491(91)90272-F [Google Scholar]
- Breed M.D, Garry M.F, Pearce A.N, Hibbard B.E, Bjostad L.B, Page R.E. The role of wax comb in honey-bee nestmate recognition. Anim. Behav. 1995;50:489–496. doi:10.1006/anbe.1995.0263 [Google Scholar]
- Breed M.D, Leger E.A, Pearce A.N, Wang Y.J. Comb wax effects on the ontogeny of honey bee nestmate recognition. Anim. Behav. 1998;55:13–20. doi: 10.1006/anbe.1997.0581. doi:10.1006/anbe.1997.0581 [DOI] [PubMed] [Google Scholar]
- Buczkowski G, Kumar R, Suib S.L, Silverman J. Diet-related modification of cuticular hydrocarbon profiles of the Argentine ant, Linepithema humile, diminishes intercolony aggression. J. Chem. Ecol. 2005;31:829–843. doi: 10.1007/s10886-005-3547-7. doi:10.1007/s10886-005-3547-7 [DOI] [PubMed] [Google Scholar]
- Crosland M.W.J. Kin recognition in the ant Rhytidoponera confusa.1. Environmental odor. Anim. Behav. 1989;37:912–919. doi:10.1016/0003-3472(89)90135-8 [Google Scholar]
- Cuvillier-Hot V, Cobb M, Malosse C, Peeters C. Sex, age and ovarian activity affect cuticular hydrocarbons in Diacamma ceylonense, a queenless ant. J. Insect Physiol. 2001;47:485–493. doi: 10.1016/s0022-1910(00)00137-2. doi:10.1016/S0022-1910(00)00137-2 [DOI] [PubMed] [Google Scholar]
- D'Ettorre P, Wenseleers T, Dawson J, Hutchinson S, Boswell T, Ratnieks F.L.W. Wax combs mediate nestmate recognition by guard honeybees. Anim. Behav. 2006;71:773–779. doi:10.1016/j.anbehav.2005.05.014 [Google Scholar]
- De Biseau J.C, Passera L, Daloze D, Aron S. Ovarian activity correlates with extreme changes in cuticular hydrocarbon profile in the highly polygynous ant, Linepithema humile. J. Insect Physiol. 2004;50:585–593. doi: 10.1016/j.jinsphys.2004.04.005. doi:10.1016/j.jinsphys.2004.04.005 [DOI] [PubMed] [Google Scholar]
- De Renobales M, Blomquist G.J. A developmental-study of the composition and biosynthesis of the cuticular hydrocarbons of Trichoplusia ni (Lepidoptera Noctuidae) Insect Biochem. 1983;13:493–502. doi:10.1016/0020-1790(83)90007-0 [Google Scholar]
- Dillwith J.W, Adams T.S, Blomquist G.J. Correlation of housefly sex-pheromone production with ovarian development. J. Insect Physiol. 1983;29:377–386. doi:10.1016/0022-1910(83)90064-1 [Google Scholar]
- Eggert A.-K, Reinking M, Müller J.K. Parental care improves offspring survival and growth in burying beetles. Anim. Behav. 1998;55:97–107. doi: 10.1006/anbe.1997.0588. doi:10.1006/anbe.1997.0588 [DOI] [PubMed] [Google Scholar]
- Fisher H.S, Rosenthal G.G. Female swordtail fish use chemical cues to select well-fed mates. Anim. Behav. 2006;72:721–725. doi:10.1016/j.anbehav.2006.02.009 [Google Scholar]
- Florane C.B, Bland J.M, Husseneder C, Raina A.K. Diet-mediated inter-colonial aggression in the Formosan subterranean termite Coptotermes formosanus. J. Chem. Ecol. 2004;30:2559–2574. doi: 10.1007/s10886-004-7950-2. doi:10.1007/s10886-004-7950-2 [DOI] [PubMed] [Google Scholar]
- Gamboa G.J, Reeve H.K, Ferguson I.D, Wacker T.L. Nestmate recognition in social wasps—the origin and acquisition of recognition odors. Anim. Behav. 1986;34:685–695. doi:10.1016/S0003-3472(86)80053-7 [Google Scholar]
- Ginzel M.D, Millar J.G, Hanks L.M. (Z)-9-Pentacosene—contact sex pheromone of the locust borer, Megacyllene robiniae. Chemoecology. 2003;13:135–141. doi:10.1007/s00049-003-0239-z [Google Scholar]
- Harris W.E, Moore P.J. Female mate preference and sexual conflict: females prefer males that have had fewer consorts. Am. Nat. 2005;165:S64–S71. doi: 10.1086/429352. doi:10.1086/429352 [DOI] [PubMed] [Google Scholar]
- Hartmann A, D'Ettorre P, Jones G.R, Heinze J. Fertility signaling—the proximate mechanism of worker policing in a clonal ant. Naturwissenschaften. 2005;92:282–286. doi: 10.1007/s00114-005-0625-1. doi:10.1007/s00114-005-0625-1 [DOI] [PubMed] [Google Scholar]
- Heinze J, Foitzik S, Hippert A, Holldobler B. Apparent dear–enemy phenomenon and environment-based recognition cues in the ant Leptothorax nylanderi. Ethology. 1996;102:510–522. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Howard R.W. Chemical ecology and biochemistry of insect hydrocarbons. Annu. Rev. Entomol. 1982;27:149–172. doi:10.1146/annurev.en.27.010182.001053 [Google Scholar]
- Howard R.W, Blomquist G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005;50:371–393. doi: 10.1146/annurev.ento.50.071803.130359. doi:10.1146/annurev.ento.50.071803.130359 [DOI] [PubMed] [Google Scholar]
- Ivy T.M, Weddle C.B, Sakaluk S.K. Females use self-referent cues to avoid mating with previous mates. Proc. R. Soc. B. 2005;272:2475–2478. doi: 10.1098/rspb.2005.3222. doi:10.1098/rspb.2005.3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.L, Blomquist G.J. Insect waxes. In: Kolattukudy P.E, editor. Chemistry and biochemistry of natural waxes. 1976. Elsevier; Amsterdam, The Netherlands: 1976. pp. 201–203. [Google Scholar]
- Koeppe J, Fuchs M, Chen T, Hunt L, Kovalick G, Briers T. The role of juvenile hormone in reproduction. In: Kerkut G, Gilbert L, editors. Comprehensive insect physiology, biochemistry and pharmacology. vol. 8. Pergamon; Oxford, UK: 1985. pp. 165–204. [Google Scholar]
- Krokos F.D, Konstantopoulou M.A, Mazomenos B.E. Alkadienes and alkenes, sex pheromone components of the almond seed wasp Eurytoma amygdali. J. Chem. Ecol. 2001;27:2169–2181. doi: 10.1023/a:1012218618218. doi:10.1023/A:1012218618218 [DOI] [PubMed] [Google Scholar]
- Leal W.S, Parra-Pedrazzoli A.L, Kaissling K.E, Morgan T.I, Zalom F.G, Pesak D.J, Dundulis E.A, Burks C.S, Higbee B.S. Unusual pheromone chemistry in the navel orangeworm: novel sex attractants and a behavioral antagonist. Naturwissenschaften. 2005;92:139–146. doi: 10.1007/s00114-004-0598-5. doi:10.1007/s00114-004-0598-5 [DOI] [PubMed] [Google Scholar]
- Liang D, Silverman J. You are what you eat: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften. 2000;87:412–416. doi: 10.1007/s001140050752. doi:10.1007/s001140050752 [DOI] [PubMed] [Google Scholar]
- Liebig J, Peeters C, Oldham N.J, Markstadter C, Hölldobler B. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc. Natl Acad. Sci. USA. 2000;97:4124–4131. doi: 10.1073/pnas.97.8.4124. doi:10.1073/pnas.97.8.4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockey K.H. Lipids of the insect cuticle—origin, composition and function. Comp. Biochem. Physiol. B. 1988;89:595–645. doi:10.1016/0305-0491(88)90305-7 [Google Scholar]
- Malosse C, Ramirezlucas P, Rochat D, Morin J.P. Solid-phase microextraction, an alternative method for the study of airborne insect pheromones (Metamasius hemipterus, Coleoptera, Curculionidae) Hrc-J. High Res. Chromatogr. 1995;18:669–670. doi:10.1002/jhrc.1240181013 [Google Scholar]
- Millar J.G. Polyene hydrocarbons and epoxides: a second major class of lepidopteran sex attractant pheromones. Annu. Rev. Entomol. 2000;45:575–604. doi: 10.1146/annurev.ento.45.1.575. doi:10.1146/annurev.ento.45.1.575 [DOI] [PubMed] [Google Scholar]
- Millar J.G, Grant G.G, McElfresh J.S, Strong W, Rudolph C, Stein J.D, Moreira J.A. (3Z,6Z,9Z,12Z,15Z)-pentacosapentaene, a key pheromone component of the fir coneworm moth, Dioryaria abietivorella. J. Chem. Ecol. 2005;31:1229–1234. doi: 10.1007/s10886-005-5813-0. doi:10.1007/s10886-005-5813-0 [DOI] [PubMed] [Google Scholar]
- Monnin T, Malosse C, Peeters C. Solid-phase microextraction and cuticular hydrocarbon differences related to reproductive activity in queenless ant Dinoponera quadriceps. J. Chem. Ecol. 1998;24:473–490. doi:10.1023/A:1022360718870 [Google Scholar]
- Müller J.K, Eggert A.K. Time-dependent shifts between infanticidal and parental behavior in female burying beetles a mechanism of indirect mother–offspring recognition. Behav. Ecol. Sociobiol. 1990;27:11–16. doi:10.1007/BF00183307 [Google Scholar]
- Müller J.K, Eggert A.-K, Elsner T. Nestmate recognition in burying beetles: the ‘breeder's badge’ as a cue used by females to distinguish their mates from male intruders. Behav. Ecol. 2003;14:212–220. doi:10.1093/beheco/14.2.212 [Google Scholar]
- Müller J.K, Braunisch V, Hwang W.B, Eggert A.K. Alternative tactics and individual reproductive success in natural associations of the burying beetle, Nicrophorus vespilloides. Behav. Ecol. 2007;18:196–203. doi:10.1093/beheco/arl073 [Google Scholar]
- Neville A.C. Springer; Berlin, Germany: 1975. Biology of the arthropod cuticle. [Google Scholar]
- Obin M.S, Vandermeer R.K. Sources of nestmate recognition cues in the imported fire ant Solenopsis invicta Buren (Hymenoptera Formicidae) Anim. Behav. 1988;36:1361–1370. doi:10.1016/S0003-3472(88)80205-7 [Google Scholar]
- Pan C.Y, Mo J.C, Cheng M.L. Influence of diet and soil on inter-colonial aggression of Coptotermes formosanus (Isoptera: Rhinotermitidae) Sociobiology. 2006;48:841–848. [Google Scholar]
- Peeters C, Monnin T, Malosse C. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. B. 1999;266:1323–1327. doi:10.1098/rspb.1999.0782 [Google Scholar]
- Peschke K, Metzler M. Cuticular hydrocarbons and female sex pheromones of the rove beetle, Aleochara curtula (Goeze) (Coleoptera: Staphylinidae) Insect Biochem. 1987;17:167–178. doi:10.1016/0020-1790(87)90157-0 [Google Scholar]
- Pukowski E. Ökologische Untersuchungen an Necrophorus F. Z. Morphol. Ökol. Tiere. 1933;27:518–586. doi:10.1007/BF00403155 [Google Scholar]
- Robinson G.E, Vargo E.L. Juvenile hormone in adult eusocial hymenoptera: gonadotropin and behavioral pacemaker. Arch. Insect Biochem. Physiol. 1997;35:559–583. doi: 10.1002/(SICI)1520-6327(1997)35:4<559::AID-ARCH13>3.0.CO;2-9. doi:10.1002/(SICI)1520-6327(1997)35:4<559::AID-ARCH13>3.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- Scott D. Sexual mimicry regulates the attractiveness of mated Drosophila melanogaster females. Proc. Natl Acad. Sci. USA. 1986;83:8429–8433. doi: 10.1073/pnas.83.21.8429. doi:10.1073/pnas.83.21.8429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M.P. Brood guarding and the evolution of male parental care In burying beetles. Behav. Ecol. Sociobiol. 1990;26:31–40. [Google Scholar]
- Scott D, Jackson L.L. The basis for control of post-mating sexual attractiveness by Drosophila melanogaster females. Anim. Behav. 1990;40:891–900. doi:10.1016/S0003-3472(05)80991-1 [Google Scholar]
- Scott M.P, Trumbo S.T, Neese P.A, Bailey W.D, Roe R.M. Changes in biosynthesis and degradation of juvenile hormone during breeding by burying beetles: a reproductive or social role? J. Insect Physiol. 2001;47:295–302. doi: 10.1016/s0022-1910(00)00116-5. doi:10.1016/S0022-1910(00)00116-5 [DOI] [PubMed] [Google Scholar]
- Sherman P.W, Reeve H.K, Pfennig D.W. Recognition systems. In: Krebs J.R, Davies N.B, editors. Behavioural ecology: an evolutionary approach. 4th edn. Blackwell Science; Oxford, UK: 1997. pp. 69–96. [Google Scholar]
- Singer T.L. Roles of hydrocarbons in the recognition systems of insects. Am. Zool. 1998;38:394–405. [Google Scholar]
- Sledge M.F, Trinca I, Massolo A, Boscaro F, Turillazzi S. Variation in cuticular hydrocarbon signatures, hormonal correlates and establishment of reproductive dominance in a polistine wasp. J. Insect Physiol. 2004;50:73–83. doi: 10.1016/j.jinsphys.2003.10.001. doi:10.1016/j.jinsphys.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Smith B.H, Breed M.D. The chemical basis for nestmate recognition and mate discrimination in social insects. In: Cardé R.T, Bell W.J, editors. Chemical ecology of insects II. Chapman and Hall; New York, NY: 1995. pp. 287–317. [Google Scholar]
- Steiner S, Mumm R, Ruther J. Courtship pheromones in parasitic wasps: comparison of bioactive and inactive hydrocarbon profiles by multivariate statistical methods. J. Chem. Ecol. 2007;33:825–838. doi: 10.1007/s10886-007-9265-6. doi:10.1007/s10886-007-9265-6 [DOI] [PubMed] [Google Scholar]
- Syvertsen T.C, Jackson L.L, Blomquist G.J, Vinson S.B. Alkadienes mediating courtship in the parasitoid Cardiochiles nigriceps (Hymenoptera: Braconidae) J. Chem. Ecol. 1995;21:1971–1989. doi: 10.1007/BF02033856. doi:10.1007/BF02033856 [DOI] [PubMed] [Google Scholar]
- Tillman J.A, Seybold S.J, Jurenka R.A, Blomquist G.J. Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 1999;29:481–514. doi: 10.1016/s0965-1748(99)00016-8. doi:10.1016/S0965-1748(99)00016-8 [DOI] [PubMed] [Google Scholar]
- Tregenza T, Buckley S.H, Pritchard V.L, Butlin R.K. Inter- and intrapopulation effects of sex and age on epicuticular composition of meadow grasshopper, Chorthippus parallelus. J. Chem. Ecol. 2000;26:257–278. doi:10.1023/A:1005457931869 [Google Scholar]
- Trumbo S.T. Interference competition among burying beetles Silphidae Nicrophorus. Ecol. Entomol. 1990a;15:347–355. [Google Scholar]
- Trumbo S.T. Reproductive benefits of infanticide in a biparental burying beetle Nicrophorus orbicollis. Behav. Ecol. Sociobiol. 1990b;27:269–274. doi:10.1007/BF00164899 [Google Scholar]
- Trumbo S.T. Infanticide, sexual selection and task specialization in a biparental burying beetle. Anim. Behav. 2006;72:1159–1167. doi:10.1016/j.anbehav.2006.05.004 [Google Scholar]
- Wakonigg G, Eveleigh L, Arnold G, Crailsheim K. Cuticular hydrocarbon profiles reveal age-related changes in honey bee drones (Apis mellifera carnica) J. Apic. Res. 2000;39:137–141. [Google Scholar]
- Whitlow, S. 2003 Chemical and behavioural investigations into species and partner recognition in burying beetles. PhD thesis. University of Freiburg, Freiburg, Germany.
- Whitlow S, Peschke K, Mueller J.K.Friend or foe: the scent of success Zoology 200210516351861 [Google Scholar]
- Zar J.H. Prentice Hall; Englewood Cliffs, NJ: 1984. Biostatistical analysis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods and results