Introduction
Relapse is the most persistent and significant problem in tobacco treatment programs, yet significant gaps remain in understanding the factors that lead individuals to resume nicotine use after the initial quit attempt. Among the factors that may contribute to relapse are the cognitive consequences of insufficient sleep. Many drug users experience sleep disruptions when they are attempting to abstain, which may interfere with their ability to resist smoking. Insufficient sleep may make it more difficult to abstain through any of a number of mechanisms. It may increase irritability, or impair attention or cognition, change cravings, affect mood, or it may increase the value of cigarettes over other rewards. In this study we utilized a laboratory procedure to study the effects of overnight sleep deprivation (SD) on mood, craving, smoking the next day, and on measures of decision making, inhibition and attention. The study modeled the human relapse situation by testing 2-day abstinent smokers after a night of SD and after a night of normal sleep (NS).
Cognitive abilities such as attention, decision making, and executive functioning degrade significantly after extended periods of wakefulness (Chee and Choo, 2004; Thomas et al., 2000, 2003, Dinges et al., 1997; Drummond et al., 1999; e.g., Harrison et al., 2000; Nilsson et al., 2005). SD elevates the expectation of gains and diminishes the effects of one’s losses following risky decisions. Further, nucleus accumbens, an area in the brain involved with the anticipation of reward, becomes selectively more active under conditions of SD during high risk-high payoff choices (Venkatraman et al, 2008). In addition to increasing sensitivity to gain and insensitivity to loss, SD also has a pronounced effect on attention. It increases both frequency and duration of lapses in attention (Doran et al., 2001), and it delays behavioral responses to salient stimuli (Chee et al, 2008). These impairments in attention and judgment after sleep loss may impair the ability of smokers to refrain from smoking during an abstinence attempt.
Abstinence from smoking, even without SD, may also impair the ability to concentrate. In nicotine-dependent individuals, tobacco deprivation can impair attention and cognitive abilities within 12 hours of overnight smoking cessation (Bell et al. 1999; Gross et al. 1993; Lyvers et al. 1994), and these deficits can be reversed with nicotine administration (Bell et al. 1999; Parrott and Roberts 1991). Pettiford and colleagues (2007) reported that following a12-hour overnight smoking abstinence, smokers were less able to inhibit responses on an antisaccade task in which they were instructed to refrain from looking at a novel stimulus. Thus, acutely abstinent smokers are impaired on measures of attention and behavioral inhibition, and these effects may be exacerbated with combined sleep and smoking deprivation.
The combined effects of SD and abstinence could increase the likelihood of smoking through several mechanisms. It may increase smoking by impairing attention or inhibitory control. Alternatively it may increase smoking by increasing the value of cigarettes over other rewards, by inducing mood states that are associated with smoking or by inducing subjective states (e.g., fatigue) that are relieved by smoking. For example, fatigue may increase the probability of smoking or of relapse because smokers know, from previous experience, that nicotine counteracts the fatigue or difficulty concentrating. Alternatively, fatigue may increase smoking through another mechanism, such as lapses in attention leading to automatic cigarette smoking.
In the present study we began to investigate the susceptibility to increased smoking after SD, using a laboratory procedure measuring smoking, craving, mood and cognitive behaviors in abstinent smokers. We hypothesized that abstinent smokers would smoke more cigarettes after SD, and that this would be related to impairments in either attention or inhibitory control. We also examined the effects of SD on subjective craving for cigarettes and mood, and evaluated the relationship between cravings, mood states and cigarette vs money choices.
Materials and methods
Participant recruitment
Healthy adults aged 18–45, who smoked 10 or more cigarettes per day (n=14) were recruited from the university and surrounding community using posters, newspaper advertisements and word-of-mouth referrals. Candidates were initially screened by telephone, and eligible individuals were scheduled for an in-person screening interview. At the interview candidates completed the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), sleep and caffeine intake questionnaires, a psychiatric symptom checklist (SCL-90; Derogatis 1983), the Michigan Alcoholism Screening Test (MAST; Selzer 1971), and a detailed drug use questionnaire. Screening also included a semi-structured psychiatric interview based on the Structured Clinical Interview for DSM-IV Disorders (SCID; First et al. 1996), an electrocardiogram and a physical examination.
Inclusion criteria were: 1) smoking 10 or more cigarettes per day, 2) habitual sleep of 6 or more hours per night, and 3) habitual caffeine consumption not more than the equivalent of 2 cups of coffee per day (200 mg/day). Subjects were excluded if they had a current medical condition requiring medication, history of or current Axis I psychiatric diagnoses (DSM-IV-TR; APA 2000), cardiovascular disease, high or low blood pressure, an abnormal electrocardiogram, history of or current substance use disorder, lack of fluency in English and less than a high school diploma or equivalent. Individuals who had irregular sleep schedules, those who usually went to sleep after midnight, who claimed habitual total sleep time to be under 6 hours, or who worked a night shift were excluded from the study. Also, eligible participants had to report that they had stayed awake overnight at least once prior to participation in the study without experiencing severe mood or motor problems.
Experiment
Design
The study used a within subject design consisting of two sessions in which subjects underwent either overnight SD or NS after 48 hours of abstinence from smoking. We chose a 48-hour period of smoking abstinence to coincide with peak withdrawal symptoms (Hughes et al, 1994). Each session consisted of 3 days. On Days 1 and 2 of the sessions subjects abstained from smoking in their normal environments. On the night of Day 2 they either had regular 8 hours of sleep at home (NS condition), or they remained in the laboratory overnight without sleeping (SD condition). The order of NS and SD condition was randomized, and the two sessions were separated by one week. On Day 3, subjects participated in a 6-hour smoking or money choice procedure in the laboratory. In this procedure they had eight opportunities to either smoke a half-length cigarette of their regular brand or receive a variable number of tokens redeemable for money (see below). Subjects also rated their mood states and completed tasks measuring impulsivity, cognition and craving. The primary outcome measures were the number of half cigarettes chosen at each token value and the total number of cigarettes smoked. Secondary outcome measures were the subjects’ self-reported craving and mood, as well as their performance on behavioral and cognitive tasks, measured on Day 3. The procedures were approved by the Institutional Review Board at The University of Chicago.
Laboratory Environment
The study was conducted in the Human Behavioral Pharmacology Laboratory in the Department of Psychiatry at The University of Chicago Hospital. The environment resembled a living room, with upholstered chairs and sofas, incandescent lighting, tables with magazines, board games, and video entertainment units. During times when participants were not participating in tasks or completing questionnaires, they were allowed to relax, read magazines, play games or watch videos, but were not allowed to study or work.
Orientation session and cigarette value procedure
Subjects first participated in an orientation session during which they signed consent forms, reviewed study procedures, practiced tasks and completed questionnaires. They completed the Brief Questionnaire of Smoking Urges (Cox, 2001), and practiced Automated Neuropsychological Assessment Metrics (ANAM) (Reeves, Kane, & Winter, 1994) (a test of cognitive performance – see below for description) and the Stop Task (a test of behavioral inhibition – see below for description).
Within the first 24 hours of the first cigarette abstinence period, each subject completed a brief computerized questionnaire to estimate the monetary value of half a cigarette for that individual. In the questionnaire, they made successive choices between differing amounts of money and half cigarettes, providing a value at which the subject was equally likely to choose the half cigarette or the money. This individualized value was used to set the monetary value of 4 tokens in the subsequent testing sessions. On the testing sessions a half cigarette would “cost” 2, 4 or 8 tokens (4 tokens corresponded to the equivalent monetary value of a half cigarette).
Smoking Abstinence Periods
For each of the two sessions, subjects were instructed to stop smoking at 9 AM on Day 1 and remain abstinent until the end of the laboratory procedure on Day 3. They were required to come to the laboratory to confirm their abstinence at 5 PM on Day 1, 9 AM on Day 2 and either 5 PM (NS condition) or 9 PM (SD condition) on Day 2. During these brief stops to the laboratory, assessments included breath carbon monoxide (CO) level, urinary cotinine and illicit drug levels, breath alcohol level (BAL), nicotine cravings (see below) and mood. A CO reading over 6 ppm or an increase in cotinine level compared to any previous cotinine level resulted in dismissal from the study. Subjects were instructed not to take any medications, alcohol, marijuana, over-the-counter medications or their usual amount of caffeine throughout each three-day session.
Sleep Conditions
During the SD session subjects remained awake in the laboratory from 9 PM until morning with the lights on, and with constant monitoring by a research assistant. Subjects were allowed to watch movies and play board games, but they were not allowed to drink coffee or take other psychoactive drugs. Upon arrival to the laboratory at 9 PM, they completed initial questionnaires, impulsivity and attention tasks (see below), and they completed initial subjective and physiological tests. They were provided with meals after completing the tasks, a snack at midnight and breakfast at 8 AM in the morning. They also completed mood, sleepiness and craving questionnaires every two hours throughout the night, and at 8 AM they completed the impulsivity and attention tasks again.
During the NS condition, subjects were instructed to spend 8 hours in bed with the lights out, in their own homes. Their compliance with this instruction was monitored with activity monitors (Actiwatch TM, Cambridge Neurotechnology, United Kingdom). They reported to the laboratory at 10 AM for the Day 3 testing procedure. In order to be accepted for the six-hour procedure, the actimeter data had to show an eight hour mean sleep efficacy of at least 85%.
Day 3 Testing Procedure
On Day 3 subjects first completed baseline measures at 10 AM, and then began the choice procedure. Baseline measures included subjects’ self-ratings of mood, sleepiness and cravings for cigarettes, as well as their CO level, blood pressure and heart rate. They also completed ANAM (a test of cognition) and the Stop Task (a test of behavioral inhibition) at 10 AM. CO level, blood pressure, heart rate and sleepiness were measured every hour during the session. The choice procedure began at 11 AM. The choice procedure has been used in two previous studies (Young et al. 2005; Acheson et al, 2007) and provides a sensitive measure of drug preferences. The choice procedure consisted of 8 choices between cigarettes and money, provided at 30 minute intervals. Subjects were given a supply of tokens at the beginning of the session. On each choice opportunity, subjects were allowed to “buy” half a cigarette using tokens set to the individualized value for each subject. Across the 8 choice trials, subjects were offered a half cigarette or tokens valued at half (2 tokens), the same (4 tokens), or double (8 tokens) the monetary value that had been determined during the orientation session. On four of the half cigarette options, the choice was between a half cigarette and four tokens, on two options the choice was between a half cigarette and two tokens (“cheap” cigarette) and on the other two the choice was between a half cigarette and eight tokens (i.e., “expensive”cigarette). The order of the varying “prices” over the eight options was either: 4, 2, 8, 4, 4, 8, 2, 4, or 4, 8, 2, 4, 4, 2, 8, 4. These orders alternated across sessions to prevent participants from anticipating the upcoming half cigarette prices. The modified cigarette versus money choice procedure used in this study had several unique features. First, the monetary value of a single cigarette was individualized for each participant to take into account individual differences in the value of money. Participants varied in the amount they were willing to pay for a cigarette, from $0.05 to $2.50 (mean $0.47). Second, the “price” of the cigarettes was varied across choice opportunities within each session, providing a measure of the sensitivity of the procedure. That is, we were able to demonstrate that choices varied systematically with the “price” of the cigarette at low (2 token), medium (4 token) and higher prices (8 token). This manipulation also decreased the predictability of the choice options across the session, discouraging participants from planning how many cigarettes to choose ahead of time. At 12:30 PM subjects completed questionnaires of mood and tobacco cravings. At the end of the choice procedure, subjects again completed the Stop Task and ANAM, as well as mood and tobacco craving ratings.
Dependent Measures
Choice
The main measure of choice was the number of occasions on which subjects chose the half cigarettes over tokens, at each of the 3 token values. In addition, we assessed the total number of half cigarettes smoked on each session.
Cigarette Craving
Brief Questionnaire of Smoking Urges (Cox, 2001) consists of 10-questions. The answers are scored from 1 (strongly disagree) to 7 (strongly agree). These questions are the bases for the two factor-derived subscales, with Factor 1 subscale reflecting desire to smoke for stimulation, and Factor 2 subscale reflecting urge to smoke to relieve nicotine withdrawal.
Impulsivity
Stop Task (Logan et al. 1984) is designed to assess the subject’s ability to inhibit a prepotent response. Subjects are instructed to respond as quickly as possible when a specific letter (Go signal) is presented on a computer screen, and to inhibit (Stop) their responses when a tone is presented very soon after the Go signal. The tone is presented on random trials and at different delays following each letter presentation. The Stop Signal Delays (SSD) are varied systematically according to the subject’s performance: the delay to the tone is adjusted until the subject inhibits (Stops) his or her responses on approximately 50% of trials. After the SSD has been adjusted to this 50% criterion, the time required for the subject to stop the go response, the Stop Signal Reaction Time (Stop RT) can be determined. The Stop RT is calculated by subtracting the final mean SSD at which the tone is presented from the mean Go Reaction Time (Go RT). This is the primary dependent measure of this task. The Go RT, or latency to respond to the letter presentation, is a secondary dependent measure, a measure of simple reaction time. Both Go RTs and Stop RT are measured in milliseconds.
Lapses of Attention Measure (de Wit, 2008) utilizes a simple reaction time task from the Automated Neuropsychological Assessment Metrics (ANAM) to determine brief lapses in attention. Automated Neuropsychological Assessment Metrics were used to detect changes in cognitive function (knowing, thinking, learning) after drug administration (Reeves et al, 1994). Subjects are required to press a key as quickly as possible upon presentation of a symbol presented on the screen at variable intervals. From the distribution of reaction times, the mean deviation of the individual reaction times from the modal reaction time is calculated for each subject. The mean deviation from the mode is equivalent to the difference between the mean and the mode of a reaction time distribution, and reflects the proportion of reaction times that are unusually long.
Mood
Profile of Mood States (POMS) (McNair et al. 1971) is an adjective checklist that is sensitive to the effects of psychoactive drugs. We used a version of the POMS consisting of 72 adjectives commonly used to describe momentary mood states. Subjects indicate how they feel at the moment in relation to each of the 72 adjectives on a 5-point scale from “not at all” (0) to “extremely” (4). Eight clusters (scales) of items have been separated empirically using factor analysis (Anxiety, Depression, Anger, Vigor, Fatigue, Confusion, Friendliness, Elation). Two additional (non-validated) scales are derived from the other scales as follows: Arousal = (Anxiety + Vigor) − (Fatigue + Confusion) and Positive Mood = Elation − Depression.
Data Analysis
The primary outcome measure was choice of smoking versus money at each token value. Secondary outcomes included measures of self-reported craving (QSU), as well as performance on the Stop Task (Stop RT in msec), lapses of attention on the reaction time task (Deviation from the Mode) and mood. All measures were analyzed using repeated measures ANOVA with two levels of experimental condition (SD and NS) and, when appropriate, time (e.g., 10 AM and 3 PM for the tasks). In order to derive a summary measure for correlations, a baseline (10 AM) value difference value was calculated by subtracting NS from SD for each secondary measure including cognition, impulsivity, cravings and mood. In order to derive a single outcome measure for cigarette choice (the primary outcome measure) the total number of cigarettes smoked during the session following NS was subtracted from the total number of cigarettes smoked after the SD condition. This method allowed us to assess the relationship between the primary and secondary outcome measures.
RESULTS
Subject Demographics (Table 1)
Table 1.
Demographic characteristics and drug use histories of the 14 participants in the study.
| Ethnicity: Caucasian/African American | 12/2 |
| Gender: Male/female | 11/3 |
| Education: High school/college degree | 3/7 |
| Full Time Student | 4 |
| Age (mean +/− SEM) | 26 (±1.8) |
| Current Drug Use (mean +/− SEM) | |
| Caffeine (drinks/week) | 7.6 (±1) |
| Alcohol (drinks/week) | 8.5 (±1.3) |
| Nicotine (cigs/week) | 112 (±6) |
| Cannabis (cigs/month) | 1.1(±0.8) |
| FTND score (mean +/− SEM) | 3.9 (±0.6) |
| Token Value* (mean +/− SEM) | $0.47 (±0.2) |
| Range of token values | $0.05 to $2.50 |
Token values correspond to the monetary value determined for each subject to be equal to the value of one forth of a cigarette.
Table 1 provides a summary of subject demographics. The mean number of cigarettes subjects smoked per week was 112.5 and the range was 73–140. The mean FTND score was 3.9, indicating that, on average, subjects were low dependence smokers. During the initial value determination, subjects valued half a cigarette to be worth the mean value of 47 cents (range $0.05 to $2.50). Individualized token values were not related to FTND scores, the number of cigarettes smoked per day, or to scores on the Frugality questionnaire.
Effects of SD on cigarette choice (Figure 1)
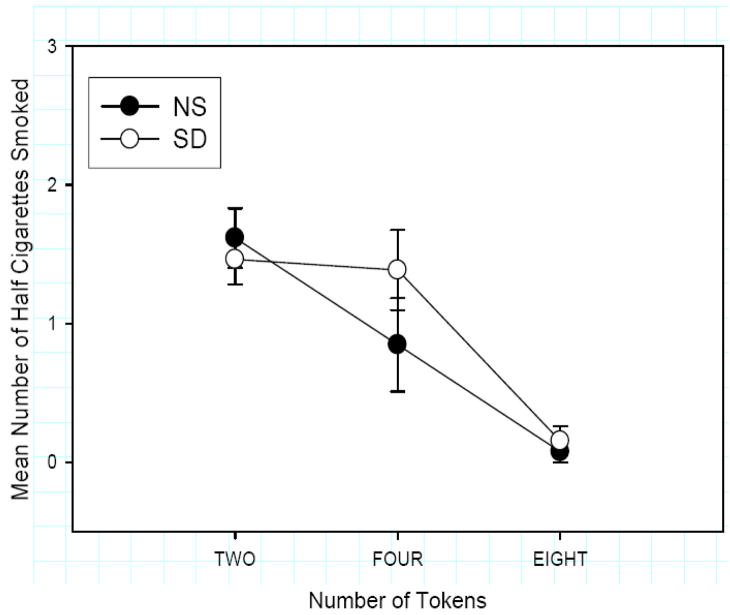
Figure 1.
Mean number (SEM) of half cigarettes smoked by token value. Subject chose more cigarettes following SD vs NS depending on the token value (Condition × Token Interaction F (1, 12) =3.4; p≤0.050).
One subject was excluded from the analysis because he never chose cigarettes on either session. The choice results for the remaining subjects (N=13) are presented in Fig 1. As expected, subjects’ choice of cigarettes varied systematically with the number of tokens available as the alternative (Main Effect of Token F(1, 12)=17.6 p≤0.001). Subjects chose the cigarette over tokens on 77% of the 2-token vs cigarette choices, 28% of the 4-token vs cigarette choices and 6% of the 8-token vs cigarette choices. This orderly relationship indicated that our pricing procedure (Young et al. 2005) is valid. Further, subjects smoked more cigarettes after sleep deprivation at the 4 token value, but not the 2 or 8 tokens (Condition x Token Interaction F (1, 12) =3.4; p=0.050). SD did not significantly increase the total number of cigarettes smoked, but the difference between total cigarettes smoked in the SD vs NS condition was positively correlated with FTND scores (r=.60, p<.25). Thus, subjects with higher FTND scores exhibited a greater increase in half cigarettes smoked during the SD compared to the NS condition.
Effect of SD on Cigarette Craving (Table 2 and Figure 2)
Table 2.
Mean (± SEM) Brief Questionnaire of Smoking Urges following SD and NS conditions. Factor 1 and Factor 2 subscales of Brief Questionnaire of Smoking Urges were not different between SD and NS conditions.
| Brief QSU Mean (±SEM) | Sleep Condition | |||||
|---|---|---|---|---|---|---|
| SD | NS | |||||
| 10:30 AM | 12:30 PM | 3:00 PM | 10:30 AM | 12:30 PM | 3:00 PM | |
| Factor 1 | 21.6 (±1.8) | 16.8 (±1.5) | 19.0 (±6.8) | 22.6 (±1.7) | 19.3 (±1.4) | 20.0 (±1.5) |
| Factor 2 | 12.0 (±2.3) | 6.5 (±1.5) | 7.3 (±1.4) | 12.1 (±2.4) | 8.3 (±1.5) | 8.0 (±1.7) |
Figure 2.
Correlation between SD-NS change in self-reported craving on Factor 1 and SD-NS change in total number of cigarettes smoked. Subjects who smoked more following SD reported more craving following SD.
SD did not significantly increase self-reported craving on either desire to smoke for stimulation (Factor 1) or urge to smoke to relieve nicotine withdrawal (Factor 2) on the QSU. Table 2 shows means for Factor 1 and Factor 2 subscales of Brief QSU at 10:30 AM (before the choice procedure), at 12:30 PM (during the choice procedure) and at 3:00 PM.
Even though SD did not significantly increase craving on the QSU, the difference in cigarette smoking between SD and NS was positively correlated with the SD-NS change in self-reported craving on Factor 1 (Figure 2; r=0.69; p=0.008). That is, subjects who exhibited the greatest increase in smoking after SD also reported greater craving after SD. There was no correlation between smoking and craving ratings on the Factor 2 subscale.
Effects of SD on impulsivity
Stop Task (Figure 3)
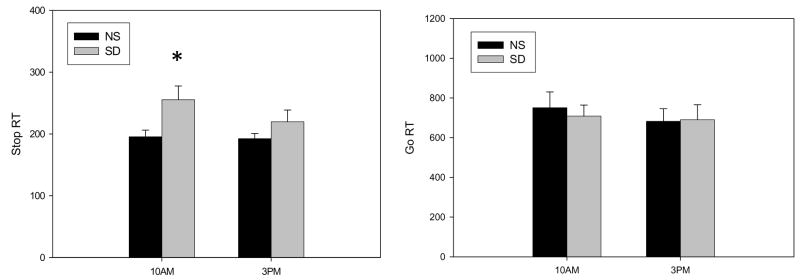
Figure 3.
The average Stop (left panel) and Go (right panel) Reaction Times (SEM) on the Stop Time Task at 10 am and 3 pm after the night if SD and NS. SD increased Stop Signal Reaction Time at 10 am in comparison to the NS condition. There was no statistically significant difference in the Go Reaction Times between SD and NS conditions.
Three subjects did not discriminate correctly between keyboard letters on the Stop Task and their data were discarded. In the remaining 11 subjects, SD significantly increased the measure of inhibition, i.e., Stop RT (Main effect of condition F(1,10) = 6.2, p =0.032; Figure 3). Post hoc comparisons revealed that Stop RT was significantly higher in the SD condition compared to the NS condition at 10 AM (p=0.022) (Mean = 248 ms compared with 188 ms). There was a marginal difference between Stop RTs following SD at 10 AM and 3 PM (p=0.052). The Go RTs (Figure 3) did not differ across the conditions at this time. Overall, across both conditions, Go RT’s were longer at the 10 AM measure compared to the 3 PM measure (Time F(1,10) = 6.3, p =0.031). Thus, SD specifically impaired response inhibition, without affecting simple reaction time. The increase in Stop RT from SD to NS was not related to the increase in total number of cigarettes smoked on these sessions. Hence, the change in response inhibition, measured by the Stop Task, was not related to the change in the cigarette choice between the two sessions.
Lapses of Attention (Table 3)
Table 3.
Performance (Mean±SEM) on the ANAM reaction time (RT) task at 10 AM and 3 PM the day after SD and NS. Deviation from the mode represents the mean deviation of each score from the modal value. The mean reaction time was significantly greater at 10 AM in the SD vs NS (**p≤0.001) and at 10 AM vs 3 PM in the SD condition (#p≤0.05). Deviation from the Mode was significantly greater at 10 AM following SD compared to the same time after NS (*p≤0.01), and was also significantly greater at 10 AM vs 3 PM in the SD condition (^p≤0.05).
| SLEEP CONDITION | RT (Mean±SEM) | Deviation from the Mode (Mean±SEM) | RT (Mean±SEM) | Deviation from the Mode (Mean±SEM) |
|---|---|---|---|---|
| 10:00 AM | 10:00 AM | 3:00 PM | 3:00 PM | |
| SD | 432(±25)** # | 116 (±23)* ^ | 344 (±14) | 56 (±9) |
| NS | 320 (±10) | 42 (±12) | 328 (±14) | 42 (±10) |
One subject’s Lapse of Attention data were lost. SD significantly increased the mean simple reaction time (Table 3; Main effect of condition F(1,12) = 11.7, p =0.005; Main Effect of time F(1,12) = 9.4, p =0.010; Condition x Time interaction F(1,12) = 16.6, p =0.002) and also deviation from the mode (Table 3; Main effect of condition F(1,12) = 6.83, p =0.023; Main Effect of time F(1,12) = 6.33, p =0.027; Condition x Time interaction F(1,12) = 7.06, p =0.021). Post hoc comparisons indicated that the Mean reaction time was significantly greater at 10 AM in the SD vs NS (p=0.001) and at 10 AM vs 3 PM in the SD condition (p≤0.003). Deviation from the Mode was also significantly greater at 10 AM following SD compared to the same time after NS (p=0.01), and was also significantly greater at 10 AM vs 3 PM in the SD condition (p=0.017). The SD vs NS differences in RT (Deviation from the mode or mean) were not correlated with the difference in total number of cigarettes smoked.
Effects of SD on mood
POMS
Mood assessment was taken at 10 AM before the choice procedure following either SD or NS. As expected, SD decreased ratings of Friendliness (F(1,13) = 12.3, p =0.004), Elation (F(1,13) = 13.4, p =0.003), Vigor (F(1,13) = 16.2, p =0.001), Arousal (F(1,13) = 51., p <0.001) and Positive mood (F(1,13) = 10.0, p =0.008). SD increased the rating of Confusion (F(1,13) = 25.7, p <0.001) and Fatigue (F(1,13) = 112.5, p <0.001). None of these effects of SD on mood (i.e., SD minus NS) were correlated with the difference in cigarette choice on SD vs NS.
CONCLUSION
In this paper, we report several interesting findings on the effects of SD in 48-hour abstinent smokers. First, subjects’ choice of cigarettes over money was dependent on the sleep condition: they chose more cigarettes after sleep deprivation. Second, subjects performed more poorly on measures of inhibition and attention after SD, and they reported the expected increases in subjective feelings of fatigue. However, there was no relationship between the increases in smoking and performance on the tasks or changes in mood states.
The main finding was that SD increased smoking, but this was not related to increases in impulsive behavior or attention. This raises the question of what processes did lead to the increase in smoking after a period of insufficient sleep. One possibility is that our measures of impulsivity and inhibition were not sufficiently sensitive to the effects of SD, and that SD increases smoking through its effects on an aspect of decision making that was not measured in this study. Another possibility is that SD in some way increases the reward value of a cigarette. For example, the reward value of smoking may increase if subjects expect that the cigarette will counter their feelings of sleepiness. Although mood states of fatigue were not related to cigarette choice, there was some support for this idea from the positive correlation between the Factor 1 scale of the QSU, reflecting the desire to smoke for stimulation, and cigarette choice. Subjects who scored higher on this scale on the SD compared to the NS condition were also more likely to smoke more in the choice procedure. Thus, SD may specifically increase the desire to smoke with the goal of reducing sleepiness. To the extent that desire to smoke is associated with smoking relapse (Killen and Fortman, 1997), this finding suggests that subjective sleepiness may increase the risk of relapse among abstinent smokers.
This study used a money-drug choice procedure (Young et al 2005; Acheson et al, 2007) in which the equivalent monetary value of a standard drink was individually determined for each participant. The individualized value ensures that the money-drug choice is comparable across individuals: an arbitrary amount of money that is fixed may be too high for some participants and too low for others. The results show that the procedure was sensitive to individual differences in monetary value and to changes in the experimental contingencies. Almost all of the subjects (13 of 14) chose cigarettes over money on at least one of the choice options, showing that the money was not always more valuable than the cigarettes. Further, subjects chose cigarettes significantly more often when the alternative choice was a small amount of money (2 tokens) and less when the alternative was greater (8 tokens). Finally, the effects of the SD manipulation appeared to be most pronounced on the token value (4 tokens) that was set to be equivalent across subjects. The 4-token value corresponded to the subjects’ initially-stated value of the half cigarette, and thus it is logical that this value was the most susceptible to change. The orderly pattern of choices in the choice procedure used here suggests that it provides an appropriate and sensitive indicator of the value of smoking.
Our finding that SD impaired performance on the measure of behavioral inhibition is inconsistent with in another recent study in which SD had no effect on Stop Task performance (Acheson et al, 2007). Stop RT was longer after overnight SD than after NS at 10 AM the following morning, while Go RT was unaffected. It is not clear why the results differ across studies, but one difference may be the smoking status of the subjects. Subjects in the present study smoked at least 10 cigarettes per day and had abstained from smoking for 48 hours at the time of testing while most subjects in the Acheson et al (2007) study were non-smokers. Smoking abstinence itself may impair inhibitory capacity (Powell et al, 2002; Pettiford et al, 2007). Notably, however, the observed impairment in behavioral inhibition in our study was not completely ameliorated by the period of cigarette smoking suggesting that the impaired Stop RT was related to SD rather than nicotine withdrawal combined with SD. Smoking and Stop RT were not correlated suggesting that impaired behavioral inhibition after insufficient sleep is not directly related to the ability to resist smoking.
In our study SD increased both mean simple reaction times and the deviations from the mode. Thus, consistent with previous studies using a variety of measures of attention (Acheson et al, 2007), SD impaired both cognitive processing and attention. We hypothesized that an impairment in attention may negatively impact the ability to abstain, because sustained attention is needed to continuously inhibit drug-taking responses. Momentary lapses in attention, caused by an environmental event such as SD, may make it more difficult to abstain from smoking. However, the present findings provide mixed support for this idea. Although SD increased smoking and impaired attention, the two measures were not correlated. Further studies are needed to evaluate psychological processes by which fatigue increases smoking. For example, smokers may increase their intake of nicotine to ameliorate their difficulties in concentrating during abstinence or they may smoke more because of lapses in attention that increase the likelihood of “automatic” or “unthinking” cigarette smoking.
Our study had several limitations. First, the sample was small, limiting our ability to detect subtle effects. There was a considerable variability across subjects in the number of cigarettes chosen and also in other measure of mood and behavior. Second, the ratio of men and women in our study was uneven, preventing us from evaluating possible sex differences in the effects of SD on smoking behavior. It is possible that men and women are differentially sensitive to the effects of SD, and it is also not known whether SD would differentially affect women at different phases of the menstrual cycle. Most importantly, the subjects included in this study may not have been heavy enough smokers to detect the SD effect. On average, subjects in this study were low in dependence (Moolchan et al, 2002). Their mean FTND scores were in the low range, and most of the subjects smoked less than a pack a day. Considering that we found a positive correlation between nicotine dependence and the size of the SD effect, it is possible that more robust effects of SD would be obtained in a more nicotine dependent sample. In addition, participants in the present study were screened for rigorous psychiatric, medical and demographic exclusion criteria, which limit the generalizability of the findings to many regular smokers. Although we intentionally chose smokers who were low caffeine consumers (less than 2 cups of coffee per day) to minimize the confounds of caffeine intake or withdrawal, it is nonetheless possible that caffeine use or abstinence may have affected sleep and cigarette smoking.
In the present pilot study we used a laboratory procedure to assess how SD affects smoking and other potentially related behaviors in abstinent smokers. In cigarette smokers, SD impaired response inhibition, increased both mean reaction time as well as lapses in attention, impaired mood and increased choice of smoking over money. However, the increase in smoking following SD was not related to increased impulsivity, deterioration in attention or mood after SD. Our results suggest that the subjects sought the stimulant effects of nicotine to counter the sleepiness induced by SD. It remains to be determined whether this effect of SD is specific to tobacco and nicotine, or whether similar findings would be observed with other drugs of abuse after SD. These questions will require additional studies.
Acknowledgments
We would like to thank Anna Asiama, Nicholas Van Dam and Christy Casnar for their excellent technical assistance. This research was supported by T32 DA007255, F32 DA024920-01, DAO2812 and DA09133.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Richards JB, de Wit H. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol Behav. 2007;91(5):579–87. doi: 10.1016/j.physbeh.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicot Tobac Res. 1999;1:45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total Sleep Deprivation. J Neuroscience. 2004;24:4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, Dinges DF. Lapsing during Sleep Deprivation Is Associated with Distributed Changes in Brain Activation. The Journal of Neuroscience. 2008;28:5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- de Wit H. Addiction Biology. [Published Online October, 13 2008]. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. The Symptom Checklist-90-revised. Minneapolis, MN: NCS Assessments; 1992. [Google Scholar]
- Derogatis LR, LipPMann RS, Covi L. SCL-90: an outpatient psychiatric rating scale-preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- Dinges DF, Pack F, WilliAs K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10:3745–3748. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for the DSM-IV Axis I Disorders. 1996. [DOI] [PubMed] [Google Scholar]
- Gross TM, Jarvik ME, Rosenblatt MR. Nicotine abstinence produces content-specific Stroop interference. Psychopharmacology (Berl) 1993;110:333–336. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of Sleep Deprivation in young adults – a model for healthy aging? Sleep. 2000;23:1067–1073. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K. The Fagerström Test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. Brit J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortman SP. Craving is associated with smoking relapse: Findings from three prospective studies. Exp Clin Psychopharmacol. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Lastovicka J, Bettencourt LA, Hughner R. Lifestyle of the Tight and Frugal: Theory and Measurement. The Journal of Consumer Research. 1999;26(1):85–98. [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- Lyvers M, Maltzman I, Miyata Y. Effects of cigarette smoking and smoking deprivation on Wisconsin Card Sorting test performance. Exp Clin Psychopharm. 1994;2:283–289. [Google Scholar]
- McNair DM, Loor M, Dropplemann LF. Profile of Mood States. San Diego: Educational and Testing Service; 1971. [Google Scholar]
- Nilsson JP, Söderström M, Karlsson AU, Lekander M, Akerstedt T, Lindroth NE, Axelsson J. Less effective executive functioning after one night’ Sleep Deprivation. J Sleep Res. 2005;14:1–6. doi: 10.1111/j.1365-2869.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Roberts G. Smoking deprivation and cigarette reinstatement: effects upon visual attention. J Psychopharmacol. 1991;5:404–409. doi: 10.1177/026988119100500435. [DOI] [PubMed] [Google Scholar]
- Pettiford J, Kozink RV, Lutz AM, Kollins SH, Rose JE, McClernon FJ. Increases in impulsivity following smoking abstinence are related to baseline nicotine intake and boredom susceptibility. Addictive Behaviors. 2007;32:2351–2357. doi: 10.1016/j.addbeh.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, Higgins S, Bickel W. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol Psychiatry. 2002;51(2):151–163. doi: 10.1016/s0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6(2):327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of Sleep Deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Sing HC, Belenky G, Holcomb HH, Mayberg HS, Dannals RF, Wagner HN, Thorne DR, Popp KA, Rowland LM, Welsh AB, Balwinski SM, Redmond DP. Neural basis of alertness and cognitive performance impairments during sleepiness II. Effects of 48 and 72 h of Sleep Deprivation on waking human regional brain activity. Thalamus Related Systems. 2003;2:199–229. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007 May 1;30(5):603–9. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- Young EM, Mahler S, Chi H, de Wit H. Mecamylamine and ethanol preference in healthy volunteers. Alcohol Clin Exp Res. 2005;29:58–65. doi: 10.1097/01.alc.0000150007.34702.16. [DOI] [PubMed] [Google Scholar]