Abstract
Rabies remains an important public health problem with more than 95% of all human rabies cases caused by exposure to rabid dogs in areas where effective, inexpensive vaccines are unavailable. Because of their ability to induce strong innate and adaptive immune responses capable of clearing the infection from the CNS after a single immunization, live-attenuated rabies virus (RV) vaccines could be particularly useful not only for the global eradication of canine rabies but also for late-stage rabies postexposure prophylaxis of humans. To overcome concerns regarding the safety of live-attenuated RV vaccines, we developed the highly attenuated triple RV G variant, SPBAANGAS-GAS-GAS. In contrast to most attenuated recombinant RVs generated thus far, SPBAANGAS-GAS-GAS is completely nonpathogenic after intracranial infection of mice that are either developmentally immunocompromised (e.g., 5-day-old mice) or have inherited deficits in immune function (e.g., antibody production or type I IFN signaling), as well as normal adult animals. In addition, SPBAANGAS-GAS-GAS induces immune mechanisms capable of containing a CNS infection with pathogenic RV, thereby preventing lethal rabies encephalopathy. The lack of pathogenicity together with excellent immunogenicity and the capacity to deliver immune effectors to CNS tissues makes SPBAANGAS-GAS-GAS a promising vaccine candidate for both the preexposure and postexposure prophylaxis of rabies.
Keywords: blood–brain barrier permeability, live-attenuated rabies virus vaccine, postexposure treatment
Rabies causes an estimated 55,000 human deaths globally each year, 23,750 of which occur in Africa (1). Moreover, 11 million people undergo rabies postexposure prophylaxis (PEP) worldwide each year. Rabies is a zoonotic disease with dogs remaining the principal host in Asia, parts of America, and large parts of Africa, and rabid dogs are the cause of most human rabies cases (2). Between 30% to 60% of the victims of dog bites are children under the age of 15 (3). Inappropriate dog vaccination programs, limited access to vaccination, and postexposure treatment of individuals that have been exposed to rabid dogs are major problems in developing countries.
Rabies virus (RV), a negative-stranded RNA virus of the rhabdoviridae family, has a relatively simple, modular genome that encodes 5 structural proteins: a RNA-dependent RNA polymerase (L), a nucleoprotein (N), a phosphorylated protein (P), a matrix protein (M), and an external surface glycoprotein (G). The N, P, and L together with the genomic RNA form the ribonucleoprotein complex (RNP). The main feature of rabies virus is neuroinvasiveness, which refers to its unique ability to invade the CNS from peripheral sites. Virus uptake, axonal transport, transsynaptic spread, and the rate of viral replication are key factors that determine the neuroinvasiveness of a RV (4–7). The regulation of viral replication also appears to be one of the important mechanisms contributing to RV pathogenesis. Pathogenic RV strains replicate at a lower rate than attenuated strains, which helps preserve the structure of neurons that is used by the viruses to reach the CNS. In addition, the lower expression levels of viral antigens, in particular the RV G, which is the major viral antigen responsible for the induction of protective immunity, hinders early detection by the host immune system (7). In contrast to wildlife RVs, most attenuated RV strains replicate very quickly and express large amounts of G, thereby inducing strong adaptive immune responses that result in virus clearance. These properties provide the basis for the use of attenuated RV strains for the pre- and PEP of rabies. A live-attenuated RV vaccine is likely to provide effective immunization with a single dose, which has practical, cost, and logistical advantages over conventional multi-dose vaccines with respect to the worldwide eradication of dog rabies. In addition, because live-attenuated RV vaccines are capable of inducing immune responses that can clear virulent RVs from the CNS (8, 9), there is the possibility that such vaccines could serve as the foundation for the treatment of early stage human rabies.
Apart from efficacy, the most important prerequisite for the use of live-attenuated RV vaccines in both preexposure and postexposure immunization against rabies is safety. In this respect, the availability of reverse genetics technology, which allows the modification of viral elements that account for pathogenicity and immunogenicity, has made the systematic development of safer and more potent modified-live rabies vaccine feasible. For example, the pathogenicity of fixed RV strains (i.e., ERA, SAD) can be completely abolished for immunocompetent mice by introducing single amino acid exchanges in their G (10), and we have shown that RVs containing a SADB19 G with an Arg333 → Glu333 mutation are nonpathogenic for adult mice after intracranial (i.c.) inoculation, and that an Asn194 → Ser194 mutation in the same gene prevents the reversion to pathogenic phenotype (10, 11). The G containing both mutations has been designated as GAS. Using the GAS gene, the single and double GAS RV variants, SPBNGAS and SPBNGAS-GAS, respectively, were constructed (10, 12). The introduction of a second G gene significantly improves the efficacy of the vaccine by enhancing its immunogenicity through higher expression of G (13). Elevated G expression is associated with the strong up-regulation of genes related to the NFκB signaling pathway, including IFN-α/β and IFN-γ (12) and increased cell death (13). Furthermore, the presence of 2 G genes also decreases substantially the probability of reversion to pathogenicity because the nonpathogenic phenotype determined by GAS is dominant over a pathogenic G that could emerge during virus growth in vivo or in vitro (14). We have now developed a RV vaccine that expresses 3 copies of GAS. This variant proves to possess unique attributes including the lack of pathogenicity for very young mice and the capacity to prevent lethal rabies encephalomyelitis even when administered after CNS infection with a highly pathogenic RV strain.
Results
Construction and in Vitro Characterization of the Triple G (GAS) Recombinant Rabies Virus SPBAANGAS-GAS-GAS.
Although RV variants containing 1 or 2 GAS genes have been shown to be very safe for normal animals (10, 14), they are pathogenic for immunodeficient mice after i.c. inoculation (see Fig. 3). Because our recent studies showed that the RV G expression level is a key factor that determines the pathogenicity of a RV, we constructed the RV variant SPBAANGAS-GAS-GAS that contains 3 copies of the GAS gene (Fig. 1). To ensure that any observations that are made with the triple GAS construct are due to an increased expression of G and not to the increased genome size, we also constructed SPBAANGAS-GAS(−)-GAS(−), in which all ATG codons of the last 2 GAS genes were scrambled (Fig. 1).
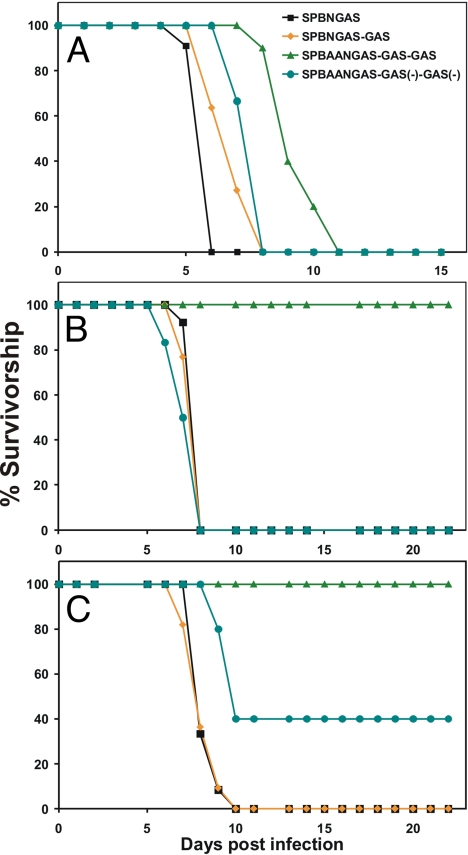
Fig. 3.
Survivorship in mice infected i.c. at day 1, day 5, and day 10 after birth with different recombinant RVs. Litters of 1- (A), 5- (B), and 10- (C) day-old Swiss–Webster mice were infected i.c. with 103 TCIU of SPBNGAS, SPBNGAS-GAS, SPBAAN-GAS-GAS-GAS or SPBAAN-GAS-GAS(−)-GAS(−) The mice were observed for 4 weeks for occurrence of clinical signs of rabies, and mortality rates were recorded daily. Three weeks after infection, blood samples were obtained from the surviving mice, and VNA titers were determined by using the rapid fluorescence inhibition test.
Fig. 1.
Schematic of the construction of recombinant RVs containing 1, 2, or 3 modified G genes. To abolish the pathogenicity, 2 amino acid substitutions were introduced into RV G (Arg333 → Glu333 and Asn194 →Ser194) resulting in GAS. In SPBAANGAS-GAS(−)-GAS(−) all ATG codons of the last 2 GAS genes were scrambled. LS, leader sequence; N, nucleoprotein; M, matrix protein; G, glycoprotein; L, RNA-dependent RNA polymerase; TS, terminal sequence; S, scrambled ATG codons. Restriction enzyme sites are described in SI Text.
The effect of triplication of the GAS gene on virus production was analyzed in the mouse neuroblastoma (NA) cell line with a single-step growth curve being examined (Fig. 2). In the single-step growth kinetics, the replication rate of SPBAANGAS-GAS-GAS was significantly lower between 12 and 24 h post infection (p.i.) than that of SPBAANGAS-GAS(−)-GAS(−) (≈1 log; P < 0.001). However, after 24 h p.i., the growth rate of SPBAANGAS-GAS-GAS increased substantially and approximated that of SPBAANGAS-GAS(−)-GAS.
Fig. 2.
Single-step growth curves for SPBAANGAS-GAS-GAS and SPBAANGAS-GAS(−)-GAS(−). NA cells were infected in duplicate at an MOI of 5, and the titers of virus in the tissue culture supernatants were determined at the indicated time points. Data are the means (±SE) of results from 3 independent experiments.*, P < 0.05; **, P < 0.01; ***, P < 0.001). FFU, focus-forming units.
The retardation in virus production during the early phase of SPBAANGAS-GAS-GAS infection was paralleled by a reduced rate of viral RNA synthesis. qRT-PCR analysis at 12 and 24 h p.i. detected significantly less viral genomic and messenger RNA in SPBAANGAS-GAS-GAS- than in SPBAANGAS-GAS(−)-GAS(−)-infected NA cells whereas at 48 h p.i., the amounts of viral RNA were similar for both viruses (Fig. S1 A and B). Fluorescence-activated “cell sorter” analysis of the surface expression of RV G was used to determine whether the differences in viral RNA synthesis rates were reflected in the levels of G protein expression and revealed higher levels of surface expression of G in SPBAANGAS-GAS(−)-GAS(−)-infected than in SPBAANGAS-GAS-GAS-infected cells at 12 h and, in particular, at 24 h p.i. However, at 48 h p.i., the G expression levels were identical for both viruses (Fig. S2).
The Triple GAS RV Variant Has Limited Pathogenicity in Suckling Mice.
To examine whether the presence of 3 GAS genes further decreases the pathogenicity in young mice of RV vaccine candidates, groups of 8–15 1-, 5-, and 10-day-old Swiss–Webster mice were inoculated intracerebrally (i.c.) with 103 focus-forming units (FFU) of SPBNGAS, SPBNGAS-GAS, SPBAANGAS-GAS-GAS, or SPBAANGAS-GAS(−)-GAS(−) and observed for occurrence of clinical signs of rabies. Although all 1-, 5-, or 10-day-old mice inoculated i.c. with SPBNGAS or SPBNGAS-GAS succumbed to infection between 6 and 10 days afterward, all 5- and 10-day-old mice infected i.c. with SPBAANGAS-GAS-GAS did not develop any clinical signs of infection and survived (Fig. 3). Moreover, although all 1-day-old mice infected with SPBAANGAS-GAS-GAS died, they died much later than SPBNGAS or SPBNGAS-GAS-infected 1-day-old mice (8–11 days after infection). Notably, 100% and 60% of the 5- and 10-day-old mice infected with SPBAANGAS-GAS(−)-GAS(−) succumbed (Fig. 3), indicating that although the increase in the genome size may somewhat contribute to the attenuation of SPBAANGAS-GAS-GAS, the strong reduction of its pathogenicity is primarily because of increased G expression.
Immunogenicity of SPBAANGAS-GAS-GAS in Young and Adult Mice.
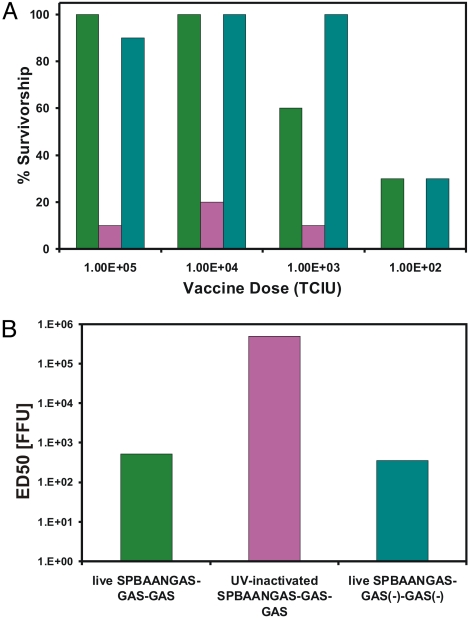
The immunogenicity of the triple G vaccine candidate was assessed in mice of different ages. Mice that were infected i.c. at day 5 or day 10 after birth produced high virus neutralizing antibody (VNA) titers by 21 days after infection (24 and 41 IU, respectively) and were completely protected against an i.c. challenge infection with DOG4 RV. These results demonstrate that, despite its decreased pathogenicity for suckling mice, SPBAANGAS-GAS-GAS is also highly immunogenic for very young mice. Adult mice that were immunized i.m. with a single dose containing 105 or 104 FFU of SPBAANGAS-GAS-GAS were completely protected against an i.c. challenge infection with DOG4 RV that killed 100% of the mock-immunized mice (Fig. 4). Notably, the ED50 of SPBAANGAS-GAS-GAS was similar to that of SPBAANGAS-GAS(−)-GAS(−) (Fig. 4). However, UV inactivation of SPBAANGAS-GAS-GAS resulted in a >1,000-fold increase in the ED50.
Fig. 4.
Preexposure immunization of SPBAANGAS-GAS-GAS, SPBAANGAS-GAS(−)-GAS(−) and UV-inactivated SPBAANGAS-GAS-GAS in Swiss–Webster mice. Groups of 10 mice were injected i.m. with 100 μL of serial 10-fold dilutions (vaccine doses: 102 to 105 TCIU) of the recombinant RVs. Three weeks after immunization, mice were infected i.c. with 100 LD50 of DOG4 RV and observed for 4 weeks. (A) The percentage of survivors in the different immunization groups at 4 weeks after virus challenge. The ED50 values (B) were calculated from the survivorship rates in the 3 vaccination groups as described in ref. 20.
Effect of SPBNAAGAS-GAS-GAS on the Outcome of an i.c. Infection with DOG4 RV in Normal and Immunodeficient Mice.
To examine whether i.c. administration of SPBNAAGAS-GAS-GAS can prevent lethal CNS infection with RV, groups of 10 6- to 8-week-old BALB/c or C57BL/6 mice were infected i.c. with 106 FFU of SPBNAAGAS-GAS-GAS, 100 50% effective doses (IC-LD50) of the highly pathogenic DOG4 RV, or a mixture of 107 FFU of SPBAANGAS-GAS-GAS and 100 IC-LD50 of DOG4 RV. Although i.c. infection with DOG4 RV alone caused 100% and 90% mortality in BALB/c or C57BL/6 mice, respectively, no mortality was seen in these mice after infection with a mixture of DOG4 RV and SPBAANGAS-GAS-GAS (Table 1.).
Table 1.
Mortality after i.c. infection of wild-type and mutant mice with SPBAANGAS-GAS-GAS (Tri GAS), DOG4 RV, or a mixture of SPBAANGAS-GAS-GAS and DOG4 RV
| Mouse strain | Mortality after i.c. virus infection |
||
|---|---|---|---|
| Tri GAS | DOG4 RV | Tri GAS + DOG4 RV | |
| Balb/C | ND | 9/9 | 0/10 |
| C57BL/6 | ND | 9/10 | 0/10 |
| B6–129-μMT−/− | 0/9 | ND | 5/5 |
| C57BL/6-MyD88−/− | 0/5 | ND | 7/7 |
| BALB/c-IFN-α/β R−/− | 0/6 | 10/10 | 11/11 |
Mice were infected i.c. with 103 FFU of SPBAANGAS-GAS-GAS, 100 ICLD50 of DOG4 RV, or a mixture of 107 FFU SPBAANGAS-GAS-GAS and 100 ICLD50 DOG4 RV. Mice were observed for 30 days, and mortality rates were recorded daily. ND, not done.
To provide preliminary insight into the nature of the immune effectors induced by SPBAANGAS-GAS-GAS that play a role in preventing a lethal i.c. infection with DOG4 RV, we coinfected mice lacking B cells (μMT−/−), or that had a defective TLR and IL-1 receptor signaling pathway (MyD88−/−), or were deficient in type I IFN responses (IFN-α/β R−/−) i.c. with DOG4 RV and SPBAANGAS-GAS-GAS. As shown in Table 1, 100% of the μMT−/−, and IFN-α/β R−/− mice succumbed to the infection with the DOG4/SPBAANGAS-GAS-GAS mixture. These data suggest that the antibody production and innate immune response are both important.
Effect of SPBAANGAS-GAS-GAS on Immune Effector Delivery to CNS Tissues.
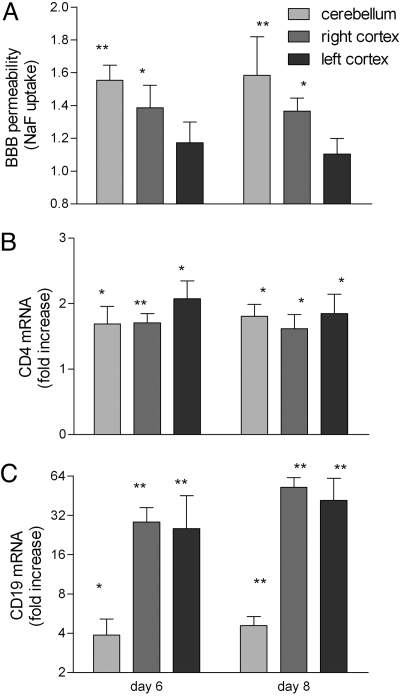
The capacity to induce the mechanisms that deliver rabies-specific immune effectors into CNS tissues is an important feature that differentiates effective vaccine variants from pathogenic rabies viruses (9). Elevated blood–brain barrier (BBB) permeability to fluid phase markers, which generally occurs between 6 and 12 days after immunization (8), is a reflection of this process. Consequently, BBB permeability to NaF was assessed in brain tissues from adult C57BL/6 mice infected i.c. with SPBAANGAS-GAS-GAS in the right cerebral cortex 6 and 8 days previously. As shown in Fig. 5A, BBB permeability to NaF is significantly elevated in the right cortex and cerebellum. Elevated expression of CD4 (Fig. 5B) and CD19 (Fig. 5A) was also detected, the latter primarily in the cortex. CD19 mRNA levels were particularly elevated, appearing at high levels in both cortical hemispheres within 6 days of infection. This indicates that immune cells are being delivered into the CNS tissues with a possible bias in B cell accumulation in the tissues where virus replication is most likely.
Fig. 5.
Induction of BBB permeability after i.c. infection with SPBAANGAS-GAS-GAS. 129/SvEv mice were injected with 107 FFU of SPBAANGAS-GAS-GAS into the right hemisphere. BBB permeability to the fluid phase marker Na-fluorescein was assessed 6 and 8 days later in the right and left cortex and in cerebellum. Levels of mRNAs specific for the T cell marker CD4 and the B cell marker CD19 in the same tissues were assessed by quantitative RT-PCR. BBB permeability is expressed as the amount of Na-fluorescein detected in infected CNS tissues normalized to the amount in uninfected CNS tissue (A). CD4 (B) and CD19 (C) mRNA levels are expressed as the fold increase in infected over the levels detected in uninfected brain tissue. Significance of differences between the signals in normal and infected tissues were assessed by the Mann–Whitney test. *, P < 0.05; **, P < 0.01.
Postexposure Efficacy of SPBAANGAS-GAS-GAS.
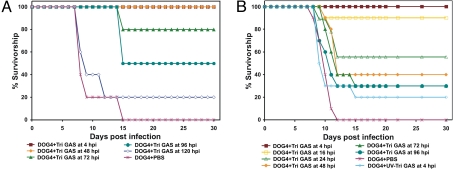
To test whether treatment with the SPBAANGAS-GAS-GAS vaccine can prevent a lethal street RV infection when administered after infection with a highly neuroinvasive RV, we first inoculated 6- to 8-week-old Swiss–Webster mice i.m. with 106 FFU of DOG4 RV. Four, 48, 72, 96, and 120 h after infection, the mice received 107 FFU of SPBAANGAS-GAS-GAS i.c. Although 100% of the mice treated with PBS developed severe rabies encephalomyelitis and succumbed to the infection, none of the mice that were treated 4 h after infection with live SPBAANGAS-GAS-GAS died (Fig. 6A) or developed clinical signs of rabies. All mice that were treated i.c. 48 h p.i. with live SPBAANGAS-GAS-GAS developed hind limb paralysis, but none of these mice died, and 60% recovered fully between 16 and 21 days p.i. Even when i.c. immunization was initiated as late as 4 days after street RV challenge, 50% of the animals survived.
Fig. 6.
Postexposure treatment with SPBNAAGAS-GAS-GAS of mice after infection with DOG RV. Groups of 10 adult Swiss–Webster mice infected i.m. with 10 IM-LD50 of DOG4 RV and treated i.c (A) or i.m. (B) at different times p.i. with SPBAANGAS-GAS-GAS as described in SI Text.
We then investigated whether the SPBAANGAS-GAS-GAS vaccine is also efficacious when administered by the i.m. route after an i.m. infection with 106 FFU of DOG4 RV. Fig. 6B shows that no mortality was seen when 108 FFU of live SPBAANGAS-GAS-GAS were injected into the masseter muscle at 4 h after infection with DOG4 RV. Notably, i.m. treatment with UV-inactivated SPBAANGAS-GAS-GAS at 4 h after infection protected only 20% of the mice, indicating that the protective activity of SPBAANGAS-GAS-GAS against a lethal RV infection of the CNS depends largely on the capacity of the vaccine virus to replicate. When i.m. treatment with SPBAANGAS-GAS-GAS was performed at 16, 24, 48, and 72 h after virus challenge, 90%, 55%, 40%, and 30% of the mice, respectively, survived. Although no clinical signs were seen in animals treated at 4 h p.i., between 50% and 90% of the mice treated i.m. at the later time points developed hind limb paralysis. Because i.c. or i.m. treatment of uninfected mice with SPBAANGAS-GAS-GAS does not cause any clinical signs, it is probable that the pathogenic RV infection had already damaged spinal cord neurons before the virus was cleared.
Discussion
Conventional inactivated multidose rabies vaccines have an extensive history in managing dog rabies in developed regions of the world. However, financial, delivery, and other considerations dictate that a single dose rabies vaccine will be required to control dog, as well as human rabies of dog origin, worldwide. This vaccine must be safe and able to confer long-lasting immunity after a single administration. We have developed the SPBAANGAS-GAS-GAS variant that meets these requirements.
To test the safety of SPBAANGAS-GAS-GAS, we assessed its pathogenicity for suckling mice as these animals are not fully immunocompetent until approximately 6 weeks of life. This experiment revealed that the pathogenicity of the triple GAS RV is considerably lower for suckling mice than that of the single and double GAS recombinant RV. The pathogenicity of the triple GAS variant is also significantly lower than that of a recombinant RV in which 2 of the 3 G genes are inactive [SPBAANGAS-GAS(−)-GAS(−)]. This strongly suggests that the higher level of attenuation of SPBAANGAS-GAS-GAS is primarily because of increased G expression rather than an increase in the size of the genome. Somewhat paradoxically, SPBAANGAS-GAS-GAS-infected cells initially express lower levels of G than SPBAANGAS-GAS(−)-GAS(−)-infected cells. We speculate that the reason for this phenomenon is that the over-expression of the G in SPBAANGAS-GAS-GAS-infected cells after primary RNA transcription, which is undetectable using available technology, results in a cellular stress response that causes the transient inhibition of virus replication (15). Despite a brief lag period in G production, the triple GAS RV variant rapidly begins to produce high levels of G protein and is highly immunogenic. It is noteworthy in this regard that 5- and 10-day-old mice infected i.c. with SPBAANGAS-GAS-GAS exhibit high levels of VNA at day 21 p.i. and are fully protected against a subsequent i.c. RV challenge infection that kills 100% of unvaccinated control mice. This finding implies that a triple GAS vaccine should be safe and effective for young dogs and present little risk for young children who may be exposed to the virus.
An important factor in controlling and eventually eradicating dog and dog-associated human rabies worldwide is the availability of a potent but affordable vaccine. Because of the ability to replicate, thereby producing relatively large amounts of antigen from a small input dose, a live-attenuated vaccine would be expected to be considerably less expensive than a killed RV vaccine product. In addition, the requirement for multiple boost doses of vaccine would be reduced. Immunization with a single dose of triple GAS vaccine containing as little as 5 × 102 live virus particles protects 50% of mice (ED50) against a lethal RV infection after with complete protection being achieved when 104 virus particles are administered. In contrast, the ED50 of UV-inactivated SPBAANGAS-GAS-GAS is >1,000 times higher than that of the live virus. This indicates that the high efficacy of the triple GAS variant depends on its ability to replicate in addition to providing insight into how much more costly a killed vaccine would be.
Our postexposure treatment experiments with mice demonstrate that lethal rabies encephalopathy can be prevented by administering live but not UV-inactivated SPBAANGAS-GAS-GAS up to several days after infection with a highly pathogenic wildlife RV strain. This suggests that the live triple GAS vaccine might also be effective for delayed rabies PEP in humans. In contrast to mice, in which disease development rapidly occurs after street virus infection (5 to 6 days p.i.) and is lethal within 2 or 3 days after the onset of clinical signs, the average incubation time of rabies in humans varies between 1 and 2 months. In addition, the disease can last several weeks from the onset of the prodromal period to the development of acute neurological disease, the progression to coma, and death. This is more than sufficient time for SPBAANGAS-GAS-GAS to induce a RV-clearing immune response, particularly because it promotes immune effector entry into infected CNS tissues.
The mechanism by which postexposure treatment with SPBAANGAS-GAS-GAS prevents a lethal encephalopathy is not exactly known. The observation that immunocompetent mice, but not mice that are deficient in B cells or have defective type I IFN, TLR, or IL-1 receptor signaling pathways, survived an i.c. infection with a mixture of DOG4 RV and SPBAANGAS-GAS-GAS strongly suggests that adaptive immune responses as well as innate immune responses are required to clear the RV from the brain. We speculate that there are 2 characteristics of SPBNAAGAS-GAS-GAS that enable it to rapidly induce an immune response capable of clearing pathogenic RV: 1) Enhanced stimulation of antiviral and proinflammatory mechanisms through the NFκB signaling pathway; and 2) overcoming the failure of pathogenic RV to trigger BBB permeability changes and the delivery of immune effectors to the CNS. In addition to the induction of innate and adaptive immune responses, the delivery of immune effectors across the BBB is necessary for clearance of RV from the CNS. Infection with attenuated but not with pathogenic RVs triggers BBB permeability changes and the invasion of immune effectors into CNS tissues (9, 16). SPBAANGAS-GAS-GAS effectively induces BBB permeability and the delivery of immune cells into CNS tissues.
The use of the highly attenuated triple GAS vaccine, which is able to induce protective immunity after a single immunization, could make global eradication of canine rabies more feasible. In addition, because of its ability to prevent the fatal outcome of the disease by overcoming immune evasion of pathogenic RVs, this vaccine may have utility for human PEP, particularly in situations where the RV has already reached the CNS tissues and current PEP regimens fail.
Materials and Methods
Viruses and Cell Lines.
The recombinant RV vaccine SPBNGAS is based on the prototype recombinant virus SPBN, which was derived from the SAD B19 cDNA clone (17). The generation of the double G variant of SPBN is described elsewhere (12, 13). All RV vaccine strains were propagated in BSR (a BHK-21 clone) (18) cells. Briefly, cells grown in DMEM (Mediatech) supplemented with 10% FBS were infected at a MOI of 0.1 and incubated for 1h at 37 °C. The inoculum was then removed, and the cells were replenished with OptiPro SFM medium (Invitrogen) supplemented with 4 mM glutamine and incubated for 72 h at 34 °C. The pathogenic RV strain DOG4, which was isolated from brain tissue of a human rabies victim, was propagated in NA as described in ref. 19.
Mice.
Six- to eight-week-old female Swiss–Webster, C57BL/6, Balb/C mice and pregnant Swiss–Webster mice were purchased from National Cancer Institute (Frederick, MD), 129/SvEv mice were purchased from Taconic Farms, and B6–129-μMT−/− were purchased from Jackson Laboratory. C57BL/6-MyD88−/− and BALB/c-IFN-α/β R−/− mice were originally obtained from Dr. S. Akira (Osaka University, Osaka, Japan) and Dr. Joan Durbin (The Ohio State University, Columbus, Ohio). All mutant mouse strains were maintained in the Thomas Jefferson University Animal Facilities, and all animal experiments were performed under Institutional Animal Care and Use Committee-approved protocols (Animal Welfare Assurance no. A3085–01).
Construction of Recombinant RV cDNA Clones and Rescue of Recombinant Viruses.
The construction of pSPBAANGAS-GAS-GAS and pSPBAANGAS-GAS(−)-GAS(−) is described in detail in the SI Text. The recombinant RVs were rescued from the cDNA clones as described (13, 17), and the correct nucleotide sequences of the inserted genes were confirmed by reverse transcriptase PCR analysis and DNA sequencing.
Infection of Mice.
One-, 5-, and 10-day-old Swiss–Webster mice were injected i.c. with 103 FFU of SPBNGAS, SPBNGAS-GAS, SPBAAN-GAS-GAS-GAS or SPBAAN-GAS-GAS(−)-GAS(−) in 5 μL PBS. One litter of 8–15 mice was used for each virus. Twenty-one days after infection, blood samples were obtained from the surviving mice and VNA titers were determined by using the rapid fluorescence inhibition test. Six 8-week-old Swiss–Webster or various mutant mice were infected i.c. under anesthesia with 5 μL PBS containing 107 FFU SPBNAAGAS-GAS-GAS, 100 50% i.c. lethal doses (IC-LD50) of DOG4 RV, or a mixture of 107 FFU of SPBNAAGAS-GAS-GAS and 100 IC-LD50 of DOG4 RV. Intramuscular (i.m.) infection of adult Swiss–Webster mice was performed under anesthesia by injecting PBS containing 10 50% i.m. lethal doses (IM-LD50) of DOG4 RV into the gastrocnemius (100 μL) or masseter (50 μL) muscles. After infection, mice were observed for 30 days for clinical signs of rabies and mortality rates were recorded daily.
Preexposure and Postexposure Prophylaxis.
For preexposure immunization against rabies, groups of 10 6- to 8-week-old female Swiss–Webster mice were inoculated i.m. with 100 μL of serial 10-fold dilutions of live recombinant RV. After 14 days, the animals were injected i.c. under isoflurane anesthesia with 5 μL containing 100 IC-LD50 of DOG4 RV. To determine the postexposure efficacy of SPBAANGAS-GAS-GAS, groups of 10 6- to 8-week-old female Swiss–Webster mice were infected i.m. (gastrocnemius muscle) with 100 μL PBS containing 10 IM-LD50 of DOG4 RV. At different times after infection ranging from 4 h to 5 days, the mice were treated either i.c. with 5 μL containing 107 FFU of SPBAANGAS-GAS-GAS or i.m. (masseter muscle) with 50 μL containing 108 FFU of SPBNAAGAS-GAS-GAS. A control group of 10 mice received 5 μL PBS i.c. and 2 other control groups of 10 mice were treated i.m. with 100 μL PBS or with 100 μL UV-inactivated SPBAANGAS-GAS-GAS. After virus challenge, mice were observed for 4 weeks for clinical signs of rabies. Mice that showed definitive clinical signs of rabies such as paralysis, tremors, and spasms were euthanized by CO2 intoxication. Survivorship rates obtained with the different vaccine dilutions for the different vaccination groups were compared, and the ED50 was calculated as described in ref. 20.
Blood–Brain Barrier (BBB) Permeability.
Fluid phase BBB permeability was assessed as described in ref. 21. Briefly, mice received 100 μL of 10% sodium fluorescein (NaF, 376 molecular weight, Sigma) in PBS i.p. and 10 min later were anesthetized, bled, and transcardially perfused with PBS/heparin (1,000 units per liter) and PBS. Brains were removed and separated into left and right cerebral cortex hemispheres and cerebellum. Brains tissues were homogenized in PBS, centrifuged, and the fluorescent marker content in the clarified supernatant determined in a Cytofluor II fluorimeter (PerSeptive Biosystems). Specific NaF content was calculated with the use of standards and uptake from the circulation into CNS tissue is expressed as (μg fluorescence CNS tissue/mg protein)/(μg fluorescence sera/μL blood) to normalize values for blood levels of the marker. The results are expressed as the level of fluorescein in the tissues with the levels detected in tissues from similarly treated normal mice taken as 1.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants R01 AI060686 (to B.D.), R01 AI065750 (to K.R.A.), and AI060005 and AI077033 (to D.C.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905640106/DCSupplemental.
References
- 1.Knobel DL, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83:360–368. [PMC free article] [PubMed] [Google Scholar]
- 2.Hampson K, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009;7:e53. doi: 10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Technical Report Series. Vol 931. Geneva: WHO; 2004. WHO Expert Consultation on Rabies 2004. [Google Scholar]
- 4.Dietzschold B, et al. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci USA. 1983;80:70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietzschold B, et al. Induction of protective immunity against rabies by immunization with rabies virus ribonucleoprotein. Proc Natl Acad Sci USA. 1987;84:9165–9169. doi: 10.1073/pnas.84.24.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morimoto K, Foley HD, McGettigan JP, Schnell MJ, Dietzschold B. Reinvestigation of the role of the rabies virus glycoprotein in viral pathogenesis using a reverse genetics approach. J Neurovirol. 2000;6:373–381. doi: 10.3109/13550280009018301. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto K, Hooper DC, Spitsin S, Koprowski H, Dietzschold B. Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J Virol. 1999;73:510–518. doi: 10.1128/jvi.73.1.510-518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- 9.Roy A, Hooper DC. Immune evasion by rabies viruses through the maintenance of blood-brain barrier integrity. J Neurovirol. 2008;14:401–411. doi: 10.1080/13550280802235924. [DOI] [PubMed] [Google Scholar]
- 10.Faber M, et al. A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J Virol. 2005;79:14141–14148. doi: 10.1128/JVI.79.22.14141-14148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietzschold ML, et al. In vitro growth and stability of recombinant rabies viruses designed for vaccination of wildlife. Vaccine. 2004;23:518–524. doi: 10.1016/j.vaccine.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Li J, McGettigan JP, Faber M, Schnell MJ, Dietzschold B. Infection of monocytes or immature dendritic cells (DCs) with an attenuated rabies virus results in DC maturation and a strong activation of the NFkappaB signaling pathway. Vaccine. 2008;26:419–426. doi: 10.1016/j.vaccine.2007.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faber M, et al. Overexpression of the rabies virus glycoprotein results in enhancement of apoptosis and antiviral immune response. J Virol. 2002;76:3374–3381. doi: 10.1128/JVI.76.7.3374-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faber M, et al. Dominance of a nonpathogenic glycoprotein gene over a pathogenic glycoprotein gene in rabies virus. J Virol. 2007;81:7041–7047. doi: 10.1128/JVI.00357-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medigeshi GR, et al. West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J Virol. 2007;81:10849–10860. doi: 10.1128/JVI.01151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy A, Phares TW, Koprowski H, Hooper DC. Failure to open the blood-brain barrier and deliver immune effectors to central nervous system tissues leads to the lethal outcome of silver-haired bat rabies virus infection. J Virol. 2007;81:1110–1118. doi: 10.1128/JVI.01964-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnell MJ, Mebatsion T, Conzelmann KK. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato M, Maeda N, Yoshida H, Urade M, Saito S. Plaque formation of herpes virus hominis type 2 and rubella virus in variants isolated from the colonies of BHK21/WI-2 cells formed in soft agar. Arch Virol. 1977;53:269–273. doi: 10.1007/BF01314672. [DOI] [PubMed] [Google Scholar]
- 19.Dietzschold B, et al. Genotypic and phenotypic diversity of rabies virus variants involved in human rabies: Implications for postexposure prophylaxis. J Hum Virol. 2000;3:50–57. [PubMed] [Google Scholar]
- 20.Habel K. In: Laboratory Techniques in Rabies. 4th Ed. Kaplan MM, Koprowski H, Meslin FX, editors. Geneva: WHO; 1996. [Google Scholar]
- 21.Hooper DC, et al. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 2000;14:691–698. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.