Abstract
Recent studies suggest a beneficial role for blocking CD103 signaling in preventing islet allograft rejection and thus Type 1 diabetes (T1D) in non-obese diabetic mice (NOD). However, antibody blockade approaches generally raise anti-microbial safety issues, necessitating additional studies to address the possible adverse effects of antibody therapy. Here we report that CD103 had no significant impact on the development of primary and memory CD8+ or CD4+ responses after acute lymphocytic choriomeningitis virus (LCMV) infection. In addition, CD103 was found to be dispensable for T1D progression in a rapid, CD8-mediated virally-induced T1D model (the rat insulin promoter– [RIP]-LCMV), suggesting that its previous efficacy in the NOD mouse model may not be related to its effect on the generation, memory conversion and/or effector function of CD8+ or CD4+ T cells. While the data does not preclude a role for CD103 in T1D in its entirety, the current study does provide much evidence to suggest that CD103 blockade may prove to be a safe intervention for autoimmunity and allo-transplantation. While in cases of rapid microbial (CD8)-driven T1D CD103 antibody blockade may not limit disease progression or severity, in mucosally-driven cases of T1D anti-CD103 antibody treatment may provide a new and safe therapeutic avenue.
Keywords: Integrin CD103 (αEβ7), Type 1 Diabetes (T1D), LCMV, Insulitis, CD8+ Effector/Memory response
Introduction
The integrin CD103 (αE β7) has been found to be expressed by subsets of CD4+ and CD8+ T cells and some dendritic cells (DCs) and can bind to E-cadherin, which is expressed on a variety of cell types including intestinal epithelial cells (IECs) and Langerhans’ cells [1–4]. While CD103 is not a homing receptor, the ability of CD103 to bind to E-cadherin-expressing epithelial cells is thought to retain lymphocytes at the epithelial surface, thus playing a role in T cell homing to the gut [5–7]. Increased CD103 expression has also been found in the lungs. While the precise role of CD103 on T cells is not fully understood, studies suggest a role for CD103 in CD8+ T cell cytotoxicity through its ability to conjugate CTL (cytotoxic T-lymphocyte) effectors to E-cadherin-expressing epithelial cells in the lungs, thereby facilitating their destruction upon virus infection [8]. Similarly, CD103 was found to be expressed by tumor-infiltrating lymphocytes (TILs), playing a major role in effective tumor cell lysis [9]. In a graft-versus-host disease (GVHD) model, the destruction of the host intestinal epithelium was also dependent on CD103 [10].
Regarding the CD4 T cell compartment, reports have linked CD103− natural regulatory T cells (nTregs) to systemic regulation and CD103+ nTregs to tissue-specific regulation according to the model systems analyzed [11–13]. For example, CD103 dependency was demonstrated in Leishmania-induced dermal inflammation but not in T-cell-induced colitis [14–16]. These observations suggest that CD103 expression is not essential for the suppression of mucosal inflammation in general and moreover, the requirement for nTreg retention in the tissue can vary by organ. Interestingly, with respect to the skin, Treg cells are particularly prevalent, where they express a unique CCR4+CD103hi phenotype. Mice with a complete loss of CCR4 in the Treg compartment developed lymphocytic infiltration and severe inflammatory disease in their skin and lungs, accompanied by peripheral lymphadenopathy [17]. Thus, selectively altering Treg cell distribution in vivo can lead to the development of tissue-specific inflammatory disease. In agreement with these observations, CD103 deficient mice develop cutaneous inflammatory disease [18].
The main cell type to express CD103, apart from T cells, is the dendritic cell (DC) [19]. CD103 is expressed on almost all lamina propria (LP) DCs, a subset of mesenteric lymph node (MLN) DCs, and only on a few splenic DCs. Gut-associated lymphoid tissue (GALT) DCs possess a unique ability to generate CCR9+ α4β7+ gut-tropic CD8+ T cells. CD103+ and CD103− GALT-DCs represent functionally distinct subsets, in which CD103 expression on DCs is a prerequisite for the generation of gut-tropic CCR9+α4β7+ CD8+ T cells [19]. Overall, CD103 is thought to be important for intraepithelial lymphocyte (IEL) localization and/or function. Furthermore, CD103 is involved in the expansion and recruitment of TCRαβ+ CD8+ IEL following microbial colonization [20]. Recently, a connection between CD103-expressing DCs and Tregs was made: CD103+ MLN and LP DCs are responsible for inducing the development of Foxp3+ Treg cells in the gut [21]. Importantly, this is dependent on TGF-β1 and the dietary metabolite, retinoic acid (RA), and influences the balance between effector and regulatory T cell activity in the intestine [22].
While little is known about the relationship between CD103 expression and Type 1 diabetes (T1D) pathogenesis, several studies suggest a possible link. Few T cells infiltrating the pancreas in NOD mice express CD103 [23]. Studies involving blocking antibodies to all integrins (α4, β2, and β7) and their respective ligands (table 1) showed a defect in T cell penetrating the pancreatic islets [24]. Similar observations were made after pancreatic islet allo-transplantation, where allografts survived indefinitely in CD103 deficient hosts [25]. CD8 cells in CD103 deficient hosts exhibited normal effector responses to donor alloantigens in vitro and trafficked normally to the graft site in vivo, but strikingly failed to infiltrate the islet allograft itself.
Table 1.
Integrin family members and their ligands.
| Integrin | Other commonly used name | Principal ligands |
|---|---|---|
| αEβ7 | CD103 | E-cadherin |
| α4β7 | LPAM-1 | MAdCAM-1 |
| α4β1 | VLA-4 | VCAM-1, fibronectin |
| αLβ2 | LFA-1 | ICAM-1,-2,-3,-4,-5 |
The previous results suggested CD103 as a target for intervening with T1D development. In order to assess its influence on T1D progression in a virally-induced T1D model, CD103 deficient mice were crossed to rat insulin promoter- lymphocytic choriomeningitis virus (RIP-LCMV) transgenic mice. Interestingly, upon acute infection with LCMV Armstrong, CD103 deficiency revealed no significant effect on LCMV-specific effector and memory CD8+ or CD4+ responses or on T1D progression. Although these results suggest a minimal effect for CD103 blockade in preventing a solely CD8-mediated virally-induced T1D, they also provide evidence for CD103 blockade safety. This is the first report to demonstrate that CD103 does not adversely effect the development of either effector or memory CD4+ or CD8+ T cell populations after an acute viral infection and as such, CD103 blockade may be proven of therapeutic value in other immune-based disorders, in which a beneficial outcome can be anticipated.
Methods
Mice
Mice deficient in CD103 (CD103−/−) were generated and backcrossed on C57BL/6 background and kindly provided by Dr Bromberg JS [20]. CD103−/− mice were crossed to RIP-GP and RIP-NP to obtain CD103+/− x RIP-GP mice. These mice were intercrossed to obtain CD103+/+, CD103+/−, and CD103− − x RIP-GP. In some experiments, non-transgenic CD103− − or +/− and +/+ littermate control mice were used. Mice were housed under specific pathogen-free conditions at La Jolla Institute for Allergy and Immunology.
Viruses
LCMV Armstrong was used throughout all experiments. 8–14-wk-old mice were infected with a single dose of 104 PFU i.p.
Blood Glucose Values
Blood glucose was monitored with OneTouch Ultra at weekly intervals. Diabetes was defined as blood glucose values (BGVs) over 300 mg/dl.
Peptides
Peptides used for viral studies were the dominant Db-restricted LCMV epitopes GP33–41 and NP396–404 as well as an I-Ab–restricted epitope GP61–80 (all from Abgent).
Immunohistochemistry
Pancreata were immersed in Tissue-Tek OCT (Bayer) and quick-frozen on dry ice. Using cryomicrotome and Superfrost Plus slides (Fisher Scientific), 6 μm tissue sections were cut. Sections were fixed with 100% acetone for 15 minutes and dried for 1 hour at room temperature, and after rehydrating in TBS, an avidin/biotin-blocking step was included (Vector Laboratories). To detect insulin and CD4 or CD8 expression in pancreatic sections, primary Abs (guinea pig anti-swine insulin from Dako [dilution 1:300], anti-CD4 (RM4.5) or anti-CD8a (Ly-2) IHC from BD-Pharmingen [dilution 1:50]) were applied to frozen tissue sections. Primary and biotinylated (Vector Laboratories) secondary antibodies were incubated with the sections for 60 min each, and color reaction was obtained by sequential incubation with avidin–peroxidase conjugate and AEC (3-amino-9-ethylcarbazole) or alkaline phosphatase conjugate and Vector Blue AP III (SK-5300) (Vector Laboratories).
Flow Cytometry
For intracellular stains, single cell suspensions were restimulated for 3 h with 1 μg/ml MHC class I–restricted viral peptides, 2 μg/ml MHC class II–restricted viral peptides in the presence of brefeldin A (Sigma-Aldrich). Cells were stained for surface expression of CD4 and CD8, fixed, permeabilized, and stained for intracellular IFNγ and TNF. DbGP33 and DbNP396 MHC class I pentamers were purchased from Proimmune. Cells were acquired on a LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software.
Statistical analysis
Data are expressed as a mean ± SD. The statistical significance of the difference between means was determined using the 2-tailed Student’s t test or the log-rank test. *, p < 0.05, **, p<0.01, ***, p<0.001.
Results
Normal Development of T1D in CD103–deficient mice
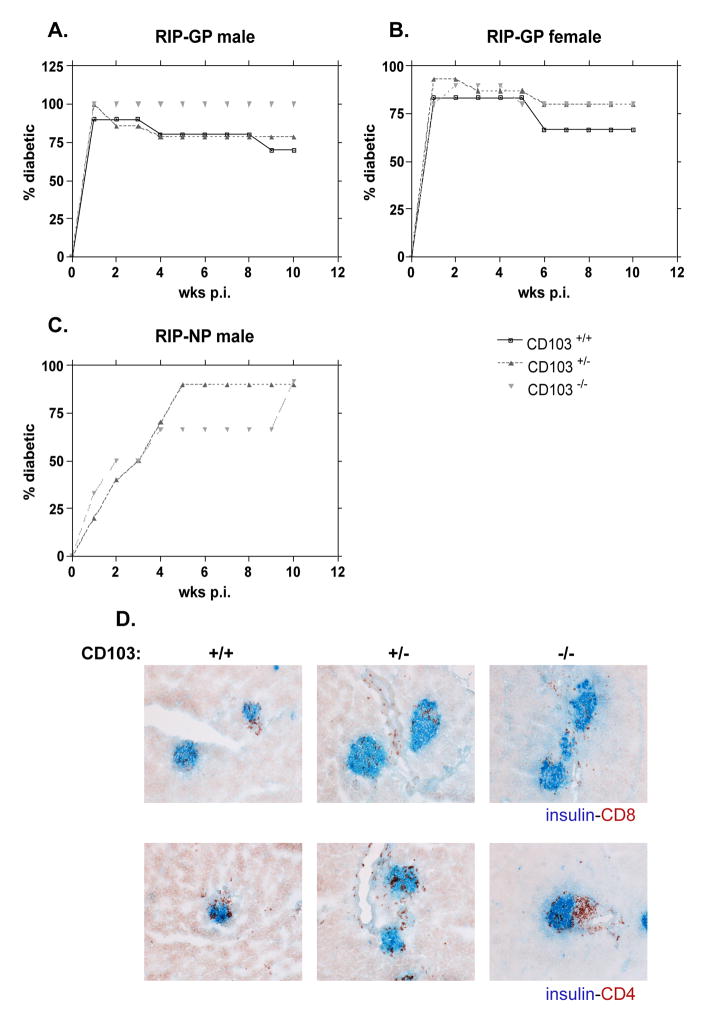
In order to examine the role for CD103 in T1D, CD103−/− mice were crossed to RIP-GP and RIP-NP transgenic mice. CD103+/+, CD103+/− or CD103−/− x RIP-GP or RIP-NP littermates were infected with 104 PFU LCMV Armstrong to induce diabetes. BGVs were measured weekly, and by 2 wks post infection (p.i.), 80–100% of RIP-GP mice, irrespective of CD103 expression and gender, were diabetic (Fig. 1A and B), with average BGVs of ~500 mg/dl. In these experiments, a small percentage of the diabetic mice reverted to normal BGVs after >4 wks. In RIP-GP and most profoundly in male, diabetes reverted in more CD103+/+ than CD103−/− x RIP-GP mice suggesting a possible role for CD103 expression in this phenomenon. Histological examination of pancreatic tissues derived from diabetic mice infected 10 wks prior showed increased CD4+ and CD8+ infiltrates, which did not differ among groups (Fig. 1D). In accordance with these findings, diabetes incidence did not differ between CD103+/− and CD103−/− x RIP-NP male mice as shown in Fig. 1C. These results show that elimination of CD103 does not result in any significant effect on diabetes onset/severity and may be partially involved in diabetes reversal.
Figure 1. CD103 has no effect on diabetes course in RIP-LCMV mice (RIP-GP and RIP-NP).
CD103+/+, CD103+/−, or CD103−/− x RIP-GP or x RIP-NP mice were infected i.p. with 104 PFU LCMV Armstrong. Incidence of diabetes in males (A) or females RIP-GP (B) or males RIP-NP (C) is shown. (RIP-GP males, n: +/+=10, +/−=14, −/−=4, females, n: +/+=5, +/−=15, −/−=10, RIP-NP males, n: +/−=10, −/−=12). Mice were considered diabetic when BGVs were >300 mg/dl. Degree of insulitis was determined after staining pancreatic sections 10 wks p.i. with insulin-CD8 or insulin-CD4 (D). One representative section of islets from each group of mice (3-4 mice per group) is shown. Histological analysis was performed in tissues from the diabetic mice.
No significant Defect in Effector CD8+ or CD4+ responses in CD103–deficient Mice
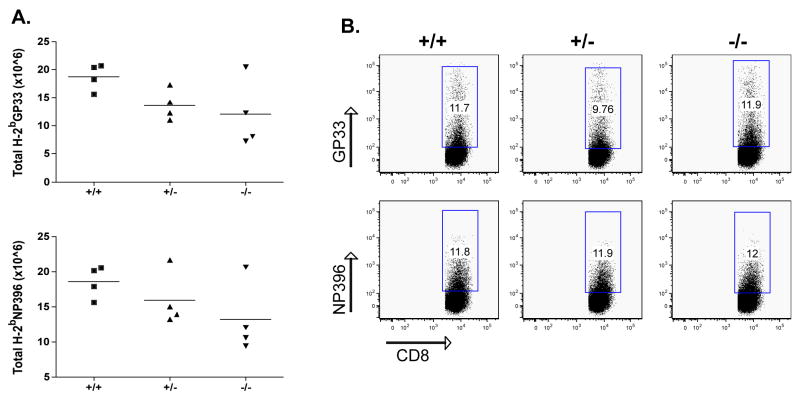
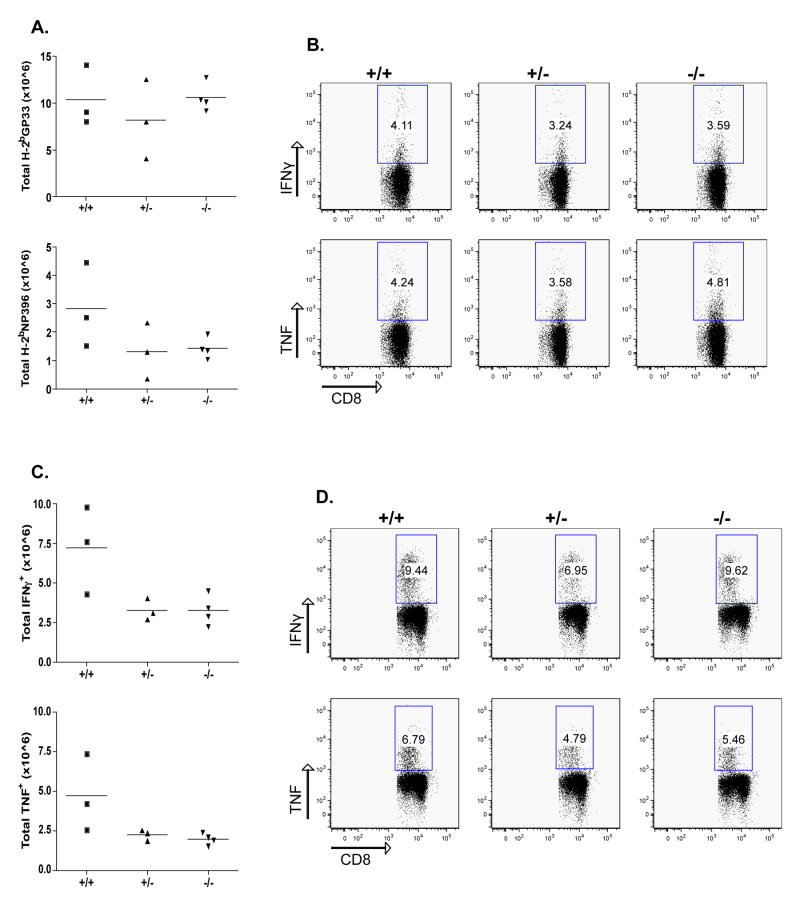
The CD8 immune response to LCMV is characterized by an initial primary expansion of LCMV-specific, IFNγ positive T cells, peaking at ~7–8 d after infection. This is followed by a contraction phase leaving a resting pool of LCMV memory cytotoxic lymphocytes (CTLs). The memory CD8+ T cells are maintained at a similar frequency for a long period of time. The frequency of LCMV-specific CD8+ lymphocytes was evaluated on day 8 after infection in the blood. As shown in Fig. 2A and B, no difference in the frequency of CD8-GP33 and NP396 pentamer positive T cells was seen in the absence of endogenous CD103 expression.
Figure 2. No reduction of effector CD8 T cells in the absence of CD103.
CD103+/+, CD103+/−, and CD103−/− mice were infected with 104 PFU LCMV and spleens were collected and analyzed 8 days after infection. Cells were stained for the presence of GP33 and NP396 pentamers and analyzed by flow cytometry. The total number of GP33 and NP396 cells was calculated after multiplication of their frequency with the total spleen cell number (A). The GP33 or NP396 cell frequency (B) was evaluated after gating in the CD8+CD19− cell population. Differences are not statistically significant.
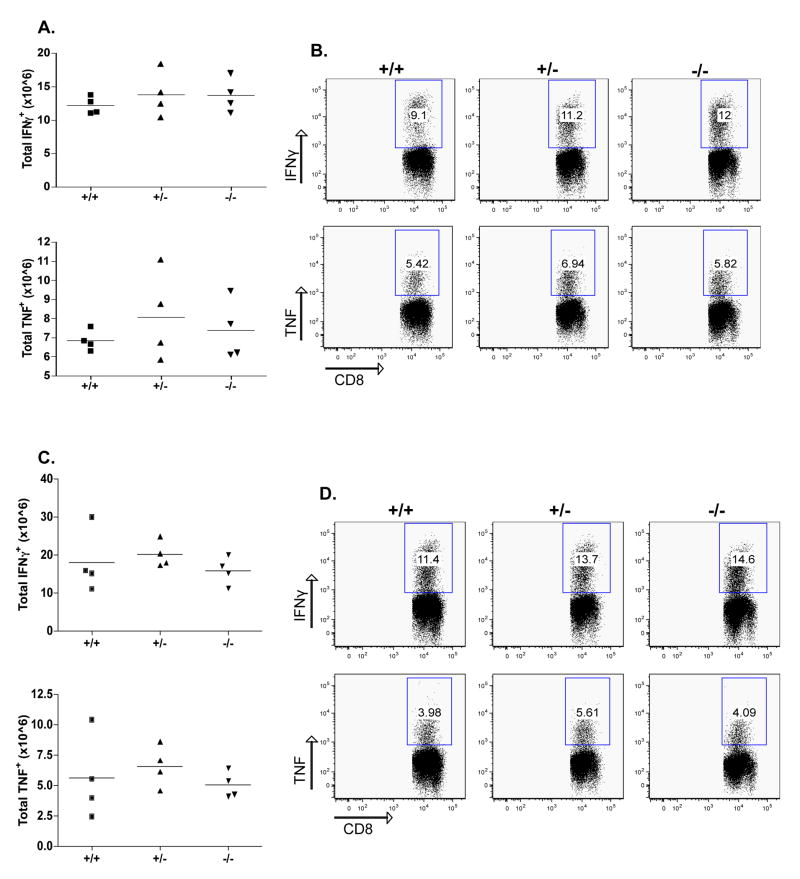
In addition, as shown in Fig. 3, intracellular cytokine staining following stimulation with class I restricted epitopes GP33 and NP396 showed no difference in IFNγ and TNF production. Compared to CD103+/+ mice, neither CD103+/− nor CD103−/− mice exhibited any differences in GP61 (class II)-specific response (Fig. 4). Thus, effector functions of LCMV-specific autoaggressive CD4+ and CD8+ lymphocytes were not different in CD103–deficient RIP-GP mice. The absence of differences in the primary CD8+ T cell response was not entirely unexpected, as LCMV-specific primary CD8+ responses occur independently of many immunological parameters, including B7, OX40, CD4 help, CD28, and CD40L [26–31].
Figure 3. No alteration of the effector CD8 T cell response in the absence of CD103.
CD103+/+, CD103+/−, and CD103−/− mice were infected with 104 PFU LCMV and spleens were collected and analyzed 8 days after infection. Spleen cells were cultured with the LCMV MHC-I peptides GP33 (A) and NP396 (B) before staining for intracellular IFNγ of TNF. The total number of antigen-specific cells per spleen was calculated by multiplying the percent CD8/IFNγ double positive cells by the total number of cells isolated from the spleen of each mouse. Representative data are from one out of two experiments. Differences are not statistically significant.
Figure 4. No alteration of the effector CD4 T cell response in the absence of CD103.
Same as Fig. 3; Spleen cells were cultured with the LCMV MHC-II peptide GP61. Differences are not statistically significant.
No Defect in Memory CD8+ or CD4+ lymphocytes in CD103–deficient Mice
Since most of the previous studies have demonstrated that the primary immune response on day 8 does not correlate with the course or incidence of diabetes development, while effector/memory cells do, the frequency of LCMV-specific memory CD8+ lymphocytes was evaluated 45 days post infection. Antiviral (‘self’) specific memory CD8+ T cells were unaffected by the lack of CD103 45 days after LCMV infection. Splenocytes were stained with H-2Db pentamers loaded with GP33 or NP396 peptides (Fig. 5A and B). In order to ensure that there was no difference in the actual numbers of LCMV-specific CD8+ memory T cells, IFNγ+ and TNFα+ cells were quantified by intracellular cytokine staining (Fig. 5C and D, GP33 data not shown).
Figure 5. No reduction of memory CD8 T cells and their response in the absence of CD103.
CD103+/+, CD103+/−, and CD103−/− mice were infected with 104 PFU LCMV and spleens were collected and analyzed 45 days after infection. A-B: Cells were stained for the presence of GP33 and NP396 pentamers and analyzed by flow cytometry. The total number of GP33 and NP396 cells was calculated after multiplication of their frequency with the total spleen number (A). The GP33 or NP396 cell frequency (B) was evaluated after gating in the CD8+CD19− cell population. C-D: Spleen cells were cultured with the LCMV MHC-I peptides GP33 (not shown) and NP396 before staining for intracellular IFNγ of TNF. The total number of antigen-specific cells per spleen was calculated by multiplying the percent CD8/IFNγ double positive cells by the total number of cells isolated from the spleen of each mouse. Representative data are from one out of two experiments. Differences are not statistically significant.
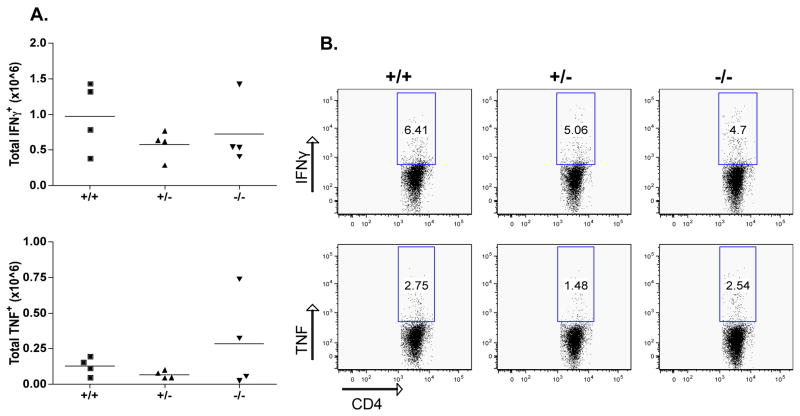
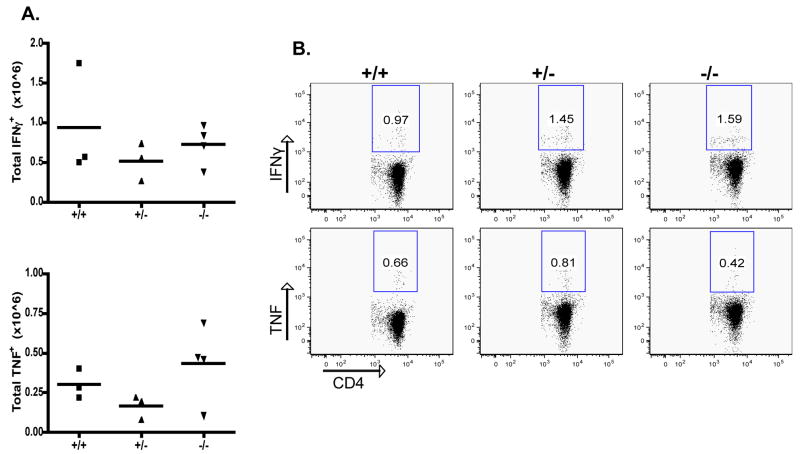
The ability of CD103−/− CD4+ memory T cells to produce IFNγ and TNF in response to GP61 re-stimulation in vitro was also examined. As shown in Fig. 6, no differences in their ability to produce either cytokine were found, correlating well with the absence of any effect of CD103 deficiency in T1D. Thus, antigen-specific CD8+ and CD4+ effector/memory responses in CD103–deficient mice were not impaired.
Figure 6. No change of the memory CD4 T cell response in the absence of CD103.
Same as Fig. 5; spleen cells were cultured with the LCMV MHC-II peptide GP61.
Discussion
CD103, initially considered to play a role almost exclusively in the GALT, was later found to participate in many diverse immune responses, including a role in CTL-mediated lung epithelial cell destruction during influenza virus infection [8], in renal allograft rejection [32], in Treg trafficking and control of Leishmania major infection [16], in GVHD [33] and colitis [34] development, etc. Relevant to T1D pathogenesis, CD103+ lymphocytes were shown to accumulate early in the pancreas of NOD mice. Blocking antibodies to α4, β2, and β7 integrins and their respective ligands resulted in a defect in the ability of T cells to penetrate the pancreatic islets and islet allo-grafts [23, 25]. These studies highlighted an important role for CD103 in mediating a crucial checkpoint in pancreatic islet destruction. In this article, a minimal role for CD103 in the course of virally-induced auto-immune diabetes was demonstrated. We provide evidence that CD103 does not influence the generation, migration, tissue entry and effector functions of LCMV-specific (‘autoreactive’) CD8+ T cells. Therefore, the beneficial effects of CD103 blockade observed in previous studies may have been mediated by mechanisms other than the control of CD4+ or CD8+ pathogenic effectors’ function.
CD103, like other integrins, provides T cells with co-stimulatory signals upon ligation with its principal ligand, E-cadherin. Interestingly, in the RIP-LCMV model, upon virus infection lymphocyte function-associated antigen (LFA-1) upregulation in pancreatic β cells and intercellular adhesion molecule (ICAM-1) upregulation on effector T cells [35, 36] may have compensated for CD103-E-cadherin ligation in T cell proliferation and effector function. Currently, there is no evidence to suggest that CD103 has unique signaling properties that cannot be substituted by LFA-1. In agreement, in the islet allograft rejection study, CD8+CD103−/− cells were able to accumulate at the graft site and were immunologically competent [25]. However, during infection with recombinant vesicular stomatitis virus, CD103 was critical for the migration of activated CD8+ T cells to the MLN and Peyer's patches at a later stage of the response, signifying its importance in gut-associated immunological events [37].
In the gut, DCs represent the population of cells in which CD103 expression is strongly related to their function. CD103+ MLN and small intestine (SI)-LP DCs express greater aldh1a2 levels compared to CD103− MLN DCs or DCs located elsewhere promoting the induction of CCR9 and Foxp3+ Treg cell differentiation through metabolizing vitamin A to RA [14, 22]. Intriguingly though, CD103−/− MLN DCs were as efficient as CD103+/+ MLN DCs at inducing CCR9 [21]. Therefore, although CD103 efficiently discriminates DCs according to their T-cell induction/differentiation capacities, CD103 expression is not requisite for acquiring and/or executing them. Although these results signify the importance of CD103 in gut-associated responses, they also explain why CD103 signaling can have variable effects depending on the system analyzed. While in the RIP-LCMV model T1D development was not significantly impaired in the absence of endogenous CD103 after viral infection, CD103 may well affect the outcome in mucosally driven cases of T1D.
Of relevance and importance to T1D, changes in the environment, including gut microflora, can influence disease pathogenesis. Recently it was shown that under specific pathogen-free housing, NOD mice lacking MyD88 protein (an adaptor for multiple innate immune receptors that recognize microbial stimuli) do not develop T1D. This is believed to be dependent on commensal microbes as germ-free MyD88-negative NOD mice developed robust diabetes due to their genetic susceptibility [38]. Our current and previously published findings suggest that molecules including the ones derived from the endemic gut microflora influence the course of autoimmune disease differently, depending on the mode of disease induction and other external parameters. Therefore, the finding that CD103 is dispensable for virally-induced T1D, does not preclude a role for CD103 in T1D in general. Additionally, and perhaps more important is the finding that CD103 blockade does not hinder the immunological response towards an acute infection, and as such has significant therapeutic potential in other settings as GVHD where CD103 clearly contributes to disease progression.
CD103 expression on MLN DCs and perhaps Tregs is of primary importance in driving T cell responses to orally administered soluble antigen [21]. Relevant to T1D, oral insulin and GAD65 administration have proven beneficial in preventing disease development in the NOD and RIP-LCMV diabetes models [39, 40 and not published]. Determining the precise role of CD103+ MLN DC–mediated antigen tolerance will likely require further analysis, especially in mice selectively depleted of this unique subset of DCs. CD103+ Tregs on the other hand, have been shown to directly migrate to the GVHD target tissue controlling inflammation even at more advanced stages of the disease [33]. In the current report CD103 deficient mice displayed an exacerbated disease course, as fewer mice reverted back to normoglycaemia after the first four weeks post infection. A defect in Treg migration and/or function within the islet infiltrate in CD103-deficient mice may have been responsible for the observed reduction in the T1D reversal rate. Whether CD103+ Tregs play any role in diabetes progression and/or reversal needs to be further evaluated. In addition, it is possible that CD103 contributed to islet regeneration through an unknown mechanism, since it is known that islet cells express E-cadherin [41].
In conclusion, our observations provide evidence that CD103 deficiency does not significantly alter CD8+ or CD4+ responses during the course of an acute viral infection and does not inhibit the ability of autoreactive T cells to infiltrate and destroy islets in a virally-induced diabetes setting. Thus, our findings suggest that while blocking CD103 activity may not be an effective treatment in preventing virally-induced CD8-mediated T1D, it may be of therapeutic value in other diseases in which CD103 is clearly linked to their progression, including perhaps in mucosally driven cases of T1D.
Acknowledgments
We would like to thank Malina McClure for excellent mouse breeding, Carmen Baca Jones for critically reading the manuscript and Shaida Omid for preparing histological sections from frozen samples. This work was supported by the Brehm coalition and by funds from U01DK. Georgia Fousteri is an American Heart Association (AHA) scholar awardee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrew DP, Rott LS, Kilshaw PJ, Butcher EC. Distribution of alpha 4 beta 7 and alpha E beta 7 integrins on thymocytes, intestinal epithelial lymphocytes and peripheral lymphocytes. Eur J Immunol. 1996;26:897–905. doi: 10.1002/eji.1830260427. [DOI] [PubMed] [Google Scholar]
- 2.Cepek KL, Parker CM, Madara JL, Brenner MB. Integrin alpha E beta 7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150:3459–3470. [PubMed] [Google Scholar]
- 3.Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- 4.Kilshaw PJ. Expression of the mucosal T cell integrin alpha M290 beta 7 by a major subpopulation of dendritic cells in mice. Eur J Immunol. 1993;23:3365–3368. doi: 10.1002/eji.1830231246. [DOI] [PubMed] [Google Scholar]
- 5.Agace WW, Higgins JM, Sadasivan B, Brenner MB, Parker CM. T-lymphocyte-epithelial-cell interactions: integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr Opin Cell Biol. 2000;12:563–568. doi: 10.1016/s0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 6.Brown DW, Furness J, Speight PM, et al. Mechanisms of binding of cutaneous lymphocyte-associated antigen-positive and alphaebeta7-positive lymphocytes to oral and skin keratinocytes. Immunology. 1999;98:9–15. doi: 10.1046/j.1365-2567.1999.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauch UG, Mueller RC, Li XY, et al. Integrin alpha E(CD103)beta 7 mediates adhesion to intestinal microvascular endothelial cell lines via an E-cadherin-independent interaction. J Immunol. 2001;166:3506–3514. doi: 10.4049/jimmunol.166.5.3506. [DOI] [PubMed] [Google Scholar]
- 8.Smyth LJ, Kirby JA, Cunningham AC. Role of the mucosal integrin alpha(E)(CD103)beta(7) in tissue-restricted cytotoxicity. Clin Exp Immunol. 2007;149:162–170. doi: 10.1111/j.1365-2249.2007.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Floc'h A, Jalil A, Vergnon I, et al. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Asady R, Yuan R, Liu K, et al. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann J, Huehn J, de la Rosa M, et al. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc Natl Acad Sci U S A. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huehn J, Siegmund K, Lehmann JC, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annacker O, Coombes JL, Malmstrom V, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banz A, Peixoto A, Pontoux C, et al. A unique subpopulation of CD4+ regulatory T cells controls wasting disease, IL-10 secretion and T cell homeostasis. Eur J Immunol. 2003;33:2419–2428. doi: 10.1002/eji.200324205. [DOI] [PubMed] [Google Scholar]
- 16.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174:5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 17.Sather BD, Treuting P, Perdue N, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schon MP, Schon M, Warren HB, Donohue JP, Parker CM. Cutaneous inflammatory disorder in integrin alphaE (CD103)-deficient mice. J Immunol. 2000;165:6583–6589. doi: 10.4049/jimmunol.165.11.6583. [DOI] [PubMed] [Google Scholar]
- 19.Johansson-Lindbom B, Svensson M, Pabst O, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schon MP, Arya A, Murphy EA, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 21.Jaensson E, Uronen-Hansson H, Pabst O, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanninen A, Salmi M, Simell O, Jalkanen S. Mucosa-associated (beta 7-integrinhigh) lymphocytes accumulate early in the pancreas of NOD mice and show aberrant recirculation behavior. Diabetes. 1996;45:1173–1180. doi: 10.2337/diab.45.9.1173. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, Issekutz TB, Wright JR., Jr Prolongation of rat islet allograft survival by treatment with monoclonal antibodies against VLA-4 and LFA-1. Transplantation. 1995;60:71–76. doi: 10.1097/00007890-199507150-00014. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Wang D, Yuan R, et al. CD103 expression is required for destruction of pancreatic islet allografts by CD8(+) T cells. J Exp Med. 2002;196:877–886. doi: 10.1084/jem.20020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreasen SO, Christensen JE, Marker O, Thomsen AR. Role of CD40 ligand and CD28 in induction and maintenance of antiviral CD8+ effector T cell responses. J Immunol. 2000;164:3689–3697. doi: 10.4049/jimmunol.164.7.3689. [DOI] [PubMed] [Google Scholar]
- 27.Bachmann MF, Schwarz K, Wolint P, et al. Cutting edge: distinct roles for T help and CD40/CD40 ligand in regulating differentiation of proliferation-competent memory CD8+ T cells. J Immunol. 2004;173:2217–2221. doi: 10.4049/jimmunol.173.4.2217. [DOI] [PubMed] [Google Scholar]
- 28.Borrow P, Tishon A, Lee S, et al. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopf M, Ruedl C, Schmitz N, et al. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 30.Suresh M, Whitmire JK, Harrington LE, et al. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 31.von Herrath MG, Yokoyama M, Dockter J, Oldstone MB, Whitton JL. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadley GA, Rostapshova EA, Gomolka DM, et al. Regulation of the epithelial cell-specific integrin, CD103, by human CD8+ cytolytic T lymphocytes. Transplantation. 1999;67:1418–1425. doi: 10.1097/00007890-199906150-00005. [DOI] [PubMed] [Google Scholar]
- 33.Zhao D, Zhang C, Yi T, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112:2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludviksson BR, Strober W, Nishikomori R, Hasan SK, Ehrhardt RO. Administration of mAb against alpha E beta 7 prevents and ameliorates immunization-induced colitis in IL-2−/− mice. J Immunol. 1999;162:4975–4982. [PubMed] [Google Scholar]
- 35.Andersson EC, Christensen JP, Scheynius A, Marker O, Thomsen AR. Lymphocytic choriomeningitis virus infection is associated with long-standing perturbation of LFA-1 expression on CD8+ T cells. Scand J Immunol. 1995;42:110–118. doi: 10.1111/j.1365-3083.1995.tb03633.x. [DOI] [PubMed] [Google Scholar]
- 36.Marker O, Scheynius A, Christensen JP, Thomsen AR. Virus-activated T cells regulate expression of adhesion molecules on endothelial cells in sites of infection. J Neuroimmunol. 1995;62:35–42. doi: 10.1016/0165-5728(95)00099-n. [DOI] [PubMed] [Google Scholar]
- 37.Lefrancois L, Parker CM, Olson S, et al. The role of beta7 integrins in CD8 T cell trafficking during an antiviral immune response. J Exp Med. 1999;189:1631–1638. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Homann D, Dyrberg T, Petersen J, Oldstone MB, von Herrath MG. Insulin in oral immune "tolerance": a one-amino acid change in the B chain makes the difference. J Immunol. 1999;163:1833–1838. [PubMed] [Google Scholar]
- 40.Ma S, Huang Y, Yin Z, et al. Induction of oral tolerance to prevent diabetes with transgenic plants requires glutamic acid decarboxylase (GAD) and IL-4. Proc Natl Acad Sci U S A. 2004;101:5680–5685. doi: 10.1073/pnas.0307420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosco D, Rouiller DG, Halban PA. Differential expression of E-cadherin at the surface of rat beta-cells as a marker of functional heterogeneity. J Endocrinol. 2007;194:21–29. doi: 10.1677/JOE-06-0169. [DOI] [PubMed] [Google Scholar]