Abstract
Parasites have been suggested to influence many aspects of host behaviour. Some of these effects may be mediated via their impact on host energy budgets. This impact may include effects on both energy intake and absorption as well as components of expenditure, including resting metabolic rate (RMR) and activity (e.g. grooming). Despite their potential importance, the energy costs of parasitism have seldom been directly quantified in a field setting. Here we pharmacologically treated female Cape ground squirrels (Xerus inauris) with anti-parasite drugs and measured the change in body composition, the daily energy expenditure (DEE) using doubly labelled water, the RMR by respirometry and the proportions of time spent looking for food, feeding, moving and grooming. Post-treatment animals gained an average 19 g of fat or approximately 25 kJ d−1. DEE averaged 382 kJ d−1 prior to and 375 kJ d−1 post treatment (p>0.05). RMR averaged 174 kJ d−1 prior to and 217 kJ d−1 post treatment (p<0.009). Post-treatment animals spent less time looking for food and grooming, but more time on feeding. A primary impact of infection by parasites could be suppression of feeding behaviour and, hence, total available energy resources. The significant elevation of RMR after treatment was unexpected. One explanation might be that parasites produce metabolic by-products that suppress RMR. Overall, these findings suggest that impacts of parasites on host energy budgets are complex and are not easily explained by simple effects such as stimulation of a costly immune response. There is currently no broadly generalizable framework available for predicting the energetic consequences of parasitic infection.
Keywords: energy, parasite, ground squirrel, resting metabolic rate, doubly labelled water, life history
1. Introduction
Infection with parasites has been suggested to influence the development of many social and evolutionary aspects of host behaviour (Thompson & Kavaliers 1994; Sheldon & Verhulst 1996; Møller et al. 1999; Zuk & Stoehr 2002). These effects may include altering their relative attractiveness to potential mates (Hamilton & Zuk 1982; Møller et al. 1999; Verhulst et al. 1999) and decisions about whether and when to breed (Buchholz 2004). At least some of these effects may be mediated via an impact of parasites on host energy budgets.
The impact of parasites on host energy budgets involves multiple mechanisms that influence both sides of the energy-balance equation. For example, infection with parasites has been shown to have suppressive effects on food intake. It has been suggested that this response by the host may be adaptive in that it may serve to reduce their exposure to ingestion of further parasites (Kyriazakis et al. 1998; Forbes et al. 2000). In at least some host–parasite systems, parasite-induced anorexia appears to be directly stimulated by metabolic by-products produced by the parasite itself (Roberts et al. 1999; Mercer et al. 2000). These effects on food intake may be amplified by reduced efficiency at absorbing food in the alimentary tract (Munger & Karasov 1989). On the energy-expenditure side of the budget, parasites may also exert multiple effects. First, there may be costs of raising an immune response to the infection (Demas et al. 1997; Svensson et al. 1998; Nilsson 2003). Second, the parasites often include invasive life stages that may cause direct tissue damage to their hosts necessitating elevated tissue repair processes (e.g. Delahay et al. 1995). Both of these effects could lead to elevations in resting metabolic rate (RMR; Giorgi et al. 2001; Ots et al. 2001; Nilsson 2003). Finally, behavioural responses to infection may be costly; for example, the time spent grooming may increase in relation to ectoparasite burden, and the energy costs of grooming may be significant (Mooring 1995; Christie et al. 2000). However, parasite infection may cause animals to have a decreased motivation to feed and this may lead them to reduce their physical activity levels (Delahay et al. 1995; Mercer et al. 2000), thus lowering their energy demands. Again this response could be adaptive to cope with the reduced energy intake.
In combination, the reduced energy availability and elevated expenditure may constrain the amount of energy that is available to be allocated to other processes and activities, such as maintenance, growth and reproduction (reviewed in Lochmiller & Deerenberg 2000). It is well established that livestock animals fed anti-parasite and anti-bacterial drugs, or animals that have been reared in germ-free environments, can have lower daily energy demands (10–30%) while they may simultaneously grow more rapidly and to a larger size (Lochmiller & Deerenberg 2000). Field studies have shown that dosing animals with anti-parasite drugs can have major effects on their livelihoods. For example, heifer cattle dosed with anti-parasite drugs spent more time grazing and grew faster than untreated animals (Forbes et al. 2000). Likewise, treated snowshoe hares (Lepus americanus) were less vulnerable to predation and survived periods of food shortage better (Murray et al. 1997).
Despite their potential importance, the energy costs of parasitism have seldom been directly quantified (but see Delahay et al. 1995; Kristan & Hammond 2004; Simon et al. 2005; Schwanz 2006) and almost never in wild animals living in their natural environment. In the current study, we determined the body composition, daily energy expenditure (DEE) and RMR of female Cape ground squirrels (Xerus inauris) both before and after chemical anti-parasite treatment that eliminated most internal and external parasites. We also observed aspects of behaviour, specifically the time spent searching for food and feeding, moving and grooming. Parasitism appears to have a dramatic impact on the reproductive success of females (M. Hillegass & J. M. Waterman, unpublished data) and a major effect on their social behaviour. For example, 40% of all social interactions may involve allogrooming, which may compromise time spent on other interactions (Waterman 1995).
2. Material and methods
(a) Study site and animals
The study site was located at the S. A. Lombard Nature Reserve (3660 ha, 18 km northwest of Bloemhoef, South Africa, 27°35′ S, 25°23′ E). Squirrels have been studied there since June 2002. The habitat consists of Cymbopogon-Themeda veld and Kalahari grasslands on a flood plain (van Zyl 1965). Mean annual precipitation is 500 mm.
Cape ground squirrels are diurnal social rodents (less than 1.0 kg) that inhabit arid environments in southern Africa (Waterman 1995). Groups are either female-based matrilineal kin groups consisting of one to three adults and up to nine related sub-adults of either sex that occupy areas around burrow clusters, or male-based bands (consisting of groups of dispersed males) that are non-territorial (Waterman 1996). Individuals reach breeding weight at approximately 11 months of age (Waterman 2002, B. Pettitt 2006, unpublished work). They suffer from chronic levels of both internal and external parasites such as the pinworm Xeroxyuris parallela, which is exclusive to X. inauris and ixodid ticks such as Rhipicephalus theileri, lice such as Neohaematopinus faurei and fleas such as Synosternus caffer, Ctenocephalides connatus and Echidnophaga bradyta (Straschil 1975; Lynch 1983; Waterman 2002).
Individuals were trapped using Tomahawk wire-mesh traps (15×15×50 cm) and marked for permanent identification using PIT tags (AVID USA). They were also freeze marked (Quick Freeze; Rood & Nellis 1980) and temporarily marked with black hair dye (Rodol D) on the body. Freeze marking allows for permanent identification whereas the black hair dye allows them to be recognized by an observer at a distance. We estimated parasite intensity (the relative number of parasites) in animals treated for parasitism and for control animals both before and after treatment. Ectoparasite intensity was estimated by combing squirrels from shoulders to tail base; three regions of the dorsal area (two lateral and a medial plane) were combed three times each. Ectoparasites (i.e. fleas, ticks and lice) were combed directly into a Petri dish containing 95% ethanol and counted. Endoparasite intensity was estimated by assessing faecal counts of endoparasite eggs. For this, we used faecal matter collected by forceps from plastic sheets that had been placed under the traps (Pettitt et al. 2007). Faecal pellets were then placed into sealed plastic bags until analysis. Following collection, faeces were weighed and 0.5 g was separated and frozen. These samples were later thawed, crushed and mixed with magnesium sulphate and allowed to ‘float’ for 5 min (McCurnin & Bassert 2002). Examinations under a compound microscope at 400× total magnification allowed for endoparasite egg (hookworm, coccidia and tapeworm) quantification. Samples were taken prior to anti-parasite treatment and one month post treatment. Parasites were also quantified for squirrels that did not receive anti-parasite treatment (control animals). The control animals were handled in exactly the same way as treatment animals, but they were not injected with ivermectin or sprayed with fipronil (below).
Squirrels were treated with 0. 1 ml of ivermectin injected subcutaneously. This drug removes both adult and larval endoparasites, as well as some ectoparasite arthropods, for up to four weeks (Campbell 1983; Heukelbach et al. 2004). The ectoparasites were also treated using a topical anti-parasitic spray (FRONTLINE, fipronil 0.29%), which is effective in killing fleas, ticks and lice for up to four weeks. Fipronil has a wide safety margin, even in squirrels, and applied topically does not past the dermis (Metzger & Rust 2002; Diaz 2005). These drugs are extremely effective at killing arthropods and have very little effect on most mammals, including squirrels, at the topical doses used (Campbell 1983; Metzger & Rust 2002). Cape ground squirrels are reproductively active throughout the year; however, we chose females that were not pregnant and had not been observed to undergo oestrus for this project.
(b) Daily energy expenditure measurements
We measured the DEE (kJ d−1) of 18 Cape ground squirrel females in June 2004 (prior to administration of ivermectin and fipronil) and 14 (of the original 18, after treatment for parasites) individuals 30 days after using the doubly labelled water (DLW) technique (Lifson & McClintock 1966; Speakman 1997, 1998). We used females because they are based around permanent burrow clusters, whereas males are more mobile and follow a roaming mating strategy (Waterman 1995). This isotopic method for determining energy expenditure has been previously validated by comparison to indirect calorimetry in a range of small mammals (Speakman 1997) and provides a measure of values over periods of several days (Speakman et al. 1994; Berteaux et al. 1996). On day 1, the animals were weighed (±0.1 g Sartorius balance) and a 0.2 ml blood sample was obtained from a cephalic vein to estimate the background isotope enrichments of 2H and 18O (Speakman & Racey 1987). The blood samples were immediately heat sealed into 2 × 100 μl glass capillaries, which were stored at room temperature. Afterwards, a known mass of DLW (665460 ppm 18O, 328410 ppm 2H) was administered (IP, 0.3 g per 100 g body weight). Syringes were weighed before and after administration (±0.0001 g, Sartorius balance) to calculate the mass of DLW injected. The blood samples were taken after 1 h to estimate initial isotope enrichments (Krol & Speakman 1999; Visser et al. 2000). Animals were recaptured 2–5 days post dose, and final blood samples (0.2 ml) were taken after whole 24 h periods (Speakman & Racey 1988) to estimate isotope elimination rates. We recaptured 100% of individuals that were injected with DLW. However, we failed to capture some individuals for measurement after pharmacological treatment (detailed below). Capillaries that contained the blood samples were vacuum distilled (Nagy 1983), and water from the resulting distillate was used to produce CO2 and H2 (methods in Speakman et al. 1990 for CO2 and Speakman & Krol 2005 for H2). The isotope ratios 18O : 16O and 2H : 1H were then analysed using gas source isotope ratio mass spectrometry (Optima, Micromass IRMS and Isochrom μG, Manchester, UK), prior to calculation of DEE (Lemen & Speakman 1997). Injectate enrichments of 18O and 2H (ppm), elimination rates (ko and kd, per day), dilution spaces (No and Nd, ml), initial and final pool sizes (ml) were calculated following Speakman (1997) (see also table 1). Energy expenditure was calculated using a single pool model as recommended for animals weighing less than 2 kg (Speakman 1993). There are several alternative approaches for the treatment of evaporative water loss in the calculation (Visser & Schekkermann 1999). We chose the assumption of a fixed evaporation of 25% of the water flux (eqn (7.17): Speakman 1997), which has been established to minimize error in a range of conditions (Visser & Schekkerman 1999; van Tright et al. 2002). In addition to DEE, we also calculated the fat content of the animals derived from the body water dilution space (Nd) as
| (2.1) |
(Wang et al. 1999; Bray et al. 2001).
Table 1.
Isotope dilution spaces and elimination rates. (Mean±1 s.d. of 18O dilution space (No, ml), 2H dilution space (Nd, ml), dilution space ratio (Nd/No), 18O elimination rate (ko, per hour), 2H elimination rate (kd, per hour) and isotope elimination ratio (ko/kd) for Cape ground squirrel females before treatment for parasites (before treatment) and after treatment (after treatment). n denotes sample sizes.)
| n | No (ml) | s.d. | Nd (ml) | s.d. | Nd/No | ko (per hour) | s.d. | kd (per hour) | s.d. | ko/kd | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| before treatment | 18 | 400 | 40 | 413 | 42 | 1.0312 | 0.0065 | 0.0020 | 0.0038 | 0.0014 | 1.8263 |
| after treatment | 14 | 408 | 42 | 411 | 41 | 1.0091 | 0.0068 | 0.0019 | 0.0044 | 0.0012 | 1.8324 |
(c) Resting metabolic rate measurements
We determined minimal oxygen consumption (ml O2 h−1) after an initial 40 min in which animals were observed to settle in a respirometry chamber. Measurements were made prior to blood sampling for DLW before and after treatment for parasites. Measurements were taken every minute for half an hour. The mean of the lowest 10 readings of oxygen consumption (ml O2 h−1) was taken when animals were seen to be at rest (Bennett et al. 1992). We used an open circuit respirometry system (Depocas & Hart 1957; Hill 1972). A metabolic chamber (16 l) was immersed in a temperature-controlled water bath (Labotec, LAUDA, Königshofen, Germany). The temperature of the water bath was maintained at 29.5±0.5°C, which is within the thermoneutral zone for this species (Haim et al. 1987). Dried air was pumped into the chamber at approximately 3.0 l min−1 so that depressions in oxygen concentration were maintained at 0.25–0.4%. The air passed through approximately 4 m of copper coil that was submerged in the water before it entered the chamber. This ensured the temperature of air that entered the chamber was the same as that of the water bath. The flow of air into the metabolism chamber was controlled by a flow regulator (Omega FMA-A2310, Stamford, CT; ±0.05 L min−1) that was placed upstream of the chamber. The concentration of oxygen in the excurrent gases was measured with an oxygen analyser (S-2A Applied Electrochemistry, AEI Technologies, inc. USA). The analyser was calibrated to an upper value in dry air (20.95% O2) prior to the measurement of each animal and to a lower value (0% O2 in N2 gas, AFROX, South Africa) every two weeks. RMR was calculated in kJ d−1 using an energy equivalence per litre of O2 consumed that is appropriate for carbohydrate (5.04 kcal l−1; Arch et al. 2006). In addition to DEE and RMR, we also calculated sustained metabolic scope (SusMS=DEE/RMR) for each animal (Drent & Daan 1980; Peterson et al. 1990; Hammond & Diamond 1997; Speakman et al. 2003).
(d) Behavioural observations
We observed squirrels from trees, vehicles or observation towers and recorded identity, location and activities every 10 min using scan sampling (Altmann 1974). Each animal was observed for at least 2 h. Observations took place three to five weeks after treatment for parasitism. Eighteen control females were observed that were not treated for parasites, while 11 females were observed that had been treated. Observations of treated and non-treated animals took place synchronously and the same individuals were not observed before and after treatment. A time-budget analysis was calculated for each individual by allocating behaviour among six categories: (i) social (all interactions between individuals), (ii) alert (vigilant postures in which the squirrels were in a vertical stance), (iii) moving (walking, running or loping), (iv) maintenance behaviour (grooming and sand bathing), (v) foraging/searching for food, (vi) feeding, and (vii) resting (sitting). Data were arcsine transformed prior to analysis (Zar 1984). Collection and analyses of data on these behaviours are described in detail by Waterman (1995).
3. Results
(a) Parasite load
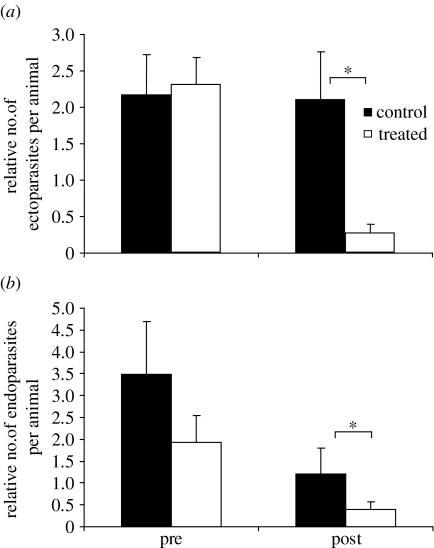
The ectoparasites collected consisted of 50.7% fleas, 24.7% ticks and 24.7% lice. Endoparasites were 27.5% roundworms, 21.7% hookworms, 8.3% coccidian and 42.5% other types. There were no differences in ectoparasite or endoparasite intensity between the control and treatment groups before treatment for parasitism (Mann–Whitney U=329, p=0.69, d.f.=36 and U=365, p=0.80, d.f.=40, respectively; figure 1). However, there were much lower levels of both ecto- and endoparasites in individuals that were treated compared with control animals (U=145, p=<0.001, d.f. =66 and U=105, p<0.001, d.f. =51, respectively).
Figure 1.
(a) Relative number of ectoparasites per animal and (b) relative number of endoparasites per animal before treatment for parasites (pre) and after treatment (post). Filled bars denote control animals; open bars denote treated animals. Error bars denote standard errors. Asterisk indicates a significant difference between groups.
(b) Body composition
Of the original 18 individuals with which we measured RMR and DEE and then treated, we recaptured 14 individuals one month later. Three of the animals that were not recaptured were not seen again at the field site (and therefore may have been predated) and one elusive individual was seen but was not recaptured. At initial capture prior to dosing, the individuals averaged 578.1 g (s.d.=61.6 g). On recapture, one month later the animals had increased in weight to on average 596.1 g (s.d.=52.1 g). The average increase in mass of 18 g marginally failed to reach significance (paired t13=1.89, p=0.057). This elevated body mass consisted entirely of fat. The fat content of the animals prior to treatment derived from their body water content was 55.3 g (s.d.=22.3), while after treatment for parasites the fat content averaged 74.6 g (s.d.=21.7), which was a significant increase (paired t13=2.15, p=0.05, table 2). The increase of 19.3 g of fat between the initial measurements and the final measurements reflects a deposition of 758 kJ, assuming an energy content for fat of 39.3 kJ g−1 (Blaxter 1989). Over the time period between the measurements, this is equivalent to a deposition of 25.2 kJ d−1.
Table 2.
Mass, energy expenditure and percent fat. (Mean ±1 s.d. of body mass (g) at initial capture, energy expenditure (DEE, kJ d−1), resting metabolic rate (RMR, kJ d−1), sustained metabolic scope (SusMS, no units) and percent fat (% fat) for Cape ground squirrel females before treatment for parasites (before treatment) and after treatment (after treatment). n denotes sample sizes.)
| before treatment | after treatment | |||
|---|---|---|---|---|
|
|
|
|||
| mean | s.d. | mean | s.d. | |
| n | 18 | 14 | ||
| body mass (g) | 578 | 62 | 596 | 52 |
| DEE (kJ d−1) | 382 | 100 | 375 | 92 |
| RMR (kJ d−1) | 173 | 50 | 217 | 39 |
| SusMS | 2.31 | 0.68 | 1.76 | 0.61 |
| % fat | 9.54 | 3.32 | 12.41 | 3.74 |
(c) Daily energy expenditure
Absolute values of DEE were not significantly different between the same individual animals before and after treatment for parasites (paired t13=0.07, p=0.95, table 2). There was no significant change in body mass of individual animals over the 2–5 day DLW experimental period for either the DLW measurement before treatment (initial mass =578.1±61.7 g, final mass =580.9±67.2 g, t13=0.68, p=0.51) or after treatment (initial mass=598.1±52.1, final mass=591.8±58.8, t13=1.28, p=0.22). DEE was however significantly related to body mass. The least-squares fitted regression
| (3.1) |
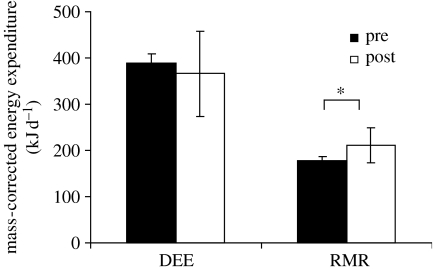
explained 31.3% of the variation in DEE (F1,30=13.28, p<0.001; data for all animals included). There was no effect of parasite infection status on DEE when body mass was entered as a covariate (ANCOVA F1,29=0.59, p=0.448; figure 2).
Figure 2.
Mass-corrected daily energy expenditure (DEE) (kJ d−1) and resting metabolic rate (RMR) (kJ d−1) of X. inauris females prior to dosing for parasitism (pre, filled bars) and after dosing (post, open bars). Values were calculated using the mean residual for each of the groups of animals added to the grand mean across all animal groups. Error bars denote the standard errors of the residual mean estimates. Asterisk indicates a significant difference between groups.
(d) Resting metabolic rate
Absolute values of RMR were significantly greater in individual animals after treatment for parasitism than before treatment (paired t13=3.09, p=0.009, table 2). On average, the RMR after treatment was 217 kJ d−1 while prior to treatment it was 173 kJ d−1. As with DEE, the RMR was related to body mass. The least-squares fitted regression
| (3.2) |
explained 43.2% of the variation in RMR (F1,30=22.80, p<0.001; data for all animals included). The significant effect of parasite infection status on RMR remained when body mass was entered as a covariate (ANCOVA F1,29=7.89, p=0.009). The RMR was significantly greater after animals were treated for parasites (figure 2).
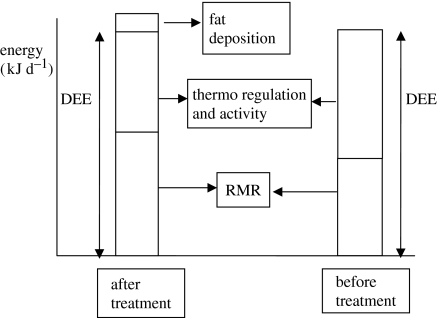
The impact of treatment for parasitism on the energy budgets of the ground squirrels in the current study is illustrated in figure 3. This block diagram shows how the total budget expanded (approx. 20 kJ d−1) after treatment for parasites, but that this effect was more than offset by the elevation in RMR amounting to approximately 40 kJ d−1. This meant that the available energy to allocate to other activities was actually reduced after treatment for parasitism. This reduction is reflected in the sustained metabolic scope, which declined from 2.31 prior to treatment to 1.76 following treatment to remove parasites.
Figure 3.
Block diagram illustrating energy allocation to DEE and RMR and fat deposition in X. inauris females before and after treatment for parasitism.
(e) Behaviour
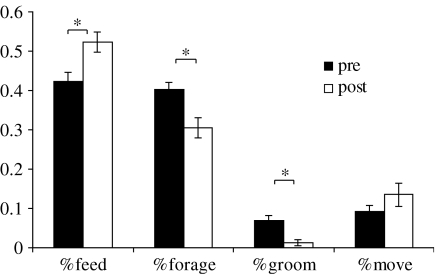
Animals spent 42% of their time feeding prior to treatment for parasites and this increased to 52% following treatment (two-sample t28=3.03, p=0.0054). By comparison, animals spent 40% of their time foraging prior to treatment and only spent 31% post treatment (t28=3.03, p=0.0051; figure 4). Animals spent significantly more time grooming at 7% prior to treatment but only spent 1% of their time post treatment (t28=4.47, p<0.001). However, there were no differences in the amount of time spent moving (running and loping) between animals before and after treatment for parasites (t28=1.17, p=0.26).
Figure 4.
Proportion of time spent feeding, foraging, grooming and moving by X. inauris females prior to anti-parasite treatment (pre: filled bars) and post treatment (post: open bars). Error bars denote standard errors. Asterisk indicates a significant difference between groups.
4. Discussion
Infection with parasites is often inferred to have significant energetic costs in both birds (e.g. Bouslama et al. 2002; Nilsson 2003; Møller & Saino 2004) and mammals (e.g. Khokhlova et al. 2002; Kristan & Hammond 2006). Since organisms may often have limited resources, the energy requirements of dealing with parasitism may compromise the energy that is available to be allocated on other things such as activity, thermoregulation or reproduction (Deerenberg et al. 1997; Svensson et al. 1998; Greenman et al. 2005). Across mammalian species, parasite species richness is positively correlated with the basal metabolic rate of their hosts (Morland & Harvey 2000). The supposition has generally been made that higher rates of metabolism are associated with greater physiological requirements necessary to cope with parasitic infection. However, direct attempts to quantify this effect have proved difficult. Relatively few studies have measured RMRs or DEEs of free-living individuals, and results have so far been mixed: parasitized individuals or those with elevated immune responses generally have lower DEE values (e.g. Moreno et al. 2001; Simon et al. 2005) and energy intakes (Delahay et al. 1995) than those that are not, but this might not always be the case (e.g. Munger & Karasov 1989; Schwanz 2006). By comparison, parasitized animals sometimes have higher RMR values (e.g. Booth et al. 1993; Delahay et al. 1995; Giorgi et al. 2001; Nilsson 2003; Manganou et al. 2006), but again this may not always be the case (e.g. Kristan 2002; Kristan & Hammond 2006). The one study that we could find that measured both RMR and DEE in the same subjects with and without parasites showed that DEE was lower in parasitized people but RMR remained unchanged (Valencia et al. 1995).
In contrast to these previous findings, in the current study we found that RMR was actually significantly higher following parasite removal. This effect was large and involved an increase of approximately 40 kJ d−1 (almost 20%), and it remained significant even after the effect of body size on RMR was removed. Why RMR should increase following parasite removal is uncertain. The greater total energy budget we observed and the accumulation of fat after parasite removal probably occurred because the animals increased their time feeding rather than their digestive efficiency (Munger & Karasov 1989), because direct observations indicated that they spent more time feeding. A primary effect of parasites in these animals therefore was probably to suppress their food intake. Other studies have also established an anorexic effect of parasite infection (Kyriazakis et al. 1998; Roberts et al. 1999; Forbes et al. 2000; Mercer et al. 2000), which appears in some parasite–host systems to be directly stimulated by metabolic by-products from the parasites themselves. Whether this is an adaptive response to infection to minimize further infection via the oral uptake of additional parasites (Kyriazakis et al. 1998) is unclear.
Several studies have suggested that individual differences in RMR may be linked to the size of the alimentary tract (Daan et al. 1989; Konarzerwski & Diamond 1995; Speakman & McQueenie 1996; Krol et al. 2003), which underpins the level of sustained energy intake (Drent & Daan 1980; Daan et al. 1990; Hammond & Diamond 1997). Hence, on the face of it, the increased RMR might be consistent with the suggestion that the post-treatment animals were eating more food. However, recent attempts to establish the energy demands associated with having larger digestive organs have indicated that such demands may in fact be a rather small component of RMR (Johnson et al. 2001; Speakman et al. 2004; Russell & Chappell 2007). Some studies have found relationships between RMR and size of the liver, but not other components of the alimentary tract (Selman et al. 2001) and yet other studies have suggested links to other metabolically active tissues such as the heart to be important (Meerlo et al. 1997). Moreover, the increase in RMR observed in the current study was more than offset by the increase in the total energy budget, so it seems unlikely that this elevation was a direct consequence of the elevated food intake on body composition. It is possible that under parasitism, animals deliberately suppressed their RMR to cope with the constrained intake levels. This effect may be adaptive to free up energy for allocation to other things. Alternatively, the effect may be a metabolic consequence of by-products produced by the parasites themselves (Richards & Edwards 2000). A final possibility is that the anti-parasite treatment elevated RMR by the stimulation of detoxification enzyme pathways such as the cytochrome P450 system (Swanepoel et al. 1999). While this could have an effect on the animals immediately post dosing, it seems unlikely that such an effect would still be evident a month later.
Whatever the cause of the elevated RMR in the post-treatment animals, the fact that total DEE remained unaffected by removal of the parasites meant that the energy budget available for allocation to other things was actually lower than when they were parasitized. This may explain why activity components of their behaviour were reduced; particularly the time spent searching for food. This would have been augmented by the reduction in the need to spend time and energy grooming owing to the absence of ectoparasites. Indeed, studies in other systems have shown that the energetic costs of grooming can be a significant portion of the daily time and energy budget (Burnett & August 1981; Christe et al. 2000; Mclean & Speakman 2000).
This study highlights the complexity of the interactions between parasites and the energetics of their hosts that depend on multiple mechanisms. A strength of this study is that it was made on free-living animals and we combined techniques to paint an almost complete picture of their energy relations in response to experimental treatment for the removal of parasites. However, our study also has several weaknesses. In particular, since we did not sacrifice the animals, we had some indication but we did not know in detail what the complete parasite load of these animals was. The animals were probably carrying multiple infections of parasites that differed between individuals. This is a disadvantage of working in the field compared with the laboratory where animals can be cleared of parasites, kept in clean environments and then experimentally re-infected with a single species. Another weakness was that for the DEE measurements we followed the animals over time but did not have a control group that was untreated. Hence some of the changes we report may not be consequences of the parasite-removal treatment but time effects. This is another potential explanation of the unexpected elevation in RMR when the parasites were removed. In spite of this, our study is among the first to attempt to quantify the impact of parasitism on energy budgets in a wild animal living in a real world setting. The findings suggest that impacts of parasites on host energy budgets are complex and are not easily explained by simple effects such as stimulation of a costly immune response. Indeed, they highlight the fact that there is no broadly generalizable framework currently available for predicting the energetic consequences of parasite infection.
Acknowledgments
Research protocols were approved by the animal ethics committees at the Universities of Central Florida and Pretoria and complied with their guidelines for animal research.
We would like to thank Johannie Caldo and Tambudzani Mulaudzi for their assistance in the field. We would also like to thank Jamie Dick and Ian Montgomery for their valuable thoughts and comments on an earlier draft of this manuscript. This research was funded in part by the National Science Foundation, USA no. 0130600 to J.M.W., the National Research Foundation, RSA to N.C.B. and the University of Pretoria to N.C.B. and M.S.
References
- Altmann J. Observational study of behaviour: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Arch J.R.S, Hislop D, Wang S.J.Y, Speakman J.R. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int. J. Obesity. 2006;30:1322–1331. doi: 10.1038/sj.ijo.0803280. [DOI] [PubMed] [Google Scholar]
- Bennett N.C, Clarke B.C, Jarvis J.U.M. A comparison of metabolic acclimation in two species of social mole-rats (Rodentia, Bathyergidae) in southern Africa. J. Arid Environ. 1992;22:189–198. [Google Scholar]
- Berteaux D, Thomas D.W, Bergeron J.-M, Lapierre H. Repeatability of daily field metabolic rate in female meadow voles (Microtus pennsylvanicus) Funct. Ecol. 1996;10:751–759. doi: 10.2307/2390510. [DOI] [Google Scholar]
- Blaxter K. Cambridge University Press; Cambridge, UK: 1989. Energy metabolism in animals and man. [Google Scholar]
- Booth D.T, Clayton D.H, Block B.A. Experimental demonstration of the energetic cost of parasitism in free-ranging hosts. Proc. R. Soc. B. 1993;253:125–129. doi: 10.1098/rspb.1993.0091. [DOI] [Google Scholar]
- Bouslama Z, Lambrechts M.M, Ziane N, Djenidi R, Chabi Y. The effect of nest ectoparasites on parental provisioning in a north-African population of the blue tit Parus caeruleus. Ibis. 2002;144:E73–E78. doi: 10.1046/j.1474-919X.2002.00070_5.x. [DOI] [Google Scholar]
- Bray G.A, DeLany J.P, Harsha D.W, Volaufova J, Champagne C.C. Evaluation of body fat in fatter and leaner 10-y-old African American and white children: the Baton Rouge children's study. Am. J. Clin. Nutr. 2001;73:687–702. doi: 10.1093/ajcn/73.4.687. [DOI] [PubMed] [Google Scholar]
- Buchholz R. Effects of parasitic infection on mate sampling by female wild turkeys (Meleagris gallopavo): should infected females be more or less choosy? Behav. Ecol. 2004;15:687–694. doi: 10.1093/beheco/arh066. [DOI] [Google Scholar]
- Burnett C.D, August P.V. Time and energy budgets for dayroosting in a maternity colony of Myotis lucifugus. J. Mammal. 1981;62:758–766. doi: 10.2307/1380597. [DOI] [Google Scholar]
- Campbell W.C. Ivermectin, an antiparasitic agent. Med. Res. Rev. 1983;13:61–79. doi: 10.1002/med.2610130103. [DOI] [PubMed] [Google Scholar]
- Christe P, Arlettaz R, Vogel P. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis) Ecol. Lett. 2000;3:207–212. doi: 10.1046/j.1461-0248.2000.00142.x. [DOI] [Google Scholar]
- Daan S, Masman D, Strijkstra A, Verhulst S. Intraspecific allometry of basal metabolic rate: relations with body size, temperature, composition, and circadian phase in the kestrel, Falco tinnunculus. J. Biol. Rhythms. 1989;4:267–283. [PubMed] [Google Scholar]
- Daan S, Masman D, Groenewold A. Avian basal metabolic rates: their association with body composition and energy expenditure in nature. Am. J. Physiol. 1990;259:R333–R340. doi: 10.1152/ajpregu.1990.259.2.R333. [DOI] [PubMed] [Google Scholar]
- Deerenberg C, Arpanius V, Daan S, Bos N. Reproductive effort decreases antibody responsiveness. Proc. R. Soc. B. 1997;264:1021–1029. doi: 10.1098/rspb.1997.0141. [DOI] [Google Scholar]
- Delahay R.J, Speakman J.R, Moss R. The energetic consequences of parasitism. Effects of a developing infection of Trichostrongylus tenuis (Nematoda) on red grouse (Lagopus lagopus scoticus): energy balance, body weight and condition. Parasitology. 1995;110:473–482. [Google Scholar]
- Depocas F, Hart J.S. Use of the Pauling oxygen analyser for measurements of oxygen consumption of animals in open-circuit system and in short-lag, closed-circuit apparatus. J. Appl. Physiol. 1957;10:388–392. doi: 10.1152/jappl.1957.10.3.388. [DOI] [PubMed] [Google Scholar]
- Demas G, Chefer V, Talan M, Nelson R. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol. 1997;273:R1631–R1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- Diaz S.L. Efficacy of fipronil in the treatment of pediculosis in laboratory rats. Lab. Anim. 2005;39:331–335. doi: 10.1258/0023677054306980. [DOI] [PubMed] [Google Scholar]
- Drent R, Daan S. The prudent parent: energetics adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Forbes A.B, Huckle C.A, Gibb M.J, Rook A.J, Nuthall R. Evaluation of the effects of nematode parasitism on grazing behaviour, herbage intake and growth in young grazing cattle. Vet. Parasitol. 2000;90:111–118. doi: 10.1016/S0304-4017(00)00218-1. [DOI] [PubMed] [Google Scholar]
- Giorgi M.S, Arlettaz R, Christe P, Vogel P. The energetic grooming costs imposed by a parasitic mite (Spinturnix myoti) upon its bat host (Myotis myotis) Proc. R. Soc. B. 2001;268:2071–2075. doi: 10.1098/rspb.2001.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenman C.G, Martin L.B, II, Hau M. Reproductive state, but not testosterone, reduces immune function in male house sparrows (Passer domesticus) Physiol. Biochem. Zool. 2005;78:60–68. doi: 10.1086/425194. [DOI] [PubMed] [Google Scholar]
- Haim A, Skinner J.D, Robinson T.J. Bioenergetics, thermoregulation and urine analysis of squirrels of the genus Xerus from an arid environment. S. Afr. J. Zool. 1987;22:45–49. [Google Scholar]
- Hamilton W.D, Zuk M. Heritable true fitness and bright birds: a role of parasites. Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Hammond K.A, Diamond J. Maximal sustained energy budgets in humans and animals. Nature. 1997;386:457–462. doi: 10.1038/386457a0. [DOI] [PubMed] [Google Scholar]
- Heukelbach J, Winter B, Wilcke T, Muehlen M, Albrecht S, Sales de Oliveira F.A, Kerr-Pontes L.R.S, Liesenfeld O, Feldmeier H. Selective mass treatment with ivermectin to control intestinal helminthiases and parasitic skin diseases in a severely affected population. Bull. World Health Organ. 2004;82:563–571. [PMC free article] [PubMed] [Google Scholar]
- Hill R.W. Determination of oxygen consumption by use of the paramagnetic oxygen analyzer. J. Appl. Physiol. 1972;33:261–263. doi: 10.1152/jappl.1972.33.2.261. [DOI] [PubMed] [Google Scholar]
- Johnson M.S, Thomson S.C, Speakman J.R. Limits to sustained energy intake II. Inter-relationships between resting metabolic rate, life-history traits and morphology in Mus musculus. J. Exp. Biol. 2001;204:1937–1946. doi: 10.1242/jeb.204.11.1937. [DOI] [PubMed] [Google Scholar]
- Khokhlova I.S, Krasnov B.R, Kam M, Burdelova N.I, Degen A.A. Energy costs of ectoparasitism: the flea Xenopsylla ramensis on the desert gerbil Gerbillus dasyurus. J. Zool. Lond. 2002;258:349–354. [Google Scholar]
- Konarzerwski M, Diamond J. Evolution of basal metabolic rate and organ masses in laboratory mice. Evolution. 1995;49:1239–1248. doi: 10.2307/2410448. [DOI] [PubMed] [Google Scholar]
- Kristan D.M. Effects of intestinal nematodes during lactation: consequences for host morphology, physiology and offspring mass. J. Exp. Biol. 2002;205:3955–3965. doi: 10.1242/jeb.205.24.3955. [DOI] [PubMed] [Google Scholar]
- Kristan D.M, Hammond K.A. Aerobic performance of wild-derived house mice does not change with cold exposure or intestinal parasite infection. Physiol. Biochem. Zool. 2004;77:440–449. doi: 10.1086/383513. [DOI] [PubMed] [Google Scholar]
- Kristan D.M, Hammond K.A. Effects of three simultaneous demands on glucose transport, resting metabolism and morphology of laboratory mice. J. Comp. Physiol. B. 2006;176:139–151. doi: 10.1007/s00360-005-0036-9. [DOI] [PubMed] [Google Scholar]
- Krol E, Speakman J.R. Isotope dilution spaces of mice injected simultaneously with deuterium, tritium and oxygen-18. J. Exp. Biol. 1999;202:2839–2849. doi: 10.1242/jeb.202.20.2839. [DOI] [PubMed] [Google Scholar]
- Krol E, Johnson M.S, Speakman J.R. Limits to sustained energy intake VIII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality. J. Exp. Biol. 2003;206:4283–4291. doi: 10.1242/jeb.00676. [DOI] [PubMed] [Google Scholar]
- Kyriazakis I, Tolkamp B.J, Hutchings M.R. Towards a functional explanation for the occurrence of anorexia during parasitic infections. Anim. Behav. 1998;56:265–274. doi: 10.1006/anbe.1998.0761. [DOI] [PubMed] [Google Scholar]
- Lemen, C. & Speakman, J. R. 1997 DLW program and DLW userguide. See http://www.abdn.ac.uk/zoology/speakman.htm.
- Lifson N, McClintock R. Theory of the turnover rates of body water for measuring energy and material balance. J. Theor. Biol. 1966;12:46–74. doi: 10.1016/0022-5193(66)90185-8. [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. doi: 10.1034/j.1600-0706.2000.880110.x. [DOI] [Google Scholar]
- Lynch, C. D. 1983 The mammals of the Orange free state. Memoir of the National Museum of Bloemfontein, pp. 1–218. Bloemfontein, Republic of South Africa: National Museum.
- Mclean J.A, Speakman J.R. Effects of body mass and reproduction on the basal metabolic rate of brown long-eared bats (Plecotus auritus) Physiol. Biochem. Zool. 2000;73:112–121. doi: 10.1086/316715. [DOI] [PubMed] [Google Scholar]
- McCurnin D.M, Bassert J.M. 5th edn. W. B. Saunders Co; Philadelphia, PA, USA: 2002. Clinical textbook for veterinary technicians. [Google Scholar]
- Manganou E, Fons R, Feliu C, Morand S. Physiological responses of insular wild black rat (Rattus rattus) to natural infection by the digenean trematode Fasciola. Parasitol. Res. 2006;99:97–101. doi: 10.1007/s00436-005-0063-1. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Bolle L, Visser G.H, Masman D, Daan S. Basal metabolic rate in relation to body composition and daily energy expenditure in the field vole, Microtus agrestis. Physiol. Zool. 1997;70:362–369. doi: 10.1086/639616. [DOI] [PubMed] [Google Scholar]
- Mercer J.G, Mitchell P.I, Moar K.M, Bissett A, Geissler S, Bruce K, Chappell L.H. Anorexia in rats infected with the nematode, Nippostrongylus brasiliensis: experimental manipulations. Parasitology. 2000;120:641–647. doi: 10.1017/S0031182099005922. [DOI] [PubMed] [Google Scholar]
- Metzger M.E, Rust M.K. Laboratory evaluation of fipronil and imidacloprid topical insecticides for control of the plague vector Oropsylla montana (Siphonaptera: Ceratophyllidae) on California ground squirrels (Rodentia: Sciuridae) J. Med. Entomol. 2002;39:152–161. doi: 10.1603/0022-2585-39.1.152. [DOI] [PubMed] [Google Scholar]
- Møller A.P, Christie P, Lux E. Parasitism, host function, and sexual selection. Q. Rev. Biol. 1999;74:3–20. doi: 10.1086/392949. [DOI] [PubMed] [Google Scholar]
- Møller A.P, Saino N. Immune response and survival. Oikos. 2004;104:299–304. doi: 10.1111/j.0030-1299.2004.12844.x. [DOI] [Google Scholar]
- Mooring M.S. The effect of tick challenge on grooming rate by impala. Anim. Behav. 1995;50:377–392. doi: 10.1006/anbe.1995.0253. [DOI] [Google Scholar]
- Moreno J, Sanz J, Merino S, Arriero E. Daily energy expenditure and cell-mediated immunity in pied flycatchers while feeding nestlings: interaction with moult. Oecologia. 2001;129:492–497. doi: 10.1007/s004420100767. [DOI] [PubMed] [Google Scholar]
- Morland S, Harvey P.H. Mammalian metabolism, longevity and parasite species richness. Proc. R. Soc. B. 2000;267:1999–2003. doi: 10.1098/rspb.2000.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger J.C, Karasov W.H. Sublethal parasites and host energy budgets: tapeworm infection in white-footed mice. Ecology. 1989;70:904–921. doi: 10.2307/1941358. [DOI] [Google Scholar]
- Murray D.L, Cary J.R, Keith L.B. Interactive effects of sublethal nematodes and nutritional status on snowshoe hare vulnerability to predation. J. Anim. Ecol. 1997;66:250–264. doi: 10.2307/6026. [DOI] [Google Scholar]
- Nagy, K. A. 1983 The doubly labelled water (3HH18O) method: a guide to its use. UCLA publication no. 12-1417. Los Angeles, CA: University of California.
- Nilsson J.A. Ectoparasitism in marsh tits: costs and functional explanations. Behav. Ecol. 2003;14:175–181. doi: 10.1093/beheco/14.2.175. [DOI] [Google Scholar]
- Ots I, Kerimov A.B, Ivankina E.V, Hõrak P. Immune challenge affects basal metabolic activity in wintering great tits. Proc. R. Soc. B. 2001;268:1175–1181. doi: 10.1098/rspb.2001.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C.C, Nagy K.A, Diamond J. Sustained metabolic scope. Proc. Natl Acad. Sci. USA. 1990;87:2324–2328. doi: 10.1073/pnas.87.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt B.A, Wheton C.J, Waterman J.M. Effects of storage treatment on fecal steroid hormone concentrations of a rodent, the Cape ground squirrel (Xerus inauris) Gen. Comp. Endocrinol. 2007;150:1–11. doi: 10.1016/j.ygcen.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Richards E.H, Edward J.P. Parasitism of (Lacanobia oleracea) Lepidoptera by the ectoparasitoid, Eulophus pennicornis, is associated with a reduction in host haemolymph phenoloxidase activity. Comp. Biochem. Physiol. B. 2000;127:289–298. doi: 10.1016/S0305-0491(00)00255-8. [DOI] [PubMed] [Google Scholar]
- Roberts H.C, Hardie L.J, Chappell L.H, Mercer J.G. Parasite-induced anorexia: leptin, insulin and corticosterone responses to infection with the nematode, Nippostrongylus brasiliensis. Parasitology. 1999;118:117–123. doi: 10.1017/S0031182098003503. [DOI] [PubMed] [Google Scholar]
- Rood J.P, Nellis D. Freeze-marking mongooses. J. Wildl. Manage. 1980;44:500–502. doi: 10.2307/3807988. [DOI] [Google Scholar]
- Russell G.A, Chappell M.A. Is BMR repeatable in deer mice? Organ mass correlates and the effects of cold acclimation and natal altitude. J. Comp. Physiol. B. 2007;177:75–87. doi: 10.1007/s00360-006-0110-y. [DOI] [PubMed] [Google Scholar]
- Schwanz L.E. Schistosome infection in deer mice (Peromyscus maniculatus): impacts on host physiology, behavior and energetics. J. Exp. Biol. 2006;209:5029–5037. doi: 10.1242/jeb.02601. [DOI] [PubMed] [Google Scholar]
- Selman C, Lumsden S, Bunger L, Hill W, Speakman J.R. Resting metabolic rate and morphology in mice (Mus musculus) selected for high and low food intake. J. Exp. Biol. 2001;204:777–784. doi: 10.1242/jeb.204.4.777. [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Simon A, Thomas D.W, Speakman J.R, Blondel J, Perret P, Lambrechts M. Impact of ectoparasitic blowfly larvae (Protocalliphora spp) on the behaviour and energetics of nestling blue tits (Parus caeruleus) J. Field Ornithol. 2005;76:402–410. [Google Scholar]
- Speakman J.R. How should we calculate CO2 production in doubly labelled water studies of animals? Funct. Ecol. 1993;7:746–750. [Google Scholar]
- Speakman J.R. Chapman and Hall; London, UK: 1997. Doubly labelled water, theory and practice. [Google Scholar]
- Speakman J.R. The history and theory of the doubly labeled water technique. Am. J. Clin. Nutr. 1998;68:932S–938S. doi: 10.1093/ajcn/68.4.932S. [DOI] [PubMed] [Google Scholar]
- Speakman J.R, Krol E. Validation of the doubly-labelled water method in a small mammal. Physiol. Biochem. Zool. 2005;78:650–667. doi: 10.1086/430234. [DOI] [PubMed] [Google Scholar]
- Speakman J.R, McQueenie J. Limits to sustained metabolic rate: the link between food intake, basal metabolic rate, and morphology in reproducing mice, Mus musculus. Physiol. Zool. 1996;69:746–769. [Google Scholar]
- Speakman J.R, Racey P.A. The equilibrium concentration of O-18 in body-water—implications for the accuracy of the doubly-labeled water technique and a potential new method of measuring RQ in free-living animals. J. Theor. Biol. 1987;127:79–95. doi: 10.1016/S0022-5193(87)80162-5. [DOI] [Google Scholar]
- Speakman J.R, Racey P.A. Consequences of non steady-state CO2 production for accuracy of the doubly labeled water technique—the importance of recapture interval. Comp. Biochem. Physiol. A. 1988;90:337–340. doi: 10.1016/0300-9629(88)91125-5. [DOI] [Google Scholar]
- Speakman J.R, Nagy K.A, Masman D, Mook W.G, Poppitt S.D, Strathearn G.E, Racey P.A. Interlaboratory comparison of different analytical techniques for the determination of O-18 abundance. Anal. Chem. 1990;62:703–708. doi: 10.1021/ac00206a011. [DOI] [Google Scholar]
- Speakman J.R, Racey P.A, Haim A, Webb P.I, Ellison G.T.H, Skinner J.D. Interindividual and intraindividual variation in daily energy-expenditure of the pouched mouse (Saccostomus campestris) Funct. Ecol. 1994;8:336–342. doi: 10.2307/2389826. [DOI] [Google Scholar]
- Speakman J.R, Krol E, Johnson M.S. The functional significance of individual variations in BMR. Physiol. Biochem. Zool. 2004;77:900–915. doi: 10.1086/427059. [DOI] [PubMed] [Google Scholar]
- Speakman J.R, Ergon T.E, Scantlebury M, Reid K.A, Cavagne L, Lambin X. Resting metabolic rate is correlated with daily energy expenditure in free-living voles (Microtus agrestis) but reflects extrinsic rather than intrinsic limits. Proc. Natl Acad. Sci. USA. 2003;100:14 057–14 062. doi: 10.1073/pnas.2235671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straschil B. Sandbathing and marking in Xerus inauris (Zimmerman, 1870) (Rodentia, Sciuridae) S. Afr. J. Sci. 1975;71:215–216. [Google Scholar]
- Svensson E, RÅberg L, Koch C, Hasselquist D. Energetic stress, immunosuppression and the costs of an antibody response. Funct. Ecol. 1998;12:912–919. doi: 10.1046/j.1365-2435.1998.00271.x. [DOI] [Google Scholar]
- Swanepoel R.E, Racey P.A, Shore R.F, Speakman J.R. Energetic effects of sublethal exposure to lindane on pipistrelle bats (Pipistrellus pipistrellus) Environ. Pollut. 1999;104:169–177. doi: 10.1016/S0269-7491(98)00196-1. [DOI] [Google Scholar]
- Thompson S.N, Kavaliers M. Physiological bases for parasite-induced alterations of host behaviour. Parasitology. 1994;109:S119–S138. doi: 10.1017/s0031182000085139. [DOI] [PubMed] [Google Scholar]
- Valencia M.E, McNeill G, Haggarty P, Moya S.Y, Pinelli A, Quihui L, Davalos R. Energetic consequences of mild Giardia intestinalis infestation in Mexican children. Am. J. Clin. Nutr. 1995;61:860–865. doi: 10.1093/ajcn/61.4.860. [DOI] [PubMed] [Google Scholar]
- van Zyl J.H.M. The vegetation of the S. A. Lombard nature reserve and its utilisation by certain antelope. Zool. Afr. 1965;1:55–71. [Google Scholar]
- Van Trigt R, Kerstel E.R.T, Neubert R.E.M, Meijer H.A.J, McLean M, G H. Visser validation of the DLW method in Japanese quail at different water fluxes using laser and IRMS. J. Appl. Physiol. 2002;93:2147–2154. doi: 10.1152/japplphysiol.01134.2001. [DOI] [PubMed] [Google Scholar]
- Visser G.H, Schekkerman H. Validation of the doubly labeled water method in growing precocial birds: the importance of assumptions concerning evaporative water loss. Physiol. Biochem. Zool. 1999;72:740–749. doi: 10.1086/316713. [DOI] [PubMed] [Google Scholar]
- Visser G.H, Dekinga A, Achterkamp B, Piersma T. Ingested water equilibrates isotopically with the body water pool of a shorebird with unrivaled water fluxes. Am. J. Physiol. 2000;279:R1795–R1804. doi: 10.1152/ajpregu.2000.279.5.R1795. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Dieleman S.J, Parmentier H.K. A tradeoff between immunocompetence and sexual ornamentation in domestic fowl. Ecology. 1999;96:4478–4481. doi: 10.1073/pnas.96.8.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner R.N, Heymsfield S.B. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am. J. Clin. Nutr. 1999;69:883–841. doi: 10.1093/ajcn/69.5.833. [DOI] [PubMed] [Google Scholar]
- Waterman J.M. The social organization of the Cape ground squirrel (Xerus inauris; Rodentia: Sciuridae) Ethology. 1995;101:130–147. [Google Scholar]
- Waterman J.M. Reproductive biology of a tropical, non-hibernating ground squirrel. J. Mamm. 1996;77:134–146. doi: 10.2307/1382715. [DOI] [Google Scholar]
- Waterman J.M. Delayed maturity, group fission and the limits of group size in female Cape ground squirrels (Sciuridae: Xerus inauris) J. Zool. Lond. 2002;256:113–120. [Google Scholar]
- Zar J.H. Prentice-Hall; New Jersey, NJ: 1984. Biostatistical analysis. [Google Scholar]
- Zuk M, Stoehr A.M. Immune defense and host life history. Am. Nat. 2002;160:S9–S22. doi: 10.1086/342131. [DOI] [PubMed] [Google Scholar]