Abstract
Setting
Laparoscopic adjustable gastric banding (LAGB) and Laparoscopic Roux-en-Y gastric bypass (LRYGB) are the two most commonly performed bariatric procedures. While both procedures likely reduce healthcare expenditures related to the resolution of comorbid conditions, they have different rates of perioperative risks and differential rates of associated weight loss.
Objective
We designed a model to evaluate the incremental cost-effectiveness (ICER) of these procedures compared to non-operative weight loss interventions and to each other.
Methods
Deterministic, payer-perspective model comparing the lifetime expected costs and outcomes of LAGB, LRYGB and non-surgical treatment. The major endpoints were survival, health related quality of life and weight loss. Life expectancy and lifetime medical costs were calculated across age, sex and body mass index (BMI) strata using previously published data.
Results
For both men and women LRYGB and LAGB were cost-effective at less than $25,000/QALY even when evaluating the full range of baseline BMI and estimates of adverse outcomes, weight loss and costs. For base-case scenarios in men (age 35, BMI 40) the ICER was $11,604 per QALY for LAGB, compared to $18,543 per QALY for LRYGB. For base-case scenarios in women (age 35, BMI 40) the ICER was $8,878 per QALY for LAGB, compared to $14,680 per QALY for LRYGB.
Conclusions
Modeled cost-effectiveness analysis showed that both operative interventions for morbid obesity, LAGB and RYGB, were cost-effective at less than $25,000, and LAGB was more cost-effective than RYGB for all the base-case scenarios.
Keywords: Laparoscopic adjustable gastric banding, Laparoscopic Roux-en-Y gastric bypass, Cost effectiveness analyses, Quality-adjusted life-years
Introduction
Obesity is one of the most common causes of preventable death with over 400,000 deaths per year, an increase of 33% over the past decade1. The total healthcare costs for obesity related-issues has been estimated to be over $90 billion per year, approximately 9% of total US healthcare expenditures2. Obesity is largely refractory to non-operative interventions3, but generally responsive to operative interventions4. Laparoscopic adjustable gastric banding (LAGB) and Laparoscopic Roux-en-Y gastric bypass (LRYGB) are the two most commonly performed bariatric procedures. The perioperative risks are lower for LAGB but patients undergoing LRYGB may have more significant weight loss in a shorter period of time4. Prior studies suggest that both procedures improve quality of life, reduce healthcare expenditures and improve life expectancy. Given the differential risk and effectiveness of the procedures the aim of this study was to evaluate the cost-effectiveness of surgical [laparoscopic Roux-en Y gastric bypass (LRYGB) and adjustable gastric banding (LAGB)] and non-surgical weight loss interventions.
Methods
Model
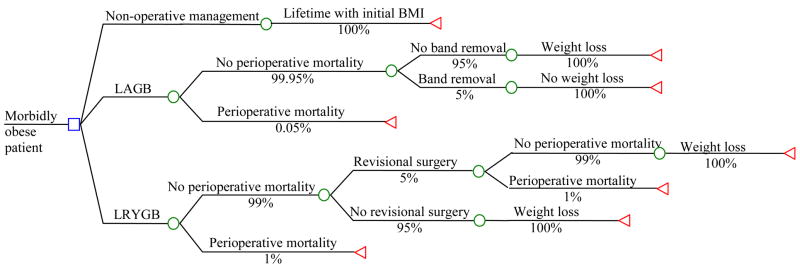
We developed a deterministic, payer-perspective decision analytic model to compare the lifetime expected costs and outcomes of LAGB, LRYGB and non-surgical treatment (Figure 1). This model was derived from a previously published model5 that involves discrete outcomes at points after surgery. In general, the model describes the possible pathways within the first three years in order to get the age and BMI of survivors at the end the three year period. Base case scenarios included morbidly obese male and female patients without obesity-related comorbidities with body mass index (BMI) 40, 50 and 60 kg/M2, ages 35, 45 and 55 years-old. Patients not undergoing operative interventions were assumed to have a stable BMI over time (rather than gaining weight as is expected) in order not to over-estimate the benefits of surgery. The major endpoints were survival and weight loss. Surviving patients could require additional surgical interventions and might undergo band removal. We assumed that 3 years after the initial operation the patient remained in his initial weight, lost weight, or died. If the band was removed, we assumed the patient remained at their initial BMI and accumulated lifetime cost and health outcomes as in the no-treatment group for that BMI. Successful patients incurred additional costs related to abdominoplasty and cholecystectomy. Postoperative complications were not modeled since they are not likely to have a long-term impact on quality of life, and modeling them would be impractical. Rather, treatment of postoperative complications was assumed into usual care medical costs associated with surgery.
Figure 1. Deterministic decision analytic model of 3 year operative and non-operative interventions for morbid obesity.
The square represents a decision node, the circles represent probability nodes and the triangles represent end nodes. The text above the lines describes the clinical event and the percentage under it represents the probability of the event.
Life expectancy and costs
Life expectancy and lifetime medical costs were calculated across age, sex and body mass index (BMI) strata using previously published data from the Framingham heart study and National Health and Nutrition Examination Survey (NHANES) III6. Since data available in these studies does not include patients with BMI greater than 37.5, we applied a simple linear approximation to these estimates to assess the effects of obesity on life expectancy and costs for BMI 40–60. We included usual care medical costs (in U.S. 2004 Dollars) associated with the surgery, including procedural fees, treatment of post-operative complications, follow-up care, and treatment of obesity-related diseases, such as coronary heart disease, stroke, type 2 diabetes, hypercholesterolemia, and hypertension. All cost estimates were adjusted for inflation; the Medical Care Component of the Consumer Price Index for All Urban Consumers was used to adjust prices, when necessary. Expected lifetime medical cost estimates were obtained from the published literature6. For the majority of the remaining costs, estimates of nationally representative hospital charges (Table 1) were obtained from the Healthcare Cost and Utilization Project and expert opinion. We obtained the cost of medications and follow-up visits from a source on wholesale drug prices7.
Table 1. Probabilities and costs over three years.
| LAGB | LRYGB | Cost | |
|---|---|---|---|
| EBWL | 55% (38–64%)20–24 | 71% (59–89%)25–35 | n/a |
| LAGB | - | - | $16,200* |
| LRYGB | - | - | $27,560* |
| Operative mortality | 0.05%8 (0–1%) | 1% (0.5–2%) 8, 36 | |
| Band adjustments | 10 adjustments | n/a | $150** |
| Revisional surgery – LRYGB | n/a | 5% (1–10%)30, 37, 38 | $10,000** |
| Revisional surgery – LAGB | 5% (2–7%)20, 39, 40 | n/a | $5,000** |
| Band removal | 5% (0–10%)** | n/a | $6,000** |
| Perioperative mortality (revision surgery) | 0.05% (0–1%)** | 1% (0.5–2%)** | |
| Minor wound infection | 2%* | 5%* | $204* |
| Major wound infection | 0.5% * | 3%* | $11,236* |
| DVT | 0.5%8 | 2.6%8 | $9,222* |
| Non fatal PE | 0.1%* | 1%* | $15,582* |
| Follow up visit (other than adjustment) | 0** | 6** | $159** |
| Dietary supplements | n/a | 100% | $7241 |
| Leak-nonoperative LRYGB | n/a | 3% (1–5%)** | $50,000** |
| Lap cholecystectomy | 7.5% (5–25%)* | 11.4% (5–30%)* | $16,000* |
| Incisional hernia repair | 0.5% (0.1–2.5%)* | 1.7% (0.1–5%)* | $14,416* |
| Abdominoplasty | 39% (35–45%)* | 39% (35–45%)* | $13,992* |
LAGB – laparoscopic adjustable gastric banding
LRYGB – laparoscopic Roux-en-Y gastric bypass
EBWL – excess body weight loss
DVT – deep venous thrombosis
PE – pulmonary embolism
n/a – non applicable
Healthcare Cost and Utilization Project (HCUP)
Expert opinion
Utilities
We assumed that a person who loses weight and drops to a lower BMI has the same health related quality of life as someone who is at that lower BMI at baseline. Utilities based on patient sex, age and BMI were derived from the 1997 National Health Interview Survey, previously published by Craig and Tseng5. Within each BMI strata utilities linearly declined with patient age and this was accounted for within the base case analyses.
Probabilities
Probabilities of clinical events and outcomes of surgical procedures were derived from a comprehensive literature review8. Base-case estimates were derived from the average of reported values. Excess body weight loss (EBWL) was estimated using studies with 36 months of follow-up. Quality-adjusted life-years (QALYs) and costs were discounted at 3%. The incremental cost-effectiveness ratio (ICER) of competing surgical strategies compared to non-surgical strategies was calculated for the full range in the target population.
Sensitivity analysis
One-way sensitivity analysis was performed for all variables in the decision model to determine the impact of uncertainty in the base-case assumptions on the costs, outcomes, and the incremental cost-effectiveness ratios when comparing the strategies LAGB versus LRYGB. The purpose of the sensitivity analysis was twofold: to investigate the robustness of the base-case estimates and to determine which factors influence the ICER, favoring LAGB vs. LRYGB. A tornado diagram was created using selected variables that had the greatest influence on the model. Several two-way sensitivity analyses were performed for combinations of particularly influential variables. The values used for the one-way sensitivity analyses for the clinical probabilities included the range that was found in the literature. When estimates were derived from expert opinion, a wide range of probabilities was used to evaluate the impact of this parameter. Costs varied by at least ± 25% of the base-case estimate to account for variation in the community.
Results
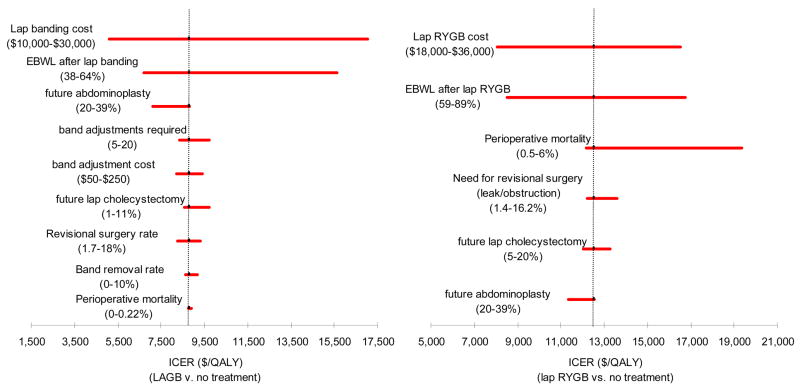
For both men and women LRYGB and LAGB were cost-effective at less than $25,000/QALY when evaluating the full range of BMI values and estimates of adverse outcomes, weight loss and costs. For base-case scenarios in men (aged 35 with BMI of 40) the ICER was $11,604 per QALY for LAGB, compared to $18,543 per QALY for LRYGB. For base-case scenarios in women (aged 35 with BMI of 40) the ICER was $8,878 per QALY for LAGB, compared to $14,680 per QALY for LRYGB. The ICER of LAGB was lower than that of LRYGB for all base-cases (men and women, ages 35, 45 and 55, BMI 40, 50 and 60) and across the full range of variables tested. In one-way sensitivity (Figure 2) analysis the ICER of LAGB was most influenced by the extent of weight loss, operation cost, and frequency of band removal. The ICER of LRYGB was most influenced by the rate of operative mortality, extent of weight loss and operation cost.
Figure 2. One-way sensitivity analysis.
One-way sensitivity analysis of the difference in incremental cost-effectiveness ratio (ICER) between the strategies of LRYGB and LABG for 45 year old female patients with BMI 40 kg/m2. The dashed vertical line represents the difference between the ICER for LRYGB and LAGB using the base-case values. The solid lines demonstrate the impact on ICER of the variables.
Discussion
Cost effectiveness analyses (CEAs) of bariatric procedures are critical given the cost of the procedures, their potential for saving future costs related to comorbid health conditions and worker productivity and the growing population of operative candidates. Economic evaluations of bariatric procedures have so far been limited9, with only one formal CEA of bariatric procedures5 evaluating RYGB, and none for LRYGB and LAGB. LAGB is increasing in popularity, has minimal operative mortality compared with LRYGB but less is known about its weight loss efficacy over time and in the community at large. Furthermore there have been only a few small, comparative studies of LAGB and LRYGB10. The main elements influencing the cost effectiveness of these procedures is associated weight loss and postoperative morbidity. Since these procedures have different rates of adverse and perhaps positive outcomes, comparing them can be problematic. CEA is an ideal methodology to balance these two sets of outcomes. Using this analytic tool probability and cost estimates associated with competing management strategies (using actual and modeled data) can be used to compare different strategies with an overall metric of cost/QALY. In this study we found that both bariatric procedures were cost-effective at less than $25,000 for all base-case scenarios. This finding was similar to those of previous CEAs5, 11 We also found that LAGB was more cost-effective than RYGB, with lower ICER compared to non-operative interventions and in certain populations LAGB was cost saving. The benefits of LAGB were related to its lower associated mortality rate and dependent on it having significant and sustained weight loss over time.
A major component of a cost-effectiveness analysis is the determination of the survival benefit of the intervention. When evaluating survival benefits of obesity surgery, the mortality rate associated with the procedure is balanced against a long-term survival benefit. In a population based study, the 30-day mortality rate of gastric bypass in Washington State was nearly 2%, twice the highest mortality rate previously reported9. However, patients surviving the first postoperative year had a significant survival benefit over non-operated patients. Researchers reported at the 2006 International Congress on Obesity16 that RYGB results in 40% less chance of mortality rate than matched non-operated cohorts. This was also exemplified in a retrospective study with 9-year follow-up that showed overall annual mortality of 1% among 154 patients that underwent RYGB compared with 4.5% annual mortality among 78 morbidly obese patients referred for RYGB who did not undergo the operation for personal or financial reasons12. Utilizing a modeled analysis of survival benefit, Pope and colleagues13 reported a 2.3–2.6 year and 3.3–3.4 year gain in life expectancy for women and men, respectively, aged 30–60 years undergoing RYGB.
On the other hand, LAGB has very low perioperative mortality (<1%, Table 1), but may have a lower extent of excess body weight loss compared to RYGB in the first 3 years after placement. In a recent retrospective series, patients that underwent LRYGB had 66% excess weight loss after 3 years versus 39.3% in LAGB14. There was no difference in excess body weight loss between LRYGB and LAGB 5 years after the operation (58.6% vs. 49%, P=0.84), however the researchers conceded that low patient follow-up at 5 years makes true comparison faulty. Two research abstracts presented at the 2006 International Congress on Obesity indicated a 62–73% reduction in mortality for LAGB patients compared to a matched cohort15. These reports suggest LAGB may extend survival in a fashion similar to RYGB. In our modeled analysis we found that despite a lower extent of weight loss in LAGB, it was more cost-effective than LRYGB. A recent systematic review of the weight loss achieved with different procedures16, showed similar percentage of excess body weight loss with LAGB and RYGB. However, most of these studies are flawed by limited number of patients with long-term follow up beyond 3 years. We assessed the cost effectiveness of these procedures with varying weight loss using a two-way sensitivity analysis (figure 3).
Figure 3. Two-way sensitivity analysis of cost-effectiveness of LAGB and LRYGB.
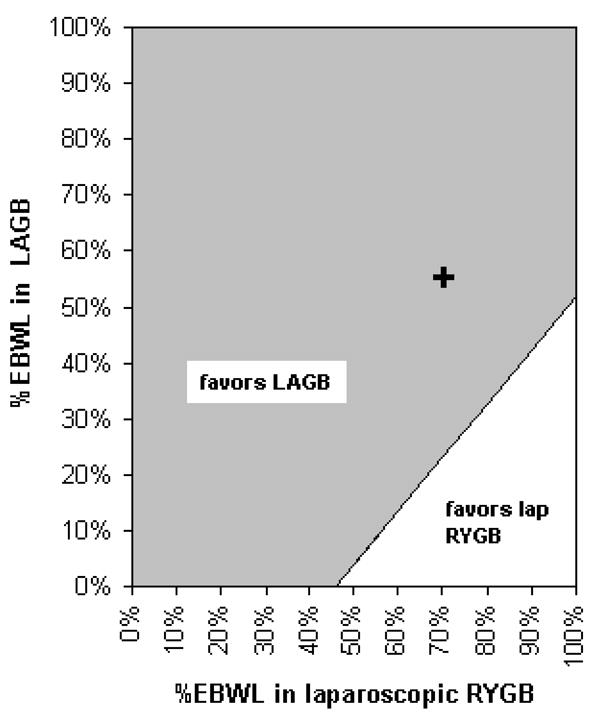
The diagram depicts the difference in the cost-effectiveness between LAGB and LRYGB with varying percentage of excess body weight loss (EBWL) achieved with these procedures, for 45-year-old women with BMI of 40. The shaded area represents EBWL values for which the difference in the cost-effectiveness of the surgical procedures favors LAGB. The line depicts scenarios where of LAGB and LRYGB yield the same cost-effectiveness. The cross represents the difference in the cost-effectiveness of these procedures using the base-case estimates
Cost is a main issue for the broader use of bariatric surgery. Although the benefits of bariatric surgery on weight reduction and the impact on obesity-related comorbidities have been shown, many health insurance companies are limiting the use of these procedures. While the cost of bariatric surgery needs to be evaluated, the cost of non-operative intervention including diet, exercise and medication also needs to be considered when directing healthcare policy. Reportedly, Americans spend over 92 billion dollars annually on obesity-related healthcare, including non-surgical interventions, all of which have been shown to be ineffective over time3. Our research group recently compared the costs of operative and non-operative interventions and found that the operative interventions are cost savings when applied to a population of morbidly obese patients17.
Clinicians and purchasers of healthcare services are engaged in a discussion to determine the best approach to the treatment of obesity. Cost and effectiveness are some of the elements that help determine this issue but all healthcare decisions should be individualized to the unique needs of the patient and practice environment. Finally, the results of a cost effectiveness analysis may not be a good argument for the health insurance companies in the United States since many insurance contracts are terminated within 3–4 years. Given that limited timeline, some have suggested that from a business perspective coverage of a bariatric procedure would be like sustaining the “upfront” costs of the operation and its complications without the long-term benefits of the reduced healthcare costs due to the weight loss and reduction in obesity related comorbidities. When taken from the broader perspective of the Federal and State governments and large employers (who finance most healthcare costs in the United States) these economic considerations are relevant to the competing crises of spiraling healthcare costs and the loss of productivity related to obesity.
This study has several limitations. Future costs, life expectancy and quality of life are based on the weight loss achieved by the procedures. While for RYGB there are studies with 7 year follow up, there are only a few studies reporting the weight loss of LAGB after 3 years. We assumed that following the initial 3 years after surgery the BMI remains stable. Weight gain after this period will reduce the cost effectiveness of the procedures. In order to make conservative estimate of the cost-effectiveness of the bariatric procedures, patients undergoing non-operative management were assumed to have a stable BMI. The estimates of life expectancy, future costs and quality of life are based on data from the NHANES III and the Framingham studies. These studies include data on BMI up to 37.5. We assumed a linear correlation between the BMI and these parameters (life expectancy, future costs and quality of life) for estimation for BMIs of 40–60. Data on life expectancy supports this linear relationship18, but data on BMI greater than 45 is limited. This may represent a conservative bias if the outcomes for patients with higher BMIs are worse than the linear projection we assumed. Furthermore, the probabilities and costs that underlie this model are not BMI or age specific because there are few reports that suggest that probabilities and costs are related to BMI or age. However, if these probabilities and costs are associated with advancing BMI or age then the model may underestimate the impact of these on patients with advanced BMI or age and the implication of this bias is difficult assess. In this study we considered the relationship between obesity and 5 chronic conditions: hypertension, hypercholesterolemia, type 2 diabetes mellitus, coronary heart disease and stroke. Results of previous research19 suggest that these conditions account for approximately 85% of the total economic burden of obesity. This may also represent a conservative bias since other comorbid conditions not considered in this model may be reduced by weight loss. Finally, although our model incorporated complications of surgery in the usual care cost calculation, rates of these complications may vary between sites and may be difficult to assess accurately in a modeled analysis
Conclusion
In conclusion, our modeled cost-effectiveness analysis showed that both operative interventions for morbid obesity, LAGB and RYGB were cost-effective at less than $25,000, and LAGB was more cost-effective than RYGB for all the base-case scenarios.
Acknowledgments
Dr Salem’s work on this project was supported in part by an undirected educational gift from Inamed Corporation (Santa Barbara, California) and by the National Institutes of Health grant number 1 R21 DK069677-01.
Footnotes
Presented at the American College of Surgeons 91st Annual Clinical Congress, October 16–20, 2005, San Francisco, CA
Disclosure There were no financial conflict of interests in conducting this study
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mokdad A, Serdula M, Dietz W, Bowman B, Marks J, Koplan J. The continuing epidemic of obesity in the United States. JAMA. 2000;284:1650–1. doi: 10.1001/jama.284.13.1650. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: how much, and who’s paying? Health Aff (Millwood) 2003 Jan-Jun;Suppl:W3-219–226. doi: 10.1377/hlthaff.w3.219. [DOI] [PubMed] [Google Scholar]
- 3.McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;139:933–49. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 5.Craig BM, Tseng DS. Cost-effectiveness of gastric bypass for severe obesity. Am J Med. 2002;113:491–8. doi: 10.1016/s0002-9343(02)01266-4. [DOI] [PubMed] [Google Scholar]
- 6.Thompson D, Edelsberg J, Colditz G, Bird A, Oster G. Lifetime health and economic consequences of obesity. Arch Inter Med. 1999;159:2177–83. doi: 10.1001/archinte.159.18.2177. [DOI] [PubMed] [Google Scholar]
- 7.Gallager S, Banasiak M, Gonzalvo J, et al. The impact of bariatric surgery on the Veterans Administration healthcare system: a cost analysis. Obes Surg. 2003;13:245–8. doi: 10.1381/096089203764467144. [DOI] [PubMed] [Google Scholar]
- 8.Chapman AE, Kiroff G, Game P, et al. Laparoscopic adjustable gastric banding in the treatment of obesity: a systematic literature review. Surgery. 2004;135:326–51. doi: 10.1016/S0039-6060(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 9.Flum D, Salem L, Broeckel Elrod J, Dellinger E, Cheadle A, Chan L. Early mortality among medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294:1903–8. doi: 10.1001/jama.294.15.1903. [DOI] [PubMed] [Google Scholar]
- 10.Jan J, Hong D, Pereira N, Patterson E. Laparoscopic adjustable gastric banding versus laparoscopic gastric bypass for morbid obesity: a single-institution comparison study of early results. J Gastrointest Surg. 2005;9:30–9. doi: 10.1016/j.gassur.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 11.Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A. The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation. Health Technol Assess. 2002;6:1–153. doi: 10.3310/hta6120. [DOI] [PubMed] [Google Scholar]
- 12.Pories W, Swanson M, MacDonald K. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetees mellitus. Ann Surg. 1995;222:339–50. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pope G, Finlayson S, Kemp J, Birkmeyer J. Life expectancy benefits of gastric bypass. Surg Innovation. 2006;13:265–73. doi: 10.1177/1553350606296324. [DOI] [PubMed] [Google Scholar]
- 14.Jan J, Hong D, Bardaro S, July L, Patterson E. Comparative study between laparoscopic adjustable gastric banding and laparoscopic gastric bypass: single-institution, 5-year experience in bariatric surgery. Surg Obes Relat Dis. 2007;3:42–51. doi: 10.1016/j.soard.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Dixon J. Survival advantage with bariatric surgery: report from the 10th International Congress on Obesity. Surg Obes Relat Dis. 2006;2:585–6. doi: 10.1016/j.soard.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien P, McPhail T, Chaston T, Dixon J. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16:1032–40. doi: 10.1381/096089206778026316. [DOI] [PubMed] [Google Scholar]
- 17.Jensen C, Flum D. The costs of nunsurgical and surgical weight loss interventins: Is an ounce of prevention really worth a pound of cure? Surg Obes Relat Dis. 2005;1:353–7. doi: 10.1016/j.soard.2005.03.215. [DOI] [PubMed] [Google Scholar]
- 18.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–93. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen N, Goldman C, Rosenquist C, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279–89. doi: 10.1097/00000658-200109000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suter M, Bettschart V, Giusti V, Heraief E, Jayet A. A 3-year experience with laparoscopic gastric banding for obesity. Surg Endosc. 2000;14:532–6. doi: 10.1007/s004640000114. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien PE, Brown WA, Smith A, McMurrick PJ, Stephens M. Prospective study of a laparoscopically placed, adjustable gastric band in the treatment of morbid obesity. Br J Surg. 1999;86:113–18. doi: 10.1046/j.1365-2168.1999.00964.x. [DOI] [PubMed] [Google Scholar]
- 22.Kellum J, DeMaria E, Sugerman H. The surgical treatment of morbid obesity. Curr Probl Surg. 1998;35:791–858. doi: 10.1016/s0011-3840(98)80009-9. [DOI] [PubMed] [Google Scholar]
- 23.De Luca M, de Werra E, Formato A, et al. Laparotomic vs laparoscopic lap-band: 4-year results with early and intermediate complications. Obes Surg. 2000;10:266–8. doi: 10.1381/096089200321643584. [DOI] [PubMed] [Google Scholar]
- 24.Dargent J. Laparoscopic adjustable gastric banding: lessons from the first 500 patients in ra single institution. Obes Surg. 1999;9:446–52. doi: 10.1381/096089299765552729. [DOI] [PubMed] [Google Scholar]
- 25.Freeman JB, Kotlarewsky M, Phoenix C. Weight loss after extended gastric bypass. Obes Surg. 1997;7:337–44. doi: 10.1381/096089297765555593. [DOI] [PubMed] [Google Scholar]
- 26.Smith SC, Goodman GN, Edwards CB. Roux-en-Y Gastric Bypass: A 7-year Retrospective Review of 3,855 Patients. Obes Surg. 1995;5:314–18. doi: 10.1381/096089295765557700. [DOI] [PubMed] [Google Scholar]
- 27.Fobi MA, Lee H, Igwe D, Jr, Stanczyk M, Tambi JN. Prospective comparative evaluation of stapled versus transected silastic ring gastric bypass: 6-year follow-up. Obes Surg. 2001;11:18–24. doi: 10.1381/096089201321454051. [DOI] [PubMed] [Google Scholar]
- 28.Howard L, Malone M, Michalek A, Carter J, Alger S, Van Woert J. Gastric bypass and vertical banded gastroplasty - a prospective randomized comparison and 5-year follow-up. Obes Surg. 1995;5:55–60. doi: 10.1381/096089295765558169. [DOI] [PubMed] [Google Scholar]
- 29.Capella JF, Capella RF. The weight reduction operation of choice: vertical banded gastroplasty or gastric bypass? Am J Surg. 1996;171:74–9. doi: 10.1016/S0002-9610(99)80077-4. [DOI] [PubMed] [Google Scholar]
- 30.Balsiger BM, Kennedy FP, Abu-Lebdeh HS, et al. Prospective evaluation of Roux-en-Y gastric bypass as primary operation for medically complicated obesity. Mayo Clin Proc. 2000;75:673–80. doi: 10.4065/75.7.673. [DOI] [PubMed] [Google Scholar]
- 31.MacLean L, Rhode B, Forse R, Nohr R. Surgery for obesity - an update of a randomized trial. Obes Surg. 1995;5:145–50. doi: 10.1381/096089295765557917. [DOI] [PubMed] [Google Scholar]
- 32.Fox SR, Oh KH, Fox K. Vertical Banded Gastroplasty and Distal Gastric Bypass as Primary Procedures: A Comparison. Obes Surg. 1996;6:421–5. doi: 10.1381/096089296765556502. [DOI] [PubMed] [Google Scholar]
- 33.Schauer P, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric byass for morbid obesity. Ann Surg. 2000;232:515–29. doi: 10.1097/00000658-200010000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalfarentzos F, Dimakopoulos A, Kehagias I, Loukidi A, Mead N. Vertical banded gastroplasty versus standard or distal Roux-en-Y gastric bypass based on specific selection criteria in the morbidly obese: preliminary results. Obes Surg. 1999;9:433–42. doi: 10.1381/096089299765552701. [DOI] [PubMed] [Google Scholar]
- 35.Sugerman H, Starkey J, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg. 1987;205:613–24. doi: 10.1097/00000658-198706000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg. 2004;1994:543–51. doi: 10.1016/j.jamcollsurg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Jones KB., Jr Experience with the Roux-en-Y gastric bypass, and commentary on current trends. Obes Surg. 2000;10:183–5. doi: 10.1381/096089200321668659. [DOI] [PubMed] [Google Scholar]
- 38.Higa K, Boone K, Ho T, Davies O. Laparoscopic Roux-en-Y gastric bypass for morbid obesity: technique and preliminary results of our first 400 patients. Arch Surg. 2000;135:1029–33. doi: 10.1001/archsurg.135.9.1029. [DOI] [PubMed] [Google Scholar]
- 39.Toppino M, Morino M, Bonnet G, Nigra I, Siliquini R. Laparoscopic surgery for morbid obesity: preliminary results from SICE registry (Italian Society of Endoscopic and Minimally Invasive Surgery) Obes Surg. 1999;9:62–5. doi: 10.1381/096089299765553809. [DOI] [PubMed] [Google Scholar]
- 40.Angrisani L, Alkilani M, Basso N, et al. Laparoscopic Italian experience with the Lap-Band. Obes Surg. 2001;11:307–10. doi: 10.1381/096089201321336656. [DOI] [PubMed] [Google Scholar]
- 41.Drug Topics Red Book. Montvale, NJ: Medical Economics Company Inc.; 2003. [Google Scholar]