Abstract
The special problems confronted by very small animals in nervous system design that may impose limitations on their behaviour and evolution are reviewed. Previous attempts to test for such behavioural limitations have suffered from lack of detail in behavioural observations of tiny species and unsatisfactory measurements of their behavioural capacities. This study presents partial solutions to both problems. The orb-web construction behaviour of spiders provided data on the comparative behavioural capabilities of tiny animals in heretofore unparalleled detail; species ranged about five orders of magnitude in weight, from approximately 50–100 mg down to some of the smallest spiders known (less than 0.005 mg), whose small size is a derived trait. Previous attempts to quantify the ‘complexity’ of behaviour were abandoned in favour of using comparisons of behavioural imprecision in performing the same task. The prediction of the size limitation hypothesis that very small spiders would have a reduced ability to repeat one particular behaviour pattern precisely was not confirmed. The anatomical and physiological mechanisms by which these tiny animals achieve this precision and the possibility that they are more limited in the performance of higher-order behaviour patterns await further investigation.
Keywords: behavioural imprecision, miniaturization, orb-weaving spiders, Araneae
1. Introduction
Very small animals confront special problems in the designs of their nervous systems that may impose limitations on their evolution and behaviour. The behaviour of some insects, for instance, is guided by more efficient but less flexible neural mechanisms than those of vertebrates (Wehner 1987; Collett & Collett 2002). Comparisons across the wide scale of insect sizes suggest that there may be lower limits for functional brain sizes. The brains of smaller insect species are smaller, but (as in vertebrates, e.g. Roth et al. 1990) they are proportionally much larger, and more densely packed with neurons (Beutel et al. 2005). The proportion of the body volume dedicated to brain tissue in very small insects is approximately 250 times greater than that in very large species: the brain of the tiny (0.2 mm long) primary larva of Mengenilla chobauti (Strepsiptera) is larger than the head (part has moved into the prothorax) and is approximately 5% of the body volume (Beutel et al. 2005), a proportion nearly double that of humans.
Because brain tissue contrasts with many other tissues in being continually active metabolically, and because the density of metabolic activity in smaller brains is likely to be greater if they have comparable information processing capabilities (Niven et al. 2007), these disproportionally large brains are probably also disproportionately costly to maintain. The developmental pattern in the brains of bees and ants also indicates that nervous tissue is relatively costly because adults add particular major subcomponents to their brains only when they are old enough to perform the tasks for which these components are used (Durst et al. 1994; Gronenberg et al. 1996; Seid et al. 2005), even when their total lifetime is only a few days (Kühn-Bühlmann & Wehner 2006). Thus, one cost of being very small is the disproportionate dedication of energy and material to a relatively large brain. Such increased relative costs may result in selection favouring reductions in behavioural capabilities: when an animal is very small, the costs associated with given behavioural capabilities may come to outweigh the benefits of these capabilities. This possibility may be of general importance because many moderate-sized invertebrates pass through very small, independent ontogenetic stages.
There are also reasons to expect that the brains of very small species should be functionally inferior, despite their relatively large size. Neuron size appears to reach a minimum (approx. 2 μm in diameter) near the lower end of the range of insects body sizes (approx. 0.9 mm) and then does not decrease further in smaller species; so very small species probably have reduced numbers of neurons (Beutel et al. 2005). Cells of this size, such as Kenyon cells, are mostly composed of nucleus, surrounded by only a thin layer of cytoplasm, so the lower limit on cell size may be imposed by nucleus size (which in turn correlates closely with genome size; see Hanken & Wake 1993). In addition, there is a lower limit of approximately 0.1 μm on the diameter of functional unmyelinated axons because stochastic opening of sodium channels in axon membranes can generate action potentials and thus increase noise in very small axons (Faisal et al. 2005). The internal substructures of the brains of very small insects may also be simpler or fewer in number (Beutel et al. 2005), and smaller insects tend to have reduced numbers of sensory organs, such as chemosensory and tactile setae, and ommatidia in their compound eyes (Rutowski 2000; Jander & Jander 2002; Spaethe & Chittka 2003; Mares et al. 2005). The dendrites in smaller brains with smaller neurons also tend to have reduced structural complexity (Wittenberg & Wang in press).
In sum, the brains of very small insects have reduced numbers of and perhaps simpler nerve cells, and evolutionary adjustments in their brain morphology (relatively large brain sizes and reduced neuron sizes) suggest that presumably costly compensatory traits have evolved to diminish the negative effects of these reductions on the animal's behaviour. The likelihood that such compensation will evolve seems especially high in tiny species which have evolved from larger ancestors that had behavioural capacities commensurate with their larger size. Two extreme possibilities suggest themselves. Tiny species may have evolved sufficient adjustments in brain size and design that allow them to have the same behavioural capacities as those of larger related species. Alternatively, very small species may be more limited behaviourally because the greater costs of a CNS with equal behavioural capabilities have made it advantageous for them to settle for reduced behavioural capacities (the ‘size limitation hypothesis’).
The present study takes advantage of the large size variation in orb-weaving spiders (weights in this study varied over five orders of magnitude) and the fact that construction of an orb web produces a detailed, easily recorded (figure 1) and precise record of hundreds of behavioural decisions to test the size limitation hypothesis. One aspect of web construction behaviour, attachment of the sticky spiral line to radii, is repeated many times over during the construction of a single orb, and thus permits especially powerful, intra-individual comparisons. This behaviour involves relatively simple behavioural actions, and perhaps also relatively low-level analyses of stimuli by the spider, so the test here is of an ambitious version of the size limitation hypothesis (more demanding behaviour may be more likely to be limited by size). The general process of sticky spiral construction is highly conserved taxonomically (Eberhard 1982; Griswold et al. 1998): the spider starts near the periphery of the orb and works inward, repeating the process illustrated in figure 2a at each radius it crosses. The space between loops of sticky spiral is determined at the moment illustrated in figure 2a(iii).
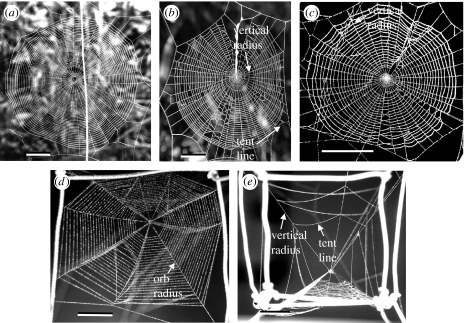
Figure 1.
Representative orbs of (a) a mature female L. mariana, (b) a mature female A. bifurca, (c) a first-instar nymph of A. bifurca and (d) a first-instar nymph of Anapisona simoni seen from directly above and (e) from the side. The scale lines are (a) 5, (b) 2, (c) 2 and (d,e) 0.5 cm.
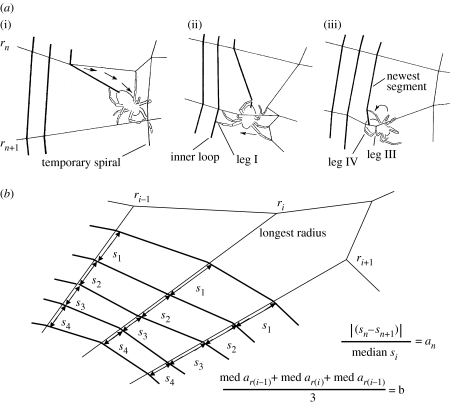
Figure 2.
(a) Diagrammatic representation of sticky spiral construction (arrows indicate movement) (after Eberhard 1982). (i) After attaching the sticky line to radius rn, the spider moves inward towards the hub and towards the next radius; (ii) after reaching the next radius (rn+1), it moves away from the hub, tapping with its extended leg I until it touches the inner loop of sticky spiral already in place; (iii) then it grasps this radius with its legs III and IV, turns its body laterally and attaches the new segment of sticky spiral line to the radius between the points gripped by tarsi III and IV. (b) Illustration of how intra-individual imprecision values were calculated. First, the absolute value of each difference between adjacent spaces between sticky spiral loops on the longest radius (ri) was divided by the median space on that radius to give a dimensionless value an (upper equation), and then the median value of a was determined for this radius (med ar(i)). The same process was repeated for the two adjacent radii, giving med ar(n−1) and med ar(n+1). These three median values were averaged to give a single imprecision value b for the web (lower equation).
I abandoned previous, perhaps hopeless attempts to quantify behavioural capabilities in terms of the difficulty to measure variable ‘complexity’ (see Vollrath (1992) and Healy & Rowe (2006) and discussion below), and focused instead on another variable that could reflect possible behavioural limitations: behavioural precision or the ability to repeat the same behaviour precisely. This trait has been hypothesized to be less developed in animals with smaller brains (Misunami et al. 2004). The logic of this approach is the following. There are several reasons to suspect that motor behaviour, coordination and orientation may be less precise in animals with reduced nervous system capabilities. Smaller insects probably often have less complete sensory information due to lower numbers of sense organs. They may also be able to perform less thorough or precise analyses of sensory information, due to lower numbers of interneurons, fewer dendrites, or fewer or more noisy connections between them; or they may have less extensive internal feedback mechanisms that reduce the behavioural imprecision that results from nervous system noise (Eberhard 1990, 2000). They may also have reduced motor precision due to smaller numbers of motor axons and less feedback information, giving less subtle control of body movements. Limitations in the precision and analysis of leg movements, in an animal like a web-building spider that uses the movements and positions of its own legs to provide sensory information, could result in additional limitations on the precision of its sensory information.
The expectation of the size limitation hypothesis was that if limits in behavioural precision are imposed by very small size, they will result in increased imprecision in sticky spiral construction behaviour in smaller spiders, or at least in especially tiny spiders; greater imprecision would be reflected as greater differences in the adjacent spaces between loops of sticky spiral. The highly regular spaces between successive loops of sticky spiral in orb webs imply that, at least in a given area of the orb (see below), a particular spacing is advantageous (Witt 1965; Eberhard 1986), and that variations between neighbouring spaces are inadvertent. An additional limitation that might be imposed by size is the speed of execution of given behaviour patterns. The expectation of the behavioural limitation hypothesis would be that to achieve a similar level of precision, the behaviour of especially small spiders might be slower.
2. Material and methods
Three species of orb-weaving spiders were used: adults of the tetragnathid Leucauge mariana which are more or less typical in size of many orb weavers (body length approximately 7 mm, wet weight approximately 50–80 mg); both adults (approx. 6 mm and 30 mg) and newly hatched spiderlings (approx. 1 mm and 0.8 mg) of the araneid Allocyclosa bifurca; and both adults (1–1.3 mm and 0.6–1.0 mg) and small nymphs (0.6 mm and less than 0.005 mg) of the anapid Anapisona simoni. The tiny size of anapids is a derived trait within orb weavers (Griswold et al. 1998). The current most probable phylogenetic hypothesis (Coddington 2005) suggests that the anapid and the tetragnathid are more closely related to each other than either is to the araneid. The validity of using behavioural comparisons between young and old spiders of the same species to test for possible handicaps of miniaturization depends on the assumption that learning does not reduce variation. This assumption is supported by the finding in other species that learning does not influence major aspects of orb construction (Petrusewiczowa 1938; Mayer 1952; Reed et al. 1970; Vollrath 1992), and by the lack of this predicted juvenile–adult difference which is documented below.
A single web built by each individual was coated with white corn starch or talcum powder and photographed at a magnification such that the web filled the field of view (figure 1). Anapisona simoni were induced to build orbs on more or less cubical wire frames (approx. 5 cm on a side for adults and 2.0–2.5 cm for early instar nymphs); the frames had short legs that were placed in shallow water in closed containers to prevent spiders from abandoning the frames. Mature female A. bifurca and L. mariana were collected by taping the anchor lines of their orbs to wire hoops, and were then allowed to build new orbs. The hoops of L. mariana (approx. 70 cm in diameter) were hung at 45° in outdoor cages (Eberhard 1987a), while those of A. bifurca (approx. 23 cm in diameter) were hung vertically indoors. The orbs of first-instar A. bifurca nymphs were built between lines of their mothers' empty orbs in hoops.
The planar orbs of A. bifurca and L. mariana were photographed perpendicular to the plane of the orb. The orbs of A. simoni differed in usually being pulled upward at the hub to form a cone (figure 1d,e). One or more radial lines usually extended upward from the hub (‘vertical radius’ in figure 1e), and a few sticky spiral lines (‘tent line’ in figure 1e) were attached to them above the orb proper. Photographs of each A. simoni web included a dorsal view and a view approximately perpendicular to the plane of the sticky spiral lines crossing the longest radius. In all three species, the spaces between loops of sticky spiral on the longest radius and on the radii on either side of this radius were measured from enlarged digital images using Scion Image software, which allowed enlargements to similar dimensions on the computer screen.
Behavioural imprecision was quantified by comparing the differences in sticky spiral spacing in each web after taking into account natural patterns of variation. The spaces between loops or turns of the sticky spiral tended to be greater on longer radii and near the edge in the orb webs of L. mariana, as well as near the hub in the orbs of A. bifurca (figure 1a–c). Thus, instead of simply using the variance in the spaces between sticky loops throughout each orb, I calculated a measure of intra-individual imprecision in the spacing of successive loops of sticky spiral (figure 2b), using orbs with at least 10 measurable spaces between attachments to the longest radius. For this radius and the radius on either side of it, I calculated the absolute differences between adjacent spaces between sticky spiral loops on the radius, standardizing these differences by dividing them by the median space between loops on that radius (figure 2b). The median of these standardized differences was taken as an estimate of the behavioural imprecision in sticky spiral spacing on that radius, and the mean of these estimates for the three radii was used to characterize imprecision in that web (figure 2b). The ‘imprecision value’ of each group was characterized by the mean of these means (table 1). This measure largely controlled for variables known to affect sticky spiral spacing such as radius length, angle with gravity, distance from the hub, body size, age and the overall amount of sticky silk available in the spider's glands (§4). All statistical tests were performed using Statistica v. 6 software.
Table 1.
Measures of behavioural imprecision in the spacing between loops of sticky spiral lines in orb webs (all means±1 s.d.). (Only those imprecision values followed by the same letter and number differ significantly (t-tests: a, p<0.05; b, p<0.01; c, p<0.001).)
| spider | no. of webs | mean no. of differences measured/radius | mean imprecision value |
|---|---|---|---|
| Anapisona simoni | |||
| first-instar nymphs | 42 | 17.4±4.3 | 0.154±0.102b1 |
| adult females | 75 | 16.1±5.4 | 0.164±0.042b2,c1 |
| adult males | 24 | 13.9±5.5 | 0.171±0.062a1 |
| Allocyclosa bifurca | |||
| first-instar nymphs | 49 | 17.9±6.3 | 0.216±0.072a1,b1,c1,c2 |
| adult females | 26 | 29.9±8.9 | 0.193±0.048b2,3 |
| Leucauge mariana | |||
| adult females | 35 | 33.8±7.6 | 0.154±0.063b3,c2 |
3. Results
The overall pattern of the imprecision values for spaces between loops of sticky spiral did not accord with expectations based on the size limitation hypothesis (table 1). Neither the smaller species nor the smaller individuals of a given species showed significant reductions in behavioural precision. In fact, the significant differences involving the tiniest spiders (first-instar A. simoni), whose tiny size seemed most likely to impose behavioural limitations, were all in the opposite direction. Their imprecision values were significantly smaller than those for the larger first-instar nymphs of A. bifurca, and not significantly different from those of the much larger adults of A. bifurca and L. mariana. The significance of this pattern is increased by the possibility that changes in spacing at different distances from the hub would result in greater differences between adjacent spaces in webs with lower numbers of loops, such as those of the anapids (table 1).
The expectation that smaller spiders might move relatively more slowly was also not met. In a small individual of the smallest species, A. simoni, the mean time between successive attachments of the sticky spiral in an orb in which sticky spiral construction was approximately one-third complete was 3.0 s (20 attachments in 60 s). In a young (1.4 mg) and an adult female of A. bifurca on orbs at a corresponding stage of construction, these times were 1.9 (80 attachments) and 3.4 s (30 attachments), respectively. In a mature female L. mariana at a similar stage, the time between attachments was smaller (1.5 s, 40 attachments), but in an even larger spider, a mature female of the araneid Gasteracantha cancriformis (100 g), the time between attachments at a corresponding stage was much longer (7.3 s, 62 attachments). Because the webs of A. simoni differ from those other species in lacking temporary spiral lines (Eberhard 1982), the smallest spiders had to travel nearly to the hub and back between each pair of attachments and were thus moving much more rapidly in terms of their body size than were the larger species.
4. Discussion
The pattern of behavioural imprecision predicted by the size limitation hypothesis clearly did not occur. There was no trend for smaller spiders, even the very tiny first-instar A. simoni, to have larger imprecision values. As noted above, this result can constitute a valid test of the effects of body size on imprecision only if learning has little or no effect on behavioural imprecision. This was indeed the case, as there were no significant differences between first-instar nymphs and adult females in A. simoni or A. bifurca, and the insignificant trends that did occur were in opposite directions in the two species.
The lack of greater behavioural imprecision of the tiny anapid documented here may not be limited to this particular species. Highly organized three-dimensional derivatives of orbs occur in species in other genera of anapids, and also in similarly small and even smaller species in the related families Symphytognathidae and Mysmenidae (Platnick & Shadab 1979; Coddington 1986; Eberhard 1987b).
Previous attempts to assess the consequences of miniaturization on behaviour that focused on behavioural complexity rather than precision yielded inconsistent and unconvincing results. Cole (1985) argued that several previous studies relating behavioural complexity and brain size (Howse 1974; Eisenberg & Wilson 1978; Harvey et al. 1980; Eisenberg 1981) were unconvincing due to the lack of objective criteria for measuring behavioural complexity. One additional citation purporting to show that smaller species of copepods have simpler behaviour (Hanken & Wake 1993) apparently resulted from use of the misleading English abstract of a French paper on ecology, where ‘valence ecologique’ was translated as ‘habits’ (Şerban 1960), and habits was taken to mean ‘behaviour’. Cole (1985) concluded that the behaviour of smaller species of ants is less ‘complex’ because he found a positive correlation between head size and behavioural repertoire (number of behavioural tasks) in nine species of as many genera. But this conclusion also suffers from serious problems in quantifying complexity: lack of clear criteria for distinguishing different behavioural tasks; the untested (and unlikely) assumption that all tasks are equally demanding with respect to neural capabilities; the untested assumption that tasks that are assigned the same name in different species (e.g. feed the larvae) are equally demanding; the untested assumption that the rate of errors in the performance of a given task does not vary between species; and the untested assumption that head size correlates with brain size in these species. In addition, appropriate controls for phylogenetic inertia (Harvey & Pagel 1991) were lacking. Comparisons of the brains of termite species were said to show the opposite correlation—species with greater behavioural complexity (in terms of nest architecture) have smaller brains with fewer neurons (Howse 1974), but again there was no clear measure of complexity. Comparison of the behavioural tasks in 10 species of Pheidole ants (suffering from similar problems in quantifying complexity) showed no correlation between repertoire size and body size in minor workers, and a weak (non-significant) negative correlation in majors (Wilson 1984). An additional anatomical study suggested that small size may not impose reduced behavioural capacities in some especially tiny insects (first-instar strepsipteran larvae, which range in size from 80 to 850 Φm long; Beutel et al. 2005). However, only very crude behavioural measures were used, such as the possession of relatively complex sense organs (assumed to imply complex neural capabilities), the ability to jump and the ability to identify and attack particular hosts.
Other very small insects obviously also accomplish the same general tasks as larger insects, and some perform apparently sophisticated analyses, such as the flexible patch-leaving decisions of some parasitoid wasps that are based on both memory and internal physiology (Vos et al. 1998; Outreman et al. 2005; Burger et al. 2006). However, it is possible that the behavioural tasks of these tiny species are somehow simplified, for instance by the use of neural tricks such as ‘matched filters’ that only work in a limited way for a narrow range of stimuli and situations (Wehner 1987), or that these insects perform their tasks less effectively (e.g. perform tasks more slowly, make more mistakes, make fewer or less precise or subtle adjustments to environmental stimuli). There are, of course, other metazoans, such as mites, that are smaller still; they apparently also have relatively large brains (Kranz 1971; Treat 1975), but their behavioural capacities are even less studied.
Studies of L. mariana and other orb weavers have shown that a relatively simple set of instructions can produce a spider-like simulated sticky spiral pattern (Eberhard 1969), but that several factors affect the spaces between loops of sticky spiral: the length of the exploratory leg I (Reed et al. 1965; Vollrath 1987), the angle of the radius with gravity (LeGuelte 1967; Witt et al. 1968; Vollrath 1992), the distance of the attachment from the hub (LeGuelte 1967; Vollrath 1992), the age of the spider (Reed et al. 1970), an internally determined ‘set point’ that is influenced by silk supplies (Eberhard 1988a), and, at least in some species, adjustments based on path integration (Eberhard 1982, 1988b). All of these factors, except the last, were held very close to constant in the analyses in this study of successive attachments to a given radius.
Current ignorance regarding the behavioural consequences of miniaturization is probably due to both the theoretical difficulty of quantifying behavioural complexity and the practical difficulty of studying behavioural refinements in especially small animals. This study offers at least partial solutions for both problems. Nevertheless, the imprecision measured here presumably includes two possible sources of inconsistent behaviour: imprecision in what the spider intended to do (perhaps the behavioural programme itself was inconsistent) and imprecision in the control of the behavioural movements involved in executing these plans. These aspects were not distinguished. In addition, the behaviour involved in determining the space between loops of sticky spiral may involve, on the execution side, processes that are relatively undemanding with respect to nervous system capacities. Placement of a new segment of sticky spiral may require only determination of the sites where legs III and IV should grasp the radius relative to the site where exploratory tapping with leg I contacted the inner loop, and of the site where the spinnerets should attach the sticky spiral line to the radius (figure 2a(iii)), plus the motor coordination necessary to attach the line at the site dictated by this information. The lack of confirmation of the size limitation hypothesis for precision in sticky spiral placement implies that miniaturization has not affected these processes, but it does not mean that the hypothesis may not hold for other behavioural processes. Further studies on possibly more demanding behavioural abilities and on the neuroanatomy of tiny orb-weaving spiders may throw further light on the size limitation hypothesis and the neural basis for their behaviour.
Acknowledgments
This research conformed to the ethical guidelines of the countries where it was performed.
I thank M. Seid and especially J. Niven for patient lessons regarding the physiology and anatomy of miniature nervous systems, G. Barrantes for advice on statistics, R. Quesada for web measurements, J. Christy, J. Niven, R. L. Rodriguez, W. Wcislo, M. J. West-Eberhard and two referees for comments on the manuscript, and STRI and UCR for financial support.
References
- Beutel R.G, Pohl H, Hunefeld F. Strepsipteran brains and effects of miniaturization (Insecta) Arthropod Struct. Dev. 2005;34:301–313. doi:10.1016/j.asd.2005.03.001 [Google Scholar]
- Burger J.M.S, Huang Y, Hemerik L, van Lenteren J.C, Vet L.E.M. Flexible use of patch-leaving decisions in a parasitoid wasp. J. Insect Behav. 2006;19:155–170. doi:10.1007/s10905-006-9014-7 [Google Scholar]
- Coddington J.A. The monophylyletic origin of the orb web. In: Shear W, editor. Spider webs behavior and evolution. Stanford University Press; Palo Alto, CA: 1986. pp. 319–363. [Google Scholar]
- Coddington, J. A. 2005 Phylogeny and classification of spiders. In Spiders of North America (eds D. Ubick, P. Paquin, P. E. Cushing, V. Roth). American Arachnological Society. See http://www.americanarachnology.org
- Cole B.J. Size and behavior in ants: constraints on complexity. Proc. Natl Acad. Sci. USA. 1985;82:8548–8551. doi: 10.1073/pnas.82.24.8548. doi:10.1073/pnas.82.24.8548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett T.S, Collett M. Memory use in insect visual navigation. Nat. Rev. Neurosci. 2002;3:542–552. doi: 10.1038/nrn872. doi:10.1038/nrn872 [DOI] [PubMed] [Google Scholar]
- Durst C, Eichmüller S, Menzel R. Development and experience lead to increased volumes of subcompartments of the honeybee mushroom body. Behav. Neural Biol. 1994;62:259–263. doi: 10.1016/s0163-1047(05)80025-1. doi:10.1016/S0163-1047(05)80025-1 [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Computer simulation of orb web construction. Am. Zool. 1969;9:229–238. [Google Scholar]
- Eberhard W.G. Behavioral characters for the higher classification of orb-weaving spiders. Evolution. 1982;36:1067–1095. doi: 10.1111/j.1558-5646.1982.tb05475.x. doi:10.2307/2408084 [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Effects of orb-web geometry on prey interception and retention. In: Shear W, editor. Spider webs behavior and evolution. Stanford University Press; Palo Alto, CA: 1986. pp. 70–100. [Google Scholar]
- Eberhard W.G. The effect of gravity on temporary spiral construction by the spider Leucauge mariana (Araneae: Tetragnathidae) J. Ethol. 1987a;5:29–36. doi:10.1007/BF02347892 [Google Scholar]
- Eberhard W.G. Orb webs and construction behavior in Anapidae, Symphytognathidae, and Mysmenidae. J. Arachnol. 1987b;14:339–356. [Google Scholar]
- Eberhard W.G. Behavioral flexibility in orb web construction: effects of silk supply in different glands. J. Arachnol. 1988a;16:303–320. [Google Scholar]
- Eberhard W.G. Memory of distances and directions moved as cues during temporary spiral construction in the spider Leucauge mariana (Araneae: Araneidae) J. Insect Behav. 1988b;1:51–66. doi:10.1007/BF01052503 [Google Scholar]
- Eberhard W.G. Imprecision in the behavior of larval fungus gnats and the origin of new behavior patterns in animals. J. Insect Behav. 1990;3:327–357. doi:10.1007/BF01052113 [Google Scholar]
- Eberhard W.G. Breaking the mold: behavioral imprecision and evolutionary innovation in Wendilgarda spiders (Araneae, Theridiosomatidae) Ethol. Ecol. Evol. 2000;12:223–235. [Google Scholar]
- Eisenberg J.G. University of Chicago Press; Chicago, IL: 1981. The mammalian radiations. An analysis of trends in evolution, adaptation and behavior. [Google Scholar]
- Eisenberg J.F, Wilson D.E. Relative brain size and feeding strategies in the Chiroptera. Evolution. 1978;32:740–751. doi: 10.1111/j.1558-5646.1978.tb04627.x. doi:10.2307/2407489 [DOI] [PubMed] [Google Scholar]
- Faisal A.A, White J.A, Laughlin S.B. Ion-channel noise places limits on the miniaturization of the brain's wiring. Curr. Biol. 2005;15:1143–1149. doi: 10.1016/j.cub.2005.05.056. doi:10.1016/j.cub.2005.05.056 [DOI] [PubMed] [Google Scholar]
- Griswold C.E, Coddington J.A, Hormiga G, Scharff N. Phylogeny of the orb-web building spiders (Araneae, Orbiculariae: Deinopidae, Araneoidea) Zool. J. Linn. Soc. 1998;123:1–99. doi:10.1006/zjls.1997.0125 [Google Scholar]
- Gronenberg W, Heeren S, Hölldobler B. Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. J. Exp. Biol. 1996;199:2011–2019. doi: 10.1242/jeb.199.9.2011. [DOI] [PubMed] [Google Scholar]
- Hanken J, Wake D.B. Miniaturization of body size: organismal consequences and evolutionary significance. Annu. Rev. Ecol. Syst. 1993;24:501–519. doi:10.1146/annurev.es.24.110193.002441 [Google Scholar]
- Harvey P.H, Pagel M.D. Oxford University Press; New York, NY: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Harvey P.H, Clutton-Brock T.H, Mace G.M. Brain size and ecology in small mammals and primates. Proc. Natl Acad. Sci. USA. 1980;77:4387–4389. doi: 10.1073/pnas.77.7.4387. doi:10.1073/pnas.77.7.4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy S.D, Rowe C. A critique of comparative studies of brain size. Proc. R. Soc. B. 2006;274:453–464. doi: 10.1098/rspb.2006.3748. doi:10.1098/rspb.2006.3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howse P.E. Design and function in the insect brain. In: Barton-Browne L, editor. Experimental analysis of insect behaviour. Springer; New York, NY: 1974. pp. 180–194. [Google Scholar]
- Jander U, Jander R. Allometry and resolution of bee eyes (Apoidea) Arthropod Struct. Dev. 2002;30:179–193. doi: 10.1016/s1467-8039(01)00035-4. doi:10.1016/S1467-8039(01)00035-4 [DOI] [PubMed] [Google Scholar]
- Kranz G.W. Oregon State University Bookstores; Corvallis, OR: 1971. A manual of acarology. [Google Scholar]
- Kühn-Bühlmann S, Wehner R. Age-dependent and task-related volume changes in the mushroom bodies of visually guided desert ants, Cataglyphis bicolor. J. Neurobiol. 2006;66:511–521. doi: 10.1002/neu.20235. doi:10.1002/neu.20235 [DOI] [PubMed] [Google Scholar]
- LeGuelte, L. 1967 Structure de la toile de Zygiella x-notata Cl. et facterus que regissent le comportement de l'araignee pendant la construction de la toile. PhD thesis, University of Nancy, France.
- Mares S, Ash L, Gronenberg W. Brain allometry in bumblebee and honey bee workers. Brain Behav. Evol. 2005;66:50–61. doi: 10.1159/000085047. doi:10.1159/000085047 [DOI] [PubMed] [Google Scholar]
- Mayer G. Untersuchengen ueber Herstellung und Struktur des Radnetz von Aranea diadema und Zilla x-notata mit besonderer Beruecksichtigung des Unterschiedes von Jungend- und Altersnetzen. Z. Tierpsychol. 1952;9:337–362. [Google Scholar]
- Misunami M, Yokohari F, Takahata M. Further exploration into the adaptive design of the arthropod “microbrain”: I. Sensory and mamory-processing systems. Zool. Sci. 2004;21:1141–1151. doi: 10.2108/zsj.21.1141. doi:10.2108/zsj.21.1141 [DOI] [PubMed] [Google Scholar]
- Niven J.E, Anderson J.C, Laughlin S.B. Energy-information trade-offs during signal transmission by single sensory receptors. PLoS Biol. 2007;5:e116. doi: 10.1371/journal.pbio.0050116. doi:10.1371/journal.pbio.0050116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outreman Y, Le Ralee A, Wajnberg E, Pierre J.S. Effects of within- and among-patch experiences on the patch-leaving decision rules in an insect parasitoid. Behav. Ecol. Sociobiol. 2005;58:208–217. doi:10.1007/s00265-004-0895-1 [Google Scholar]
- Petrusewiczowa E. Beobachtungen ber den Bau des Netzes der Kreuzspinne. Trav. Inst. Biol. Univ. Wilno. 1938;9:1–25. [Google Scholar]
- Platnick N.I, Shadab M. A review of the spider genera Anapisona and Pseudanapis (Araneae, Anapidae) Am. Mus. Novitat. 1979;2672:1–20. [Google Scholar]
- Reed C.F, Witt P.N, Jones R.L. The measuring function of the first legs of Araneus diadematus Cl. Behaviour. 1965;25:98–118. doi: 10.1163/156853965x00129. [DOI] [PubMed] [Google Scholar]
- Reed C.F, Witt P.N, Scarboro M.B, Peakall D.B. Experience and the orb web. Dev. Psychobiol. 1970;3:251–265. doi: 10.1002/dev.420030406. doi:10.1002/dev.420030406 [DOI] [PubMed] [Google Scholar]
- Roth G, Rottluff B, Grunwald W, Hanken J, Linke R. Miniaturization in plethodontid salamanders (Caudata: Plethodontidae) and its consequences for the brain and visual system. Biol. J. Linn. Soc. 1990;40:165–190. [Google Scholar]
- Rutowski R.L. Variation of eye size in butterflies: inter- and intraspecific patterns. J. Zool. Lond. 2000;252:187–195. [Google Scholar]
- Seid M.A, Harris K.M, Traniello J.F. Age-related changes in the number and structure of synapses in the lip region of the mushroom bodies in the ant Pheidole dentata. J. Comp. Neurobiol. 2005;488:269–277. doi: 10.1002/cne.20545. doi:10.1002/cne.20545 [DOI] [PubMed] [Google Scholar]
- Şerban M. La néotenie et la problème de la taille ches les Copépodes. Crustaceana. 1960;1:77–83. [Google Scholar]
- Spaethe J, Chittka L. Interindividual variation of eye optics and single object resolution in bumblebees. J. Exp. Biol. 2003;206:3447–3453. doi: 10.1242/jeb.00570. doi:10.1242/jeb.00570 [DOI] [PubMed] [Google Scholar]
- Treat A. Comstock Publishers Association; Ithaca, NY: 1975. Mites of moths and butterflies. [Google Scholar]
- Vollrath F. Spiders with regenerated legs can build normal webs. Nature. 1987;328:247–248. doi:10.1038/328247a0 [Google Scholar]
- Vollrath F. Analysis and interpretation of orb spider exploration and web-building behavior. Adv. Stud. Behav. 1992;21:147–199. [Google Scholar]
- Vos M, Hemerik L, Vet L.E.M. Patch exploitation by the parasitoids Cotesia rubecula and Cotesia glomerulata in multi-patch environments with different host distributions. J. Anim. Ecol. 1998;67:774–783. doi:10.1046/j.1365-2656.1998.00239.x [Google Scholar]
- Wehner R.‘Matched filters’—neural models of the external world J. Comp. Physiol. A 1611987511–531.doi:10.1007/BF006036593316619 [Google Scholar]
- Wilson E.O. The relation between caste ratios and division of labor in the ant genus Pheidole (Hymenoptera: Formicidae) Behav. Ecol. Sociobiol. 1984;16:89–98. doi:10.1007/BF00293108 [Google Scholar]
- Witt P.N. Do we live in the best of all possible worlds? Spider webs suggest an answer. Perspect. Biol. Med. 1965;8:475–487. doi: 10.1353/pbm.1965.0056. [DOI] [PubMed] [Google Scholar]
- Witt P.N, Reed C.F, Peakall D.B. Springer; New York, NY: 1968. A spider's web. Problems in regulatory biology. [Google Scholar]
- Wittenberg, G. M. & Wang, S. S.-H. In press. Evolution of dendrites. In Dendrites (eds M. Spruston & G. Stuart), 2nd edn. New York, NY: Oxford University Press.