Abstract
Blur induced by uncorrected astigmatism during early development can result in amblyopia, as evidenced by reduced best-corrected vision relative to normal, in measures of grating acuity, vernier acuity, contrast sensitivity across a range of spatial frequencies, recognition acuity, and stereoacuity. In addition, uncorrected astigmatism during early development can result in meridional amblyopia (MA), or best-corrected visual deficits that are greater for, or are present only for, specific stimulus orientations. Astigmatism-related amblyopia can be successfully treated with optical correction in children as old as school age, but the amblyopia may not be completely eliminated with optical treatment alone, and the age at which optical treatment is most effective has yet to be determined. Future research on determining the period of susceptibility of the visual system to negative effects of uncorrected astigmatism and exploration of alternative or complimentary treatment methods, in addition to optical correction, are warranted.
Keywords: astigmatism, meridional amblyopia, visual development, amblyopia treatment
Classic animal studies have demonstrated that deprivation of visual experience for stimuli of certain orientations during a critical period of development results in reduced response for stimuli of those orientations that persists after the deprivation is eliminated.1,2 A similar phenomenon, clinically referred to as meridional amblyopia (MA), occurs in humans. Research conducted primarily in the 1970s and ‘80s demonstrated that orientation-dependent blur induced by uncorrected astigmatism during early development results in orientation-dependent visual deficits that persist despite optical correction or emmetropization of the astigmatism.3–11 The orientation for which subjects demonstrate poorer acuity is typically consistent with the stimulus orientation for which they experienced greater visual degradation when astigmatism was uncorrected.3–6,10
Few studies were added to the literature on MA after these early studies,12–14 presumably due to the low prevalence of astigmatism in the general population, until recent years when partnership with the Tohono O’odham Nation, a Native American tribe with a high prevalence of high bilateral with-the-rule astigmatism,15–20 allowed researchers to conduct large-scale studies of preschool- and school-aged children with high astigmatism.21–26 These studies evaluated the effects of bilateral astigmatism on visual development, as well as the effectiveness of optical treatment of astigmatism-related deficits, utilizing reliable and valid methods of measuring refractive error27 and an age-matched non-astigmatic control group of children with which to compare best-corrected monocular visual performance in bilaterally astigmatic children. The present paper summarizes what we have learned from these and previous studies of astigmatism-related amblyopia. In the summary that follows, reduced best-corrected vision relative to normal is referred to as amblyopia, and best-corrected visual deficits that are greater for, or are present only for, specific stimulus orientations is referred to as meridional amblyopia (MA).
Patterns of Blur and Uncorrected Astigmatism
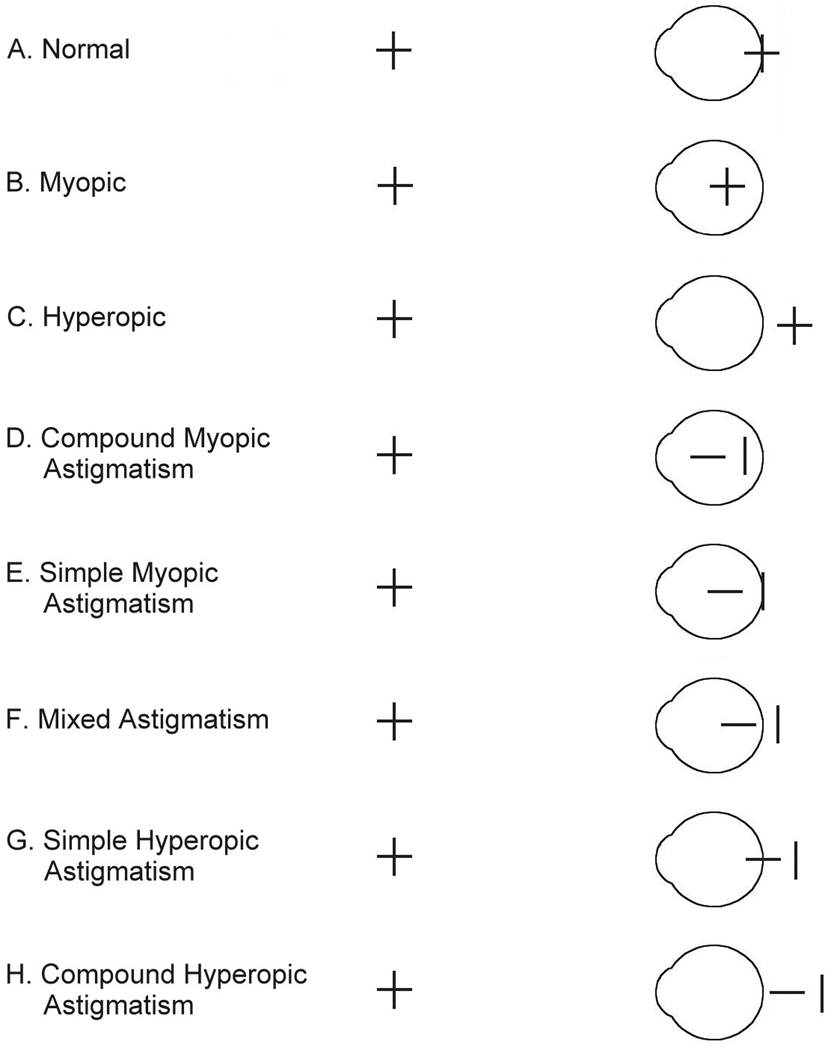
Mitchell and colleagues asserted that the pattern of MA shown by an astigmatic adult could be predicted from the astigmatic focal plane that is most often out of focus.4 Figure 1 illustrates the differential focusing of vertical and horizontal stimulus orientations, presented at distance, in non-astigmatic eyes and in eyes with different types of uncorrected with-the-rule astigmatism when the eyes are in their unaccommodated state. In non-astigmatic eyes (Figure 1A–C), focal planes of all stimulus orientations come into focus at the same location, but in uncorrected astigmatic eyes (Figure 1D–H), orthogonal focal planes come into focus at different locations. When viewing stimuli at distance, the more myopic focal plane is most out of focus for myopic astigmats (Figure 1D–E), and the myopic focal plane is likely to be most out of focus for mixed astigmats (Figure 1F) because they can accommodate to focus the hyperopic, but not the myopic, focal plane. When viewing stimuli at near, myopic and mixed astigmats may be to accommodate to either focal plane, depending on viewing distance and amount of myopia. However, it is likely that the myopic and mixed astigmats illustrated in Figure 1 experience more frequent degradation of input for horizontal stimuli, and would show meridional amblyopia for horizontal stimuli.
Figure 1.
Illustration of the location relative to the retina that horizontal and vertical line stimuli come into focus when accommodation is relaxed in non-astigmatic eyes (A–C) and in astigmatic eyes with with-the-rule astigmatism (greatest curvature in the vertical meridian, plus cylinder axis 90°, minus cylinder axis 180°) (D–H) (From Harvey et al., 2007).22
Predictions are more complicated for hyperopic astigmats (Figure 1G–H), as they are typically capable of accommodating to bring either focal plane into focus. However, it is most likely that they either accommodate to bring the less hyperopic focal plane into focus, since it requires the least effort, or that they accommodate between the two focal planes, so that both are equally out of focus. Data from small samples of subjects indicate that hyperopic astigmats tend to accommodate to bring the less hyperopic focal plane into focus.6,28 However, there is also data to suggest that when performing a task that requires good acuity (reading an eye chart), some hyperopic astigmats accommodate between the two extreme focal planes.28 Thus, depending on patterns of accommodation, hyperopic astigmats may experience the more hyperopic focal plane most often out of focus and therefore show meridional amblyopia for stimuli corresponding to the more hyperopic focal plane,4 or they may often experience both focal planes equally out of focus, in which case they may not be at risk for developing meridional amblyopia (reduced vision for one orientation relative to the orthogonal orientation), but they may be still be at risk for developing amblyopia (i.e., vision that is not necessarily reduced for a specific stimulus orientation, but is reduced relative to normal).
Meridional Amblyopia
Grating Acuity
Early studies found MA for grating stimuli that was consistent with the predictions outlined above, i.e., best-corrected (BC) grating acuity for the stimulus orientation that is likely to be most out of focus when the astigmatism is uncorrected tended to be reduced relative to grating acuity for the orthogonal orientation.3,4,10 Recent studies of Tohono O’odham children showed the predicted patterns of MA for myopic and mixed astigmats, but they did not find evidence of MA in hyperopic astigmats (relative to meridional differences in grating acuity in age matched non-astigmatic control groups), 21,22,25 However, BC grating acuity was not normal for children with any type of astigmatism. For myopic/mixed and hyperopic astigmats, grating acuity was reduced for both stimulus orientations relative to grating acuity in the non-astigmatic control group.21,22,25 One possible reason for the discrepancy between studies in terms of observing MA in hyperopic astigmats is that there may be individual differences in accommodation patterns, and thus differences in patterns of blur experienced by hyperopic astigmats. Some uncorrected hyperopic astigmats may tend to bring the less hyperopic meridian into focus and others may accommodate to focus between the two focal planes, leaving both stimulus orientations out of focus. Alternatively, it is possible that there are differences between myopic/mixed and hyperopic astigmats in this population with respect to changes in refractive error, and therefore changes in patterns of astigmatic blur experienced, during development. Predicted patterns of meridional deficits were based on refractive error at the time of testing, which may or may not have been the same throughout development. However, longitudinal data on refractive error throughout early development was not available on the children in these studies, and therefore we cannot be certain that patterns of blur experienced were consistent throughout early development.
Vernier Acuity
Early studies reported that astigmatism in infancy or early childhood can lead to MA for vernier stimuli.4,10 However, recent studies of astigmatic Tohono O’odham school-age children showed that, relative to an age matched non-astigmatic control group, there was no significant MA for vernier stimuli, although there was amblyopia for vernier acuity as BC vernier acuity was reduced relative to normal across stimulus orientations.21,25 The finding of significant MA for grating stimuli, but no MA for vernier stimuli in myopic/mixed astigmats, is of particular interest as it suggests that the meridional deprivation that results in MA for grating acuity does not have the same orientation specific effects on the neural mechanisms responsible for vernier acuity, but does nonetheless influence development of vernier acuity, since vernier acuity was reduced relative to normal in children with myopic/mixed astigmatism.
The reason for the discrepancy across studies with regard to observing MA for vernier acuity in astigmatic subjects is not clear. It is possible that stimulus differences across studies may account for differences in findings with regard to observing MA in measurements of vernier acuity. Our studies used stationary single line vernier stimuli with no gap at the vernier offset, whereas studies in which MA was observed in measurements of vernier acuity differed in that they used stimuli with a gap at the vernier offset,4 or included motion in the vernier stimuli.10 A study of vernier acuity performance for different forms of vernier stimuli under conditions of induced astigmatic blur would allow us to determine if meridional blur is sufficient to induced meridional differences in vernier acuity, and to determine if stimulus variables might have been the cause of differences in findings of MA across studies. Such studies may also provide us with further insight into the mechanisms involved with vernier acuity.
Another interpretation of the data is that MA for vernier stimuli may have been present in the Tohono O’odham children earlier in development but later resolved via mechanisms similar to those responsible for improvements in vernier acuity through perceptual learning once the children reached an age at which they began to read and spend more time performing fine perceptual tasks. Research has shown that development of vernier acuity continues later into childhood than grating acuity,29 and is amenable to improvement through alterations in visual experience, i.e., perceptual learning, into adulthood in amblyopes.30,31 Independence of grating acuity and vernier acuity deficits might be assessed in future studies by determining if grating acuity deficits and MA for grating acuity stimuli persist when vernier acuity deficits are reduced or eliminated through perceptual learning
Contrast Sensitivity
Early studies of adult astigmats found MA in contrast sensitivity for grating stimuli.3–7 MA was present across a range of spatial frequencies, with more prominent deficits for high spatial frequency stimuli.5,7 Recent studies of Tohono O’odham school-age children found reduced BC contrast sensitivity across stimulus orientations for middle and high spatial frequency stimuli (6.0 and 18.0 cy/deg) in astigmats relative to a non-astigmatic control group, but not for low spatial frequency stimuli (1.5 cy/deg). MA was not observed for low, middle, or high spatial frequency contrast sensitivity stimuli.21,25 The finding of no MA for high spatial frequency grating contrast sensitivity stimuli in children in whom MA for grating acuity was observed (myopic/mixed astigmats) suggests that the meridional deprivation does not have the same orientation specific effects on development of contrast sensitivity that is does on development of grating acuity, although it does appear to influence development of contrast sensitivity, relative to normal, in astigmatic children, as high spatial frequency contrast sensitivity was reduced across stimulus orientation, relative to the non-astigmatic control group.
Astigmatism and Recognition Acuity Deficits
Several studies of members of the Tohono O’odham Nation have reported reduced recognition acuity in adults32 and in children,15,21,22,25,32 including children as young as 3 to 5 years of age.22 In school-age children with ≥ 1.00 diopter of astigmatism, mean BC letter acuity was reduced by approximately 2 logMAR lines relative to BC letter acuity in an age matched group on non-astigmatic children from the same population (mean logMAR acuity was 0.27 for myopic/mixed astigmats, 0.25 for hyperopic astigmats, and 0.04 for non-astigmats), with a reduction of almost one 0.1 logMAR for each diopter of astigmatism.25
Astigmatism and Stereoacuity Deficits
There are data indicating that astigmatic blur reduces stereoacuity,33 suggesting that bilateral astigmatic blur may be sufficient to disrupt normal stereoacuity. However, the available data on the relation between uncorrected astigmatism and the development of stereoacuity are limited to an early case study4 and a more recent study of Tohono O’odham children which are limited to children with bilateral with-the-rule astigmatism. 21,25 The study of Tohono O’odham school-age children reported reduced BC stereoacuity (measured with the Randot Preschool Stereoacuity Test) in astigmatic children without strabismus or anisometropia, relative to a non-astigmatic control group.21,25 It is likely that these BC stereoacuity deficits are the result of reduced high spatial frequency input (due to presence of amblyopia) to stereoacuity mechanisms. However, it is also possible that some of the stereoacuity deficits observed may have been an artifact of meridional magnification caused by the optical correction.
Sensitive Period for Development of Astigmatism-related Amblyopia
As summarized above, uncorrected astigmatism in early childhood is associated with a wide range of visual deficits, including resolution acuity for grating stimuli, recognition acuity, contrast sensitivity, vernier acuity, and stereoacuity. However, there is relatively little direct evidence documenting the sensitive period for development of these deficits, i.e., the period of time during infancy or childhood when the presence of uncorrected astigmatism leads to the development of astigmatism-related amblyopia. Two studies failed to find astigmatism-related MA for grating stimuli in myopic and hyperopic astigmatic infants less than one year of age, suggesting that MA does not develop prior to age one year.34,35 Given the grating acuity limits of infants, these results are consistent with studies of older astigmatic children and adults in which there was less prominent or no MA observed for mid to low spatial frequency stimuli.5,7,21,25 In addition, failure to observe MA in infants less than one year of age does not necessarily mean that astigmatism in infancy does not lead to the later appearance of astigmatism-related amblyopia. For example, a study that followed children longitudinally from infancy to school age found MA for vernier stimuli in non-astigmatic children that was associated with their astigmatism at age six to 24 months, but not with astigmatism present before or after this age range.10 Several studies have shown that MA and deficits for grating stimuli9,11,22 and deficits for recognition acuity22 are present by the time astigmatic children reach three to five years of age, and deficits for vernier stimuli, contrast sensitivity for grating stimuli, and stereoacuity stimuli are present by the time astigmatic children reach five years of age.21,25 Thus, the available data suggest that the sensitive period for development of astigmatism-related amblyopia begins between 6 months and some time prior to three to five years of age. Data are not available to indicate the timing of the end of the sensitive period. Longitudinal studies of refractive error and BC acuity are needed to allow us to define more specifically when the visual system is susceptible to the detrimental effects uncorrected astigmatism.
Treatment of Astigmatism-Related Amblyopia
In current clinical practice, astigmatism-related amblyopia in the absence of anisometropia, strabismus, and other ocular abnormalities is treated by providing the individual with clear visual input through optical correction of the astigmatism. Until recently, there was little prospective research on the sensitive period for successful optical treatment of astigmatism-related amblyopia. Early retrospective studies reported that astigmatic adults who received optical correction prior to seven years of age did not show evidence of MA,4,8 suggesting that the sensitive period for successful treatment is prior to age seven years. These findings are supported by the results of a case study in which MA in a 34-month-old astigmatic child was eliminated after three months of optical correction,9 and by the results of a prospective study in which the majority of the children in the sample who had astigmatism (≥ 1.00 D) that had resolved by age two years did not show evidence of MA when tested at age four years.11 However, data indicating that MA was present in 5- to 11-year-old children in whom astigmatism had emmetropized by age two years suggests that the sensitive period for successful treatment may end even earlier, i.e., prior to age two years.10
A prospective study of three to five-year-old astigmatic Tohono O’odham children revealed no significant improvement in grating acuity or recognition acuity and no significant reduction in MA, relative to a change in a non-astigmatic control group, after four months of spectacle correction, supporting the hypothesis that the sensitive period for successful treatment ends by age three years.23 However, a recent study found that astigmatic kindergarten children who received optical correction in preschool had significantly better BC recognition acuity than did astigmatic kindergarten children who did not receive optical correction prior to kindergarten, suggesting that initiating optical treatment in preschool can reduce astigmatism-related amblyopia.36 Furthermore, several recent studies have provided evidence that astigmatism-related amblyopia can be successfully treated beyond age 3 years. A prospective study of older (school-aged) Tohono O’odham children found that six weeks of spectacle correction resulted in a reduction in astigmatism-related amblyopia, measured by BC grating acuity, recognition acuity, vernier acuity, contrast sensitivity, and stereoacuity, and that the reduction was similar in younger children (4 to 7 years of age) and older children (8 to 13 years of age).24,26 However, even after a full year of spectacle correction, visual function in astigmatic children still did not reach normal levels, as defined by BC vision in a group of non-astigmatic age-matched children from the same population.24,26 Finally, a study of treatment of bilateral refractive amblyopia in 3-to 10-year-old children, most of whom received optical treatment only, reported that for the children in their sample with bilateral astigmatism, binocular acuity improved by an average of 3.5 to 5.4 lines after one year of treatment.37
Given these findings of significant reduction in amblyopia following optical treatment in school-age children, 24,26,37 it is likely that failure to find a reduction in amblyopia in the 3- to 5-year-old Tohono O’odham children23 was due factors other than lack of plasticity. For example, variability in treatment compliance across studies and across age groups (i.e., poorer compliance with spectacle wear in younger children) may have contributed to the failure to see a treatment effect in the preschool children with just four months of treatment. It is also possible that there are differences in short-term treatment effectiveness due to differences in typical visual experience and visual tasks performed by younger and older children (i.e., older children performing more demanding near tasks may have increased treatment effectiveness).
Unanswered Questions
Over the past 35 years, much has been learned about the developing visual system as a result of studies of astigmatism-related amblyopia. However, many questions remain regarding the specific mechanisms and timing of the development and successful treatment of astigmatism-related amblyopia. Several areas of research remain open, including:
Sensitive period for development of astigmatism-related amblyopia and the complex interactions between development of MA and refractive error changes during development Existing studies suggest that MA can develop if astigmatism is present some time between age six months and three years.9–11,22,35,36 More detailed longitudinal studies examining refractive error and BC acuity will provide us with the necessary data to more clearly define the sensitive period of susceptibility to the detrimental effects of uncorrected astigmatism on visual development, and will allow us to determine the appropriate timing for astigmatism screening and for spectacle prescribing for astigmatic children.
Conditions necessary for development of MA. Some studies reported MA in hyperopic astigmats,3,4,10 but others reported equally reduced acuity across stimulus orientation in hyperopic astigmats.22,25 Studies examining individual differences in patterns of accommodation in uncorrected astigmatism along with longitudinal data on refractive error might provide further insight into the varying results in the existing literature, and the conditions necessary for development of MA.
Effects of interocular differences in meridional blur on development. Much of the data summarized here are from members of a specific population in which high astigmatism, when it occurs, is almost always with-the-rule and essentially equal in amount and type of astigmatism between eyes. However, there are data that suggest that much could be learned from studies that might examine the effects of interocular differences in meridional blur on visual development in children with astigmatism. For example, Hirsch and Spinelli found that when the two eyes of a kitten had exposure to stimuli of different orientations (horizontal versus vertical), each eye could only activate cortical units with receptive fields of the stimulus orientation that it had experienced during development, whereas in a normally reared cat, receptive fields of all orientations could be activated by each eye.2 Abrahamsson and Sjöstrand found an increased risk for amblyopia in children with oblique astigmatism, most of whom had differences in axis between eyes of about 90 degrees (i.e., forming an A or V pattern) and suggested that the increased risk for amblyopia may be related to the dissimilarity of input to the two eyes.14
Methods of improving effectiveness of treatment of astigmatism-related amblyopia. Existing prospective studies indicate that optical correction results in reduction in amblyopia over time in astigmatic school-aged children,24,26,37 but even after a full year of optical correction, meridional differences in vision are not reduced and vision does not reach normal levels.24,26 These findings suggest that exploration of alternative or complementary treatments of astigmatism-related amblyopia and studies that more closely examine treatment compliance are warranted. For example, a common and effective treatment for unilateral amblyopia penalizes the fellow eye, forcing the amblyope to use the amblyopic eye. For meridional amblyopes, it is conceivable that optical penalization of the less amblyopic stimulus orientation could produce some improvement over time in BC vision for the more amblyopic orientation (K. Tarczy-Hornoch, personal communication, 2005). Another possible treatment for the residual astigmatism-related amblyopia that persists after a period of optical correction is perceptual learning. Recent studies have shown that perceptual learning, in which the ability to discriminate fine differences between visual stimuli improves a result of practice or experience, can improve visual performance in adult and child amblyopes.38 MA may prove to be an interesting model with which to examine the effects of targeting specific patterns of amblyopic deficits for treatment through perceptual learning.
Determining the most effective ages for successful prevention and optical treatment of astigmatism-related amblyopia. Little is known about treatment of astigmatism-related amblyopia prior to age three years, by which time astigmatism-related amblyopia has developed.9–11,22 Would earlier optical correction avert the development of astigmatism-related amblyopia, and result in better outcome than providing treatment once a child develops amblyopia?
CONCLUSIONS
In summary, the existing literature on development of astigmatism-related amblyopia has provided us with insight into how patterns of early visual input, often fairly subtle, are reflected in the developing visual brain. Studies of treatment of astigmatism-related amblyopia have allowed us to better understand how altering patterns of visual input through optical correction can result in changes in neural responsiveness to visual stimuli, and how this responsiveness varies across age and across different visual functions.
It has been 35 years since Mitchell, Freeman, and their colleagues first documented their cases of meridional amblyopia in astigmatic adults.3,4 Since then, several research groups have focused on the study of this phenomenon, and have provided valuable information about the effect of early astigmatism on visual development.5–14,21,22,25,34,35 As a result of recent collaboration with the Tohono O’odham Nation, the first large-scale cross-sectional and prospective studies of astigmatic children aimed at better understanding the nature and treatability of astigmatism-related amblyopia have been conducted.21–26 These studies have significantly increased our understanding of astigmatism-related amblyopia, but are limited to a specific population of individuals with bilateral with-the-rule astigmatism. Further research on astigmatism-related amblyopia can provide insight in terms of our basic understanding of normal development and capacity for plasticity across age, will allow development of treatment guidelines that are based on controlled clinical studies, and may lead to improvement in treatment success through the introduction and evaluation of new treatment options.
ACKNOWLEDGMENTS
This research was supported by grants from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD (EY11155 (Joseph M. Miller, MD) and EY13153 (EMH)), and Research to Prevent Blindness, New York, NY (Unrestricted grant to University of Arizona Department of Ophthalmology and Vision Science (Joseph M. Miller, MD), Career Development Award (EMH)).
The author thanks Velma Dobson, PhD, for her encouragement in preparing this manuscript and her valuable feedback and comments on earlier drafts.
REFERENCES
- 1.Blakemore C, Cooper GF. Development of the brain depends on the visual environment. Nature. 1970;228:477–478. doi: 10.1038/228477a0. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch HV, Spinelli DN. Visual experience modifies distribution of horizontally and vertically oriented receptive fields in cats. Science. 1970;168:869–871. doi: 10.1126/science.168.3933.869. [DOI] [PubMed] [Google Scholar]
- 3.Freeman RD, Mitchell DE, Millodot M. A neural effect of partial visual deprivation in humans. Science. 1972;175:1384–1386. doi: 10.1126/science.175.4028.1384. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell DE, Freeman RD, Millodot M, Haegerstrom G. Meridional amblyopia: evidence for modification of the human visual system by early visual experience. Vision Res. 1973;13:535–558. doi: 10.1016/0042-6989(73)90023-0. [DOI] [PubMed] [Google Scholar]
- 5.Mithcell DE, Wilkinson F. The effect of early astigmatism on the visual resolution of gratings. J Physiol. 1974;243:739–756. doi: 10.1113/jphysiol.1974.sp010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman RD. Asymmetries in human accomodation and visual experience. Vision Res. 1975;15:483–492. doi: 10.1016/0042-6989(75)90025-5. [DOI] [PubMed] [Google Scholar]
- 7.Freedman RD, Thibos LN. Contrast sensitivity in humans with abnormal visual experience. J Physiol. 1975;247:687–710. doi: 10.1113/jphysiol.1975.sp010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobb SR, MacDonald CF. Resolution acuity in astigmats: evidence for a critical period in the human visual system. Br J Physiol Opt. 1978;32:38–49. [PubMed] [Google Scholar]
- 9.Mohindra I, Jacobson SG, Held R. Binocular visual form deprivation in human infants. Doc Ophthalmol. 1983;55:237–249. doi: 10.1007/BF00140811. [DOI] [PubMed] [Google Scholar]
- 10.Gwiazda J, Bauer J, Thorn F, Held R. Meridional amblyopia does result from astigmatism in early childhood. Clin Vis Sci. 1986;1:145–152. [Google Scholar]
- 11.Atkinson J, Braddick O, Robier B, Anker S, Ehrlich D, King J, Watson P, Moore A. Two infant vision screening programmes: prediction and prevention of strabismus and amblyopia from photo- and videorefractive screening. Eye. 1996;10(Pt 2):189–198. doi: 10.1038/eye.1996.46. [DOI] [PubMed] [Google Scholar]
- 12.Abrahamsson M, Fabian G, Andersson AK, Sjostrand J. A longitudinal study of a population based sample of astigmatic children. I. Refraction and amblyopia. Acta Ophthalmol (Copenh) 1990;68:428–434. doi: 10.1111/j.1755-3768.1990.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 13.St John R. Contrast detection and orientation discrimination thresholds associated with meridional amblyopia. Vision Res. 1997;37:1451–1457. doi: 10.1016/s0042-6989(96)00290-8. [DOI] [PubMed] [Google Scholar]
- 14.Abrahamsson M, Sjostrand J. Astigmatic axis and amblyopia in childhood. Acta Ophthalmol Scand. 2003;81:33–37. doi: 10.1034/j.1600-0420.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 15.Kerschner RM, Brick DC. Prevalence of high corneal astigmatism in Papago school children. Invest Ophthalmol Vis Sci. 1984;25 Suppl.:217. [Google Scholar]
- 16.Tyszko RM, Dobson V, Miller JM, Harvey EM. Characteristics of astigmatism prevalent in a preschool population of Native American children. Invest Ophthalmol Vis Sci. 1998;39:S281. [Google Scholar]
- 17.Dobson V, Miller JM, Harvey EM. Corneal and refractive astigmatism in a sample of 3-to 5-year-old children with a high prevalence of astigmatism. Optom Vis Sci. 1999;76:855–860. doi: 10.1097/00006324-199912000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Dobson V, Miller JM, Harvey EM, Sherrill DL OSA Technical Digest Series. Vision Science and Its Applications 1999. Vol. 1. Washington, DC: Optical Society of America; 1999. Prevalence of astigmatism, astigmatic anisometropia, and glasses wearing among preschool- and school-age Native American children; pp. 177–180. [Google Scholar]
- 19.Miller JM, Dobson V, Harvey EM, Sherrill DL. Comparison of preschool vision screening methods in a population with a high prevalence of astigmatism. Invest Ophthalmol Vis Sci. 2001;42:917–924. [PubMed] [Google Scholar]
- 20.Harvey EM, Dobson V, Miller JM. Prevalence of high astigmatism, eyeglass wear, and poor visual acuity among Native American grade school children. Optom Vis Sci. 2006;83:206–212. doi: 10.1097/01.opx.0000214333.84822.71. [DOI] [PubMed] [Google Scholar]
- 21.Harvey EM. Visual development and plasticity in children. Dissertation Abstracts International. 2002;63(12b):6115. [Google Scholar]
- 22.Dobson V, Miller JM, Harvey EM, Mohan KM. Amblyopia in astigmatic preschool children. Vision Res. 2003;43:1081–1090. doi: 10.1016/s0042-6989(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 23.Harvey EM, Dobson V, Miller JM, Sherrill DL. Treatment of astigmatism-related amblyopia in 3-to 5-year-old children. Vision Res. 2004;44:1623–1634. doi: 10.1016/j.visres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Harvey EM, Dobson V, Clifford-Donaldson CE, Miller JM. Optical treatment of amblyopia in astigmatic children: the sensitive period for successful treatment. Ophthalmology. 2007;114:2293–2301. doi: 10.1016/j.ophtha.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Harvey EM, Dobson V, Miller JM, Clifford-Donaldson CE. Amblyopia in astigmatic children: patterns of deficits. Vision Res. 2007;47:315–326. doi: 10.1016/j.visres.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey EM, Dobson V, Miller JM, Clifford-Donaldson CE. Changes in visual function following optical treatment of astigmatism-related amblyopia. Vision Res. 2008;48:773–787. doi: 10.1016/j.visres.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvey EM, Miller JM, Dobson V, Tyszko R, Davis AL. Measurement of refractive error in Native American preschoolers: validity and reproducibility of autorefraction. Optom Vis Sci. 2000;77:140–149. doi: 10.1097/00006324-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Harvey EM, Dobson V, Miller JM, Clifford CE. Accommodation in uncorrected astigmatic children. Invest Ophthalmol Vis Sci. 2003;44 E-Abstract 2727. [Google Scholar]
- 29.Skoczenski AM, Norcia AM. Development of VEP Vernier acuity and grating acuity in human infants. Invest Ophthalmol Vis Sci. 1999;40:2411–2417. [PubMed] [Google Scholar]
- 30.Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proc Natl Acad Sci U S A. 1996;93:6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levi DM, Polat U, Hu YS. Improvement in Vernier acuity in adults with amblyopia. Practice makes better. Invest Ophthalmol Vis Sci. 1997;38:1493–1510. [PubMed] [Google Scholar]
- 32.Dobson V, Tyszko RM, Miller JM, Harvey EM OSA Technical Digest Series. Vision Science and its Applications 1996. Vol. 1. Washington, DC: Optical Society of America; 1996. Astigmatism, amblyopia, and visual disability among a Native American population; pp. 139–142. [Google Scholar]
- 33.Chen SI, Hove M, McCloskey CL, Kaye SB. The effect of monocularly and binocularly induced astigmatic blur on depth discrimination is orientation dependent. Optom Vis Sci. 2005;82:101–113. doi: 10.1097/01.opx.0000153251.39450.b4. [DOI] [PubMed] [Google Scholar]
- 34.Gwiazda J, Mohindra I, Brill S, Held R. Infant astigmatism and meridional amblyopia. Vision Res. 1985;25:1269–1276. doi: 10.1016/0042-6989(85)90042-2. [DOI] [PubMed] [Google Scholar]
- 35.Teller DY, Allen JL, Regal DM, Mayer DL. Astigmatism and acuity in two primate infants. Invest Ophthalmol Vis Sci. 1978;17:344–349. [PubMed] [Google Scholar]
- 36.Dobson V, Clifford-Donaldson CE, Green TK, Miller JM, Harvey EM. Optical treatment reduces amblyopia in astigmatic children who receive spectacles prior to kindergarten. Ophthalmology. 2009;116 doi: 10.1016/j.ophtha.2008.11.013. in press. [DOI] [PubMed] [Google Scholar]
- 37.Wallace DK, Chandler DL, Beck RW, Arnold RW, Bacal DA, Birch EE, Felius J, Frazier M, Holmes JM, Hoover D, Klimek DA, Lorenzana I, Quinn GE, Repka MX, Suh DW, Tamkins S. Treatment of bilateral refractive amblyopia in children three to less than 10 years of age. Am J Ophthalmol. 2007;144:487–496. doi: 10.1016/j.ajo.2007.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levi DM, Li RW. Improving the performance of the amblyopic visual system. Philos Trans R Soc Lond B Biol Sci. 2009;364:399–407. doi: 10.1098/rstb.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]