Abstract
Transgenic crops producing Bacillus thuringiensis (Bt) toxins kill some key insect pests and can reduce reliance on insecticide sprays. Sustainable use of such crops requires methods for delaying evolution of resistance by pests. To thwart pest resistance, some transgenic crops produce 2 different Bt toxins targeting the same pest. This “pyramid” strategy is expected to work best when selection for resistance to 1 toxin does not cause cross-resistance to the other toxin. The most widely used pyramid is transgenic cotton producing Bt toxins Cry1Ac and Cry2Ab. Cross-resistance between these toxins was presumed unlikely because they bind to different larval midgut target sites. Previous results showed that laboratory selection with Cry1Ac caused little or no cross-resistance to Cry2A toxins in pink bollworm (Pectinophora gossypiella), a major cotton pest. We show here, however, that laboratory selection of pink bollworm with Cry2Ab caused up to 420-fold cross-resistance to Cry1Ac as well as 240-fold resistance to Cry2Ab. Inheritance of resistance to high concentrations of Cry2Ab was recessive. Larvae from a laboratory strain resistant to Cry1Ac and Cry2Ab in diet bioassays survived on cotton bolls producing only Cry1Ac, but not on cotton bolls producing both toxins. Thus, the asymmetrical cross-resistance seen here does not threaten the efficacy of pyramided Bt cotton against pink bollworm. Nonetheless, the results here and previous evidence indicate that cross-resistance occurs between Cry1Ac and Cry2Ab in some key cotton pests. Incorporating the potential effects of such cross-resistance in resistance management plans may help to sustain the efficacy of pyramided Bt crops.
Keywords: evolution, genetically engineered crops, transgenic crops, Bt cotton
To reduce insecticide sprays, cotton and corn have been genetically engineered to produce proteins from Bacillus thuringiensis (Bt) that kill some key insect pests. First grown commercially in 1996, transgenic Bt crops covered 46 million hectares (ha) worldwide in 2008 (1). Evolution of resistance by pests is the primary threat to the continued efficacy of Bt crops (2–6). Analysis of monitoring data suggests that the refuge strategy, which requires non-Bt host plants near Bt crops to promote survival of susceptible pests, has delayed pest resistance to Bt crops (4, 7). Although Bt crops have remained effective against most targeted pest populations, 3 cases have been reported of field-evolved pest resistance to Bt crops that produce only 1 toxin (4, 7–11). The first case is bollworm (Helicoverpa zea) resistance to Cry1Ac in Bt cotton in the southeastern United States (4, 8, 9). Although an attempt to challenge this initial report considered only a subset of the relevant data (12), more comprehensive analyses revealed compelling evidence including 14 field populations with >100-fold resistance to Cry1Ac, increased survival on leaves of Bt cotton plants, and control problems in the field (4, 8, 9, 13). The two more recent cases are resistance of fall armyworm (Spodoptera frugiperda) to Cry1F in Bt corn in Puerto Rico (10) and stem borer (Busseola fusca) to Cry1Ab in Bt corn in South Africa (11).
To delay pest resistance, some second-generation Bt crops produce 2 distinct Bt toxins that are active against the same pest. This approach, which is called a “pyramid,” is expected to delay pest resistance most effectively when selection for resistance to 1 of the toxins does not cause cross-resistance to the other toxin (14). Other factors favoring success of pyramided Bt crops are abundant refuges of non-Bt host plants near Bt crops and the following conditions for each toxin in the pyramid: functionally recessive inheritance of resistance, low initial resistance allele frequency, and fitness costs associated with resistance (3, 14–16).
The most widely used pyramided Bt crop is cotton producing Cry1Ac and Cry2Ab, which was registered in December 2002 (http://www.epa.gov/pesticides/biopesticides/pips/pip_list.htm) and planted on more than 1 million ha in the United States in 2006, 2007, and 2008 (www.monsanto.com/pdf/investors/2008/2008_biotech_acres.pdf). Cross-resistance between Cry1Ac and Cry2Ab has been presumed unlikely, because these toxins differ substantially in amino acid sequence and bind to different target sites in the larval midgut (17–20). Both Cry1Ac and Cry2Ab are active against some key lepidopteran pests, including pink bollworm (Pectinophora gossypiella), a major cotton pest in the southwestern United States and in many other countries (21). Bt cotton producing only Cry1Ac has been exceptionally effective against pink bollworm in Arizona; susceptibility to Cry1Ac has not decreased in field populations of this pest despite more than a decade of exposure (22–25). Previous work showed that laboratory selection of pink bollworm with Cry1Ac yielded high levels of resistance to Cry1Ac, but little or no cross-resistance to Cry2A toxins (26–28; http://www.epa.gov/scipoly/sap/meetings/2006/october/unnithan_et_al_04_cry2ab_baselines.pdf). Here we show, however, that laboratory selection of pink bollworm with Cry2Ab yielded resistance to Cry2Ab and cross-resistance to Cry1Ac. We term this “asymmetrical cross-resistance” because selection with Cry2Ab caused cross-resistance to Cry1Ac, but selection with Cry1Ac did not cause cross-resistance to Cry2Ab. Asymmetrical cross-resistance could be important for resistance management but has received little attention previously. In particular, most previous tests for cross-resistance include selection with only 1 of the 2 toxins being evaluated and thus cannot detect asymmetrical cross-resistance (2, 18).
Results
Effects of Selection with Cry2Ab on Susceptibility to Cry2Ab, Cry1Ac, and Cry2Aa.
Laboratory selection of pink bollworm strains BX-R1 and BX-R2 with Cry2Ab in diet yielded resistance of both strains to Cry2Ab, as well as cross-resistance to Cry1Ac and Cry2Aa (Tables 1 and 2, and Fig. 1). Confirming previous results (26, 27), the AZP-R strain selected with Cry1Ac had little or no cross-resistance to Cry2Ab or Cry2Aa (Tables 1 and 2). Relative to the susceptible strain APHIS-S, the maximum resistance ratios for BX-R1 were 240 for Cry2Ab and 420 for Cry1Ac. Maximum resistance ratios for BX-R2 were 74 for Cry2Ab and 21 for Cry1Ac. Relative to their hybrid parent strain BX-H, maximum increases in LC50 values were 110-fold for Cry2Ab and 190-fold for Cry1Ac for BX-R1, and 44-fold for Cry2Ab and 9.2-fold for Cry1Ac for BX-R2. Relative to BX-H, selection with Cry2Ab increased survival of BX-R1 and BX-R2 exposed to a diagnostic concentration (10 μg toxin/mL diet) of Cry2Ab, Cry1Ac, or Cry2Aa (Tables 1 and 2). For BX-R1, survival at the diagnostic concentration of Cry2Ab was slightly higher in the F22–24 generations (92%) than in the F16–17 generations (88%), even though the LC50 of Cry2Ab was lower in the F22–24 generations (27 μg toxin/mL diet) than in the F16–17 generations (99 μg toxin/mL diet) (Table 1). The consistently high survival at the diagnostic concentration indicates that resistance did not decrease substantially.
Table 1.
Responses of pink bollworm to Bt toxins Cry1Ac and Cry2Ab in diet bioassays
| Strain | Dates | Gens.* | n | LC50 (95% FL)† | RR‡ | Survival, %§ |
|---|---|---|---|---|---|---|
| Cry2Ab | ||||||
| BX-R1 | Feb. 2005 | F16–17 | 786 | 99 (52–530) | 240 | 88 |
| BX-R1 | Sept.–Nov. 2005 | F22–24 | 700 | 27 (23–32) | 64 | 92 |
| BX-R2 | Feb. 2005 | F5 | 334 | 4.2 (3.2–5.3) | 10 | 23 |
| BX-R2 | Sept.–Oct. 2005 | F12–13 | 647 | 31 (24–40) | 74 | 83 |
| BX-H | Feb. 2005 | F40 | 435 | 0.94 (0.84–1.0) | 2.2 | 0 |
| BX-H | Aug. 2005 | F47 | 400 | 0.70 (0.58–0.83) | 1.7 | 0 |
| AZP-R | Feb. 2005 | F72 | 408 | 0.76 (0.65–0.88) | 1.8 | 0 |
| AZP-R | Aug. 2005 | F78 | 449 | 0.80 (0.70–0.89) | 1.9 | 0 |
| APHIS-S | Feb. 2005 | NA | 424 | 0.42 (0.28–0.55) | 1 | 0 |
| APHIS-S | Aug. 2005 | NA | 250 | 0.42 (0.31–0.52) | 1 | 0 |
| Cry1Ac | ||||||
| BX-R1 | Feb. 2005 | F15–16 | 640 | 590 (330–1400) | 420 | 94 |
| BX-R1 | Sept.–Oct. 2005 | F22–24 | 544 | 600 (420–730) | 180 | 94 |
| BX-R2 | Feb. 2005 | F5 | 777 | 3.7 (1.2–8.0) | 2.6 | 24 |
| BX-R2 | Sept.–Oct. 2005 | F12–13 | 589 | 69 (26–130) | 21 | 59 |
| BX-H | Mar. 2005 | F40–41 | 599 | 3.1 (1.3–5.7) | 2.2 | 9.3 |
| BX-H | Aug. 2005 | F47 | 405 | 7.5 (3.7–14) | 2.3 | 12 |
| AZP-R | Dec. 2004 | F70–71 | 496 | 2100 (1800–2500) | 1500 | 100 |
| APHIS-S | Dec. 2004 | NA | 356 | 1.4 (1.1–1.7) | 1 | 0 |
| APHIS-S | July 2005 | NA | 240 | 3.3 (1.9–4.1) | 1 | 5.7 |
*Generations tested in bioassays. NA, not available.
†Concentration killing 50% with 95% fiducial limits in parentheses, in μg of toxin per ml of diet.
‡Resistance ratio, the LC50 of a strain divided by the LC50 of APHIS-S from the same time period.
§Survival at 10 μg of toxin per ml of diet, adjusted for control mortality; sample size for each survival estimate ranged from 60 to 380 (mean = 120).
Table 2.
Responses of pink bollworm to Bt toxin Cry2Aa in diet bioassays
| Strain | Gens.* | n | LC50 (95% FL)† | RR‡ | Survival, %§ |
|---|---|---|---|---|---|
| BX-R1 | F24–25 | 513 | 8.4 (6.3–11) | 25 | 43 |
| BX-R2 | F14 | 509 | 7.9 (6.0–10) | 24 | 41 |
| BX-H | F50–51 | 420 | 0.61 (0.47–0.77) | 1.8 | 0 |
| AZP-R | F76–78 | 410 | 0.95 (0.70–1.2) | 2.9 | 1.9 |
| APHIS-S | NA | 398 | 0.33 (0.27–0.40) | 1 | 0 |
All bioassays with Cry2Aa were started during November to December 2005.
*Generations tested in bioassays. NA, not available.
†Concentration killing 50% with 95% fiducial limits in parentheses, in μg of Cry2Aa per ml of diet.
‡Resistance ratio, the LC50 of a strain divided by the LC50 of APHIS-S.
§Survival at 10 μg of Cry2Aa per ml of diet, adjusted for control mortality; sample size for each survival estimate ranged from 38 to 71 (mean = 58).
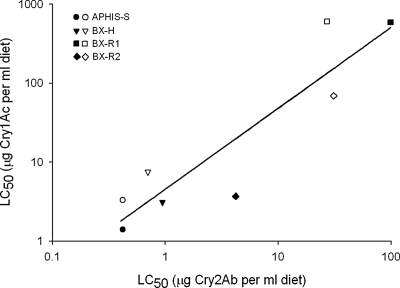
Fig. 1.
Effect of selection with Cry2Ab on resistance to Cry1Ac. Solid symbols correspond to LC50 values from the first set of bioassay dates (December 2004 to March 2005), and open symbols correspond to LC50 values from the second set of bioassay dates (July to November 2005; Table 1). A significant portion of the variation in the logarithm of LC50 of Cry1Ac is explained by variation in the logarithm of LC50 of Cry2Ab (y = 1.02x + 1.52, R2 = 0.83, df = 6, P = 0.0015).
Selection with Cry2Ab also increased survival in response to simultaneous exposure to Cry2Ab and Cry1Ac in diet tests. On diet treated with a combination of 10 μg Cry2Ab plus 100 μg Cry1Ac/mL diet, survival was 75% for BX-R1 (F17, n = 65) versus 0% for BX-H (F41, n = 30, Fisher's exact test, P < 0.0001). Similarly, survival at 10 μg Cry2Ab plus 10 μg Cry1Ac per mL diet was 72% for BX-R1 (F22) and 64% for BX-R2 (F12) compared with 0% for BX-H (F49) and AZP-R (F81) (n = 60 larvae per strain, Fisher's exact test, P < 0.0001 for each of 4 pairwise comparisons, see Materials and Methods).
Inheritance of Resistance to Cry2Ab.
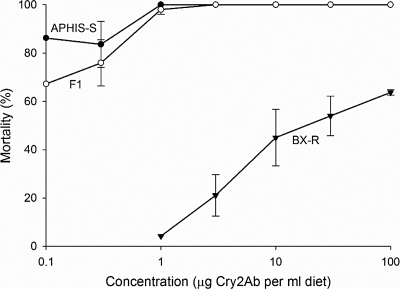
Results of a cross between the resistant BX-R strain and the susceptible APHIS-S strain showed that resistance to Cry2Ab was inherited as a completely recessive trait at concentrations of 3, 10, 30, and 100 μg Cry2Ab/mL diet (h = 0) (Fig. 2). As the concentration of Cry2Ab decreased, resistance was slightly less recessive (Fig. 2). Results did not differ between the F1 hybrid progeny from the 2 reciprocal crosses (BX-R females × APHIS-S males and APHIS-S females × BX-R males), indicating that the resistance was autosomally inherited.
Fig. 2.
Responses to Cry2Ab of pink bollworm larvae from a resistant strain (BX-R), a susceptible strain (APHIS-S), and their F1 progeny (BX-R X APHIS-S). Each point represents mean mortality (with standard error bars) of 40–160 larvae fed diet treated with Cry2Ab, adjusted for control mortality (see Materials and Methods). Inheritance was completely recessive at 3, 10, 30, and 100 μg Cry2Ab/mL diet (h = 0) and somewhat less recessive at lower concentrations (h = 0.021, 0.089–0.097, and 0.19–0.23, at 1, 0.3, and 0.1 μg Cry2Ab/mL diet, respectively, see Materials and Methods).
Survival on Bolls of Bt Cotton and Non-Bt Cotton.
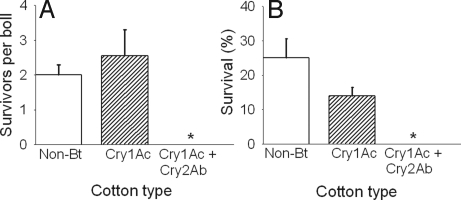
Despite their increased survival on diet treated with Cry2Ab and Cry1Ac, BX-R1 larvae did not survive on cotton bolls producing both of these toxins (Fig. 3). In greenhouse boll bioassays, survival of BX-R1 larvae did not differ significantly between non-Bt cotton (2.0 larvae per boll, n = 15 bolls) and cotton producing only Cry1Ac (2.6 larvae per boll, n = 20 bolls; Mann-Whitney U-test, P = 0.68). However, no larvae survived on 110 bolls of cotton producing Cry1Ac and Cry2Ab, which was significantly lower than survival on the 2 other types of cotton (Mann-Whitney U-test, P < 0.0001). These results were confirmed in laboratory boll bioassays where survival was 25% on non-Bt cotton, 14% on cotton producing Cry1Ac, and 0% on cotton producing Cry2Ab and Cry1Ac (n = 50 or 60 neonates infesting 5 or 6 bolls, respectively, for each type of cotton). As with the greenhouse boll bioassays, survival in laboratory boll bioassays did not differ significantly between non-Bt cotton and cotton producing only Cry1Ac (Fisher's exact test, P = 0.12), but survival on cotton producing both toxins was significantly lower than survival on the other 2 types of cotton (Fisher's exact test, P < 0.0001).
Fig. 3.
Survival of pink bollworm larvae from a resistant strain (BX-R1) on bolls of cotton with no Bt toxins (non-Bt), Cry1Ac only, or Cry1Ac and Cry2Ab. Means are shown with standard errors. Asterisks show no survival on bolls producing both Cry1Ac and Cry2Ab. (A) Greenhouse. (B) Laboratory. See Results for details.
Discussion
Together with previous results, the data reported here imply that cross-resistance between Bt toxins Cry1Ac and Cry2Ab was asymmetrical in the pink bollworm strains examined. Whereas previous laboratory selection of pink bollworm with Cry1Ac produced little or no cross-resistance to Cry2A toxins (26, 27), selection with Cry2Ab here yielded up to 420-fold cross-resistance to Cry1Ac (Table 1). As far as we know, asymmetrical cross-resistance between Cry1 and Cry2 toxins has not been reported before. For example, in the cotton pest Helicoverpa armigera, selection with Cry1Ac did not cause cross-resistance to Cry2Ab and selection with Cry2Ab did not cause cross-resistance to Cry1Ac (29–32). However, most previous studies could not detect asymmetrical cross-resistance, because insects selected with Cry1 toxins were tested for cross-resistance to Cry2 toxins, but the reciprocal experiment was not done (2, 18).
Although the mechanism of pink bollworm's asymmetrical cross-resistance between Cry1Ac and Cry2Ab remains to be determined, the available evidence excludes some explanations. In pink bollworm strains selected with Cry1Ac including AZP-R, resistance to Cry1Ac is tightly linked with mutations in a gene encoding a cadherin protein that binds Cry1Ac in the larval midgut (33–35). Because the strains selected with Cry1Ac had little or no cross-resistance to Cry2A toxins (Table 1, and refs. 26, 27), we infer that the cadherin mutations alone do not confer high levels of cross-resistance to Cry2A toxins. Furthermore, given that Cry1Ac and Cry2Ab bind to different sites in the larval midgut of pink bollworm (19), it is unlikely that mutations affecting any single toxin-binding protein confer resistance to Cry2Ab and cross-resistance to Cry1Ac. We hypothesize that the observed resistance to Cry2Ab requires resistance alleles at 2 or more loci, including the cadherin locus (or a locus linked with the cadherin locus) and at least 1 locus not linked with the cadherin locus. If so, selection with Cry2Ab could increase the frequency of cadherin mutants as well as resistance alleles at 1 or more independent loci affecting susceptibility to Cry2Ab. Conversely, selection with Cry1Ac alone could increase the frequency of cadherin mutants without affecting the frequency of alleles at other loci conferring resistance to Cry2Ab. Additional experiments are needed to test this and other hypotheses.
For several reasons, the type of asymmetrical cross-resistance between Cry1Ac and Cry2Ab seen in our laboratory selection experiments does not threaten control of pink bollworm by pyramided Bt cotton in the field. First, the conditions of our experiments differed markedly from conditions in the field. In particular, the laboratory selection experiments with Cry2Ab in diet started with a hybrid strain of pink bollworm (BX-H) that had close to 10% survival at a diagnostic concentration of Cry1Ac (10 μg toxin/mL diet) (Table 1). In contrast, after a decade of exposure to cotton producing only Cry1Ac, survival at the diagnostic concentration of Cry1Ac was essentially 0% in field-derived strains of pink bollworm (24, 25). Second, laboratory-selected strains of pink bollworm showed recessive inheritance of resistance to high concentrations of Cry2Ab (Fig. 2) and Cry1Ac (22, 28), which favors sustained efficacy of pyramided Bt cotton. Third, pink bollworm larvae from the BX-R1 strain selected with Cry2Ab did not survive on bolls of Bt cotton producing Cry1Ac and Cry2Ab, despite their high levels of resistance to both toxins in diet bioassays and survival on Bt cotton bolls producing only Cry1Ac. This pattern probably reflects the 160-fold higher toxin concentration in bolls for Cry2Ab (792 μg toxin/g) versus Cry1Ac (4.95 μg toxin/g in bolls producing Cry1Ac only and bolls producing both toxins) (36). The concentration of Cry2Ab in bolls was 8 to 29 times higher than the LC50 values of Cry2Ab for BX-R1 (Table 1). Conversely, the LC50 values of Cry1Ac for BX-R1 (Table 1) were more than 100 times greater than the concentration of Cry1Ac in bolls (Table 1). Thus, it seems likely that the Cry2Ab in bolls killed BX-R1 larvae, but the Cry1Ac did not.
Whereas selection with Cry1A toxins caused little or no cross-resistance to Cry2A toxins in several cases (18, 29–31, 37), notable exceptions involve the major U.S. cotton pests Heliothis virescens and Helicoverpa zea. Laboratory selection of H. virescens strain CP73–3 with Cry1Ac caused 50-fold resistance to Cry1Ac and 53-fold cross-resistance to Cry2Aa (38). Selection with Cry1Ac also caused some cross-resistance to Cry2Aa in the KCB and YHD2 strains of H. virescens (39, 40). Genetic linkage analysis identified loci in H. virescens that contributed to resistance to both Cry1Ac and Cry2Aa (41). However, as in pink bollworm and H. armigera (30), H. virescens resistance to Cry2Aa was not caused by the cadherin gene linked with Cry1Ac resistance (41). Also similar to pink bollworm, field populations of H. virescens have remained susceptible to Cry1Ac despite many years of exposure to Bt cotton producing only Cry1Ac (9). Slower processing of protoxin to activated toxin was identified in 2 of 3 strains of H. virescens with resistance to Cry1Ac and Cry2Aa (42). Although resistance was similar to the protoxin and activated toxin forms of Cry1Ab in pink bollworm strain AZP-R (26), more work is needed to determine if altered processing of protoxin contributes to the asymmetrical cross-resistance seen here.
As in some strains of H. virescens, resistance to Cry1Ac was genetically correlated with resistance to Cry2Aa in field populations of H. zea sampled during 2000 (43). In addition, responses to Cry1Ac and Cry2Ab were genetically correlated in field populations of H. zea sampled during 2001 and 2002 (44). An independent study showed that for 61 populations of H. zea tested from 2002 to 2004, the LC50 values of Cry2Ab were positively correlated with those of Cry1Ac (45). Although H. zea has been exposed to cotton producing only Cry1Ac since 1996 and some field populations have evolved resistance to Cry1Ac (4, 8–9), cotton producing Cry1Ac and Cry2Ab was registered in December 2002 and first exceeded 1 million ha planted in the United States in 2006. Thus, cross-resistance to Cry2Ab caused by resistance to Cry1Ac is a plausible explanation for the observed positive correlation between LC50 values for Cry1Ac and Cry2Ab (45). An alternative scenario is that the positive correlation was caused by selection first for resistance to Cry1Ac followed by selection for resistance to Cry2Ab. Survival on cotton with both toxins would be more likely for individuals with Cry2Ab resistance alleles if they were already resistant to Cry1Ac (14). However, because cotton producing both Cry1Ac and Cry2Ab was relatively rare from 2002 to 2004, the positive correlation between LC50 values for Cry1Ac and Cry2Ab detected during this period probably resulted from cross-resistance, rather than sequential resistance to the 2 toxins. Cross-resistance is more problematic in H. zea than in pink bollworm or H. virescens, because H. zea showed nonrecessive inheritance of resistance to Cry1Ac and Cry2Aa (43, 46), relatively low inherent susceptibility to Cry1Ac and Cry2Ab (36), and survival in the field on cotton plants producing Cry1Ac and Cry2Ab (47).
As the second and future generations of insecticidal transgenic crops are deployed, crops with pyramids of toxins such as Cry1Ac and Cry2Ab will become increasingly widespread. Indeed, pyramided Bt corn with Cry1A.105 and Cry2Ab was registered in the United States in 2008 (www.epa.gov/EPA-IMPACT/2008/July/Day-24/i16947.htm). To maximize the benefits of pyramided crops, it will be important to apply insights gained from experiments and field experience. The results reported here suggest that it may be useful to perform separate selection experiments with each of the toxins in a pyramid to fully delineate patterns of cross-resistance. Evidence to date suggests that the ideal conditions for pyramid performance may not always be attained. In particular, despite the dissimilarity in structure and toxin-binding sites for Cry1Ac and Cry2Ab, cross-resistance occurs between these toxins in some key target pests. Previous results suggest that refuges of host plants that do not produce Bt toxins can delay pest resistance (4, 7). To determine the optimal abundance of such refuges for pyramided Bt crops, it may be useful to account for the potential effects of cross-resistance between the toxins in pyramids.
Materials and Methods
Insect Strains.
We used 6 strains of pink bollworm: APHIS-S, AZP-R, BX-H, BX-R1, BX-R2, and BX-R. APHIS-S is a susceptible strain that had been reared for >20 years without exposure to Bt toxins (22). AZP-R is a Cry1Ac-resistant strain that was started by pooling survivors of exposure to Cry1Ac in diet from 10 strains derived in 1997 from Arizona cotton fields (22). AZP-R had been selected repeatedly with 10 or 100 μg Cry1Ac/mL diet, yielding >1,000-fold resistance to Cry1Ac, but little cross-resistance to Cry2Aa or Cry2Ab (27, 28). BX-H is a hybrid strain that included a mixture of Cry1Ac-resistant and susceptible individuals (34). BX-R1 and BX-R2 were started with individuals from BX-H and selected for resistance to Cry2Ab as described below. After BX-R1 and BX-R2 achieved substantial resistance to Cry2Ab, 875 pupae from these 2 strains were pooled to create BX-R in December 2006. Larvae of all strains were reared on wheat germ diet (48).
Bt Toxins.
We used the protoxin form of Bt toxins in all experiments. The source of Cry1Ac was MVP II obtained from Dow Agrosciences (28). The Cry2Ab in the inheritance experiment was produced by a recombinant acrystalliferous strain of Bt subsp. kurstaki (HD73 cry-) that was transformed with the cry2Ab gene from strain HD1 of Bt subsp. kurstaki (49). For all other experiments, Cry2Ab2 was obtained from Monsanto Inc. in powder from transgenic corn. The powder was made by grinding freeze-dried leaves of corn (Mon84006) that produced Cry2Ab2. Cry2Aa2 provided by William Moar (Auburn University, Auburn, AL) was purified from transformed Eschericia coli that produced Cry2Aa protoxin inclusion bodies (27). We refer to Cry2Ab2 as Cry2Ab and Cry2Aa2 as Cry2Aa because the amino acid sequence is the same for Cry2Ab2 and Cry2Ab, as well as for Cry2Aa2 and Cry2Aa (http://www.lifesci.sussex.ac.uk/home/Neil_Crickmore/Bt/).
Diet Bioassays and Selection.
We measured susceptibility to Cry2Ab, Cry1Ac, and Cry2Aa protoxins by using bioassays in which the toxins were incorporated in diet at 6–8 concentrations, neonates were put individually on diet, and survival was recorded after 21 days at 29 ± 2 °C (22, 27, 28). We also measured susceptibility to combinations of Cry1Ac (10 or 100 μg toxin/mL diet) and Cry2Ab in diet (10 μg toxin/mL diet). The BX-R1 and BX-R2 strains were started with individuals from BX-H and selected for resistance by putting neonates on diet treated with Cry2Ab, using the methods for bioassays described above. In each generation, survivors of exposure to Cry2Ab were reared to continue each strain. As susceptibility decreased, the Cry2Ab concentrations used for selection were increased (1–56 μg Cry2Ab/mL diet).
Greenhouse and Laboratory Boll Bioassays.
BX-R1 (F21) larvae were tested in the greenhouse and laboratory on bolls of non-Bt cotton (Deltapine 436 RR), cotton-producing Cry1Ac (Deltapine 33B), and cotton-producing Cry1Ac and Cry2Ab (Deltapine 424 BGII/RR). We grew all cotton plants in the greenhouse at the University of Arizona Campus Agricultural Center (27). In greenhouse boll bioassays, bolls ≈3 weeks old were infested by placing pieces of paper bearing >50 eggs of BX-R1 under the bracts of bolls (34). Survival was recorded after 15–27 days. In laboratory boll bioassays, bolls 3–4 weeks old were removed from plants grown in the greenhouse, infested in the laboratory by putting 10 neonates from BX-R1 on each boll, and held at 29 ± 2 °C and 16-h light/8-h dark. Survival was recorded after 21 days.
Inheritance of Resistance to Cry2Ab.
To evaluate dominance of resistance, maternal effects, and sex linkage, we used diet bioassays with Cry2Ab protoxin as described above to test APHIS-S, BX-R, and the F1 hybrid progeny resulting from crosses between BX-R females and APHIS-S males and the reciprocal cross (APHIS-S females × BX-R males) (28). The entire experiment was replicated in 2 different time periods starting in April and May 2007.
Statistical Analysis.
We estimated the concentration of toxin killing 50% of larvae (LC50) and its 95% fiducial limits from diet bioassay data by using the program POLO (50). LC50 values with nonoverlapping 95% fiducial limits are significantly different. Resistance ratios were calculated as the LC50 of a strain divided by the LC50 of the susceptible APHIS-S strain tested during the same time period. We also calculated adjusted mortality at individual toxin concentrations, including a diagnostic concentration of 10 μg toxin/mL diet, as: 100% − adjusted survival, where adjusted survival equals [survival (%) on treated diet divided by survival (%) on diet without toxin] × 100%. In some cases, larvae were tested with bioassays in 2 to 3 consecutive generations, and data were pooled across generations to yield robust estimates of susceptibility parameters. To test the hypothesis that selection with Cry2Ab caused cross-resistance to Cry1Ac, we used linear regression with the logarithm of LC50 of Cry2Ab as the explanatory variable and the logarithm of LC50 of Cry1Ac as the response variable (51). To test the hypothesis that selection with Cry2Ab yielded resistance to a combination of Cry1Ac and Cry2Ab, we used Fisher's exact test to make the following pairwise comparisons of survival between strains: BX-R1 versus BX-H, BX-R2 versus BX-H, BX-R1 versus AZP-R, and BX-R2 versus AZP-R.
We estimated dominance (h) (52) based on adjusted survival for APHIS-S, BX-R, and their F1 hybrid progeny at each of 7 concentrations: 0.1, 0.3, 1, 3, 10, 30, and 100 μg Cry2Ab/mL diet. Values of h range from zero (completely recessive) to 1 (completely dominant). The highest concentrations tested against APHIS-S were 1, 3, and 10 μg Cry2Ab/mL diet, which all caused 100% adjusted mortality. To calculate h for 30 and 100 μg Cry2Ab/mL diet, we assumed that these higher concentrations also killed 100% of APHIS-S. The lowest concentration tested against BX-R was 1 μg Cry2Ab/mL diet, which caused 4.2% adjusted mortality. To calculate h for 0.1 and 0.3 μg Cry2Ab/mL diet, we assumed that these lower concentrations killed zero to 4.2% of BX-R, which generated a range of values for h at 0.1 and 0.3 μg Cry2Ab/mL diet.
Acknowledgments.
We thank Timothy Dennehy for initiating this research when he was at the University of Arizona. We thank Robert Biggs, Al Mazza, and the staff of the Extension Arthropod Resistance Management Laboratory for technical assistance, and Mark Sisterson and David Onstad for comments on the manuscript. We thank Monsanto for Cry2Ab, William Moar (Auburn University, Auburn, AL) for Cry2Aa, and Dow for Cry1Ac. The research was supported by U.S. Department of Agriculture-National Research Initiative Grants 2006-35302-17365 and 2008-35302-0390.
Footnotes
Conflict of interest statement: Although preparation of this article was not supported by organizations that may gain or lose financially through its publication, the authors have received support for other research from Monsanto, Cotton Inc., the Cotton Foundation, and the Arizona Cotton Growers Association. B.E.T. is a coauthor of a patent application filed with the World Intellectual Property Organization on engineering modified Bt toxins to counter pest resistance, which is related to published research [(2007) Science 318:1640–1642].
This article is a PNAS Direct Submission.
References
- 1.James C. ISAAA Briefs. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications; 2008. Global status of commercialized biotech/GM crops: 2008. 39-2008. [Google Scholar]
- 2.Tabashnik BE. Evolution of resistance to Bacillus thuringiensis. Annu Rev Entomol. 1994;39:47–79. [Google Scholar]
- 3.Gould F. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu Rev Entomol. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- 4.Tabashnik BE, Gassmann AJ, Crowder DW, Carrière Y. Insect resistance to Bt crops: Evidence versus theory. Nat Biotechnol. 2008;26:199–202. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- 5.Soberón M, et al. Engineering modified Bt toxins to counter insect resistance. Science. 2007;318:1640–1642. doi: 10.1126/science.1146453. [DOI] [PubMed] [Google Scholar]
- 6.Meihls LN, et al. Increased survival of western corn rootworm on transgenic corn within three generations of on-plant greenhouse selection. Proc Natl Acad Sci USA. 2008;105:19177–19182. doi: 10.1073/pnas.0805565105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabashnik BE. Delaying insect resistance to transgenic crops. Proc Natl Acad Sci USA. 2008;105:19029–19030. doi: 10.1073/pnas.0810763106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luttrell RG, et al. Resistance to Bt in Arkansas populations of cotton bollworm. In: Richter DA, editor. Proceedings of the 2004 Beltwide Cotton Conferences; January 5–9, 2004; San Antonio, TX. Memphis, TN: National Cotton Council of America; 2004. pp. 1373–1383. [Google Scholar]
- 9.Ali MI, Luttrell RG, Young SY., III Susceptibilities of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) populations to Cry1Ac insecticidal protein. J Econ Entomol. 2006;99:164–175. doi: 10.1093/jee/99.1.164. [DOI] [PubMed] [Google Scholar]
- 10.Matten SR, Head GP, Quemada HD. How governmental regulation can help or hinder the integration of Bt crops into IPM programs. In: Romeis J, Shelton AM, Kennedy GG, editors. Integration of Insect-Resistant Genetically Modified Crops within IPM Programs. New York: Springer; 2008. pp. 27–39. [Google Scholar]
- 11.Van Rensburg JBJ. First report of field resistance by stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S African J Plant Soil. 2007;24:147–151. [Google Scholar]
- 12.Moar W, et al. Field-evolved resistance to Bt toxins. Nat Biotechnol. 2008;26:1072–1074. doi: 10.1038/nbt1008-1072. [DOI] [PubMed] [Google Scholar]
- 13.Tabashnik BE, Gassmann AJ, Crowder DW, Carrière Y. Field-evolved resistance to Bt toxins. Nat Biotechnol. 2008;26:1074–1076. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J-Z, et al. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proc Natl Acad Sci USA. 2005;102:8426–8430. doi: 10.1073/pnas.0409324102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould F, et al. Impact of small fitness costs on pest adaptation to crop varieties with multiple toxins: A heuristic model. J Econ Entomol. 2006;99:2091–2099. doi: 10.1603/0022-0493-99.6.2091. [DOI] [PubMed] [Google Scholar]
- 16.Gassmann A, Carrière Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis. Annu Rev Entomol. 2009;54:147–163. doi: 10.1146/annurev.ento.54.110807.090518. [DOI] [PubMed] [Google Scholar]
- 17.Tabashnik BE, et al. Cross-resistance of diamondback moth indicates altered interactions with domain II of Bacillus thuringiensis toxins. Appl Environ Entomol. 1996;62:2839–2844. doi: 10.1128/aem.62.8.2839-2844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferré J, Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu Rev Entomol. 2002;47:501–533. doi: 10.1146/annurev.ento.47.091201.145234. [DOI] [PubMed] [Google Scholar]
- 19.González-Cabrera J, Escriche B, Tabashnik BE, Ferré J. Binding of Bacillus thuringiensis toxins in resistant and susceptible strains of pink bollworm (Pectinophora gossypiella) Insect Biochem Mol Biol. 2003;33:929–935. doi: 10.1016/s0965-1748(03)00099-7. [DOI] [PubMed] [Google Scholar]
- 20.Hernández-Rodríguez CS, Van Vliet A, Bautsoens N, Van Rie J, Ferré J. Specific binding of Bacillus thuringiensis Cry2 insecticidal proteins to a common site in the midgut of Helicoverpa species. Appl Environ Microbiol. 2008;74:7654–7659. doi: 10.1128/AEM.01373-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henneberry TJ, Naranjo SE. Integrated management approaches for pink bollworm in the southwest United States. Integrated Pest Management Rev. 1998;3:31–52. [Google Scholar]
- 22.Tabashnik BE, et al. Frequency of resistance to Bacillus thuringiensis in field populations of pink bollworm. Proc Natl Acad Sci USA. 2000;97:12980–12984. doi: 10.1073/pnas.97.24.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrière Y, et al. Long-term regional suppression of pink bollworm by Bacillus thuringiensis cotton. Proc Natl Acad Sci USA. 2003;100:1519–1523. doi: 10.1073/pnas.0436708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabashnik BE, Dennehy TJ, Carrière Y. Delayed resistance to Bt cotton in pink bollworm. Proc Natl Acad Sci USA. 2005;102:15389–15393. doi: 10.1073/pnas.0507857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabashnik BE, et al. DNA screening reveals pink bollworm resistance to Bt cotton remains rare after a decade of exposure. J Econ Entomol. 2006;99:1525–1530. doi: 10.1603/0022-0493-99.5.1525. [DOI] [PubMed] [Google Scholar]
- 26.Tabashnik BE, Liu YB, deMaagd RA, Dennehy TJ. Cross-resistance of pink bollworm (Pectinophora gossypiella) to Bacillus thuringiensis toxins. Appl Environ Microbiol. 2000;66:4582–4584. doi: 10.1128/aem.66.10.4582-4584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabashnik BE, et al. Control of resistant pink bollworm by transgenic cotton with Bacillus thuringiensis toxin Cry2Ab. Appl Environ Microbiol. 2002;68:3790–3794. doi: 10.1128/AEM.68.8.3790-3794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabashnik BE, et al. Inheritance of resistance to Bt toxin Cry1Ac in a field-derived strain of pink bollworm (Lepidoptera: Gelechiidae) J Econ Entomol. 2002;95:1018–1026. doi: 10.1603/0022-0493-95.5.1018. [DOI] [PubMed] [Google Scholar]
- 29.Akhurst RJ, James W, Bird LJ, Beard C. Resistance to the Cry1Ac ∂-endotoxin of Bacillus thuringiensis in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) J Econ Entomol. 2003;96:1290–1299. doi: 10.1603/0022-0493-96.4.1290. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Yu L, Wu Y. Disruption of a cadherin gene associated with resistance to Cry1Ac ∂-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. Appl Environ Microbiol. 2005;71:948–954. doi: 10.1128/AEM.71.2.948-954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo S, et al. Cross-resistance studies of Cry1Ac-resistant strains of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry2Ab. J Econ Entomol. 2007;100:909–915. doi: 10.1603/0022-0493(2007)100[909:csocso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Mahon RJ, Olsen KM, Garsia KA, Young SR. Resistance to Bacillus thuringiensis toxin Cry2Ab in a strain of Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. J Econ Entomol. 2007;100:894–902. doi: 10.1603/0022-0493(2007)100[894:rtbttc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Morin S, et al. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc Natl Acad Sci USA. 2003;100:5004–5009. doi: 10.1073/pnas.0831036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabashnik BE, et al. Association between resistance to Bt cotton and cadherin genotype in pink bollworm. J Econ Entomol. 2005;98:635–644. doi: 10.1603/0022-0493-98.3.635. [DOI] [PubMed] [Google Scholar]
- 35.Fabrick JA, Tabashnik BE. Binding of Bacillus thuringiensis toxin Cry1Ac to multiple sites of cadherin in pink bollworm. Insect Biochem Mol Biol. 2007;37:97–106. doi: 10.1016/j.ibmb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Sivasupramaniam S, et al. Toxicity and characterization of cotton expressing Bacillus thuringiensis Cry1Ac and Cry2Ab2 proteins for control of lepidopteran pests. J Econ Entomol. 2008;101:546–554. doi: 10.1603/0022-0493(2008)101[546:tacoce]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, et al. Mechanism of resistance to Bacillus thuringiensis toxin Cry1Ac in a greenhouse population of the cabbage looper, Trichoplusia ni. Appl Environ Microbiol. 2007;73:1199–1207. doi: 10.1128/AEM.01834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gould F, et al. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc Natl Acad Sci USA. 1992;89:7986–7988. doi: 10.1073/pnas.89.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gould F, Anderson A, Reynolds A, Bumgarner L, Moar W. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J Econ Entomol. 1995;88:545–1559. [Google Scholar]
- 40.Jurat-Fuentes JL, Gould FL, Adang MJ. Dual resistance to Bacillus thuringiensis Cry1Ac and Cry2Aa toxins in Heliothis virescens suggests multiple mechanisms of resistance. Appl Environ Microbiol. 2003;69:5898–5906. doi: 10.1128/AEM.69.10.5898-5906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gahan LJ, et al. Genetic basis of resistance to Cry1Ac and Cry2Aa in Heliothis virescens (Lepidoptera: Noctuidae) J Econ Entomol. 2005;98:1357–1368. doi: 10.1603/0022-0493-98.4.1357. [DOI] [PubMed] [Google Scholar]
- 42.Karumbaiah L, Oppert B, Jurat-Fuentes JL, Adang MJ. Analysis of midgut proteinases from Bacillus thuringiensis-susceptible and -resistant Heliothis virescens (Lepidoptera: Noctuidae) Comp Biochem Physiol B. 2007;146:139–146. doi: 10.1016/j.cbpb.2006.10.104. [DOI] [PubMed] [Google Scholar]
- 43.Burd AD, Gould F, Bradley JR, Van Duyn JW, Moar WJ. Estimated frequency of nonrecessive Bt resistance genes in bollworm, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in eastern North Carolina. J Econ Entomol. 2003;96:127–142. doi: 10.1093/jee/96.1.137. [DOI] [PubMed] [Google Scholar]
- 44.Jackson RE, Gould F, Bradley JR, Jr, Van Duyn JW. Genetic variation for resistance to Bacillus thuringiensis toxins in Helicoverpa zea (Lepidoptera: Noctuidae) J Econ Entomol. 2006;99:1790–1797. doi: 10.1603/0022-0493-99.5.1790. [DOI] [PubMed] [Google Scholar]
- 45.Ali MI, Luttrell RG. Susceptibility of bollworm and tobacco budworm (Lepidoptera: Noctuidae) to Cry2Ab2 insecticidal protein. J Econ Entomol. 2007;100:921–931. doi: 10.1603/0022-0493(2007)100[921:sobatb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Burd AD, Bradley JR, Jr, Van Duyn JW, Gould F. Resistance of bollworm, Helicoverpa zea, to Cry1A(c) toxin. In: Dugger P, Richter DA, editors. Proceedings of the Beltwide Cotton Conference Volume 2; Memphis, TN: National Cotton Council; 2000. pp. 923–926. [Google Scholar]
- 47.Jackson RE, Bradley JR, Jr, Van Duyn JW, Gould F. Comparative production of Helicoverpa zea (Lepidoptera: Noctuidae) from transgenic cotton expressing either one or two Bacillus thuringiensis proteins with and without insecticide oversprays. J Econ Entomol. 2004;97:1719–1725. doi: 10.1603/0022-0493-97.5.1719. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y-B, Tabashnik BE, Meyer SK, Carrière Y, Bartlett AC. Genetics of pink bollworm resistance to Bacillus thuringiensis toxin Cry1Ac. J Econ Entomol. 2001;94:248–252. doi: 10.1603/0022-0493-94.1.248. [DOI] [PubMed] [Google Scholar]
- 49.Bah A, Van Frankenhuyzen K, Brousseau R, Masson L. The Bacillus thuringiensis Cry1Aa toxin: Effects of trypsin and chymotrypsin site mutations on toxicity and stability. J Invert Pathol. 2004;85:120–127. doi: 10.1016/j.jip.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 50.LeOra Software. POLO-PC: A User's Guide to Probit or Logit Analysis. Berkeley, CA: LeOra Software; 1987. [Google Scholar]
- 51.SAS Institute. JMP 5.1. Version 5. Cary, NC: SAS Institute; 2004. [Google Scholar]
- 52.Liu YB, Tabashnik BE. Inheritance of resistance to Bacillus thuringiensis toxin Cry1C in the diamondback moth. Appl Environ Microbiol. 1997;63:2218–2223. doi: 10.1128/aem.63.6.2218-2223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]